Abstract
A major challenge to the effective treatment of injured cardiovascular tissues is the promotion of endothelialization of damaged tissues and implanted devices. For this reason, there is a need for new biomaterials that promote endothelialization to enhance vascular repair. The goal of this work was to develop antibody-modified polysaccharide-based hydrogels that could selectively capture endothelial progenitor cells (EPCs). We showed that CD34 antibody immobilization on hyaluronic acid (HA) hydrogels provides a suitable surface to capture EPCs. The effect of CD34 antibody immobilization on EPC adhesion was found to be dependent on antibody concentration. The highest level of EPC attachment was found to be 52.2 cells per mm2 on 1% HA gels modified with 25 μg mL−1 antibody concentration. Macrophages did not exhibit significant attachment on these modified hydrogel surfaces compared to the EPCs, demonstrating the selectivity of the system. Hydrogels containing only HA, with or without immobilized CD34, did not allow for spreading of EPCs 48 h after cell seeding, even though the cells were adhered to the hydrogel surface. To promote spreading of EPCs, 2% (w/v) gelatin methacrylate (GelMA) containing HA hydrogels were synthesized and shown to improve cell spreading and elongation. This strategy could potentially be useful to enhance the biocompatibility of implants such as artificial heart valves or in other tissue engineering applications where formation of vascular structures is required.
Introduction
Millions of people suffer from the effects of cardiovascular diseases.1 For this reason, there is significant research to generate approaches to treat cardiovascular ailments. For example, the development of stents has generated significant improvement in patient outcomes.2,3 Furthermore, by using tissue engineering approaches it may be possible to generate artificial tissue constructs that could be used to replace diseased or damaged tissues. In both of these approaches, and indeed for virtually any implanted biomaterial, the interaction of the host with the tissue of interest is of great importance.
A current limitation with artificial cardiovascular devices is that without appropriately engineered surfaces, they do not effectively promote vascular healing and may lead to thrombosis and other complications. One approach to increase the biocompatibility of artificial implants is to form a monolayer of endothelial cells on their surface.4 A potentially powerful cell source for endothelialization of biomaterials is circulating EPCs in the blood stream.5–9 EPCs have previously been reported to hasten surgical healing, re-epithelialization, angiogenesis and vascularization events.10–15 For this reason, direct capture of circulating EPCs from the blood could be an attractive strategy to achieve in vivo endothelialization of cardiovascular grafts and tissue engineering scaffolds.4 Therefore, EPC capture approaches are promising strategies for endothelialization of blood-contacting implants, cardiovascular stents and artificial heart valves. As an example, stents are often coated with antibodies, which have affinities against surface antigens of EPCs.2,16,17 For instance, anti-human CD34 antibody has been immobilized on stainless steel stents to capture EPCs in vivo as CD34 is naturally present on the cell surface of EPCs.2,3,16–19 These implants indicated that the use of EPC capturing stents improved re-endothelialization in clinical studies.2 We hypothesized that the combination of non-adhesive polysaccharide gels, such as hya-luronic acid (HA) with CD34 antibodies, could be a useful approach to synthesize materials that can selectively capture EPCs. These materials could potentially be useful for a number of biomedical applications ranging from vascularizing tissue-engineering scaffolds to endothelializing stents.
Biodegradable anti-coagulant substrates are attractive materials for surface coatings on artificial implants. HA is a negatively charged polysaccharide and important component of the extracellular matrix (ECM) of many tissues.20–22 HA has also been reported to play a significant role in wound healing processes.20,23,24 In addition, HA takes part in cell attachment and signaling events by interacting with cell surface receptors. An interesting aspect of HA or other negatively charged poly-saccharides (like heparin) is that they are also non-thrombogenic and have been used as anti-coagulants.25–28 Therefore, they could be used as a coating material to decrease blood coagulation on a material.
Polysaccharide-based hydrogels have previously been used by Thebaud et al. to provide support for EPC attachment.29 In most cases, however, HA based gels and HA coated surfaces have been shown to be non-biofouling.30–32 Due to its non-adhesive features, HA is a suitable biomaterial to prevent non-specific adhesion of cells in the body. Since HA is prevalently found in vascular tissues and heart valves, it has the potential to produce biocompatible materials for cardiovascular tissue engineering. Furthermore, due to the highly negative charge of HA, it can be easily integrated into layer-by-layer approaches for coating surfaces.
The overall goal of this study was to develop a new material that could be used to promote the endothelialization of materials in the body through the merger of non-biofouling polysaccharide based hydrogels with antibodies that could selectively capture circulating EPCs. Here we developed a material that merges the desirable properties of HA hydrogels with an antibody coating that could be used to recruit EPCs from the blood to hasten re-endothelialization for implants such as artificial tissues or stents. We report that CD34 antibody immobilization on HA-hydrogels provides a suitable surface to selectively capture EPCs as a subset of these cells expresses CD34 antigens on their surfaces. Finally, we determined suitable conditions to promote capturing and spreading of EPCs on HA-based hydrogels in two-dimensions (2D) by incorporating collagen-based materials with HA.
