Abstract
Current vision science adaptive optics systems use near infrared wavefront sensor ‘beacons’ that appear as red spots in the visual field. Colored fixation targets are known to influence the perceived color of macroscopic visual stimuli(Jameson, D. and Hurvich, L. M., 1967. Fixation-light bias: an unwanted by-product of fixation control. Vis. Res. 7, 805 – 809.), suggesting that the wavefront sensor beacon may also influence perceived color for stimuli displayed with adaptive optics. Despite its importance for proper interpretation of adaptive optics experiments on the fine scale interaction of the retinal mosaic and spatial and color vision, this potential bias has not yet been quantified or addressed. Here we measure the impact of the wavefront sensor beacon on color appearance for dim, monochromatic point sources in 5 subjects. The presence of the beacon altered color reports both when used as a fixation target as well as when displaced in the visual field with a chromatically neutral fixation target. This influence must be taken into account when interpreting previous experiments and new methods of adaptive correction should be used in future experiments using adaptive optics to study color.
Keywords: Color appearance, fixation light bias, adaptive optics psychophysics, color naming
1. Introduction
Adaptive optics can remove nearly all the effects of the eye’s aberrations while displaying arbitrary stimuli to the retina, making this technology a promising tool not only for studying the impact of the eye’s aberrations on vision (Dalimier et al., 2007; Liang et al., 1997; Lundstrom et al., 2007; Marcos et. al, 2008; Piers et al., 2007; Sawides et al., 2010; Yoon and Williams, 2002) but its retinal and neural limits as well (Brainard et al., 2008; Hofer et al., 2005; Rossi et al., 2007; Rossi and Roorda, 2010a, 2010b). Within this latter category, adaptive optics allows experimental investigation of the topography of the retinal mosaic and its impact on color as well as spatial vision (Brainard et al., 2008; Hofer et al., 2005). All vision science adaptive optics systems use near infrared wavefront measuring ‘beacons’ that, despite having near infrared wavelengths, are visible and salient in the visual field. This is potentially problematic since it has long been known that colored fixation lights can impact the perceived hue of a visual stimulus. For example, Jameson and Hurvich (1967) demonstrated a marked difference in the reported appearance of large (44’) colored fields with changes in fixation target color from red to blue. Given their findings, it is possible that the red wavefront beaconmay also impact the perceived hue of visual stimuli displayed with adaptive optics.
We sought to determine whether the presence of a dim, red wavefront beacon impacts reported color appearance when viewing monochromatic, small spot stimuli. Since the wavefront beacon can be used as a fixation point (for non-foveal stimuli- as in the experiments described by Hofer et al., 2005) or displaced from the visual axis (typical when presenting foveal stimuli) we performed experiments to assess the perceptual impact in both situations. If the adaptive optics wavefront beacon impacts perceived color then this bias must be taken into account when interpreting previous experiments and new methods of adaptive correction should be developed and used in future experiments involving color.
2. Methods
2.1 Subjects
Five subjects participated, subjects 1, 2, and 5 were authors; subjects 2, 3 and 4 were inexperienced observers, and subjects 3 and 4 were naïve to the purposes of the study. Subjects 1,3 and 5 were female and subjects 2 and 4 were male. All subjects had normal vision correctable to at least 20/20 and normal color vision as assessed by the HRR 4th edition plates. The research followed the tenets of the Declaration of Helsinki and all subjects gave informed consent after an explanation of the study procedure and any possible risks. All study procedures were approved by the Institutional Review Board of the University of Houston.
2.2 Apparatus and Stimuli
Subjects used hue-scaling to rate the appearance of brief (6 msec), monochromatic (580 nm), small spot stimuli. Stimuli were created by illuminating a 25 micron pinhole, subtending ~0.2’, with a white-light LED. (Subject 5 acquired data with an older stimulus configuration using a weaker broadband halogen light and 34 msec duration, data acquired in subjects 1 and 2 with both configurations showed no observable differences in color reports.) Stimulus wavelength was chosen based on previous research (Cicerone and Nerger, 1989; Krauskopf and Srebro, 1965) to maximize variability in color appearance and was controlled either with a monochromator (subject 5) or with a narrow band (10 nm bandwidth) interference filter (subjects 1–4). Since effective adaptive optics correction cannot be achieved without the wavefront beacon, stimuli were displayed with conventional refraction through a 2 mm artificial pupil. These stimuli have retinal size of ~1’ full-width at half-maximum (fwhm), which is roughly the smallest stimulus size achievable with conventional optical means.
Stimuli were monocularly presented to the central fovea after at least 10 minutes of dark adaptation in an otherwise completely dark visual field, save for 2 dim fixation dots presented in Maxwellian view. Subjects fixated midway between these two dots, which were separated by 2.25°. This fixation target was chosen to minimize interference at the fovea without introducing large fixation errors. Reported accuracy of fixation for similar targets is 3 – 6’ (Rattle, 1969), which is not significantly different from that reported by Ditchburn (1973) for central fixation targets. Subjects wore their habitual spectacle correction, or were corrected with either trial lenses and/or by translating a movable stage in a Badaloptometer. No dilating agents were used, since subjects’ natural pupils were always larger than the 2 mm artificial pupil. This small pupil size also provided a large depth of focus, minimizing the potential impact of accommodative drifts and fluctuations on the retinal stimulus profile. The negligible influence of accommodative fluctuations under these conditions was confirmed in one subject (subject 1) by calculating the retinal stimulus profile as a function of time from time-resolved wavefront measurements.
To assess the applicability of our findings for stimuli even smaller than possible with conventional optics (i.e. those produced with adaptive optics), one subject (subject 5) acquired data under two additional conditions: 1. with foveal stimuli displayed through a 6 mm artificial pupil with static (or open-loop) adaptive optics correction of higher order aberrations, and 2. with conventionally refracted stimuli presented through a 2 mm artificial pupil 1° from fixation, where stimulus and cone size are more comparable. The subject foveated a single fixation dot in this second condition.
In the first condition (aberrations corrected, foveal stimuli) the subject’s pupil was dilated (1% tropicamide) to allow aberration correction over a 7 mm pupil. Static (or open-loop) aberration correction was achieved with direct-slope (Jiang and Li, 1990) control of a 97 channel deformable mirror (Xinetics) run in closed-loop until the root-mean-square wavefront error over a 7 mm pupil was below 0.12 microns. At this point the mirror was held fixed, allowing the wavefront beacon to be turned off. Correction was updated prior to the start of each new trial block. Static correction of the eye’s aberrations as described here is known to be less effective than dynamic closed-loop aberration correction due to the inherent instability of the eye’s optics (Hofer et al., 2001). Calculations from temporally resolved wavefront measurements acquired with static correction in this subject (subject 5) indicated a relatively stable retinal stimulus profile of ~0.5’ fwhm.
In the second condition (conventionally refracted stimuli presented 1° from fixation) all trials took place in the 4–11 minute interval following a full bleach of rod and cone pigment (the ‘cone plateau’) to minimize any potential impact of rods. Bleaching was achieved with a diffused halogen source (color temperature ~1900K) of ~7.4 log photopic Troland-seconds, a strength calculated to bleach at least 99% of both L and M cone pigment according to Rushton’s pigment kinetics equations (1968). These stimulus conditions closely mimic those used in the study reported by Hofer et al. (2005).
