Abstract
Purpose/Objective(s)
Conformal radiation therapy in the post-prostatectomy setting requires accurate setup and localization of the prostatic fossa. In this series, we report prostate bed localization and motion characteristics using data collected from implanted radiofrequency transponders.
Materials and Methods
The Calypso 4D Localization System uses three implanted radiofrequency transponders for daily target localization and real-time tracking throughout a course of radiation therapy. We reviewed the localization and tracking reports of 20 patients who received ultrasound-guided placement of Calypso transponders within the prostate bed prior to a course of intensity modulated radiation therapy at Fox Chase Cancer Center.
Results
At localization, prostate bed displacement relative to bony anatomy exceeded 5 mm in 9% of fractions in the Anterior-Posterior (A-P) direction, and 21% of fractions in the Superior-Inferior (S-I) direction. The three-dimensional vector length from skin marks to Calypso alignment exceeded 1 cm in 24% of all 652 fractions with available setup data. During treatment, the target exceeded the 5 mm tracking limit for at least 30 seconds in 11% of all fractions, generally in the A-P or S-I directions. In the A-P direction, target motion was twice as likely to move posteriorly, towards the rectum, than anteriorly. 15% of all treatments were interrupted for repositioning, and 70% of patients were repositioned at least once during their treatment course.
Conclusion
Set-up errors and motion of the prostatic fossa during radiotherapy are nontrivial, leading to potential undertreatment of target and excess normal tissue toxicity if not taken into account during treatment planning. Localization and real-time tracking of the prostate bed via implanted Calypso transponders can be used to improve the accuracy of plan delivery.
Keywords: post-prostatectomy, tracking, localization, motion
Introduction
Conformal radiation in the post-prostatectomy setting has been shown to reduce GI and GU toxicity compared to conventional techniques (1, 2). Intensity Modulated Radiation Therapy (IMRT) allows for excellent coverage of the target while sparing adjacent critical structures, such as the bladder and rectum, by creating sharp dose falloffs along tight margins. Accurate delivery of highly conformal plans demands precise daily localization and alignment. Day to day variation in rectal and bladder filling can result in significant displacement of prostate bed clinical target volume (CTV) and departure from planned dose distribution (3).
Several investigators have published their experiences with various localization techniques, including conebeam CT (CBCT) (4), ultrasound (5, 6), orthogonal imaging with alignment to surgical clips (7), or implanted gold seeds (8). While these studies have described the characteristics of inter-fractional displacement of the prostate bed relative to bony anatomy, there is as of yet, no detailed investigation of prostate bed movement during treatment delivery.
The Calypso 4D localization system (Calypso Medical Technologies, Seattle, WA, USA) uses a detector array for localization and continuous real-time tracking of implanted radiofrequency beacons (9). Its use in the setting of intact prostate has been well-documented by multiple institutions, with detailed analyses of inter- and intra-fraction prostate gland motion (10-13). At our institution we have a large experience using Calypso beacons in the prostate bed for localization and tracking of post-prostatectomy radiation treatments (14). In this study, we present our Calypso experience in the post-prostatectomy setting, including positional stability of the transponders and setup uncertainties. This is the first study to describe intra-fractional motion of the prostate bed throughout a course of IMRT.
Materials and Methods
We identified the charts of 20 patients who had undergone post-prostatectomy radiation treatment to the prostate bed following implantation of electromagnetic transponders for daily localization and tracking. Data for analysis was obtained by retrospective review of each patient's treatment record.