Experimental
Materials
Gelatin, methacrylic anhydride, 3-(trimethoxysilyl) propyl methacrylate, 3-(N-morpholino) propanesulfonic acid sodium salt and N-hydroxysuccinimide were purchased from Sigma-Aldrich (St Louis, MO). Sodium hyaluronate was obtained from Lifecore Biomedical (Chaska, MN). N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride was purchased from Thermo Scientific (Rockford, IL). 2-Hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959) was obtained from Ciba (Florham Park, NJ). Mouse antibodies to CD31 and von Willebrand Factor, FITC and Rhodamine labeled antibodies to mouse IgG were supplied by Abcam (Cambridge, MA). Mouse antibodies to CD34 and its FITC labeled analog were obtained from BioLegend (San Diego, CA). Calcein AM and Phalloidin (Alexa Fluor 594) stains were purchased from Invitrogen (Carlsbad, CA). 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Vector Laboratories (Burlingame, CA). Pre-cleaned microscope glass slides were purchased from Fisher Scientific (Waltham, MA). Medium for EPCs and macrophages and their components were purchased from Gibco-Invitrogen (Carlsbad, CA) and ATCC (Manassas, VA), respectively. All reagents were used as supplied without further purification.
Production of photocrosslinkable HA precursors
We synthesized methacrylated HA as reported before.33 Briefly, we added 1 gram of sodium hyaluronate to 100 mL of water. After obtaining a homogenous solution we placed 1 mL of methacrylic anhydride, then adjusted the pH of the mixture to 8– 9 and maintained it in the same range throughout the reaction. The reaction was carried out on ice for 24 h. We then dialyzed the final product against deionized water for 3 days at 4 °C and then lyophilized the resulting solution for 3 days. 1H NMR analysis indicated a degree of methacrylation of 16%.
Production of HA-based hydrogels
We prepared 1% (w/v) methacrylated HA in PBS solution containing 0.5% (w/v) photoinitiator. This prepolymer solution was kept at 80 °C until the HA completely dissolved. To enable spreading of EPCs to endothelialize the surface we also made hydrogels containing 1% (w/v) methacrylated HA, 2% (w/v) methacrylated gelatin and 0.5% (w/v) photoinitiator. After complete dissolution of solid components we exposed prepolymer solutions to UV-light for 180 s at 5.5 mW cm°2 between two untreated glass slides with 1 mm thick spacers. Following photopolymerization, the two glass slides were separated and 4 mm diameter hydrogel samples were punched using biopsy punches. A thin HA layer was coated on 3-(trimethoxysilyl) propyl methacrylate (TMSPMA) treated glass slides to attach the punched hydrogel samples. After this step we carried on with antibody immobilization.
Confirmation of covalent attachment on HA hydrogels
To conjugate CD34 antibody on the surface of gels, we incubated HA hydrogels in 3-(N-morpholino) propanesulfonic acid (MES) buffer (pH 5.6) for 30 min. After removing the buffer, the 0.1 M N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide (EDC) and 0.2 M N-hydroxysuccinimide (NHS) mixture were added to activate the carboxylate groups on HA hydrogels. This mixture was incubated on a shaker for 1 h and then the EDC/NHS solution was removed. Activated hydrogels were washed in PBS for 10 min. Upon removal of PBS, 1, 10 or 25 μg mL−1 FITC conjugated or non-labeled anti-human CD34 antibody was added on the top surface of the hydrogels and the resulting material was incubated at 4 °C overnight. We then kept these hydrogels in PBS overnight to allow for desorption of nonspecifically adsorbed antibodies. All experiments were carried out in triplicate. Dimensionless fluorescent intensity values were obtained utilizing ImageJ analysis by dividing the fluorescence with the background value and plotted against antibody concentration.
Characterization of antibody modified hydrogels
The modified gels were characterized by using an inverted Nikon fluorescent microscope (Melville, NY) to analyze the amount of fluorescence from the FITC labeled antibodies. Fluorescent intensities of images were examined using ImageJ and compared with the negative controls, which did not include the antibody incubation step. For further confirmation we incubated the CD34 antibody modified gels with a Rhodamine-labeled secondary antibody and analyzed them with a fluorescent microscope.
Cell cultures
Human EPCs were isolated from infant cord blood and kindly provided by Dr Joyce Bischoff’s lab. Macrophage P388D1 cell line was provided by Dr Blaine Pfeifer. The EPCs were cultured in EBM-2 culture media and macrophages using RPMI-1640 media with 10% Fetal Bovine Serum (FBS) supplement. The medium for cell cultures was changed every two to three days. All cell cultures were maintained in a humidified incubator at 37 °C and 5% carbon dioxide. EPCs of passage number 7 were harvested by trypsinization and then counted with a hemacytometer to obtain a density of 4.1 × 104 cells per cm2 for cell seeding experiments. We utilized EPCs with the same passage number throughout out experiments due to possible decrease in the level of CD34 expression for higher passage numbers.