2.3 Psychophysics
2.3.1 Procedure
We performed two sets of experiments to assess the impact of the adaptive optics wavefront beacon (840 nm, 1 microWatt) on reported color appearance of dim, monochromatic, point stimuli. In Experiment 1 we sought to determine whether color reports are impacted when using a red fixation target, as is the case when using the beacon as a fixation point with non-foveal stimuli. In Experiment 2 we sought to determine whether the influence of the wavefront beacon can be avoided or diminished by displacing the beacon in the visual field and using a chromatically neutral stimulus to guide fixation.
In Experiment 1, subjects performed blocks of trials alternating between red and white fixation target conditions. In Experiment 2 a white fixation target was used to guide fixation and the wavefront beacon was displaced from the visual axis by ~1.25° (horizontal displacement for foveal stimuli, vertical displacement for subject 5 when viewing stimuli at 1°). Subjects then performed blocks of trials alternating between beacon absent and beacon present conditions. Each block consisted of 50–100 trials, including blanks, at 4 or 5 intensity levels spanning the psychometric function. Appearance of these stimuli is known to depend somewhat on both intensity and the subjective detection criterion (Koenig and Hofer, in press). Testing at multiple intensities allows disambiguation of the impact of fixation target, or beacon related hue bias from any potential shifts due to differences in either sensitivity or criterion across conditions. Stimulus timing and intensity were controlled by a custom Matlab program (The Mathworks, Natick, MA) incorporating Psychophysics Toolbox routines (Brainard, 1997; Pelli, 1997). Data collection was typically spread over 2–4 days and most subjects performed 600–700 trials per condition. Prior to the start of the experiment, a series of frequency of seeing data was acquired to select the most appropriate stimulus intensities and to appropriately focus the subject. Subjects used these preliminary sessions to practice rating color appearance, and the ratings data from these sessions were discarded.
2.3.2 Hue-scaling response framework
Subjects rated the color appearance of each stimulus, if seen, by distributing 10 key presses among 5 categories: white, green, blue, yellow, or red; in any manner they felt best reflected the appearance of the stimulus on each trial (Koenig and Hofer, in press). Subjects were instructed to rate stimuli according to apparent hue and saturation and not apparent brightness. A stimulus appearing saturated green, for example, would be rated by placing all 10 key presses in the green category, whereas a moderately desaturated orange might be rated as 3 red, 2 yellow, and 5 white. In the event that a ‘colorless’ or ‘indescribable’ stimulus was seen (Bouman and Walraven, 1957; Krauskopf, 1978; Hofer et al., 2005) subjects were instructed to use the white category. Stimuli rated purely white (all ten key presses in the white category) therefore reflected all stimuli without a discernable hue, including both ‘colorless’ or ‘indescribable’ stimuli as well as those appearing white or gray.
Trials were self-paced and subjects entered color ratings either with a game controller or a small handheld numeric keypad. In both cases each key press was accompanied by an auditory tone, with the final key press sounding a distinct ‘completion’ tone. Subjects were unable to proceed to the next stimulus until completing the requisite number of key presses. Only certain keys were recognized during color rating, and unexpected key presses were accompanied by an error tone and flagged as a mistake. Finally, subjects were able to intentionally flag individual trials by means of an additional key press in the event that a known rating mistake occurred. Rating mistakes were excluded from analysis.
2.4 Data Analysis
Frequencies of seeing were computed at each stimulus intensity for each subject and condition. No significant differences in sensitivity or criterion were observed for any subject in either the red vs. white fixation target or beacon present vs. beacon absent conditions. The impact of the granularity of the cone mosaic should be most evident with these stimuli near the detection threshold (Brainard et al., 2008; Hofer et al., 2005; Krauskopf, 1978) and prior work with similar stimuli has suggested a qualitative shift in color appearance for stimuli with frequencies of seeing above ~85% (Hofer et al., 2005). Consequently, we restricted our data analysis to stimuli with frequencies of seeing between 20 and 85%, which generally included 2 or 3 stimulus intensities per subject (actual numbers of stimuli seen and retained for data analysis are reported in tables 1, 2, and 3).
Table 1.
Hue-scale data for threshold (20–85% seen), monochromatic (580 nm), point stimuli with white or red fixation targets (experiment 1). Shown is the mean rating (after the arcsin transform) for all seen spots for each subject in each of the 5 categories (white, red, green, blue, and yellow) plus or minus the standard error. Ratings for each category are on a scale from 0–10. ‘Percent achromatic’ is the percent of seen stimuli, on a scale of 0–100, that were rated only as white (a rating of 10 in the white category). ‘Mean saturation’ is the average saturation (sum of all ratings other than white) for all seen stimuli on a scale of 0–100. Stimuli were presented foveally for subjects 1–4, and at 1° for subject 5.
| White fixation target | |||||
|---|---|---|---|---|---|
| subject 1 | subject 2 | subject 3 | subject 4 | subject 5 (1) | |
| number seen | 297 | 407 | 359 | 72 | 411 |
| percent achromatic | 42.4 ± 2.9 | 54.8 ± 2.5 | 48.7 ± 2.6 | 77.8 ± 5.0 | 35.8 ± 2.4 |
| mean saturation | 35.3 ± 2.3 | 37.3 ± 2.2 | 47.8 ± 2.6 | 7.3 ±1.6 | 25.6 ± 1.2 |
| mean white rating | 6.51 ± 0.23 | 6.30 ± 0.22 | 5.22 ± 0.26 | 9.41 ±0.16 | 7.44 ± 0.12 |
| mean red rating | 0.39 ± 0.08 | 0.38 ± 0.08 | 0.64 ± 0.12 | 0.03 ± 0.03 | 0.94 ± 0.09 |
| mean green rating | 1.34 ± 0.12 | 0.33 ± 0.08 | 2.98 ± 0.24 | 0 | 0.73 ± 0.09 |
| mean blue rating | 1.58 ± 0.14 | 2.82 ± 0.21 | 0.08 ± 0.04 | 0.18 ± 0.07 | 0.73 ± 0.07 |
| mean yellow rating | 0.45 ± 0.07 | 0.19 ± 0.05 | 1.08 ± 0.16 | 0.51 ± 0.15 | 0.36 ± 0.05 |
|
Red fixation target | |||||
| subject 1 | subject 2 | subject 3 | subject 4 | subject 5 (1) | |
| number seen | 336 | 314 | 331 | 76 | 429 |
| percent achromatic | 37.2 ± 2.6 | 54.4 ± 2.8 | 51.1 ± 2.8 | 51.3 ± 5.8§ | 20.7 ± 2.0‡ |
| mean saturation | 32.1 ± 1.9 | 36.5 ± 2.4 | 44.2 ± 2.6 | 15.5 ± 2.0# | 39.9 ± 1.4‡ |
| mean white rating | 6.82 ± 0.19 | 6.35 ± 0.24 | 5.58 ± 0.26 | 8.45 ± 0.20# | 6.01 ± 0.14‡ |
| mean red rating | 0.85 ± 0.12# | 1.26 ± 0.17‡ | 0.92 ± 0.15 | 0.54 ± 0.14# | 2.22 ± 0.14‡ |
| mean green rating | 0.95 ± 0.10# | 0.12 ± 0.05* | 2.29 ± 0.22* | 0.03 ± 0.03 | 1.01 ± 0.10* |
| mean blue rating | 1.30 ± 0.12 | 1.98 ± .21# | 0.18 ± 0.07 | 0* | 0.58 ± 0.06 |
| mean yellow rating | 0.38 ± 0.06 | 0.29 ± 0.07 | 1.04 ± 0.16 | 0.99 ± 0.18* | 0.55 ± 0.06# |
Superscripts indicate significant differences in the two conditions, with
p<0.05,
p<0.01,
p<0.001, and
p < 0.0001 (two-tailed z-test).
