Abstract
To examine the role of limb posture on vascular conductance during rapid changes in vascular transmural pressure, we determined brachial (n = 10) and femoral (n = 10) artery post-occlusive reactive hyperemic blood flow (RHBF, ultrasound/Doppler) and vascular conductance in healthy humans with each limb at three different positions – horizontal, up and down. Limb posture was varied by raising or lowering the arm or leg from the horizontal position by 45°. In both limbs, peak RHBF and vascular conductance was highest in the down or horizontal position and lowest in the up position (arm up 338 ± 38, supine 430 ± 52, down 415 ± 52 ml/min, P < 0.05; leg up 1208 ± 88, supine 1579 ± 130, down 1767 ± 149 ml/min, P < 0.05). In contrast, the maximal dynamic fall in blood flow following peak RHBF (in ml/s/s) in both limbs was highest in the limb down position and lowest with the limb elevated (P < 0.05). These data suggest that the magnitude and temporal pattern of limb reactive hyperemia is in part related to changes in vascular transmural pressure and independent of systemic blood pressure and sympathetic control.
Keywords: blood flow, vascular conductance, arm/leg reactive hyperemia, posture
Introduction
Blood vessels respond to increased and decreased transmural pressure by vasoconstriction and vasodilation, respectively (Bayliss 1902; Carlsson et al. 1987; Davis and Hill 1999; Johnson 1980, 1986; Rowell 1986). This pressure-sensitive mechanism may differ among different vascular beds (Schubert and Mulvany 1999), is calcium-dependent (Davis and Hill 1999), modulated by neural, metabolic and endothelial factors (Davis and Hill 1999; Lott et al. 2002; Schubert and Mulvany 1999), and serves to adjust vascular resistance in response to changes in perfusion pressure.
In contrast to numerous animal studies (Izzard et al. 1996; Matrougui et al. 1998; Mohrman and Sparks 1974; Nurkiewicz and Boegehold 1998), and despite their physiologic importance, few reports investigated the effects of altered vascular transmural pressure on skeletal muscle blood flow in humans. In prior studies, head-up tilt (Imadojemu et al. 2001; Jepsen and Gaehtgens 1995), and external pressure devices (Lott et al. 2004; Lott et al. 2002) have been employed to alter vascular transmural pressure. In these studies, changes in autonomic neural tone and potential effects on muscle metabolism likely played a confounding role.
The present study was undertaken to examine reactive hyperemia in the arm and leg of healthy humans at three different limb positions – horizontal, up and down (i.e., at, above or below the level of the heart). Under resting conditions, variable limb positions alter hydrostatic and hence vascular transmural pressure with little effect on central hemodynamics. Post-occlusive reactive hyperemic blood flow (RHBF) is a well-established and reproducible index of peripheral vascular function (Patterson and Whelan 1955; Rowell 1986; Taylor et al. 1992) and is thought to be due to release of vasoactive metabolites and the transient reduction (during ischemia) of vascular distension. We reasoned that upon reestablishment of blood flow post occlusion the vascular distending pressure would rise to higher levels in the limb down than limb up position. Accordingly, we postulated that differences in the dynamic fall of hyperemia during the recovery from ischemia in the three conditions would in part reflect the impact of altered transmural pressure on vascular tone.
Methods
Young and healthy subjects (10 men, 10 women, age 26 ± 1 yr; height 170 ± 2 cm; weight 67 ± 2 kg) participated in two separate protocols performed on separate days (Arm: n = 10; Leg: n = 10). None was a smoker and none took any cardiovascular medications. The experiments were carried out in a quiet, dimly-lit laboratory at a temperature of 21–24°C. Caffeine, alcohol and exercise were avoided 24 hrs before testing. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and written informed was consent obtained.
Heart rate (HR) was monitored by 2-lead electrocardiogram. Blood pressure was measured on the non-experimental forearm by the volume clamp method (Finapres, Ohmeda, Madison, WI) and was verified with an automated sphygmomanometer (Dinamap, Critikon; Tampa, FL) at the beginning and end of the study. HR and blood pressure were measured continuously and recorded at 100 Hz using a PowerLab system (ADInstruments, Castle Hill, Australia). Mean arterial pressure (MAP) was determined electronically from the blood pressure trace.