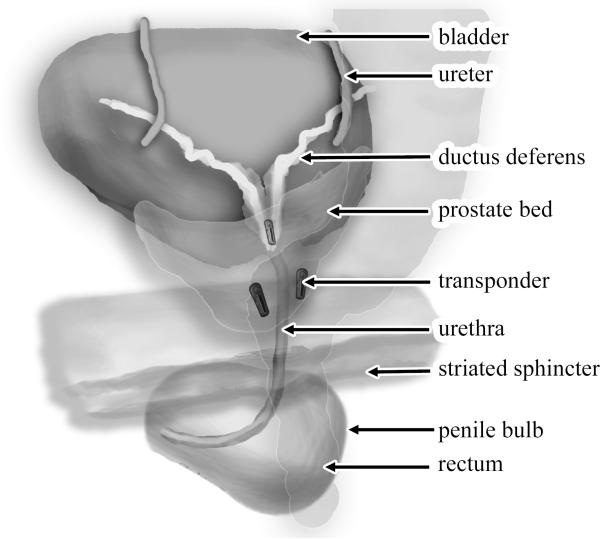
A detailed description of our implantation and simulation technique has been previously published (14), but is briefly summarized as follows. Patients were instructed to come to all simulations and treatments with an empty rectum and a full bladder. All patients were simulated and treated in the supine position. A customized thermoplastic foam cast was used to aid patient immobilization and positioning. On the day of implantation, an MRI simulation was performed in the Department of Radiation Oncology before the beacon placement procedure. Three radiofrequency beacons were implanted into the prostate bed by one of our urologic oncologists using ultrasound guidance (Figure 1). One week later, a non-contrast CT simulation was obtained in our radiation department, and fused with the MRI simulation. At simulation, the treatment isocenter is placed within the prostate bed and, guided by lasers aligned to the simulator isocenter, triangulation tattoos are placed on the skin surface. The prostate bed, bladder, rectum, femoral head and small bowel volumes are determined and contoured by the treating physician using the RTOG postoperative prostate volume guidelines (15). The three radiofrequency beacons are identified and contoured on the simulation CT, and are designated right, left, and apex beacons according to their relative positions. The locations of the beacons on the simulation CT define a baseline set of coordinates for the Calypso system to determine target isocenter shifts for daily localization and tracking. Details of our post-prostatectomy IMRT treatment planning technique have been published (16) but are briefly summarized as follows: a CTV → PTV expansion of 5 mm posteriorly and 8 mm in all other directions is applied to the prostate bed. An IMRT plan is generated by a dosimetrist using a treatment planning system. Prior to treatment, a physicist performs a QA procedure which includes comparison of calculated to delivered relative dose distribution as measured on a 2-D ion chamber array. All patients in this series received a total dose of 68 Gy delivered in 34 daily fractions to the prostate bed.
Figure 1.
Schematic of Calypso beacons implanted within prostate bed. Illustration created by Nicholas Zaorsky, M.D.
At the start of each fraction, patients are initially positioned by laser alignment of skin surface tattoos. The Calypso detector array was then placed over the patient's pelvis and used to align the target isocenter with the linear accelerator (LINAC) isocenter, via localization of the three implanted transponders. Of 680 total fractions delivered, 652 had initial setup data available for analysis. The recorded positioning offsets are the sum of the setup error and motion of the prostate bed relative to bony anatomy. In addition to documenting daily positioning errors, the Calypso system also records the rotational deviations (roll, pitch, yaw) and intertransponder distances within the target.
Every week, orthogonal kV electronic portal imaging was obtained several seconds after alignment by Calypso. For 87 treatment fractions, each electronic portal image (EPI) was overlayed with its corresponding digitally reconstructed radiograph (DRR) from CT simulation using the Mosaiq software system (IMPAC Medical Systems, Sunnyvale, CA). Prostate bed motion was estimated by measurement of the three dimensional shifts needed to align pelvic bony anatomy between the EPI and DRR. Setup errors, calculated by subtracting prostate bed shifts from initial positioning offsets, were determined for the 80 fractions with both EPI and initial offsets.
During treatment delivery, the Calypso system continuously tracks, records, and displays in real-time the x-, y-, and z-position of the target, at a rate of approximately 10 observations per second (10 Hz). Tracking limits of 5 mm from the machine isocenter were imposed by our institution, reflecting the 5 mm CTV to PTV expansion during treatment planning. In response to target excursions exceeding 5 mm in any direction, therapists could interrupt treatment and reposition the patient before resuming treatment. Treatment interruption or delay without repositioning (i.e. manual gating) was not routinely performed.