All subjects rated spots significantly more red or less green with the red fixation target. Some subjects also rated spots less blue (subject 2 and 4) or more yellow and more saturated (subject 4 and 5) with the red fixation target.
Table 2.
Hue-scale data for threshold (20–85% seen), monochromatic (580 nm), point stimuli with a white fixation target and the red wavefront correcting beacon either present or absent ~1.25° from the visual stimulus (experiment 2). Shown is the mean rating (after the arcsine transform) for all seen spots for each subject in each of the 5 categories (white, red, green, blue, and yellow) plus or minus the standard error. Ratings for each category are on a scale from 0–10. ‘Percent achromatic’ is the percent of seen stimuli, on a scale of 0–100, that were rated only as white (a rating of 10 in the white category). ‘Mean saturation’ is the average saturation (sum of all ratings other than white) for all seen stimuli on a scale of 0–100. Stimuli were presented foveally. Data also shown for subject 5 with foveal stimuli displayed with a static adaptive optics correction and for stimuli displayed at 1°. Subject 4 did not participate in this experiment.
| Beacon absent | ||||||
|---|---|---|---|---|---|---|
| subject 1 | subject 2 | subject 3 | subject 5 (1) | subject 5 (fov) | subject 5 (fov, AO) | |
| number seen | 25 | 87 | 336 | 304 | 218 | 330 |
| percent achromatic | 52.0 ± 10.2 | 17.2 ± 4.1 | 43.8 ± 2.7 | 34.9 ± 2.7 | 47.7 ± 3.4 | 49.4 ± 1.6 |
| mean saturation | 28.8 ± 8.1 | 68.8 ± 4.2 | 43.8 ± 2.3 | 28.1 ± 1.4 | 27.5 ± 2.1 | 24.9 ± 1.6 |
| mean white rating | 7.12 ± 0.81 | 3.12 ± 0.42 | 5.62 ± 0.23 | 7.19 ± 0.14 | 7.25 ± 0.21 | 7.51 ± 0.16 |
| mean red rating | 0.16 ± 0.11 | 0.82 ± 0.28 | 0.68 ± 0.12 | 1.17 ± 0.12 | 0.54 ± 0.09 | 0.62 ± 0.08 |
| mean green rating | 1.10 ± 0.34 | 0.22 ± 0.11 | 2.05 ± 0.20 | 1.02 ± 0.11 | 1.26 ± 0.17 | 0.57 ± 0.09 |
| mean blue rating | 1.52 ± 0.52 | 5.28 ± 0.49 | 0.30 ± 0.08 | 0.59 ± 0.07 | 0.97 ± 0.14 | 1.26 ± 0.13 |
| mean yellow rating | 0.25 ± 0.14 | 0.57 ± 0.19 | 1.37 ± 0.17 | 0.37 ± 0.05 | 0.11 ± 0.04 | 0.17 ± 0.04 |
|
Beacon present | ||||||
| subject 1 | subject 2 | subject 3 | subject 5 (1) | subject 5 (fov) | subject 5 (fov, AO) | |
| number seen | 26 | 97 | 374 | 310 | 203 | 286 |
| percent achromatic | 30.8 ± 9.4 | 4.1 ± 2.0# | 43.0 ± 2.6 | 33.5 ± 2.5 | 44.3 ± 3.5 | 48.6 ± 3.0 |
| mean saturation | 46.9 ± 7.8 | 83.8 ± 2.8# | 46.2 ± 2.3 | 57.2 ± 2.1‡ | 28.8 ± 2.2 | 26.5 ± 1.8 |
| mean white rating | 5.31 ± 0.78 | 1.62 ± 0.28# | 5.38 ± 0.23 | 7.03 ± 0.21 | 7.12 ± 0.22 | 7.35 ± 0.18 |
| mean red rating | 1.05 ± 0.55 | 0.32 ± 0.16 | 0.54 ± 0.10 | 1.14 ± 0.12 | 0.76 ± 0.12 | 0.78 ± 0.11 |
| mean green rating | 2.53 ± 0.57* | 2.00 ± 0.35‡ | 2.34 ± 0.20 | 1.19 ± 0.11 | 1.29 ± 0.18 | 0.76 ± 0.11 |
| mean blue rating | 1.35 ± 0.38 | 4.61 ± 0.46 | 0.23 ± 0.06 | 0.60 ± 0.06 | 0.84 ± 0.13 | 1.11 ± 0.13 |
| mean yellow rating | 0.16 ± 0.11 | 1.47 ± 0.27# | 1.54 ± 0.17 | 0.38 ± 0.05 | 0.16 ± 0.05 | 0.19 ± 0.04 |
Superscripts indicate significant differences in the two conditions, with
p<0.05,
p<0.01, and
p < 0.0001 (two-tailed z-test).
Green ratings where significantly higher for subjects 1 and 2 when the beacon was present than when it was absent.
Table 3.
Hue-scale data for subjects 1 and 2 when viewing foveal, parathreshold (>85% seen), monochromatic (580 nm), point stimuli with a white fixation target and the red wavefront correcting beacon either present or absent ~1.25° from the visual stimulus (experiment 2). Shown is the mean rating (after the arcsine transform) for all seen spots in each of the 5 categories (white, red, green, blue, and yellow) plus or minus the standard error. Ratings for each category are on a scale from 0–10. ‘Percent achromatic’ is the percent of seen stimuli, on a scale of 0–100, that were rated only as white (a rating of 10 in the white category). ‘Mean saturation’ is the average saturation (sum of all ratings other than white) for all seen stimuli on a scale of 0–100.
| Beacon absent | Beacon present | |||
|---|---|---|---|---|
| subject 1 | subject 2 | subject 1 | subject 2 | |
| number seen | 90 | 310 | 90 | 343 |
| percent achromatic | 33.3 ± 5.0 | 15.8 ± 2.1 | 22.1 ± 4.4 | 4.1 ± 1.1‡ |
| mean saturation | 28.2 ± 3.0 | 62.4 ± 2.1 | 33.5 ± 3.0 | 77.9 ± 1.6‡ |
| mean white rating | 7.18 ± 0.30 | 3.76 ± 0.21 | 6.65 ± 0.30 | 2.20 ± 0.16‡ |
| mean red rating | 0.33 ± 0.09 | 0.44 ± 0.10 | 0.27 ± 0.09 | 0.64 ± 0.12 |
| mean green rating | 1.03 ± 0.19 | 0.73 ± 0.12 | 2.02 ± 0.26# | 1.40 ± 0.14§ |
| mean blue rating | 0.51 ± 0.14 | 3.96 ± 0.24 | 0.43 ± 0.12 | 3.76 ± 0.24 |
| mean yellow rating | 1.17 ± 0.22 | 1.15 ± 0.13 | 1.07 ± 0.20 | 2.03 ± 0.15‡ |
Superscripts indicate significant differences in the two conditions, with
p<0.005,
p<0.001, and
p < 0.0001 (two-tailed z-test).
Green ratings were significantly higher for both subjects when the beacon was present than when it was absent.
Mean ratings were computed for each subject and condition after performing an arcsine transform (Abramov et al., 2009) to insure appropriately distributed variance. Ratings were inspected for each subject as a function of stimulus intensity, and while mean hue was generally found to shift with increasing intensity (most notably decreasing in blueness and increasing in yellowness with smaller changes in the red-green balance), any differences in the red vs. white target or beacon absent vs. beacon present conditions were consistent at all intensities examined. Data for all the threshold stimulus intensities (20–85% frequency of seeing) was subsequently binned to allow easier visualization of the impact of the colored fixation target, or beacon, on reported color appearance. Two-tailed z-tests were then used to assess the significance of any differences in mean ratings across conditions, the validly of this method for our data was confirmed with permutation tests (Good, 2005).
