Abstract
Purpose
To determine if local recurrence of prostate cancer after radiation therapy occurs at the same site as the primary tumor before treatment, using longitudinal MR imaging and MR spectroscopic imaging to assess dominant tumor location.
Materials and Methods
This retrospective study was HIPAA compliant and approved by our Committee on Human Research. We identified all patients in our institutional prostate cancer database (1996 onwards) who underwent endorectal MR imaging and MR spectroscopic imaging before radiotherapy for biopsy-proven prostate cancer and again at least 2 years after radiotherapy (n = 124). Two radiologists recorded the presence, location, and size of unequivocal dominant tumor on pre- and post-radiotherapy scans. Recurrent tumor was considered to be at the same location as the baseline tumor if at least 50% of the tumor location overlapped. Clinical and biopsy data was collected on all patients.
Results
Nine patients had unequivocal dominant tumor on both pre- and post-radiotherapy imaging, with mean pre- and post-radiotherapy dominant tumor diameter of 1.8 cm (range, 1 to 2.2) and 1.9 cm (range, 1.4 to 2.6), respectively. The median follow-up interval was 7.3 years (range, 2.7 to 10.8). Dominant recurrent tumor was at the same location as dominant baseline tumor in 8 of the 9 patients (89%).
Conclusion
Local recurrence of prostate cancer after radiation usually occurs at the same site as the dominant primary tumor at baseline, suggesting supplementary focal therapy aimed at enhancing local tumor control would be a rational addition to management.
Keywords: prostate cancer, cancer recurrence, radiotherapy, MR imaging, MR spectroscopic imaging
Introduction
Prostate cancer killed an estimated 32,050 American men in 2010, second only to lung cancer as a cause of cancer deaths [1]. That said, many men die with prostate cancer rather than of it, and the major challenge in the management of this malignancy is stratifying patients with aggressive cancer who need definitive treatment from those with indolent disease who may be candidates for active surveillance. The decision to treat is not trivial. Standard definitive treatment by radical prostatectomy or radiation therapy targets the whole gland and carries substantial risks of incontinence and impotence because of damage to adjacent structures. In recent years, focal therapy has been suggested as providing a middle ground between the definitive but morbid options of radical prostatectomy or radiation and the minimalist approach of active surveillance [2–6]. The recent emergence of endorectal MR-guided focused ultrasound surgery as an investigational method of prostate cancer ablation provides a novel and exciting approach to focal therapy that promises precisely targeted tissue necrosis with real-time monitoring by MR thermometry [7]. The use of focal therapy as the primary treatment modality for prostate cancer has generated considerable discussion and controversy, primarily related to patient selection and the choice of ablative technique. In addition to being a potential primary treatment modality, focal therapy can also be used to supplement standard treatment. Examples include dose escalation at the site of dominant primary tumor during intensity modulated external beam radiation therapy or brachytherapy [8–11]. However, focal therapy of prostate cancer, either as an isolated or supplementary treatment, only makes sense if cancer recurs or persists within the prostate in the same place as the initial dominant cancer subjected to focal treatment. The rationale for focal therapy collapses if tumor recurs at a different site within the prostate. However, very few studies have examined the relationship between dominant tumor site at baseline and at recurrence [12, 13]. Combined endorectal MR imaging and MR spectroscopic imaging has become a well established and accurate method for the localization of dominant tumor within the prostate, both before and after radiation therapy [14–17]. Therefore, we undertook this study to determine if local recurrence of prostate cancer after radiation therapy occurs at the site as primary tumor before treatment, using longitudinal MR imaging and MR spectroscopic imaging to assess the dominant tumor location at baseline and recurrence.
Materials and methods
Subjects
This was a retrospective single institutional study approved by our institutional Committee on Human Research with waiver of the requirement for informed consent. The study was compliant with the Health Insurance Portability and Accountability Act. We performed a computerized search of our institutional prostate cancer patient database and identified all patients who underwent baseline endorectal MR imaging and MR spectroscopic imaging of the prostate for biopsy-proven cancer prior to radiotherapy between 1996 and 2006 and again at least two years after radiotherapy (n = 124).