Characterization of cultured cells
The EPCs with passage number 7 were analyzed for their expression of CD34 by immunostaining. Briefly, after harvesting cells, they were washed with PBS buffer containing 0.1% sodium azide. The cells were then resuspended in the same buffer and incubated with FITC-labeled CD34 antibody for 1 h. We washed the cells and analyzed them with flow cytometry. Furthermore, we immunostained the cells by following standard procedures for CD31 and vWF expression at 48 h. To immunostain, cells were fixed with paraformaldehyde, incubated with bovine serum albumin (BSA) to block non-specific binding and permeabilized by Triton X-100. The samples were then incubated with primary and secondary antibodies for 15 min respectively by 1/100 dilution. The cells were washed with PBS and analyzed with an inverted fluorescent microscope to obtain phase-contrast and fluorescent images. DAPI and Phalloidin stainings were carried out following manufacturers’ standard protocols.
Capturing of EPCs on CD34 coated HA substrates
To test the adhesion of EPCs and macrophages on the CD34 conjugated gels, cells were seeded at a density of 4.1 × 104 cells per cm2 of varying concentrations of CD34 antibody coating or controls without antibody modification. Two different CD34 antibody concentrations (10 and 25 μg mL−1) were utilized to study the attachment of EPCs on the antibody-modified hydrogels. Macrophages were chosen as the negative control as they did not express CD34 on their cell surface as determined from the flow cytometry analysis (see ESI†). We tested the adhesion of both cell types at various times after seeding (5 min, 1 h, 3 h and 6 h). At each time point the non-adherent cells were gently washed from the surface and the remaining cells were visualized and quantified. To characterize the viability of the cells, which were captured on HA-based hydrogels Calcein AM staining was utilized. Then the amount of attachment was visualized by utilizing fluorescent imaging and quantified by ImageJ program. To test the spreading of captured EPCs we used a 1% HA and 2% GelMA combination to produce the hydrogel surface. Then we carried out the antibody modification in the same way explained above. Spreading and elongation of EPCs were quantified by calculating the shape index with the following formula:
where S represents the shape index, A is the area and P is the perimeter of the cell.
Statistical analysis
To analyze the antibody immobilization and EPC attachment data we used standard statistical software by GraphPad Prism (Version 4.02, La Jolla, CA). All experiments were run in triplicate. The differences between groups were analyzed by using one-way ANOVA, two-way ANOVA and Bonferroni tests. All data are presented as mean × standard deviation (SD). p-values that were less than 0.05 were considered significant. * represents p < 0.05, whereas ** and *** represent p < 0.01 and <0.001, respectively.
Results and discussion
Synthesis and characterization of photocrosslinkable HA hydrogels conjugated with CD34 antibody
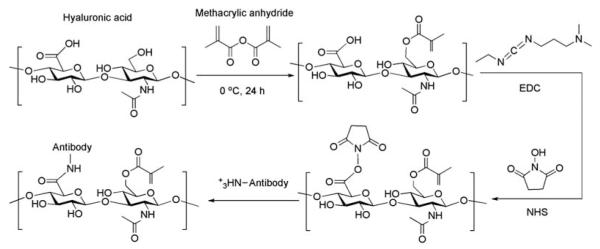
In this study HA-based hydrogels were conjugated with CD34 antibodies to render them selectively adhesive to EPCs. Fig. 1 demonstrates the scheme to develop photocrosslinked HA-based hydrogels with covalently conjugated anti-human CD34 antibodies. Covalent immobilization of the antibody was performed by following standard amine coupling methods using EDC/NHS conjugation process. Photocrosslinkable HA was synthesized as reported through a methacrylation reaction.33 These hydrogels have been previously shown to be cell compatible and capable of forming tissue-like structures of various geometries.31 Also, we have previously characterized their properties such as the presence of the methacrylate groups in the modified gels by 1H NMR analysis. The schematic in Fig. 1 represents only one of the possible outcomes for the chemical modification processes we performed. Methacrylation of the alcohol groups at position 6 in the second sugar moieties does not take place with full efficiency for all repeating units of HA. For instance, the degree of methacrylation for HA was determined to be 16% by NMR analysis in this study. Likewise, covalent immobilization of anti-CD34 antibody does not take place in all repeating units in HA polymer.
Fig. 1.
Schematic of the process to develop photocrosslinked HA-based hydrogels with covalently conjugated anti-human CD34 antibodies. Standard amine coupling strategy was followed for covalent antibody immobilization on the surface of hydrogels. Methacrylic anhydride was first used to attach the photoactivatable functional groups on hyaluronic acid. Then EDC/NHS was utilized to activate the carboxyl substituents for immobilization of CD34 antibodies. Neither methacrylation reaction nor covalent antibody immobilization process takes place with full efficiency.