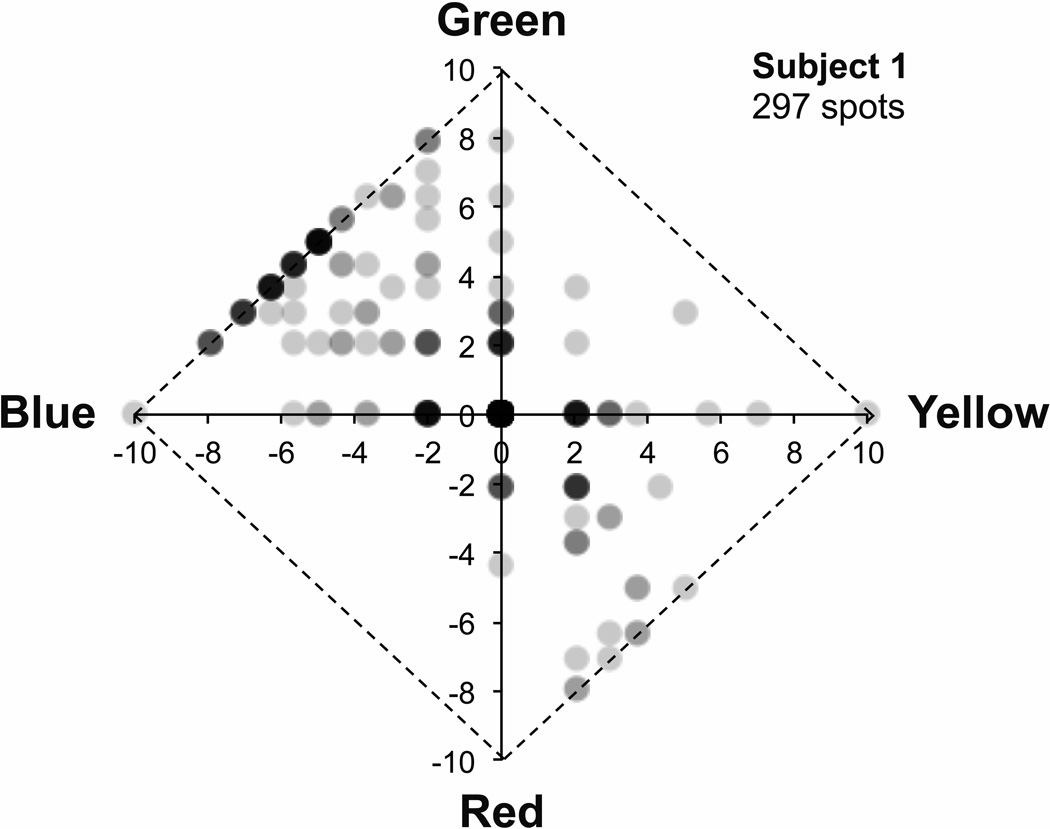
In addition to computing and plotting mean ratings in each color category, data were also visualized with Uniform Appearance Diagrams (also described by Abramov et al., 2009) by plotting the difference in green and red ratings on the y axis and the difference in yellow and blue ratings on the x axis. Figure 1 shows one example of the variation in color appearance of individual 580 nm foveal small spot stimuli for one subject (subject 1). Stimuli that appeared achromatic fall at the origin and stimuli that appeared completely saturated lie along the edges. The variation in color appearance shown in this diagram is typical of small, threshold, foveally presented monochromatic stimuli and similar to that previously reported (Krauskopf and Srebro, 1965; Koenig and Hofer, in press). Stimuli varied markedly in both hue and saturation, were most commonly blue-green or orange, were never purple or violet, and were only rarely yellow-green.
Figure 1.
Uniform Appearance Diagram illustrating typical variation in appearance of monochromatic (580 nm), threshold (20–85% seen), foveally viewed point stimuli for one subject (subject 1). The green minus the red rating (after the arcsine transform) is plotted vs the yellow minus the blue rating for each of 297 seen stimuli. Stimuli rated as purely white fall at the origin, while stimuli rated as purely colored (saturated) lie along the edges (diagonal lines) of the diagram. The weight of each point indicates the number of stimuli with that rating, with the darkest points representing the most stimuli. The variation in color appearance reported by this subject is typical, with stimuli often appearing white, green, blue-green, blue, yellow, red, or orange; seldom appearing yellow-green; and rarely appearing purple or violet.
3. Results
3.1 Experiment 1: red vs white fixation targets
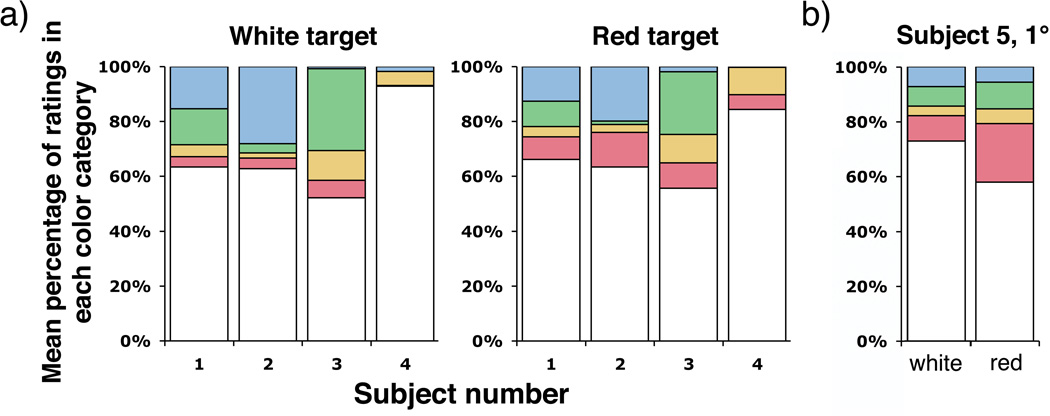
Figures 2 and 3 show that all subjects rated the monochromatic yellow point stimuli redder, on average, when the fixation target was red than when it was white (see also Table 1). Figure 2, which presents the distribution of ratings reported in each color category, shows that the average red rating significantly increased, the average green rating significantly decreased, or both, for all subjects with the red compared with white fixation target. An alternative way of viewing the data is presented in figure 3, which shows the mean ratings for each subject plotted on the Uniform Appearance Diagram. The data displayed in these figures includes all threshold stimuli seen at frequencies of seeing from 20 to 85%, with an average frequency of seeing of ~65%.
Figure 2.
A. Distribution of color ratings among the 5 categories: red, yellow, green, blue, and white; for 580 nm, threshold (20–85% seen), foveally presented point stimuli viewed when fixating red or white targets. B. Distribution of color ratings among the 5 color categories for subject 5, who viewed 580 nm, threshold (20–85% seen) stimuli at 1° while foveating a red or white fixation dot. Red ratings increased for all subjects when using the red fixation target than with the white target. The magnitude of the effect was roughly equal for all subjects, including subject 5 who used a foveal fixation point and more eccentric stimuli.
Figure 3.