Brachial and femoral arterial mean blood velocity (MBV) was measured by duplex ultrasound (ATL 5000, Philips Medical System, Bothell, WA, USA) (Newcomer et al. 2004). A 12–5 MHz transducer was positioned 1–2 cm proximal to an occlusion cuff on the arm or leg and measurements were made at an insonation angle of ≤60° and recorded via PowerLab system. Vessel diameter was measured in a longitudinal view at end-diastole, during baseline and at the end of the 3-min RHBF protocol by determining the distance between the near and far intima-media interfaces and was averaged. Brachial and femoral artery blood flow (ml/min) was the product of cross-sectional area of the vessel (πr2, cm2) and MBV (cm/s). Vascular conductance (C) was blood flow/MAP (ml/min/mmHg).
Experimental Design
The experimental methodology was identical in the arm and leg and all measurement were made with the subjects supine. Pneumatic cuffs were positioned around the wrist and the upper arm or around the ankle and thigh, respectively. Limb ischemia was produced by inflating the arm or thigh cuffs to 200 mmHg after inflating the wrist or ankle cuffs to minimize the contribution of skin blood flow in the hand or foot to RHBF (Lenders et al. 1991).
To assure repeatability of RHBF, a priming maneuver was performed by inflating the arm or thigh cuffs to 200 mmHg for 5 min (Patterson and Whelan 1955). Following a 10-min recovery period, the wrist or ankle cuff was inflated for 1 min before baseline blood flow measurements which were averaged over 3–5 min. The thigh or arm cuffs were then inflated to 200 mmHg for 5 and 10 min, respectively. Because of greater discomfort we chose a shorter ischemia period for the leg. Whereas the duration of ischemia affects the time course of reactive hyperemia, peak RHBF does not differ between 5, 10 or 20 min of ischemia (Carlsson et al. 1987). The thigh or upper arm cuff was then rapidly deflated and MBV was measured 5 and 15 s after cuff release and every 15 s thereafter for 3 min. The highest value was considered peak RHBF and occurred consistently within 15 s after restoration of blood flow. After measurements of vessel diameter at the end of the 3 min period, the wrist or ankle cuff was deflated to allow for a 15 min recovery period (Carlsson et al. 1987). Once flow returned to baseline and blood pressure and HR remained stable, limb position was altered between horizontal, up or down conditions with the sequence randomized. Raising or lowering the arm or leg was achieved at 45° from the horizontal plane.
Data Analysis and Statistics
The slope (ml/s/s) of the RHBF response was derived within its linear portion between the coordinates of peak flow and flow at time t = 30 s, thus representing the maximum rate of dissipation of the hyperemia from its peak. The recovery half-time of RHBF (t1/2 peak, s) was defined as the time form the onset of hyperemia to one-half of the peak flow value. One-way repeated measures analysis of variance was used to examine the effects of limb position on peak conductance, the slope of reactive hyperemic flow, MAP and HR. Where appropriate, paired comparisons were made with the paired t-test. The results are presented as mean ± SE. A P-value of < 0.05 was considered statistically significant.
Results
Baseline HR and MAP did not differ significantly between pre- and post-RHBF and between the three positions studied for both arm and leg (Table 1). Basal blood flow was similar in the three positions (arm up 29 ± 4; horizontal 33 ± 4; down 29 ± 3 ml/min; leg up 238 ± 34; horizontal 214 ± 25; down 191 ± 21 ml/min). Femoral and brachial artery diameters did not differ between baseline and post-RHBF as well as across limb positions (up, horizontal and down).
Table 1.