The average molecular weight of HA that we used in our experiments was 53 kDa, which is considered in the low molecular weight range. It was reported in the literature that low molecular weight HA possesses lower water absorption features compared to its high molecular weight analogs.34
Characterization of covalent CD34 antibody immobilization
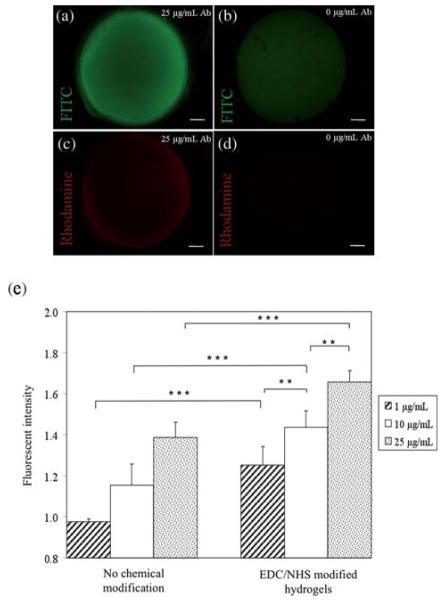
In the current set of experiments, we confirmed the conjugation of the antibody on HA hydrogels by performing fluorescence analysis. Immobilization of three different concentrations (1, 10 and 25 μg mL−1) of FITC-labeled CD34 antibodies was performed along with a negative control without the antibody incubation step. The fluorescent images for 25 μg mL−1 CD34 antibody and the control are shown in Fig. 2a and b. The fluorescent image demonstrating the absorbance from FITC labeled anti-human CD34 antibody modified HA hydrogels is given in Fig. 2a. In addition, the adsorption of CD34 antibody onto the HA hydrogel without such chemical modification was investigated by omitting the EDC/NHS activation step of the reaction. Fig. 2b represents the negative control sample without antibody conjugation. Some degree of non-specific protein adsorption was observed as expected for the non-modified HA hydrogels but this was significantly less than their chemically modified analogs. A Rhodamine-labeled antibody, with specific affinity to the immobilized CD34, was used for further confirmation for covalent immobilization (Fig. 2c and d). A small amount of nonspecific antibody adsorption was observed on the non-modified hydrogel. These figures together suggest that the CD34 antibody was covalently conjugated to the surface of the hydrogels. To further characterize these findings quantification of fluorescence intensity of the resulting HA-based antibody conjugated gels was performed by using ImageJ Software. The resulting data was then processed by using standard statistical tests (ANOVA and Bonferroni comparisons) to evaluate statistical significance of the results (Fig. 2e). Dimensionless fluorescent intensity values, which were obtained by dividing the fluorescence with the background value, are given in Fig. 2e. The background value is described as the fluorescence intensity measured for control hydrogels. To obtain these values, control hydrogels were treated in the exact same conditions and incubated with only buffer without antibody in the final step. A non-chemically modified hydrogel was used as a control for the “non-chemical modification” group whereas an EDC/NHS treated hydrogel was used for the “EDC/NHS modified hydrogels” group. Based on these calculations, chemically modified surfaces with 25 mgmL−1 CD34 produced a fluorescence intensity of 1.66 ± 0.06 compared to the non-modified surface with an intensity of 1.39 ± 0.08. Once the the × concentration of CD34 antibody was decreased to 10 μg mL−1 on the modified hydrogel the fluorescent intensity changed to 1.44 ± 0.08, which was significantly different from the 1 μg mL−1 antibody dose (p < 0.01).
Fig. 2.
Characterization of antibody conjugated HA hydrogels. (a) Fluorescent images of FITC labeled anti-human CD34 antibody modified HA-based hydrogel demonstrating the fluorescent absorbance, (b) negative control with no antibody conjugation. It was shown that there was some degree of non-specific protein adsorption without the antibody coating as expected, but this was measured to be significantly less compared to the antibody modified gel surface, (c) Rhodamine labeled secondary anti mouse antibody on FITC-CD34 modified hydrogel confirmed the antibody immobilization on the hydrogel surface, and (d) negative control with no antibody conjugation. There was some degree of nonspecific antibody adsorption on the non-modified hydrogel, (e) statistical analysis for the quantification of fluorescent intensity on hydrogels. Chemical modification of the hydrogel surface was found to be creating a significantly higher effect on the amount of immobilized CD34 antibodies than the non-modified analog (error bars: ±SD, **p < 0.01, and ***p < 0.001). The scale bars represent 500 μm.
When the antibody concentration was further reduced for the same surface the fluorescence intensity was significantly decreased to 1.25 ± 0.09 (p < 0.01). The fluorescence intensity was significantly higher on the modified surface for the 1 μg mL−1 CD34 dose as compared to the negative control without antibody immobilization (p < 0.001). This decreasing trend for fluorescence intensity with respect to decreasing antibody concentration can be attributed to the number of available substituents of the antibody interacting with the activated functional groups on the surface of HA gels. Fluorescence intensity for the negative control (0 μg mL−1 antibody concentration) is not shown in Fig. 2e as it was used as the background value.