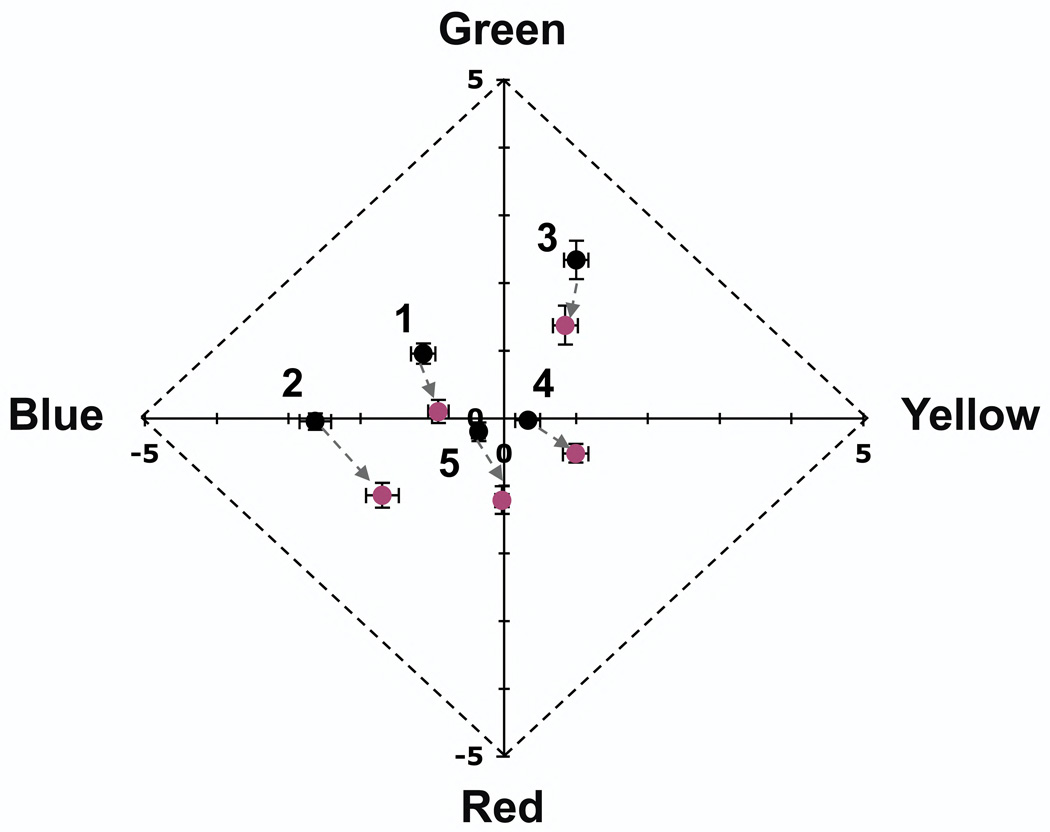
Mean hue is significantly redder for each subject when viewing foveally presented (1° for subject 5) 580 nm stimuli with red fixation targets (reddish points) than with white fixation targets (black points). Points are labeled with subject number and represent the average of seen stimuli (Table 1), error bars are plus or minus 1 standard error. Note that only the central portion of the Uniform Appearance Diagram is shown.
The magnitude of the hue shift caused by fixating the red target was comparable for all subjects, despite significant individual differences in mean hue. That the same hue shift was also found in subject 5, who viewed a stimulus presented at 1°, suggests that decreasing the size of the stimulus relative to the underlying photoreceptors does not lessen the impact of fixation target color on perceived hue. The similarity of the results of subject 5, who foveated a fixation point, to those of the other subjects, who fixated midway between two fixation dots, also suggests that the impact of the fixation target is insensitive to the retinal position of the elements comprising the target. It is also important to note that the hue shift caused by fixating a red target showed no evidence of decreasing with decreased fixation target intensity (data not shown), and in fact these data were taken with dim fixation dots just bright enough for accurate fixation. Fixating a red target, as when fixating the wavefront sensor beacon, causes the color appearance of tiny monochromatic yellow (580 nm) stimuli to become more similar to the beacon color (redder). Interestingly, this shift in appearance is opposite to that reported by Jameson and Hurvich (1967), whose subjects rated the appearance of their larger (44’) suprathreshold yellow (578 nm) stimuli as less red and less yellow when viewed with red than with blue fixation dots.
3.2 Experiment 2: beacon present vs beacon absent
3.2.1 Threshold stimuli
Displacing the beacon from the visual axis is a typical strategy used in adaptive optics psychophysical experiments to mitigate its impact (Putnam et al., 2005; Chen et al., 2006, 2007). We sought to determine whether employing this strategy with a chromatically neutral fixation target would eliminate the impact of the beacon on the color appearance. Figures 4 and 5 show that 2 of 4 subjects rated threshold (frequency of seeing from 20 to 85%, average frequency of seeing ~50%) monochromatic yellow point stimuli greener, on average, when the wavefront correcting beacon was present ~1.25° from the stimulus in the visual field. Figure 4, which presents the distribution of ratings placed in each color category, shows a significant increase in green ratings for subjects 1 and 2 when the beacon is present, compared with absent (see also Table 2). This was accompanied by an increase in overall saturation (decrease in white ratings). The magnitude of the hue shift for these subjects, shown in Figure 5, was roughly equal and opposite to that caused by fixating a red target.
Figure 4.
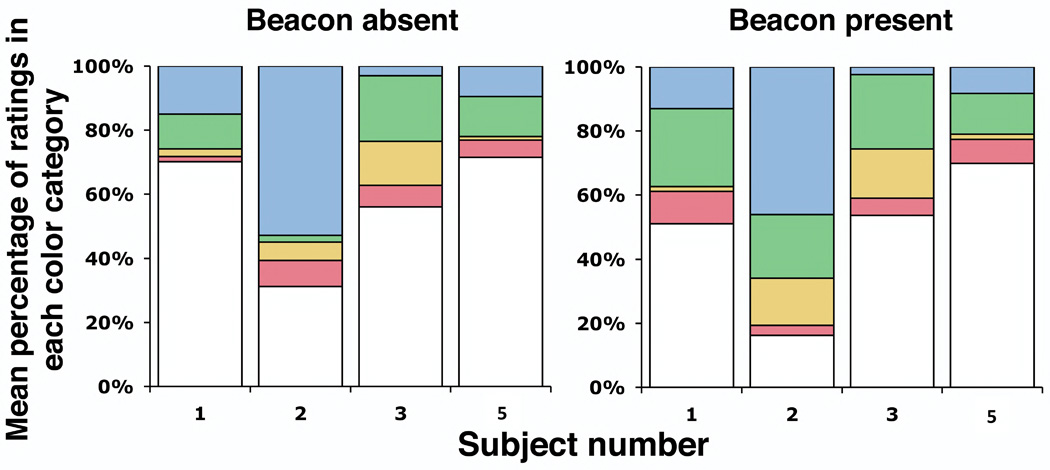
Distribution of color ratings among the 5 categories: red, yellow, green, blue, and white; for 580 nm, threshold (20–85% seen), foveally presented point stimuli with and without the correcting beacon in the visual field (1.25° from the stimulus). Color reports were not significantly different for subjects 3 and 5 in the two conditions, but green ratings increased significantly for subjects 1 and 2. This increase in green ratings was accompanied by an increase in overall saturation.
Figure 5.