Imaging technique
MR studies were performed on a 1.5-Tesla whole body MR scanner (Signa; GE Medical Systems, Milwaukee, WI). Patients were scanned in a supine position using the body coil for excitation and a pelvic phased array coil (GE Medical Systems, Milwaukee, WI) in combination with a commercially available balloon-covered expandable endorectal coil (Medrad, Pittsburgh, PA) for signal reception. MR imaging sequences acquired included thin-section high spatial resolution axial and coronal T2-weighted fast spin-echo images of the prostate and seminal vesicles with the following parameters: TR/effective TE 5000/96 ms, echo train length = 16, slice thickness = 3 mm, interslice gap = 0 mm, field of view = 14 cm, matrix 256 × 192, anteroposterior frequency encoding (to prevent obscuration of the prostate by endorectal coil motion artifact), and 3 excitations. After review of the axial T2-weighted images, an MR spectroscopic imaging volume was selected to maximize coverage of the prostate and minimize the inclusion of periprostatic fat and rectal air. Three-dimensional MR spectroscopic imaging data were acquired using a water and lipid suppressed double-spin echo Point-Resolved Spectroscopy (PRESS) sequence that utilized spectral spatial pulses for the two 180° excitation pulses. The spectral-spatial pulses allowed for sharp volume selection as well as frequency selection in order to reduce the water resonance and suppress lipid resonances. Data sets were acquired as 16 × 8 × 8 phase encoded spectral arrays (1024 voxels with a spatial resolution of 0.24–0.34 cc), TR/TE 1000/130 ms, and a 17-minute acquisition time. MR spectroscopic imaging data were overlaid on the corresponding axial T2-weighted images, including the raw spectra and the choline to creatine ratio and the choline plus creatine to citrate ratio. The total examination time was 1 hour, including coil placement and patient positioning.
Image interpretation
Two radiologists (FVC and ACW), with 15 and 6 years of experience, respectively, in the interpretation of MR imaging and MR spectroscopic imaging of the prostate, reviewed all images at a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium). Readers were aware that patients had biopsy-proven prostate cancer treated with radiotherapy, but were unaware of all other clinical and histopathologic findings. Readers initially reviewed the post-radiotherapy MR images, and, by consensus, recorded the presence, location, and size of unequivocal dominant recurrent tumor. After a 4 week “wash-out” interval, readers reviewed the pre-radiotherapy MR images of those patients with unequivocal dominant recurrent tumor identified on the initial dataset review (n = 17) and, by consensus, recorded the presence, location, and size of unequivocal dominant baseline tumor. Readers did not review the baseline scans of patients without identifiable recurrent tumor, since the primary purpose of our study was to compare location for those patients with dominant tumor on both pre- and post-radiotherapy imaging. We opted to review the post-radiotherapy scans first, on the assumption that less patients would have clear-cut tumor after radiation than before. Readers only reviewed the post or pre radiotherapy scans during the first and second review sessions, respectively, so that the findings on the post or pre radiotherapy scans did not influence interpretation of the companion study. Unequivocal dominant tumor was considered present if there was a clear-cut measureable mass on T2-weighted MR imaging with gross metabolic abnormality at MR spectroscopic imaging [18–20]. Gross metabolic abnormality was defined as a choline peak of greater magnitude than the citrate peak at baseline, and as isolated choline elevation after radiotherapy [21–28]. We used this stringent definition of dominant tumor so that tumor was identified and located with a high level of confidence. Dominant tumor location was recorded as present or absent in each of eight standard regions (right base, left base, right midgland, left midgland, right apex, left apex, right central gland, and left central gland). Tumor size at T2-weighted MR imaging was recorded as the maximal axial diameter of the tumor mass. Tumor volume was recorded at MR spectroscopic imaging by multiplying the number of spectral voxels with unequivocal malignant metabolism by the volume of the voxel.
Data and Statistical Analysis
Based on the imaging interpretation described above, 17 patients had unequivocal dominant recurrent tumor on post-radiotherapy imaging, and 9 of these patients had unequivocal dominant tumor at baseline pre-radiotherapy imaging. The principal investigator (--) reviewed all available medical records and recorded pertinent clinical, imaging, and histopathological data on the 9 patients with unequivocal dominant tumor on both pre- and post-radiotherapy imaging. Recurrent tumor was considered to be at the same location as the baseline tumor if at least 50% of the tumor location overlapped, based on the presence or absence of tumor in the eight regions described above. That is, if a tumor was present in four regions on post-radiotherapy imaging, then at least two of those regions had to be tumor-containing at baseline for recurrence to be considered as occurring in the same location as baseline tumor. Statistical analysis was performed using commercially available software (Microsoft Excel, Redmond, WA). Proportions were calculated for the number of patients with local recurrence in the same location, the regions of the prostate most frequently containing tumor, and the frequency of cancer per region in pre- and post-radiotherapy images.