A significant concentration effect was also observed on the non-modified hydrogels, with fluorescence intensities ranging from 1.39 ± 0.08 to 0.98 ± 0.01 (p-values 0.05–0.001). In these experiments, increasing the antibody concentration provided significantly higher immobilization for CD34 antibodies (p < 0.001). These results demonstrate that chemical modification of the hydrogel surface produced a statistically significant improvement in antibody attachment. EDC/NHS modification significantly enhanced the amount of CD34 immobilized for all conditions with 1, 10 or 25 μg mL−1 antibody concentration (p < 0.001). Although there was still fluorescent absorbance on the hydrogels without chemical modification, as expected, the quantification demonstrated significantly smaller intensities.
Characterization of captured EPCs
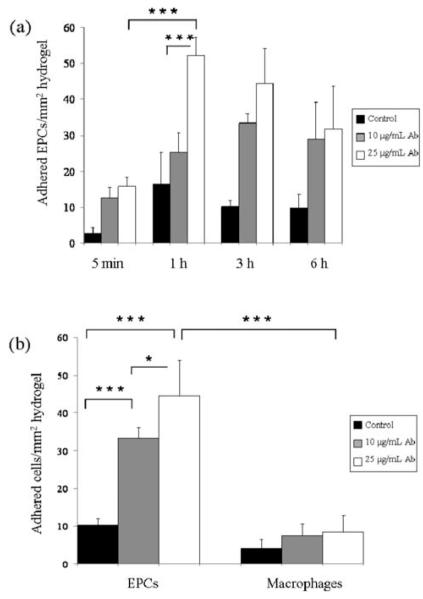
Two different CD34 antibody doses (10 and 25 μg mL−1) were tested to study the attachment of EPCs at a seeding density of 4.1 × 104 cells per cm2 on antibody-modified hydrogels. Fig. 3a–d shows captured cells on HA-based hydrogels stained with Calcein AM for the intracellular esterase activity, a measure of cell viability. Quantification of the amount of captured EPCs and macrophages on HA hydrogels at different times was carried out using ImageJ software (Fig. 4a and b). The highest number of EPCs per mm2 area was obtained as 52.2 ± 5.0 on 25 μg mL−1 CD34 antibody modified hydrogels at 1 h. In ×contrast, the number of adhered EPCs on control hydrogels without CD34 antibody modification was significantly lower. There was no statistical difference in terms of the number of attached EPCs with times greater than 1 h (Fig. 4a). However, it was determined that increasing the antibody concentration significantly increased cell attachment. In comparison to the 25 μg mL−1 CD34 antibody modified hydrogels that captured 52.2 ± 5.0 EPCs per mm2 after 1 h incubation, there was roughly a 2-fold decrease in the number of adhered cells per mm2 (25.3 ± 5.3) at the 10 μg mL−1 CD34 antibody dose for the same time point (p-value < 0.001). The increase in the number of adhered cells with increasing antibody concentration suggests the possibility of capturing more EPCs by modifying the hydrogel surface with a higher amount of CD34 antibodies. EPC attachment was determined to be significantly higher after 1 h seeding as compared to 5 min time point (Fig. 4a). However, increasing the cell capture time longer than 1 h did not significantly increase the number of adhered EPCs. Macrophages, which were used as a negative control, did not show statistically significant attachment as compared to the EPCs. Macrophage attachment was found to be significantly less than that of EPCs (p < 0.001) (Fig. 4b). This result was expected, as based on the flow cytometry results macrophages do not express CD34 on their cell surfaces. After 3 h cell seeding, only 8.5 ± 4.2 macrophages per mm2 were observed to be adhered compared to EPCs (44.5 ± 9.5) at 25 μg mL−1 CD34 antibody concentration (p < 0.001). Furthermore, no significant difference in macrophage cell attachment was observed with respect to decreasing CD34 antibody concentration (8.5 ± 4.2 and 7.5 ± 3.1 for 25 μg mL−1 and 1 μg mL−1 concentration respectively). Non-modified hydrogels demonstrated even less binding, with only 4.1 ± 3.1 bound macrophages per mm2.
Fig. 3.