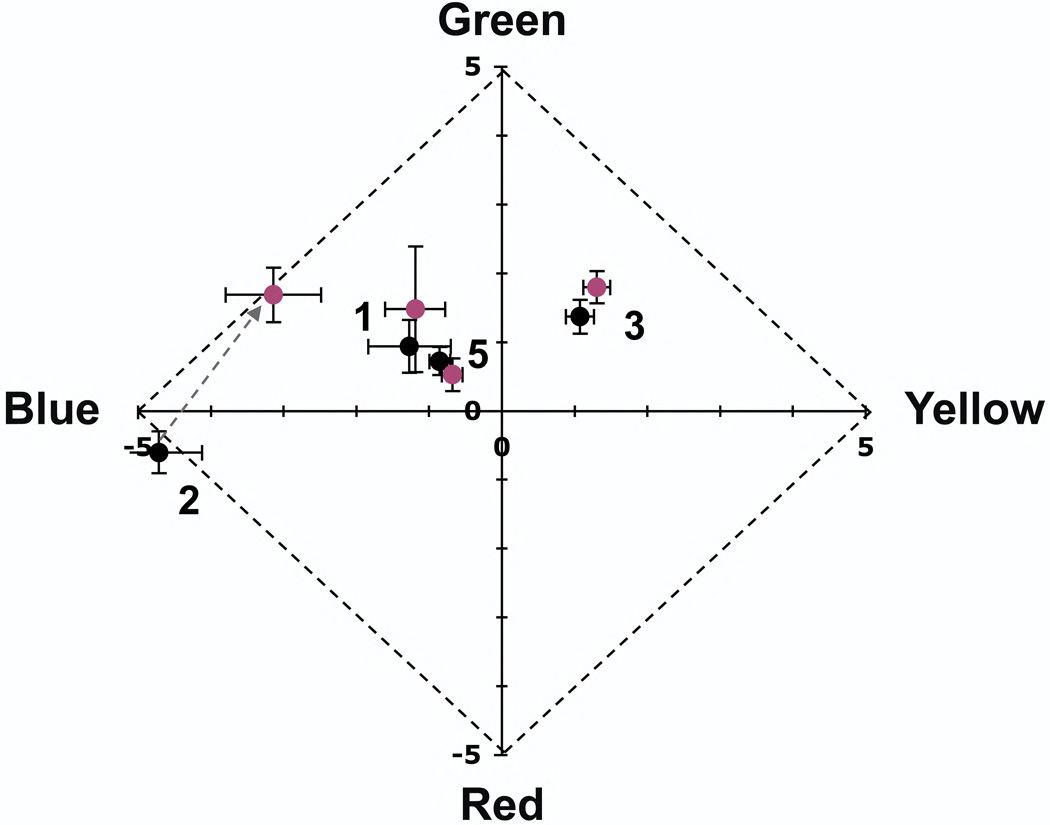
Mean hue is significantly greener for 2 subjects (1 and 2) when viewing foveally presented, threshold, 580 nm stimuli with the correcting beacon present (reddish points) than without (black points). Mean hue was not significantly different for the other two subjects (3 and 5- only data for foveal stimuli displayed with conventional refraction are shown for subject 5, results with static aberration correction and stimuli presented at 1° were similar). Points are labeled with subject number and represent the average of all seen stimuli (Table 2), error bars are plus or minus 1 standard error. Note that only the central portion of the Uniform Appearance Diagram is shown.
Although color reports for subject 5 were not significantly different in the beacon present vs beacon absent conditions, they did differ for foveal stimuli displayed with conventional optics compared with those displayed with a static aberration correction (Table 2). There were significantly fewer green ratings when viewing the smaller stimuli produced with aberration correction. Assessing the significance and the generalizability of this finding require further study.
3.2.2 Parathreshold stimuli
We took additional data with brighter stimuli in subjects 1 and 2, the subjects for whom the wavefront beacon caused a significant increase in the reported green component of the stimulus, to determine whether the beacon’s impact might lessen with small increases in stimulus intensity (Fig. 6). Subject 1 viewed stimuli ~0.45 log units above threshold (bright enough to be seen 100% of the time), while subject 2 viewed stimuli at a frequency of seeing of 89%. A shift in the green direction was still observed for both subjects, with no apparent decrease in magnitude. It is possible that larger increases in stimulus intensity may diminish the impact of the beacon for these subjects, however very bright stimuli cannot be effectively confined at the receptor scale and will be less effective for probing the impact of the granularity of the retinal mosaic on vision. We conclude that at least some subjects are significantly impacted by the presence of the beacon when viewing small threshold stimuli, even when the beacon is not fixated, and that the technique of using a chromatically neutral fixation target and displacing the beacon in the visual field is not generally sound.
Figure 6.
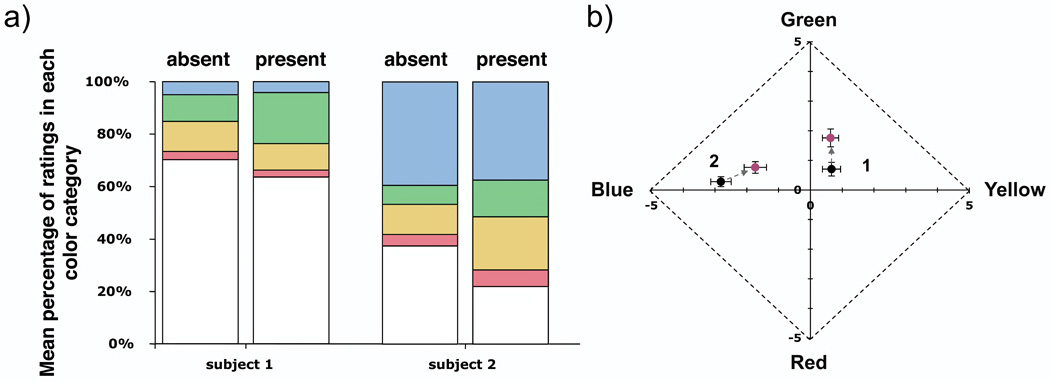
Mean hue is significantly greener for 2 subjects (1 and 2) when viewing foveally presented, parathreshold (subject 1: 100% frequency of seeing, 0.45 log units above threshold; subject 2: 89% frequency of seeing), 580 nm stimuli with the correcting beacon present in the visual field than without the beacon. A) Distribution of color ratings among the 5 categories: red, yellow, green, blue, and white; shows increased green ratings accompanied by an increase in overall saturation (reduced white ratings). B) The magnitude of the hue shift is similar to that seen in the same two subjects with dimmer stimuli. Data with more intense stimuli were not acquired for other subjects. Points are labeled with subject number and represent the mean of all seen spots plus or minus 1 standard error. Note that only the central portion of the Uniform Appearance Diagram is shown.
4. Discussion
4.1 Color appearance of small foveal stimuli
Our data show significant individual differences in color appearance of foveal, monochromatic, point stimuli, with these differences occurring in both the red-green and blue-yellow color directions. That most subjects reported a significant blue component to the color appearance of these stimuli, despite the paucity of S cones in the central fovea and their relative insensitivity at 580 nm, supports prior work suggesting an M cone contribution to the sensation of blueness (Brainard et al., 2008; DeValois and DeValois, 1993; Drum, 1989; Hofer et al., 2005; Schirillo and Reeves, 2001; see also the work of Knoblauch and Shevell, 2001, which cautions against the general association offixed hues with specific cone types). Individual differences in the blue-yellow direction may be partially due to differences in the criterion for deciding whether or not stimuli were seen.