Results
Nine patients had unequivocal dominant tumor on both pre- and post-radiotherapy imaging. The mean baseline patient age was 67 years (range, 55–74). All patients underwent external beam radiotherapy with a mean dose of 75.9 Gy (range, 74–79). Six patients received adjuvant hormonal therapy after external beam radiotherapy. The median follow-up interval was 7.25 years (range, 2.7 to 10.8). Dominant recurrent tumor was at the same location as dominant baseline tumor in 8 of the 9 patients (89%). Additional clinical and demographic details for these 9 patients are shown in Table 1. A representative example is illustrated in Figures 1. The one patient who recurred at a site distant to the dominant tumor identified at baseline is shown in Figure 2.
Table 1.
Clinical and demographic details of nine patients with unequivocal dominant tumor on both pre- and post- radiotherapy imaging.
| # | Age at baseline (years) | Baseline Gleason score, serum prostate specific antigen level, and clinical stage | Baseline T2 diameter (cm) | Baseline MR spectral tumor volume (cm3) | Radiation dose (Gy) | Follow-up interval (years) | Post- treatment serum prostate specific antigen level | Recurrent T2 diameter (cm) | Recurrent MR spectral tumor volume (cm3) | Post- treatment biopsy | Baseline and recurrent tumor at same location |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | 3+3, 9.1, N/A | 2.1 | 1.44 | N/A | 2.7 | 4.1 | 2.2 | 2.7 | Positive | Yes |
| 2 | 58 | N/A, 6.5, T3 | 2.1 | 5.95 | 79 | 3.9 | 0.6 | 1.6 | 3.36 | N/A | Yes |
| 3 | 67 | N/A | 2.1 | 1.87 | 75.6 | 10.8 | 0.1 | 1.6 | 0.8 | N/A | Yes |
| 4 | 69 | 5+4, N/A, N/A | 1.5 | 1.92 | 74 | 3.4 | N/A | 1.6 | 4.59 | Positive | Yes |
| 5 | 72 | 3+4, 8.9, N/A | 2 | 0.96 | 76 | 7.2 | 10.1 | 2.4 | 1.68 | Positive | Yes |
| 6 | 55 | 3+3, 10.5, T3B | 2.2 | 1.87 | 75.6 | 7.8 | 10.3 | 1.9 | 2.56 | N/A | Yes |
| 7 | 67 | 4+3, 12.7, T3A | 1.4 | 1.53 | 77 | 7.6 | 2.3 | 1.4 | 1.19 | Positive | Yes |
| 8 | 67 | 4+3, 6.8, T3A | 1.6 | 1.7 | 74 | 7.3 | 1.2 | 1.5 | 0.63 | Positive | No |
| 9 | 74 | 4+3, 3.4, T2A | 1 | 0.45 | N/A | 7.9 | 3.6 | 2.6 | 2.87 | Positive | Yes |
Figure 1.
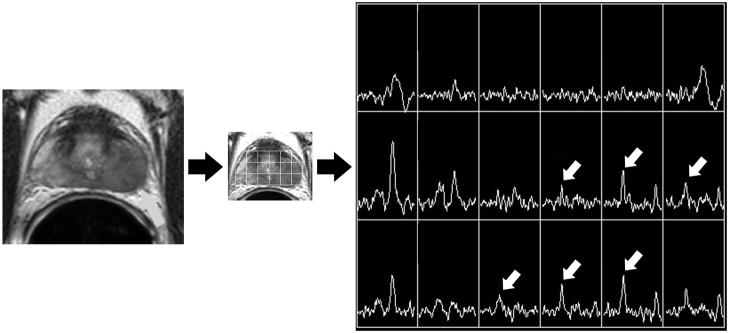
Figure 1A. Photomontage of axial T2-weighted MR image, overlaid MR spectral grid, and corresponding MR spectra array in a 58 year old with recently diagnosed prostate cancer. A large mass-like focus (grey arrow) of reduced T2 signal intensity in the peripheral zone of the left mid-gland is associated with multiple voxels demonstrating high choline peaks (white arrows). This was considered the dominant tumor location.