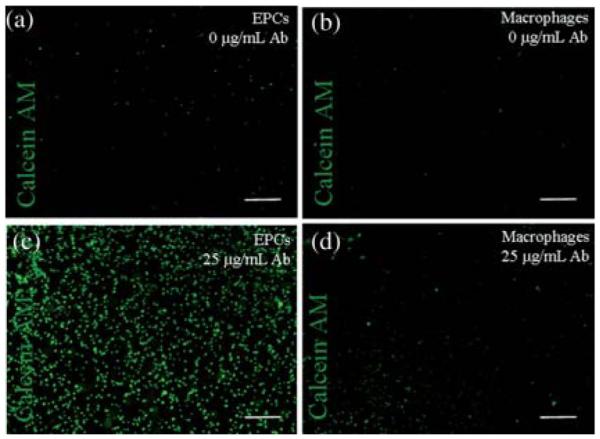
Cell adhesion at a seeding density of 4.1 × 104 cells per cm2 on surface modified HA-hydrogels at 1 h. (a) EPC attachment on control hydrogel without CD34 antibody modification was found to be low, (b) macrophage attachment on control hydrogel without CD34 antibody modification was low, (c) 25 μg mL−1 CD34 antibody modified hydrogel allowed capture of significant amount of EPCs, and (d) macrophage attachment on 25 μg mL−1 CD34 antibody modified hydrogel was significantly less compared to the amount of adhered EPCs. The scale bars represent 400 μm.
Fig. 4.
Quantification of the amount of captured EPCs and macrophages on HA-based hydrogels at different times. (a) Quantification of cell attachment on CD34 antibody immobilized gels. EPC attachment was found to be significantly higher after 1 h seeding compared to 5 min time point. After 1 h, though, there was no statistical difference in terms of adhered EPCs with respect to increasing time. (b) Comparison of the amount of captured EPCs and macrophages on HA hydrogels at 3 h. No significant difference was observed for macrophage attachment at different antibody concentrations for the same time point. In addition, macrophages adhered significantly less on the hydrogels compared to EPCs (error bars: ±SD, * p < 0.05, and ***p < 0.001).
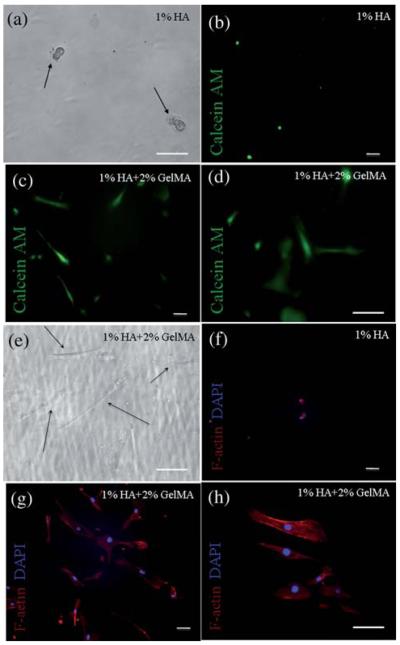
In addition to cell attachment tests, spreading and elongation of EPCs on HA-based hydrogels were also determined at 24 h (Fig. 5a–h). Phase-contrast image for adhered EPCs is shown on 1% (w/v) HA hydrogels in Fig 5a. This data demonstrated that gels containing only HA do not allow for spreading of EPCs following adhesion. In that case cells remained rounded on the hydrogel surface. To improve the spreading behavior, we combined 2% (w/v) GelMA with 1% (w/v) HA to produce hydrogels for cell attachment.
Fig. 5.
Spreading and elongation of EPCs on surface-modified HA hydrogels after 24 h seeding. (a) Phase-contrast image for adhered EPCs on 1% HA hydrogel, EPCs remained rounded on the hydrogel surface which contained only HA, (b) fluorescent image for Calcein AM stained EPCs on CD34 antibody modified 1% HA hydrogel at 24 h indicated no spreading on the non-adherent gel, (c and d) fluorescent images for Calcein AM stained EPCs on CD34 antibody modified (1% HA + 2% GelMA) hydrogel at 24 h, GelMA containing surface allowed spreading of EPCs, (e) phase-contrast image of elongated EPCs on (1% HA + 2% GelMA) hydrogel. EPCs continued spreading on the GelMA containing HA surfaces, (f) fluorescent image for Phalloidin and DAPI staining for EPCs on CD34 antibody modified 1% HA hydrogel at 24 h, and (g and h) EPCs on CD34 antibody modified (1% HA + 2% GelMA) hydrogels at 24 h. The scale bars represent 100 μm.
The phase-contrast image for adhered EPCs on GelMA containing surfaces is given in Fig. 5e. Addition of GelMA allowed EPCs to spread on combination hydrogels after they were captured. Adhered cells were also stained with Calcein AM to display their spread morphology. Fluorescent image for Calcein AM stained EPCs on CD34 antibody modified 1% HA hydrogels at 24 h is shown in Fig. 5b. EPCs adhered and elongated on CD34 antibody modified 1% HA and 2% GelMA combination hydrogels (Fig. 5c and d). Phalloidin staining further demonstrated the spreading and elongation of EPCs. The fluorescent images for Phalloidin staining of F-actin in EPCs are given in Fig. 5f–h. These images demonstrate the actin filament distribution throughout the cells. Cells were counterstained with DAPI to visualize the nuclei. There was no spreading observed on gels containing HA only, however, 1% HA and 2% GelMA combination hydrogels promoted spreading of EPCs (Fig. 5g and h). Shape index for cells attached to both type of hydrogels was calculated using ImageJ, where a shape factor of 1 indicated a perfect circle and 0 a straight line. Cells on gels containing only HA produced an average shape index of 0.962 ± 0.016, whereas cells attached to 1% HA and 2% GelMA gels produced an index of 0.336 ± 0.060 implying significantly higher spreading for the combination hydrogels (p < 0.001 with standard t-test).