4.3 Implications for AO psychophysics
Fixating a red target, as when fixating the adaptive optics wavefront correcting beacon, causes a robust shift in the appearance of threshold, 580 nm, point stimuli toward the beacon hue. This effect occurred in all subjects regardless of naiveté or the amount of prior psychophysical experience. That the same effect was also observed subject 5, who viewed stimuli at 1° while foveating a single fixation dot, suggests that the fixation hue bias is not strongly sensitive to the geometry of the fixation target and is unlikely to be lessened by decreasing the size of the stimulus relative to the underlying photoreceptors. We observed a similar effect in preliminary experiments when using a large (1°), dim, red concurrent imaging flash, as is required to localize individual retinal stimuli in either a flood-illuminated system (Putnam et al., 2005) or adaptive optics confocal scanning ophthalmoscopes (Arathon et al., 2007; Rossi and Roorda, 2010a). This suggests that the imaging fields required with these stimulus localization strategies will also interfere with color judgments until it becomes possible to image with wavelength and intensity combinations that are not visible to subjects.
An opposite and roughly equal shift towards the green direction occurs in some, but not all, subjects when the beacon is not fixated but present in the visual field ~1.25° from the stimulus. For these subjects, one of which was naïve and the other highly experienced, the effect was not reduced by modest increases in stimulus brightness. It may be possible to eliminate this effect by further displacing the beacon in visual field, but this will not allow effective adaptive optics correction since the quality of correction decreases with increasing separation between the beacon and the system axis (Bedggood et al., 2008).
The differing impact on perceived stimulus color when fixating a red target compared with having a red spot (the wavefront beacon) displaced in the visual field (with the same distance between the stimulus and target/beacon in both cases) indicates the effect is not purely retinal or adaptational and suggests a central component. One possibility is that subjects viewing a colored fixation target attend to its color, which may increase its perceptual salience (Blaser et al., 1999; Tseng et al., 2004, 2010). That reported appearance was, on average, more similar to the fixation target color is consistent with this account, although somewhat in contradiction to findings that color appearance itself is not altered by attention (Prinzmetal et al., 1998), at least for large, suprathreshold stimuli.
Another potential strategy for reducing the impact of the wavefront sensor beacon (or imaging light in the case of a concurrent localization experiment) on color appearance is to perform experiments on a bright white background, just bright enough to mask the red imaging or beacon light. Another variant of this strategy would be to precisely superpose a green field on top of the red light. We explored the former strategy in one subject (subject 1) and found a significant difference in color reports for stimuli presented on light and dark backgrounds (Fig. 7), with reported color appearance for threshold stimuli viewed on the white background becoming more similar to that expected for macroscopic stimuli. This suggests that even if a masking or hue canceling strategy is feasible it may not be desirable, as it does not appear to produce conditions favorable for probing the granularity of the retinal mosaic.
Figure 7.
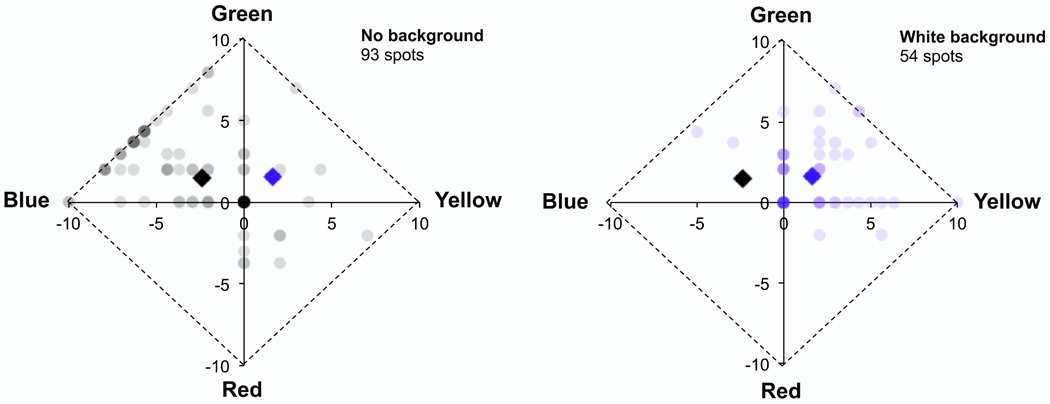
Impact of a white background (1.16 Trolands) on color reports for one subject (subject 1) with threshold (percent seen < 85%), foveal, 580 nm point stimuli. The distribution of color reports is different for stimuli displayed to the dark adapted fovea (left) than stimuli displayed on a white background (right). As in Fig. 1, the weight of each dot reflects the number of stimuli rated similarly. Stimulus intensity was adjusted to maintain threshold performance in both conditions. Variability decreases on the bright background and mean hue (represented by the black and blue diamonds, error bars are plus or minus one standard error and are smaller than the size of the markers) shifts significantly in the yellow direction, which is the expected hue for a macroscopic stimulus of this wavelength. As in Figs 1,3,5, and 6, stimuli were displayed through a 2 mm artificial pupil with conventional refraction and color rating data were arcsine transformed prior to plotting.
5. Conclusions
Small, dim colored lights in the visual field significantly impact perceived hue of threshold small spot stimuli and the nature of this impact depends on whether the lights are serving as fixation targets or simply present in the visual field. When fixating a chromatically neutral target, the additional presence of the red beacon either had no impact or shifted appearance of a 580 nm small spot stimulus in the green direction, with the magnitude and significance of the effect varying considerably across subjects. A more robust and dramatic impact occurred when fixating a red target, with appearance for the same stimuli shifting markedly in the red direction. We conclude that it is inappropriate to use visible wavefront beacons, or imaging lights, in color-related adaptive optics experiments, especially with threshold stimuli. Ideally, alternative methods, such as longer wavelength beacons or wavefront sensorless schemes (Biss et al., 2007; Booth, 2007; Hofer et al., 2011; Zommer et al., 2006), will be developed and employed for psychophysical experimentation.
Highlights.