Mean Arterial Pressure (MAP) and Heart Rate (HR) Responses to Reactive Hyperemic Blood Flow (RHBF) and to Changes of Limb Position
| Limb Position | Up | Horizontal | Down | |||
|---|---|---|---|---|---|---|
| Pre-RHBF | Post-RHBF | Pre-RHBF | Post-RHBF | Pre-RHBF | Post-RHBF | |
| MAP (mmHg) | ||||||
| Arm | 88 ± 3 | 90 ± 3 | 83 ± 3 | 85 ± 3 | 86 ± 3 | 87 ± 3 |
| Leg | 95 ± 4 | 95 ± 4 | 93 ± 4 | 101 ± 8 | 94 ± 3 | 96 ± 3 |
| HR (bpm) | ||||||
| Arm | 58 ± 2 | 59 ± 2 | 60 ± 2 | 61 ± 2 | 61 ± 3 | 60 ± 2 |
| Leg | 64 ± 1 | 62 ± 3 | 64 ± 4 | 63 ± 3 | 63 ± 3 | 63 ± 5 |
Data are mean ± SE. There was no difference in MAP and HR between pre- and post-RHBF and across limb positions (P = NS).
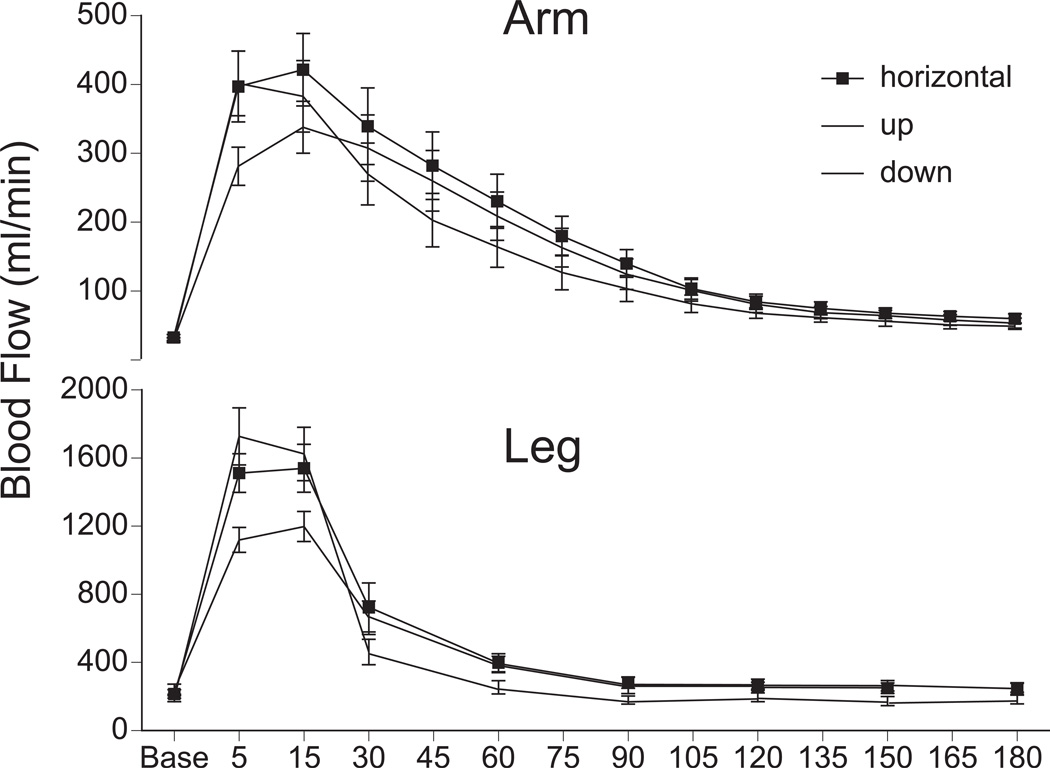
The RHBF responses from both arm (n=10) and leg (n=10) and at each position are displayed in Figure 1. Peak RHBF was lowest with the limb up compared to the horizontal or down positions (Table 2). Regardless of limb position, the peak RHBF response in the leg was lower than in the arm (arm up 1176 ± 178; horizontal 1320 ± 152; down 1397 ± 167 %; leg up 481 ± 80; horizontal 726 ± 109; down 916 ± 151 %; P < 0.05).
Figure 1.