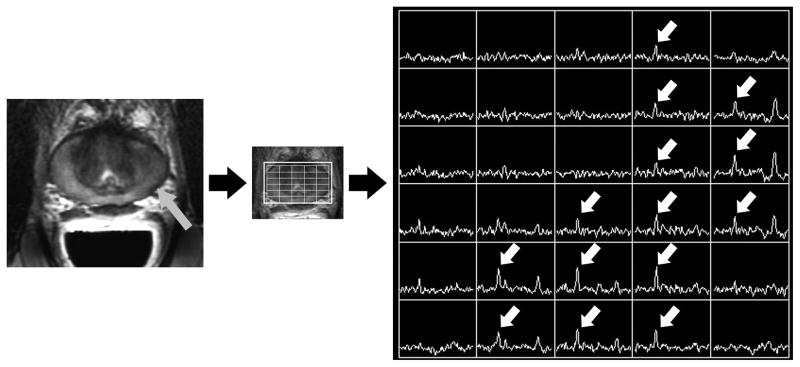
Figure 1B. Photomontage of axial T2-weighted MR image, overlaid MR spectral grid, and corresponding MR spectra array obtained 4 years later, after treatment with external beam radiotherapy. Reduced T2 signal intensity (grey arrow) in the peripheral zone of the left mid-gland is associated with multiple voxels demonstrating high choline peaks (white arrows). This was considered the dominant recurrent tumor location, and matches the dominant location seen at baseline imaging.
Figure 2.
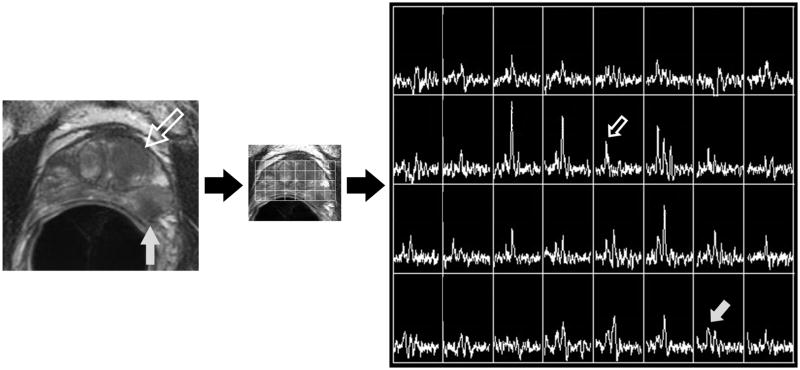
Figure 2A. Photomontage of axial T2-weighted MR image, overlaid MR spectral grid, and corresponding MR spectra array in a 67 year old with recently diagnosed prostate cancer. A large mass-like focus (large hollow white arrow) of reduced T2 signal intensity in the left central gland is associated with isolated choline elevation (small hollow white arrow white arrow). Readers considered this the dominant tumor location. A second smaller focus (large grey arrow) of reduced T2 signal intensity in the peripheral zone of the left mid-gland associated with relative choline elevation (small grey arrow) was not considered the dominant tumor location.
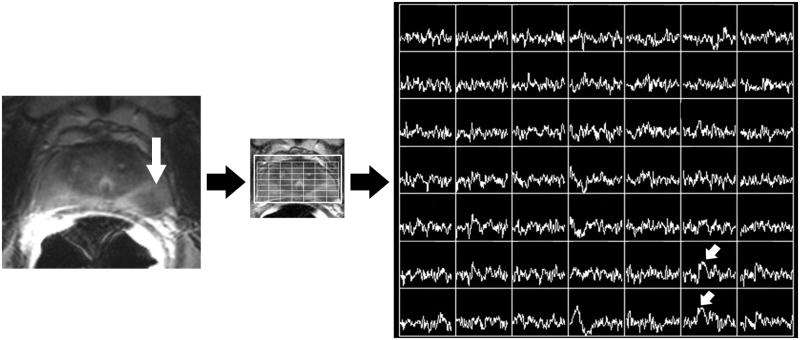
Figure 2B. Photomontage of axial T2-weighted MR image, overlaid MR spectral grid, and corresponding MR spectra array obtained 7 years later, after treatment with external beam radiotherapy. Reduced T2 signal intensity (large white arrow) in the peripheral zone of the left mid-gland is associated with high choline peaks (small white arrows). This was considered the dominant recurrent tumor location. This does not match the dominant location seen at baseline imaging, but in retrospect does correspond to the secondary tumor focus seen in Figure 2A.