Of the 638 fractions with available tracking reports, 487 fractions had available detailed tracking data enabling calculation of the Area Under the Curve (AUC) for target deviations in the x-, y- and z-directions. The AUC is a cumulative measurement of target deviation, and is calculated by integrating the position of the target in excess of the tracking limit over time (Figure 1e). For example, a unidirectional target deviation of 6 mm lasting 10 seconds would have an AUC of 1 (0.1 cm outside of tracking limits × 10 seconds), as would a deviation of 1 cm for 2 seconds (0.5 cm outside tracking limits × 2 seconds). A fraction was considered to have significant intra-fraction motion if the tracking limit was exceeded for at least 30 seconds (10), or if the AUC was ≥ 1.
Treatment planning margins were calculated using the van Herk formalism for 95% CTV coverage in 90% of patients (17).
Results
Stability of Calypso Beacons
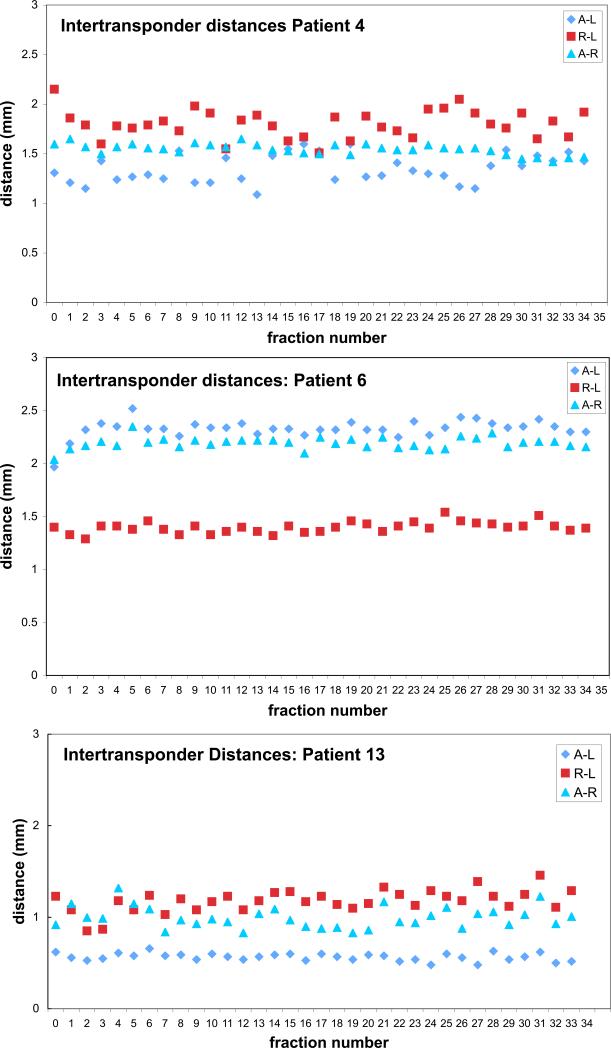
Beacon loss via urinary voiding occurred in two patients who were among the first at our institution to undergo post-prostatectomy Calypso implantation. Of the remaining 18 patients, intertransponder distances were recorded at the time of simulation and weekly thereafter (a total of 441 data points), with standard deviations calculated for every axis of each patient. The mean change in measured intertransponder distances relative to simulation was 1.3 mm (range: 0-7.7 mm). The mean standard deviation of intertransponder distances was 1.0 mm. Intertransponder positional stability was comparable between the left, right, and apical beacons. Daily intertransponder distances were also recorded for three patients, as shown in Figure 2.
Figure 2.
Intertransponder distances at simulation and over a 34-fraction course of treatment for 3 patients in our series
Daily Rotational Offsets
Daily setup rotational offsets were recorded for the 18 patients with three detectable beacons. Over 573 treatments, wide variation in daily setup was seen along all three rotational axes. Average (+/- SD) pitch, roll, and yaw were 1.9 +/- 10.6 degrees, 2.0 +/- 8.3 degrees, and -0.1 +/- 6.2 degrees, respectively.