Reactive hyperemic blood flow as a function of time, at three limb positions – horizontal, up and down. Upper Panel: Arm, 10 min occlusion at the brachial artery (n = 10); Lower Panel: Leg, 5 min occlusion at the femoral artery (n = 10).
Table 2.
Peak Reactive Hyperemic Blood Flow (RHBF), Recovery Half-time, and Slope of RHBF in the Arm and Leg as a Function of Limb Position
| Limb Position | Up | Horizontal | Down | |
|---|---|---|---|---|
| Peak RHBF (ml/min) | Arm | 338 ± 38* | 430 ± 52 | 415 ± 52 |
| Leg | 1208 ± 88* | 1579 ± 130 | 1767 ± 149 | |
| Recovery Half-time (s) | Arm | 73 ± 5 | 65 ± 3 | 51 ± 3† |
| Leg | 54 ± 5 | 46 ± 4 | 38 ± 4† | |
| Slope of RHBF (ml/s/s) | Arm | −0.03 ± 0.02* | −0.08 ± 0.02 | −0.12 ± 0.02 |
| Leg | −0.53 ± 0.08* | −0.81 ± 0.12 | −0.89 ± 0.07 |
Data are mean ± SE.
P < 0.05 compared to “horizontal” and “down”;
P < 0.05 compared to “horizontal” and “up”.
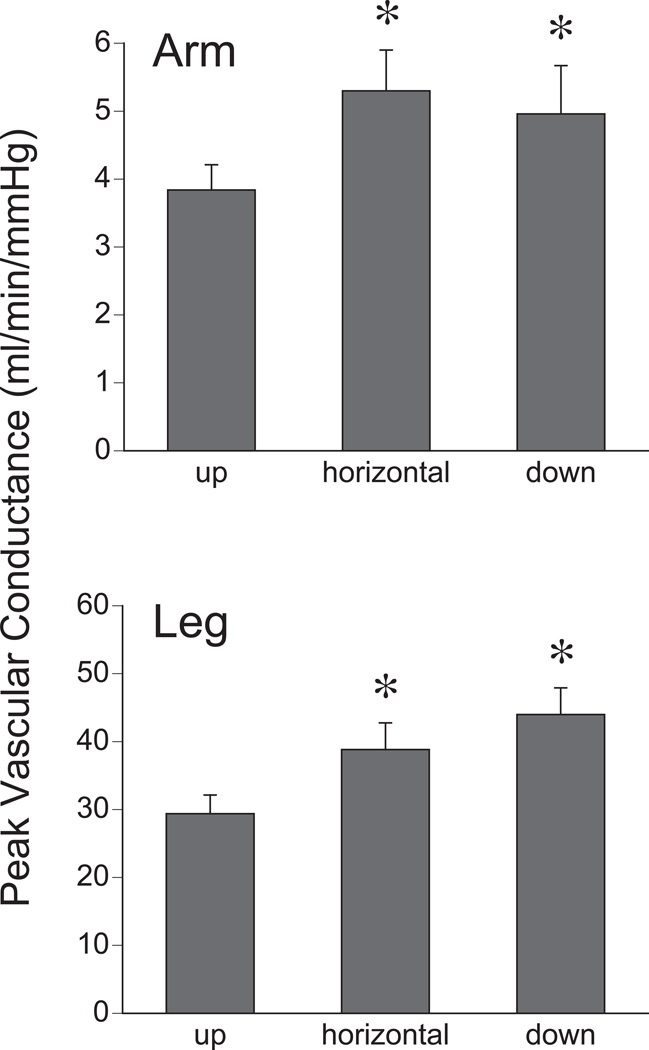
The vascular conductance (C) responses from both arm (n=10) and leg (n=10) and at each limb position are shown in Figure 2. Peak C was reduced with the limb up compared to horizontal or limb down positions (arm up 3.8 ± 0.4, horizontal 5.3 ± 0.6, down 4.9 ± 0.7 ml/min/mmHg; leg up 29.4± 2.7, horizontal 38.8 ± 3.9, down 44.0 ± 3.9 ml/min/mmHg; P < 0.05). Figures 1 and 2 demonstrate that the RHBF responses for the arm and leg follow similar trends at varying limb positions.