Discussion
To our knowledge, this is the first study to confirm with both MR imaging and MR spectroscopic imaging that post-radiation recurrence of prostate cancer within the prostate gland usually occurs at the same site as the primary tumor. This result has important implications for focal therapy, since it would support supplementary focal treatment aimed at the dominant primary tumor at baseline in order to enhance tumor control. Such focal therapy could be delivered using radiation dose escalation with conformal been radiotherapy or modifications of brachytherapy seed distribution or with focal ablative techniques such as cryosurgery, focused ultrasound surgery, or radio-frequency ablation. All these approaches are based on the assumption that enhanced treatment of the dominant primary tumor will improve local tumor control, and while this assumption makes intuitive sense, it has not been previously validated using the advanced metabolic imaging capabilities of MR spectroscopic imaging.
Two prior studies have reported the use of imaging to evaluate the location of tumor recurrence within the prostate after radiation. Cellini et al. found that local recurrence of prostate cancer after radiation corresponded to the site of primary tumor in 12 of 12 patients using transrectal ultrasound and MR imaging for tumor localization. The imaging modality for each of these 12 patients was not presented, the definition of tumor was not explicitly stated, and the definition of what constituted “the same location” was also not described. Pucar et al. found that local recurrence of prostate cancer after radiation therapy occurred at the site of primary tumor based on pre-and post radiation MR imaging in 9 of 9 patients, as confirmed by salvage prostatectomy findings. The correlation with whole-mount salvage prostatectomy pathology is a particular strength of this latter study. However, it is noteworthy that imaging findings were evaluated by a single reader and it is not mentioned in the publication if this reader had a “washout” interval between evaluating the pre-and post radiation studies. If not, it is possible that identification of obvious tumor on the pre-radiation study unduly influenced the localization of tumor on the post radiation studies. Examination of the very subtle tumors identified after radiation in the published figures would suggest this may have been a confounding factor. We believe our more rigorous methodology, including a strict definition of what we considered tumor and how we defined the same location, means that our results may be more representative of the histopathological truth. For example, we found 1 patient who recurred within the prostate outside the site of dominant primary tumor, even though the prior investigators did not report this in any of their patients. In addition, MR spectroscopic imaging was not included in either study, and our mean follow-up interval of 87 months is longer than either of the prior studies with mean follow-up intervals of 45 and 54.5 months.
Our study has several limitations. First, it was a single institutional retrospective study, with all of the inherent potential for bias. Second, because we only included patients with gross and unequivocal tumor on both pre-and post radiation MR imaging and MR spectroscopic imaging. As such, it is likely that our final study population of 9 patients was skewed to those with bigger and more aggressive disease, which might be more radioresistant and likely to recur or persist at the same site as the primary tumor. Be that as it may, the clinical implication of our study would appear unchanged, since these are the same tumors that would likely benefit from additional focal therapy. Third, our study lacks histological proof of tumor location at baseline or recurrence. Of course, the ideal reference standard of radical prostatectomy histopathology would be unavailable for baseline tumor localization in a study of patients undergoing radiation therapy. We also opted not to use transrectal ultrasound biopsy results for tumor localization, since these are notoriously inaccurate [29]. Our definition and localization of tumor on imaging is also supported by other similar studies [13,14,27,29–34]. While imperfect, we believe concordant MR imaging and MR spectroscopic imaging, particularly when confined to gross and unequivocal abnormalities, represents a reasonable standard of reference in the setting of pre-and post radiation tumor localization.
In conclusion, local recurrence of prostate cancer after radiation usually occurs at the same site as the dominant primary tumor at baseline, suggesting supplementary focal therapy aimed at enhancing local tumor control would be a rational addition to management.