Setup
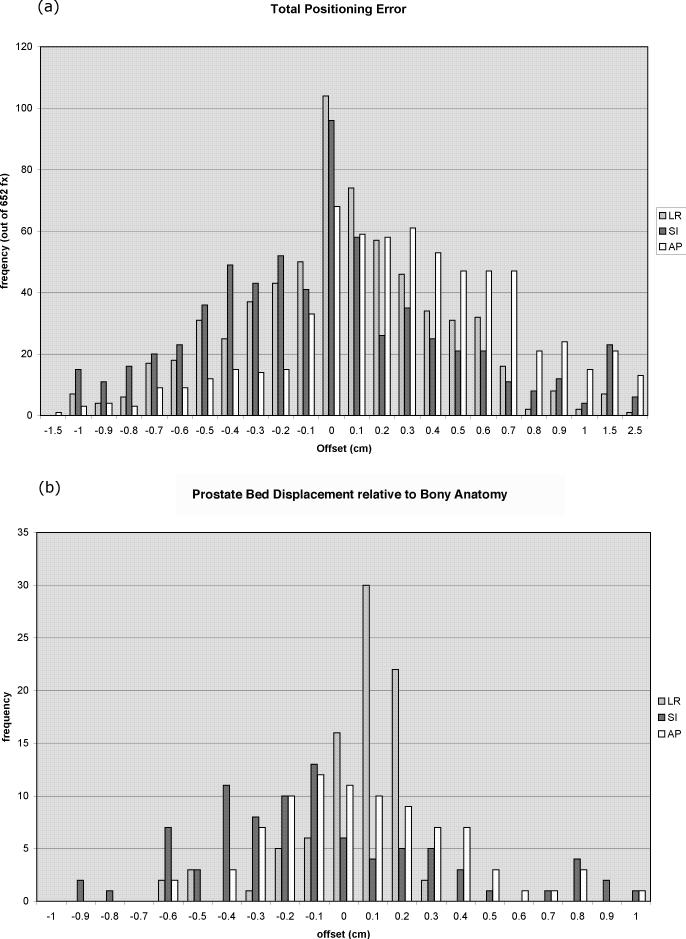
Average magnitudes of daily positioning error, prostate bed motion, and setup errors are reported in Table 1. Daily positioning errors were recorded for 652 fractions, and represent the sum of setup error and prostate bed displacement relative to bony anatomy. Histograms for daily positioning error in the Left-Right (L-R), Superior-Inferior (S-I), and Anterior-Posterior (A-P) axes can be seen in Figure 3a. The A-P (z) axis, along which the prostate bed-rectal interface lies, had particularly large offsets, with 38/652 (5.8%) errors exceeding 1 cm. The three-dimensional offset vector exceeded 1 cm in 157/652 (24%) and 1.5 cm in 36/652 (5.5%) cases. The systematic and random components of the setup error are reported in Table 1e (17). The Van Herk margin for daily alignment to skin markings was calculated to be 9 mm L-R, 13 mm S-I, and 15 mm A-P (17).
Table 1.
Positioning Errors
| # fractions | L-R | S-I | A-P | 3D magnitude | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean magnitude | ± SD | Mean magnitude | ± SD | Mean magnitude | ± SD | Mean | ± SD | ||
| Total Positioning Error | 652 | 3.0 | 4.1 | 3.8 | 5.2 | 4.0 | 4.9 | 7.7 | 4.0 |
| Prostate Bed Displacement | 87 | 1.3 | 1.8 | 3.6 | 4.2 | 2.5 | 3.2 | 5.1 | 2.6 |
| Setup Error | 80 | 3.9 | 5.2 | 4.1 | 5.2 | 4.1 | 4.7 | 8.1 | 4.2 |
Figure 3.