Figure 2.
Peak vascular conductance during reactive hyperemia as a function of limb position. Upper Panel: Arm; Lower Panel: Leg. *P < 0.05 compared to “up”.
In the arm, peak flow was reached at 5 or 15 s at both horizontal and down positions. In the arm up position, in all subjects peak flow was reached only by 15 s. In the leg, peak flow was reached between 5 to 15 s in the leg up, horizontal and leg down positions. Figure 1 highlights the differences in dynamic fall of the hyperemic response between arm and leg at varying positions. Recovery half-time was shortest with the limb down followed by horizontal and up positions and the slope of the RHBF response was steeper with the arm/leg down compared to horizontal or up positions (Table 2).
Discussion
To characterize the effects of changes in transmural pressure on reactive hyperemic blood flow (RHBF), we studied brachial and femoral RHBF responses at three different limb positions – horizontal, up and down. For any given limb position, the percent increase in RHBF above baseline was higher in the brachial than in the femoral artery. However, trends across limb positions in the arm and leg were similar. Peak RHBF was highest with the limb down or horizontal, and lowest with the limb up, suggesting a relationship of peak RHBF with hydrostatic pressure in the limb. In contrast, the recovery from peak RHBF (expressed as the negative slope of flow over time, or as the time from peak to one half of peak flow), was fastest with the limb down and slowest with the limb up. These data suggest that increased vascular transmural pressure, which is highest with the limb down, enhances vascular tone, whereas limb elevation, by reducing transmural pressure, attenuates it.
By design we intended to alter vascular transmural pressure but not the metabolic vasodilator stimulus evoked by ischemia in the limb. Since lowering of the limb reduces arterial and venous pressures equally (Levick and Michel 1978), perfusion pressure does not change whereas it may decrease slightly in the limb up position once the veins collapse. In addition, the absence of changes in blood pressure and/or heart rate speak against a significant engagement by our study maneuvers of the arterial baroreflex and the sympathetic nervous system. Because ischemia duration was the same for each limb in the three positions, the metabolic stimulus was assumed to be the same. Lastly, because the sequence was randomized, the findings cannot be explained by a sequence effect. Therefore, the most likely explanation for the positional differences in RHBF and its recovery is the effect of altered transmural pressure on vascular tone.
Regardless of limb position, peak RHBF was greater in the arm than leg (arm-to-leg ratio, up ~2.4:1, supine ~1.8:1, down ~1.5:1). Using various methodologies, similar differences in peak vasodilator responses between the arm and the leg have been reported (Imadojemu et al. 2001; Lott et al. 2005; Newcomer et al. 2004; Newcomer et al. 2005). However, because in this study the duration of ischemia and the subjects were different in the arm and leg protocols, direct comparisons between the arm and leg should be made with great caution.
In humans, myogenic autoregulation or the Bayliss effect (Bayliss 1902) has been demonstrated in various vascular beds, including the mesenteric, cerebral, renal retinal and coronary circulations (Johnson 1959; Muller et al. 1996; Schubert and Mulvany 1999) and skeletal muscle vascular myogenic mechanisms likely play a role in regulating blood flow with changing posture. Moreover, altered myogenic responses may impact numerous conditions including pregnancy (Veerareddy et al. 2002), ischemia and reperfusion (Cipolla et al. 1997), diabetes mellitus (Arima and Ito 2003), hypertension (Feihl et al. 2006), autonomic dysfunction and orthostatic intolerance (Kooijman et al. 2007).