According to the flow cytometry results only 6% of the EPCs used expressed CD34 antigens. Although, at first the number of adhered EPCs per mm2 may appear to exceed the theoretical maximum, once the data was normalized based on the number of captured cells on control hydrogels we obtained values only slightly higher than the number of cells expected. This may implicate that there may be a small degree of non-specific cell attachment on these hydrogels. This condition could potentially be improved by addition of P-selectin, which was reported to have specific affinity for progenitor cells. In the future we will include P-selectin in addition to CD34 antibody to improve the specificity of this method.
Although we did not study non-specific attachment of macrophages on 2% (w/v) GelMA including 1% HA hydrogels, we think that there would be a higher number of macrophages adhering to the surface of hydrogels as compared to only HA-containing gels as gelatin possesses cell adhesive functionalities. However, CD34 antibody modified hydrogel surfaces would promote the EPC binding through specific antibody–antigen interactions. For this reason, we expect to observe a significant difference in the number of adhered EPCs and macrophages on GelMA containing HA-based hydrogels.
In addition to capturing EPCs on the surface modified hydrogels, we also carried out immunostaining for the expression of endothelial cell markers CD31 and von Willebrand Factor (vWF) 48 h after seeding on 1% HA and 2% GelMA combination hydrogels. CD31 is expressed on the cell surface of EPCs4 whereas the more specific endothelial marker vWF is expressed once they start differentiating into endothelial cells.35,36 These results showed that the EPCs expressed CD31 and vWF onantibody modified, GelMA containing HA hydrogels (data not shown) confirming the suitability of the HA-based substrate. This result supports our hypothesis that CD34 antibody modified HA hydrogels could possibly promote formation of an endothelial layer once seeded with EPCs.
Taken together, the data suggest that CD34 antibody decorated HA-based hydrogels may be a useful material to capture EPCs. EPC capture strategies could be useful in the treatment of cardiovascular injuries to recruit EPCs for enhancing re-endothelialization events.11,12
In future work, we will study capture of EPCs on anti-CD34 antibody immobilized HA-based hydrogels under controlled flow conditions. We anticipate that capture efficiency will be different for both EPCs and macrophages under shear stress compared to static conditions.
Conclusion
Here we synthesized hydrogels made from photocrosslinkable HA and conjugated CD34 antibodies to enable selective capturing of EPCs on the hydrogels. We developed these antibody decorated HA-based hydrogels to enhance attachment of EPCs on the surface of the hydrogels. Chemical modification of the hydrogel surface significantly enhanced the amount of covalently immobilized CD34 antibody. We determined that higher antibody concentrations could be used to enhance the capture of EPCs on surface decorated polysaccharide-based hydrogels. As expected, we did not observe significant cell attachment for macrophages, which do not express CD34 on their cell surfaces. Hydrogels containing only HA did not promote spreading of EPCs 48 h after cell seeding by maintaining their round shapes. To enhance spreading of EPCs 2% (w/v) GelMA was added to the content of the hydrogels, which improved cell spreading and elongation. This strategy could potentially be useful to endothelialize artificial grafts especially inside blood vessel walls and in the treatment stage of cardiovascular diseases to recruit EPCs to decrease re-endothelialization time. Such capture strategies could be useful in cardiovascular tissue engineering, formation of vascular structures and engineering heart valves. The development of HA-based hydrogels with controllable cell–biomaterial interactions that could capture specified cell types to aid in endothelialization of cardiovascular implants as well as tissue engineered constructs might solve long standing problems in biomaterials and tissue engineering and open up new avenues of research.
Acknowledgements
This paper was supported by the National Institutes of Health (EB008392; DE019024; HL092836), National Science Foundation (DMR0847287), the Institute for Soldier Nanotechnologies, and the US Army Corps of Engineers. The authors acknowledge Dr Joyce Bischoff and her lab for providing the EPCs for this study. We also acknowledge Dr Blaine Pfeifer and his lab for providing the macrophages for our experiments.
Footnotes
Electronic supplementary information (ESI) available: Data on flow cytometry analysis of EPCs and macrophages for their expression of CD34. See DOI: 10.1039/c0sm00508h
This paper is part of a joint Soft Matter and Journal of Materials Chemistry themed issue on Tissue Engineering. Guest editors: Molly Stevens and Ali Khademhosseini.
Notes and references
- 1.American Heart Association [accessed 5/13/10]; http://www.americanheart.org/presenter.jhtml?identifier = 4478.