Histograms of (a) daily initial target offsets in three dimensions for a total of 652 fractions and (b) prostate bed motion displacement to bony anatomy for a total of 87 fractions
Prostate bed motion, represented by the difference between Calypso and kV imaging localization, was greatest in the S-I and A-P directions, as shown in the histogram (Figure 3b). A small error, secondary to prostate bed motion between the time of Calypso localization and kV imaging, is introduced by this method. Based on visual examination of the initial part of tracking curves, we estimate that in most cases this error is within one millimeter. Prostate bed motion exceeded 5 mm a total of 8/87 (9%) times in the AP-direction, and 18/87 (21%) times in the SI-direction. The Van Herk margin for daily alignment to bony anatomy were calculated to be 5 mm L-R, 13 mm S-I, and 9 mm A-P (17).
Real-time Tracking
During treatment, the 5-mm tracking threshold was exceeded, in any direction, in 206 of 638 fractions (32%), with the majority of displacements occurring along the S-I and A-P axes (Table 2). Eighteen (90%) patients exceeded the 5 mm threshold at least once during their treatment course. The target exceeded threshold for at least 30 seconds in 16 patients (80%) and 73 fractions (11%), again, mostly along the S-I and A-P axes (Table 2). The AUC was ≥ 1 in 51 (11%) of 487 fractions total (Table 2). Target excursion was greater than 1 centimeter in 13 (2%) instances in the A-P direction and 20 (3%) instances in the S-I direction. Directionality of excursions was about equal in the L-R and S-I directions. There was an increased tendency for posterior target motion in the A-P direction, with maximal excursion trending toward the rectum in 77% of all fractions. In this direction, excursions exceeding 5 mm were twice as likely to be posterior (n=106) than anterior (n=53). Systematic and random components of intra-fractional motion can be found in Table 1e (17).
Table 2.
3-Dimensional Target Excursions during IMRT Delivery
| Left-Right | Superior-Inferior | Anterior-Posterior | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 5 mm Tracking Limit Exceeded ≥ 1 second (out of 638 fractions) | 10 | 1.6% | 176 | 28% | 159 | 25% |
| 5 mm Tracking Limit Exceeded ≥ 30 seconds (out of 638 fractions) | 1 | < 0.5% | 44 | 7% | 31 | 5% |
| Target Excursion ≥ 1 cm (out of 638 fractions) | 0 | 0 | 20 | 3% | 13 | 2% |
| Area Under Curve ≥ 1 (out of 487 fractions) | 1 | <0.5% | 39 | 8% | 30 | 6% |
A total of 96 of 638 treatments (15%) were interrupted for patient repositioning. Of these, 48 occurred before 30 seconds outside tracking threshold had elapsed, including 5 fractions where the target was approaching but had not yet exceeded tracking limits. Had realignment not occurred, it is likely that the target would have exceeded tracking limits for the remainder of that day's treatment duration, meaning that, for this series, potentially significant deviations could have occurred in 121 (19%) treatments.
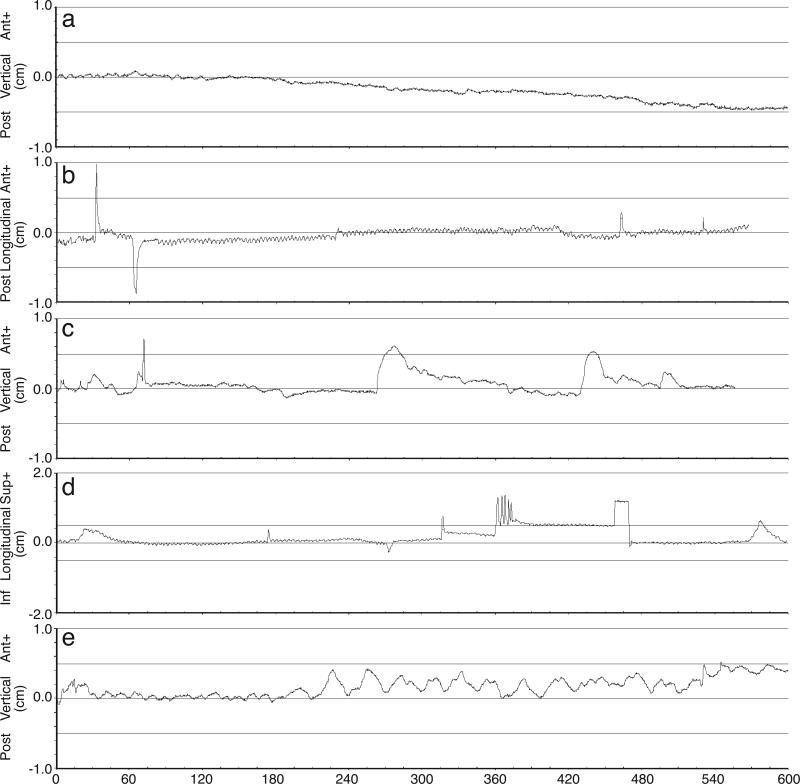
In fractions with target excursion exceeding the 5mm tracking limit, the pattern of motion was reviewed and categorized as (a) continuous drift, (b) high frequency excursions, (c) transient excursions, (d) persistent excursion, or (e) erratic behavior (10). Representative examples from our dataset are shown in Figure 4. Of the total 126 fractions reviewed and categorized, the most common behavior was continuous drift (63 instances) with target displacements predominantly in the inferior and posterior directions. Two-thirds of treatments exhibiting continuous drift were interrupted for repositioning, the greatest proportion of any of the motion behavior categories. The second-most common behavior described was ‘transient excursion’, of which 5 out of 35 treatments were interrupted for realignment. Target motion exhibiting erratic motion or high frequency excursions were unlikely to prompt repositioning.