Several limitations of our study should be acknowledged. Because the duration of ischemia was shorter in the leg than the arm (5 vs. 10 min), differences in RHBF between arm and leg could be due to differences in ischemia duration. However, in the forearm, peak RHBF is similar for arterial occlusions of 5, 10 and 20 min (Carlsson et al. 1987). Conversely, increasing ischemia duration prolongs the time course of RHBF (Carlsson et al. 1987). Therefore, we did not compare recovery half-times between arm and leg in our study. Because heart rate and blood pressure did not change, we assumed that baroreflex activity and sympathetic vasoconstrictor tone were not altered by changing limb position. However, we did not measure muscle sympathetic nerve activity. It has been shown that non-hypotensive reductions in aortic volume may trigger baroreflex-mediated hemodynamic adjustments that escape detection by conventional means (Taylor et al. 1995). In addition, it has been reported that a transient increase in cardiac output is induced by passive limb exercise in the absence of a metabolic vasodilator stimulus and may be reflex in origin (McDaniel et al. 2010). We cannot exclude the possibility that such a neural mechanism modified the hyperemia responses in our study. It is well established that in high flow conditions during whole body exercise competition between the leg and arm vascular beds evokes sympathetic vasoconstriction (Secher and Volianitis 2006). We should also note that RHBF could modify vascular tone via shear stress and flow-mediated dilation. However, since peak RHBF was greatest in the limb down and horizontal positions, we would have expected this mechanism to attenuate rather than accentuate the dynamic fall in blood flow during limb down RHBF. We acknowledge that our flow calculation likely underestimated absolute RHBF because we did not account for the transient vessel expansion induced by the hyperemia. However, because this error was likely similar within subjects and among differing limb positions, we doubt that it affected the conclusions. Lastly, we cannot rule out that limb position related differences in the hyperemia responses could be modified by the venoarteriolar reflex, an axonal reflex that results in upstream arteriolar constriction in response to venous distention and is thought to play a prominent role in the skin (Durand et al. 2004; Jepsen and Gaehtgens 1995; Okazaki et al. 2005) and at venous pressures >l25 mmHg (Henriksen 1991).
Conclusion
Our data demonstrate that changes in transmural vascular pressure induced by altering arm and leg position relative to the level of the heart importantly influence the magnitude and time course of reactive hyperemia in limbs of humans. Because these effects were independent of detectable changes in systemic pressure and central sympathetic control, they appear to be primarily due to autoregulation. In addition to compensating for fluctuations of perfusion pressure, the ability to autoregulate limb blood flow in response to postural changes likely plays an important role in blood pressure homeostasis.
Acknowledgements
The authors would like to thank Michael Herr for excellent technical support, Kristen Gray for statistical expertise, and Jennifer Stoner for expert manuscript preparation. This work was supported by NIH grants P01 HL077670, R01 HL068699, NIH/NCRR grant M01 RR010732 and C06 RR016499 and Pennsylvania Tobacco Settlement Funds – Penn State College of Medicine.
References
- Arima S, Ito S. The mechanisms underlying altered vascular resistance of glomerular afferent and efferent arterioles in diabetic nephropathy. Nephrol Dial Transplant. 2003;18:1966–1969. doi: 10.1093/ndt/gfg263. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the artieral wall to changes of internal pressure. J Physiol (London) 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson I, Sollevi I, Wennmalm A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperemia. J Physiol (London) 1987;389:147–161. doi: 10.1113/jphysiol.1987.sp016651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Durand S, Zhang R, Cui J, Wilson TE, Crandall CG. Evidence of a myogenic response in vasomotor control of forearm and palm cutaneous microcirculations. J Appl Physiol. 2004;97:535–539. doi: 10.1152/japplphysiol.01299.2003. [DOI] [PubMed] [Google Scholar]
- Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol Scand. 1991;603:33–39. [PubMed] [Google Scholar]