- 2.Aoki J, Serruys PW, van Beusekom H, Ong ATL, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJB. J. Am. Coll. Cardiol. 2005;45:1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 3.Klomp M, Beijk MAM, de Winter RJ. Expert Rev. Med. Devices. 2009;6:365–375. doi: 10.1586/erd.09.16. [DOI] [PubMed] [Google Scholar]
- 4.Avci-Adali M, Paul A, Ziemer G, Wendel HP. Biomaterials. 2008;29:3936–3945. doi: 10.1016/j.biomaterials.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Shirota T, He HB, Yasui H, Matsuda T. Tissue Eng. 2003;9:127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, vanderZee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Schatteman GC, Dunnwald M, Jiao CH. Am. J. Physiol.: Heart Circ. Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- 8.Woywodt A, Bahlmann FH, de Groot K, Haller H, Haubitz M. Nephrol., Dial., Transplant. 2002;17:1728–1730. doi: 10.1093/ndt/17.10.1728. [DOI] [PubMed] [Google Scholar]
- 9.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 10.Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FWH, Schoen FJ, Mayer JE, Bischoff J. Am. J. Physiol.: Heart Circ. Physiol. 2004;287:H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cogle CR, Scott EW. Exp. Hematol. 2004;32:885–890. doi: 10.1016/j.exphem.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Murasawa S, Asahara T. Physiology. 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 14.Zampetaki A, Kirton JP, Xu QB. Cardiovasc. Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 15.Plouffe BD, Kniazeva T, Mayer JE, Murthy SK, Sales VL. FASEB J. 2009;23:3309–3314. doi: 10.1096/fj.09-130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silber S. Minerva Cardioangiol. 2006;54:1–3. [PubMed] [Google Scholar]
- 17.Szmitko PE, Kutryk MJB, Stewart DJ, Strauss MH, Verma S. Can. J. Cardiol. 2006;22:1117–1119. doi: 10.1016/s0828-282x(06)70947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Exp. Mol. Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 19.Felschow DM, McVeigh ML, Hoehn GT, Civin CI, Fackler MJ. Blood. 2001;97:3768–3775. doi: 10.1182/blood.v97.12.3768. [DOI] [PubMed] [Google Scholar]
- 20.Tian WM, Zhang CL, Hou SP, Yu X, Cui FZ, Xu QY, Sheng SL, Cui H, Li HD. J. Controlled Release. 2005;102:13–22. doi: 10.1016/j.jconrel.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Manasek FJ. Circ. Res. 1976;38:331–337. doi: 10.1161/01.res.38.5.331. [DOI] [PubMed] [Google Scholar]
- 22.Markwald RR, Fitzharris TP, Bernanke DH. J. Histochem. Cytochem. 1979;27:1171–1173. doi: 10.1177/27.8.479561. [DOI] [PubMed] [Google Scholar]
- 23.West DC, Hampson IN, Arnold F, Kumar S. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 24.Chen WYJ, Abatangelo G. Wound Repair Regener. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 25.Luthra AK, Sandhu SS. 7 034 061 US Pat. 2000
- 26.Verheye S, Markou CP, Salame MY, Wan B, King SB, Robinson KA, Chronos NAF, Hanson SR. Arterioscler., Thromb., Vasc. Biol. 2000;20:1168–1172. doi: 10.1161/01.atv.20.4.1168. [DOI] [PubMed] [Google Scholar]
- 27.Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. J. Biomed. Mater. Res. 2004;71:172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 28.Mason M, Vercruysse KP, Kirker KR, Frisch R, Marecak DM, Prestwich CD, Pitt WG. Biomaterials. 2000;21:31–36. doi: 10.1016/s0142-9612(99)00129-5. [DOI] [PubMed] [Google Scholar]
- 29.Thebaud NB, Pierron D, Bareille R, Le Visage C, Letourneur D, Bordenave L. J. Mater. Sci.: Mater. Med. 2007;18:339–345. doi: 10.1007/s10856-006-0698-1. [DOI] [PubMed] [Google Scholar]
- 30.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, Langer R, Burdick JA. J. Biomed. Mater. Res., Part A. 2006;79a:522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 32.Khademhosseini A, Chung BG. IEEE/NIH Life Science Systems and Applications Workshop; Maryland. 2009. [Google Scholar]
- 33.Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noh I, Kim G-W, Choi Y-J, Kim M-S, Park Y, Lee K-B, Kim I-S, Hwang S-J. Biomed. Mater. (Bristol, U. K.) 2006;1:116–123. doi: 10.1088/1748-6041/1/3/004. [DOI] [PubMed] [Google Scholar]
- 35.Rabelink TJ, de Boer HJ, de Koning EJP, van Zonneveld A-J. Arterioscler., Thromb., Vasc. Biol. 2004;24:834–838. doi: 10.1161/01.ATV.0000124891.57581.9f. [DOI] [PubMed] [Google Scholar]
- 36.Liew A, Barry F, O’Brien T. BioEssays. 2006;28:261–270. doi: 10.1002/bies.20372. [DOI] [PubMed] [Google Scholar]