Figure 4.
Representative tracking curves demonstrating (a) continuous drift, (b) high frequency excursions, (c) transient excursions, (d) persistent excursion (note that patient was repositioned at t ≈ 470 seconds), and (e) erratic behavior
Discussion
This is the first study to provide an in-depth analysis of prostate bed motion during a fraction of radiotherapy treatment. Using implanted radiofrequency transponders, the Calypso system allows for both initial localization and real-time tracking of the prostate bed without additional radiation exposure.
The precision of any fiducial-based localization system is limited by the positional stability of each fiducial marker with respect to its surroundings. A previous investigation found the positional variation of gold fiducials implanted within the prostate bed to fall within the 0.9 mm measurement uncertainty of the kV imaging-based measurement system (8). In our study, we found that relative change in transponder position commonly exceeded 1 mm, well in excess of the sub-millimeter measurement uncertainty of the Calypso system (9). As seen for the three representative patients plotted in Figure 2, intertransponder distances appear to vary randomly over time. If the beacons were migrating within the soft tissues of the prostate bed, intra-transponder distances would be expected to demonstrate a pattern of progressive change over the course of treatment rather than arbitrary fluctuation. The observed pattern of intertransponder distances, rather, is likely due to prostate bed deformation by bladder and rectal filling.
In contrast to intact prostate, which is not significantly deformed by bladder or rectal filling (18), the prostate bed is a volume delineated largely by the position of the bladder and rectum and is therefore subject to distortion secondary to bladder and rectal volume changes. In a series of 10 post-prostatectomy patients treated at Thomas Jefferson University Hospital, Showalter identified rectal and bladder volumes and wall positions on 166 pretreatment CBCT images [4], used for daily localization to bony anatomy. Daily variations in organ filling resulted in corresponding changes in the distance between bladder and rectum, effectively displacing and deforming the prostate bed volume (4).
This deformation may also explain the apparent increased daily rotational variability of the prostate bed when compared to intact prostate (12) (Table 2e). Rotational offset, calculated from positional displacement of the three beacons around the L-R, S-I, and A-P axes, has been shown in phantom studies to be accurate to within 1 degree (12). Small changes in relative fiducial position, however, can dramatically affect the measured rotation (19), introducing an error. Rotational misalignment results in corresponding geometrical offset (12), for which proper accounting requires an accurate measurement of the actual rotation of the prostate bed. Adaptation of existing methods of accounting for variations in relative fiducial positions (19, 20) to the Calypso system is an intriguing topic for further investigation.