- Imadojemu VI, Lott MEJ, Gleeson K, Hogeman CS, Ray CA, Sinoway LI. Contribution of perfusion pressure to vascular resistance response during head-up tilt. Am J Physiol Heart Circ Physiol. 2001;281:H371–H375. doi: 10.1152/ajpheart.2001.281.1.H371. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Bund SJ, Heagerty AM. Myogenic tone in mesenteric arteries from spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 1996;270:H1–H6. doi: 10.1152/ajpheart.1996.270.1.H1. [DOI] [PubMed] [Google Scholar]
- Jepsen H, Gaehtgens P. Postural vascular response vs. sympathetic vasoconstriction in human skin during orthostasis. Am J Physiol Heart Circ Physiol. 1995;269:H53–H61. doi: 10.1152/ajpheart.1995.269.1.H53. [DOI] [PubMed] [Google Scholar]
- Johnson PC. Myogenic nature of increase in intestinal vascular resistance with venous pressure elevation. Circ Res. 1959;7:992–999. doi: 10.1161/01.res.7.6.992. [DOI] [PubMed] [Google Scholar]
- Johnson PC. The myogenic response. In: Bohr DF, Somlyo AP, Sparks HV Jr, Geiger SR, editors. Handbook of Physiology - The Cardiovascular System: Vascular Smooth Muscle, Section 2, Volume II. Bethesda: American Physiological Society; 1980. pp. 409–442. [Google Scholar]
- Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- Kooijman M, de Hoog M, Rongen GA, van Kuppevelt HJ, Smits P, Hopman MT. Local vasoconstriction in spinal cord-injured and able-bodied individuals. J Appl Physiol. 2007;103:1070–1077. doi: 10.1152/japplphysiol.00053.2007. [DOI] [PubMed] [Google Scholar]
- Lenders J, Janssen G-J, Smits P, Thien T. Role of the wrist cuff in forearm plethysmography. Clin Sci. 1991;80:413–417. doi: 10.1042/cs0800413. [DOI] [PubMed] [Google Scholar]
- Levick JR, Michel CC. The effects of position and skin temperature on the capillary pressures in the fingers and toes. J Physiol. 1978;274:97–109. doi: 10.1113/jphysiol.1978.sp012136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott M, Hogeman C, Herr M, Huang K, Sinoway L. Comparison of brachial and femoral artery myogenic responses in young subjects. Med Sci Sports Exerc. 2005;37:S224. (Abstract) [Google Scholar]
- Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R586–R591. doi: 10.1152/ajpregu.00612.2003. [DOI] [PubMed] [Google Scholar]
- Lott MEJ, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood flow velocity dynamics in humans. J Appl Physiol. 2002;93:2137–2146. doi: 10.1152/japplphysiol.00443.2002. [DOI] [PubMed] [Google Scholar]
- Matrougui K, Schiavi P, Guez D, Henrion D. High sodium intake decreases pressure-induced (myogenic) tone and flow- induced dilation in resistance arteries from hypertensive rats. Hypertension. 1998;32:176–179. doi: 10.1161/01.hyp.32.1.176. [DOI] [PubMed] [Google Scholar]
- McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol. 2010;108:76–84. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol. 1974;227:531–535. doi: 10.1152/ajplegacy.1974.227.3.531. [DOI] [PubMed] [Google Scholar]
- Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res. 1996;32:668–678. [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol (London) 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol. 2005;289:H308–H315. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Boegehold MA. High dietary salt alters arteriolar myogenic responsiveness in normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 1998;275:H2095–H2104. doi: 10.1152/ajpheart.1998.275.6.H2095. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Fu Q, Martini ER, Shook R, Conner C, Zhang R, Crandall CG, Levine BD. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1354–R1359. doi: 10.1152/ajpregu.00804.2004. [DOI] [PubMed] [Google Scholar]
- Patterson GC, Whelan RF. Reactive hyperaemia in the human forearm. Clin Sci. 1955;14:197–209. [PubMed] [Google Scholar]
- Rowell LB. Regulation During Physical Stress. New York: Oxford University Press; 1986. Human Circulation. [Google Scholar]
- Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- Secher NH, Volianitis S. Are the arms and legs in competition for cardiac output? Med Sci Sports Exerc. 2006:1797–1803. doi: 10.1249/01.mss.0000230343.64000.ac. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. 'Non-hypotensive' hypovolaemia reduces ascending aortic dimensions in humans. J Physiol (London) 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. 1992;86:1789–1799. doi: 10.1161/01.cir.86.6.1789. [DOI] [PubMed] [Google Scholar]
- Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2226–H2233. doi: 10.1152/ajpheart.00593.2002. [DOI] [PubMed] [Google Scholar]