Adaptive optics wavefront correcting beacons alter stimulus color reports
The nature of the impact depends on whether or not subjects fixate the beacon
Valid color appearance measures require adaptive optics without a visible beacon
Acknowledgements
Research supported by: NIH RO1 EY019069, T35 EY07088, P30 EY07551, and the University of Houston STAR scholar program. We thank Lukasz Sterkowicz, Hope Queener, Chris Kuether, and Chueh Ting for assistance with software and the stimulus apparatus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
H. J. Hofer, University of Houston, College of Optometry, 4901 Calhoun Rd., Houston, Tx, 77204, hhofer@optometry.uh.edu
J. Blaschke, University of Houston, College of Optometry, 4901 Calhoun Rd., Houston, Tx, 77204
J. Patolia, University of Houston, College of Optometry, 4901 Calhoun Rd., Houston, Tx, 77204
D. E. Koenig, University of Houston, College of Optometry, 4901 Calhoun Rd., Houston, Tx, 77204
References
- Abramov I, Gordon J, Chan H. Color appearance: Properties of the uniform appearance diagram derived from hue and saturation scaling. Atten. Percept. Psychophys. 2009;71:632–643. doi: 10.3758/APP.71.3.632. [DOI] [PubMed] [Google Scholar]
- Arathorn DW, Yang Q, Vogel CR, Zhang Y, Tiruveedhula P, Roorda A. Retinally stabilized cone-targeted stimulus delivery. Opt. Express. 2007;15:13731–13744. doi: 10.1364/oe.15.013731. [DOI] [PubMed] [Google Scholar]
- Bedggood P, Daaboul M, Ashman R, Smith G, Metha A. Characteristics of the human isoplanatic patch and implications for adaptive optics retinal imaging. J. Biomed. Opt. 2008;13:024008. doi: 10.1117/1.2907211. [DOI] [PubMed] [Google Scholar]
- Biss DP, Webb RH, Zhou Y, Bifano TG, Zamiri P, Lin CP. An adaptive optics biomicroscope for mouse retinal imaging. Proc. SPIE. 2007;6467:646703. [Google Scholar]
- Booth MJ. Adaptive optics in microscopy. Phil. Trans. R. Soc. A. 2007;365:2829–2843. doi: 10.1098/rsta.2007.0013. [DOI] [PubMed] [Google Scholar]
- Blaser E, Sperling G, Lu ZL. Measuring the amplification of attention. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11681–11686. doi: 10.1073/pnas.96.20.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Seeing Perceiving. 1997;10:433–436. [PubMed] [Google Scholar]
- Brainard DH, Williams DR, Hofer H. Trichromatic reconstruction from the interleaved cone mosaic: Bayesian model and the color appearance of small spots. J. Vis. 2008;8:1–23. doi: 10.1167/8.5.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman MA, Walraven PA. Some color naming experiments for red and green lights. J. Opt. Soc. Am. 1957;47:834–839. doi: 10.1364/josa.47.000834. [DOI] [PubMed] [Google Scholar]
- Chen L, Kruger PB, Hofer H, Singer B, Williams DR. Accommodation with higher order monochromatic aberrations corrected with adaptive optics. J. Opt. Soc. Am. A. 2006;23:1–8. doi: 10.1364/josaa.23.000001. [DOI] [PubMed] [Google Scholar]
- Chen L, Artal P, Gutierrez D, Williams DR. Neural compensation for the best aberration correction. J. Vis. 2007;7:1–9. doi: 10.1167/7.10.9. [DOI] [PubMed] [Google Scholar]
- Cicerone CM, Nerger JL. The relative numbers of long-wavelength-sensitive to middle-wavelength-sensitive cones in the human fovea centralis. Vis. Res. 1989;29:115–128. doi: 10.1016/0042-6989(89)90178-8. [DOI] [PubMed] [Google Scholar]
- Dalimier E, Dainty C, Barbur J. Effects of higher-order aberrations on contrast acuity as a function of light level. J. Mod. Opt. 2007;55:791–803. [Google Scholar]
- De Valois RL, De Valois KK. A multi-stage color model. Vis. Res. 1993;33:1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW. Eye-movements and visual perception. Oxford: Clarendon Press; 1973. [Google Scholar]
- Drum B. J. Opt. Soc. Am. A. Vol. 6. 1989. Hue signals from short- and middle-wavelength-sensitive cones; pp. 153–157. [DOI] [PubMed] [Google Scholar]
- Good PI. Resampling methods: a practical guide to data analysis. 3rd edition. Boston: Birkhauser; 2005. [Google Scholar]
- Hofer H, Chen L, Yoon GY, Singer B, Yamauchi Y, Williams DR. Opt. Express. Vol. 8. 2001. Improvement in retinal image quality with dynamic correction of the eye’s aberrations; pp. 631–643. [DOI] [PubMed] [Google Scholar]
- Hofer H, Singer B, Williams DR. Different sensations from cones with the same photopigment. J. Vis. 2005;5(5):444–454. doi: 10.1167/5.5.5. [DOI] [PubMed] [Google Scholar]
- Hofer H, Sredar N, Li C, Porter J. Wavefront sensorless adaptive optics ophthalmoscopy in the human eye. Opt. Express. 2011;19:14160–14171. doi: 10.1364/OE.19.014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson D, Hurvich LM. Fixation-light bias: an unwanted by-product of fixation control. Vis. Res. 1967;7:805–809. doi: 10.1016/0042-6989(67)90043-0. [DOI] [PubMed] [Google Scholar]
- Jiang W, Li H. Hartmann-Shack wavefront sensing and control algorithm. Proc. SPIE. 1990;1271:82–93. [Google Scholar]
- Koenig DK, Hofer HJ. Do color appearance judgments interfere with detection of small threshold stimuli? J. Opt. Soc. Am. A. doi: 10.1364/JOSAA.29.00A258. doc ID 153890 (posted 22 November 2011, in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch K, Shevell SK. Relating cone signals to color appearance: Failure of monotonicity in yellow/blue. Vis. Neurosci. 2001;18:901–906. doi: 10.1017/s0952523801186062. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Srebro R. Spectral sensitivity of color mechanisms: derivation from fluctuations of color appearance near threshold. Science. 1965;150:1477–1479. doi: 10.1126/science.150.3702.1477. [DOI] [PubMed] [Google Scholar]
- Krauskopf J. On identifying detectors. In: Armington JC, Krasukopf J, editors. Visual psychophysics and physiology. New York: Academic Press; 1978. [Google Scholar]
- Liang J, Williams DR, Miller DT. J. Opt. Soc. Am. A. Vol. 14. 1997. Supernormal vision and high-resolution retinal imaging through adaptive optics; pp. 2884–2892. [DOI] [PubMed] [Google Scholar]
- Lundstrom L, Manzanera S, Prieto PM, Ayala DB, Gorceix N, Gustafsson J. Effect of optical correction and remaining aberrations on peripheral resolution acuity in the human eye. Opt. Express. 2007;15:12654–12661. doi: 10.1364/oe.15.012654. [DOI] [PubMed] [Google Scholar]
- Marcos S, Sawides L, Gambra E, Dorronsoro C. Influence of adaptive-optics ocular aberration correction on visual acuity at different luminances and contrast polarities. J. Vis. 2008;8:1–12. doi: 10.1167/8.13.1. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Seeing Perceiving. 1997;10:437–442. [PubMed] [Google Scholar]
- Piers PA, Manzanera S, Prieto PM, Gorceix N, Artal P. Use of adaptive optics to determine the optimal ocular spherical aberration. J. Cataract. Refract. Surg. 2007;33:1721–1726. doi: 10.1016/j.jcrs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W, Amiri H, Allen K, Edwards T. Phenomenology of attention: I. Color, location, orientation, and spatial frequency. J. Exp. Psychol. Hum. Percept. Perform. 1998;24:261–282. [Google Scholar]
- Putman NM, Hofer H, Doble N, Chen L, Carroll J, Williams DR. The locus of fixation and the foveal cone mosaic. J. Vis. 2005;5:632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- Rattle JD. Effect of target size on monocular fixation. OpticaActa. 1969;16:183–192. doi: 10.1080/713818162. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Weiser P, Tarrant J, Roorda A. Visual Performance in Emmetropia and Low Myopia After Correction of High Order Aberrations. J. Vis. 2007;7:14. doi: 10.1167/7.8.14. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Roorda A. The relationship between visual resolution and cone spacing in the human fovea. Nat. Neurosci. 2010a;13:156–157. doi: 10.1038/nn.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, Roorda A. Is visual resolution after adaptive optics correction susceptible to perceptual learning? J. Vis. 2010b;10:1–14. doi: 10.1167/10.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WAH, Henry GH. Bleaching and regeneration of cone pigments in man. Vis. Res. 1968;8:617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]
- Schirillo J, Reeves A. Color-naming of M-cone incremental flashes. Color Res. Application. 2001;26:132–140. [Google Scholar]
- Tseng CH, Vidnyanszky Z, Papathomas T, Sperling G. Attention-based long-lasting sensitization and suppression of colors. Vis. Res. 2010;50:416–423. doi: 10.1016/j.visres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Yoon GY, Williams DR. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J. Opt. Soc. Am. A. 2002;19:266–275. doi: 10.1364/josaa.19.000266. [DOI] [PubMed] [Google Scholar]
- Zommer S, Ribak EN, Lipson SG, Adler J. Simulated annealing in ocular adaptive optics. Opt. Lett. 2006;31:939–941. doi: 10.1364/ol.31.000939. [DOI] [PubMed] [Google Scholar]