The positional difference between the treatment (machine) and target (prostate bed) isocenters at initial setup is the sum of setup error (misalignment of patient to machine) and prostate bed displacement within the patient. In our dataset, there was no correlation between prostate bed displacement and total positioning offsets in any direction, and a robust correlation between positioning and setup errors (Figure 2e). These results imply that the setup error is more significant component of positioning error than prostate bed displacement, and are consistent with the findings of previous investigators (7, 8).
While not the major contributor to positioning error, prostate bed motion must be considered when assigning treatment margins. In our series, prostate bed motion exceeded the 5-mm margin in the A-P direction for 9% of all 87 fractions. Prostate bed motion greater than 5 mm in the A-P direction was found to occur in 3.7% of 163 fractions by Schiffner (8), and in 10% of 384 fractions by Sandhu [7], using orthogonal kV imaging of implanted gold seed fiducials and surgical clips, respectively, to localize the prostate bed. Based on his analysis of localization CBCTs, Showalter recommended PTV margins of up to 10.2 mm at the posterior rectal wall when aligning to bony anatomy (4), which is close to the 9 mm calculated here. In practice, institutions will need to determine their planning margins based on their own random and systemic errors related to the simulation and treatment planning process, patient population, patient setup, and equipment.
Precise daily alignment of the target prior to each treatment is no guarantee that it will remain aligned, or even within the planning margin of error, for the duration of that treatment. We observed that real-time motion characteristics of the prostate bed appeared to be similar to those of the intact prostate, and are both likely the consequence of bowel and bladder filling. In the current series, target motion exceeded the 5-mm tracking limit for at least 30 seconds and resulted in repositioning in 11% and 15% of all fractions, respectively. A large multi-institutional study of Calypso in the setting of intact prostate found that the 5mm tracking limit was exceeded for >30 seconds in 15% of 1157 fractions (10). Repositioning occurred 8% of the time; any intervention, which in that series included treatment pauses and gating in addition to repositioning, occurred in 11% of all fractions [10]. As with intact prostate [10], the longitudinal and vertical offsets typically moved together throughout each individual treatment, whereas the lateral offsets were the most stable. 77% of the time, the target moved posteriorly towards the rectum, suggesting that bladder rather than rectal filling may have a greater impact on target displacement during treatment. Repeated posterior target drift may result in possible underdosing of the posterior aspect of the prostate bed PTV.
The degree to which any individual patient benefited from real-time tracking is related to how often that tracking resulted in treatment interruption or repositioning. In our series, 30% of patients made it through their course of radiation treatment without any interruptions for repositioning. Only five of twenty (25%) patients were repositioned more than five times over their treatment course. One patient demonstrated a daily posterior and inferior target drift, and was repositioned for over 90% of his treatments. In the absence of real-time tracking, these patients may have benefited from larger treatment margins. Unfortunately, identifying those patients who would benefit most from real-time tracking or margin expansion is just about as difficult as measuring the magnitude of that benefit.
The effect of intra-fraction motion on treatment delivery and resulting clinical outcomes is difficult to quantify. It is dependent not only on the magnitude and duration of target displacement, but also on the beam configurations and Monitor Units delivered during that interval, as well as the dose-response characteristics of the tissues within the treatment field. For simplicity, in this study a target excursion was considered significant if, in any dimension, the target exceeded the tracking limit for at least 30 seconds [10] or if the AUC was ≥1.
Much larger patient numbers and several years of follow-up are needed to estimate the actual impact of intra-fraction motion on tumor control and toxicity outcomes.
Conclusion
Set-up errors due to differences in positioning relative to bony anatomy and displacement of the prostatic fossa during radiotherapy are substantial. This can result in undertreatment of target and overtreatment of surrounding critical structures, and must be taken into account when prescribing treatment margins. Calypso-based localization and real-time tracking of the prostate bed is a feasible method to ensure precise delivery of highly conformal radiation treatment plans.
Supplementary Material
Supplementary Figure Captions
Figure 1e: Graphic representation of AUC value
Figure 2e: Scatter plots demonstrating the relationship between total positioning error and its components, prostate bed displacement relative to bony anatomy and setup error.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors would like to thank Nicholas Zaorsky, MD, Karen Trush, and Keith Fraser for their artistic contributions.