Acknowledgments
Work supported by NIH/NIBIB T32 Training Grant T32 EB001631 (EA), NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 (ACW) and NIH Grant Number 1S10RR028911-01 (FVC). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
There is no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. p. 23. [Google Scholar]
- 2.Pouliot J, Kim Y, Lessard E, et al. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2004;59:1196–1207. doi: 10.1016/j.ijrobp.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 3.van Lin EN, Futterer JJ, Heijmink SW, et al. IMRT boost dose planning on dominant intraprostatic lesions: Gold marker–based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys. 2006;65:291–303. doi: 10.1016/j.ijrobp.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky MJ, Cohen G, Zakian KL, et al. Intraoperative conformal optimization for transperineal prostate implantation using magnetic resonance spectroscopic imaging. Cancer J. 2000;6:249–255. [PubMed] [Google Scholar]
- 5.Xia P, Pickett B, Vigneault E, et al. Forward or inversely planned segmental multileaf collimator IMRT and sequential tomotherapy to treat multiple dominant intraprostatic lesions of prostate cancer to 90 Gy. Int J Radiat Oncol Biol Phys. 2001;51:244–254. doi: 10.1016/s0360-3016(01)01643-1. [DOI] [PubMed] [Google Scholar]
- 6.Cellini N, Morganti AG, Mattiucci GC, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 7.Pucar D, Hricak H, Shukla-Dave A, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69:62–9. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 8.Mueller-Lisse UG, Vigneron DB, Hricak H, et al. Localized prostate cancer: effect of hormone deprivation therapy measured by using combined three-dimensional H-1 MR spectroscopy and MR imaging— clinicopathologic case-controlled study. Radiology. 2001;221:380–390. doi: 10.1148/radiol.2211001582. [DOI] [PubMed] [Google Scholar]
- 9.Parivar F, Hricak H, Shinohara K, et al. Detection of locally recurrent prostate cancer after cryosurgery: evaluation by transrectal ultrasound, magnetic resonance imaging, and three-dimensional proton magnetic resonance spectroscopy. Urology. 1996;48:594–599. doi: 10.1016/S0090-4295(96)00250-6. [DOI] [PubMed] [Google Scholar]
- 10.Parivar F, Kurhanewicz J. Detection of re- current prostate cancer after cryosurgery. Curr Opin Urol. 1998;8:83–86. doi: 10.1097/00042307-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Coakley FV, Teh HS, Qayyum A, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy (Preliminary experience) Radiology. 2004;233:441–448. doi: 10.1148/radiol.2332032086. [DOI] [PubMed] [Google Scholar]
- 12.Menard C, Smith IC, Somorjai RL, et al. Magnetic resonance spectroscopy of the malignant prostate gland after radiotherapy: A histopathologic study of diagnostic validity. Int J Radiat Oncol Biol Phys. 2001;50:317–323. doi: 10.1016/s0360-3016(01)01480-8. [DOI] [PubMed] [Google Scholar]
- 13.Wefer AE, Hricak H, Vigneron DB, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000;164:400–404. [PubMed] [Google Scholar]
- 14.Murat FJ, Poissonnier L, Gelet A, et al. Recurrent prostate cancer after radiotherapy – salvage treatment by high intensity focused ultrasound. Eur Oncol Dis. 2006;1:64–6. [Google Scholar]
- 15.Kara T, Akata D, Akyol F, et al. The value of dynamic contrast-enhanced MRI in the detection of recurrent prostate cancer after external beam radiotherapy: correlation with transrectal ultrasound and pathological findings. Diagn Interv Radiol. 2011 Mar;17(1):38–43. doi: 10.4261/1305-3825.DIR.3079-09.1. [DOI] [PubMed] [Google Scholar]
- 16.Pucar D, Shukla-Dave A, Hricak H, et al. Prostate cancer: correlation of MR imaging and MR spectroscopy with pathologic ndings after radiation therapy-initial experience. Radiology. 2005;236:545–53. doi: 10.1148/radiol.2362040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haider MA, Chung P, Sweet J, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:425–430. doi: 10.1016/j.ijrobp.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Westphalen AC, Kurhanewicz J, Cunha RM, et al. T2-weighted endorectal magnetic resonance imaging of prostate cancer after external beam radiation therapy. Int Braz J Urol. 2009;35:171–180. doi: 10.1590/s1677-55382009000200007. discussion 181–182. [DOI] [PMC free article] [PubMed] [Google Scholar]