Conflict of Interest Notification: No actual or potential conflicts of interest exist.
References
- 1.Cozzarini C, Fiorino C, Ceresoli GL, et al. Significant correlation between rectal DVH and late bleeding in patients treated after radical prostatectomy with conformal or conventional radiotherapy (66.6-70.2 Gy). Int J Radiat Oncol Biol Phys. 2003;55:688–694. doi: 10.1016/s0360-3016(02)04117-2. [DOI] [PubMed] [Google Scholar]
- 2.Teh BS, Mai WY, Augspurger ME, et al. Intensity modulated radiation therapy (IMRT) following prostatectomy: more favorable acute genitourinary toxicity profile compared to primary IMRT for prostate cancer. Int J Radiat Oncol Biol Phys. 2001;49:465–472. doi: 10.1016/s0360-3016(00)01474-7. [DOI] [PubMed] [Google Scholar]
- 3.Fiorino C, Foppiano F, Franzone P, et al. Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiother Oncol. 2005;74:187–195. doi: 10.1016/j.radonc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Showalter TN, Nawaz AO, Xiao Y, et al. A cone beam CT-Based Study for Clinical Target Definition Using Pelvic Anatomy During Postprostatectomy Radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:431–436. doi: 10.1016/j.ijrobp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Chinnaiyan P, Tomee W, Patel R, et al. 3D-ultrasound guided radiation therapy in the post-prostatectomy setting. Technol Cancer Res Treat. 2003;2:455–458. doi: 10.1177/153303460300200511. [DOI] [PubMed] [Google Scholar]
- 6.Paskalev K, Feigenberg S, Jacob R, et al. Target localization for post-prostatectomy patients using CT and ultrasound image guidance. J Appl Clin Med Phys. 2005;6:40–49. doi: 10.1120/jacmp.v6i4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandhu A, Sethi R, Rice R, et al. Prostate bed localization with image-guided approach using on-board imaging: reporting acute toxicity and implications for radiation therapy planning following prostatectomy. Radiother Oncol. 2008;88:20–25. doi: 10.1016/j.radonc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Schiffner DC, Gottschalk AR, Lometti M, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:610–619. doi: 10.1016/j.ijrobp.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Balter JM, Wright JN, Newell LJ, et al. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:933–937. doi: 10.1016/j.ijrobp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Li HS, Chetty IJ, Enke CA, et al. Dosimetric consequences of intrafraction prostate motion. Int J Radiat Oncol Biol Phys. 2008;71:801–812. doi: 10.1016/j.ijrobp.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Li JS, Jin L, Pollack A, et al. Gains from real-time tracking of prostate motion during external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2009;75:1613–1620. doi: 10.1016/j.ijrobp.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Langen KM, Willoughby TR, Meeks SL, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Canter D, Greenberg RE, Horwitz EM, et al. Implantation of electromagnetic transponders following radical prostatectomy for delivery of IMRT. Can J Urol; 17:5365–5369. [PubMed] [Google Scholar]
- 15.Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 76:361–368. doi: 10.1016/j.ijrobp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz E, Sharma N, Ruth K, et al. The Role of Adjuvant and Salvage Post-Prostatectomy IMRT or 3DCRT: The Fox Chase Experience. Int J Radiat Oncol Biol Phys. 2010;78:S333–S334. [Google Scholar]
- 17.van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 18.Nichol AM, Brock KK, Lockwood GA, et al. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007;67:48–56. doi: 10.1016/j.ijrobp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Aubry JF, Beaulieu L, Girouard LM, et al. Measurements of intrafraction motion and interfraction and intrafraction rotation of prostate by three-dimensional analysis of daily portal imaging with radiopaque markers. Int J Radiat Oncol Biol Phys. 2004;60:30–39. doi: 10.1016/j.ijrobp.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys. 2002;29:334–344. doi: 10.1118/1.1448823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure Captions
Figure 1e: Graphic representation of AUC value
Figure 2e: Scatter plots demonstrating the relationship between total positioning error and its components, prostate bed displacement relative to bony anatomy and setup error.