Abstract
The following report describes novel methodology for the rapid synthesis of unique conformationally constrained norstatine analogs of potential biological relevance. A PADAM (Passerini reaction – Amine Deprotection – Acyl Migration reaction) sequence is followed by a TFA-mediated microwave-assisted cyclization to generate the final benzimidazole isostere of the norstatine scaffold in moderate to good yields. The applicability of this solution phase methodology to the preparation of a small collection of compounds is discussed.
Keywords: multicomponent reactions, Passerini reaction, peptidomimetics, benzimidazole, protease inhibitor
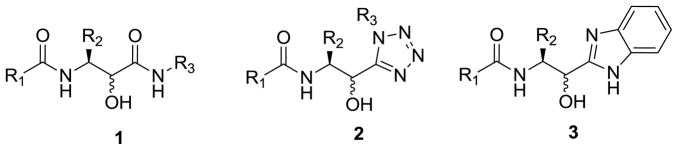
Aspartic proteases are a group of enzymes involved in a plethora of biological processes including the development and progression of a variety of diseases, such as HIV infection, inflammation and cancer.1–3 Possessing this variety of therapeutic potential, they have become attractive targets over the last twenty years. As such, these enzymes catalyze the amide bond hydrolysis of peptidic substrates, a process which proceeds via a classical ‘tetrahedral intermediate’, often targeted as a key motif to mimic for new inhibitor design. Indeed, it is typically found that incorporation of a secondary hydroxyl group enables access to this key interaction, although interestingly over the last 10 years amines have also been shown to play this role. 2–6 Accordingly the hydroxyl group is typically found on a poly-peptidic moiety linked together with different heterocycles.2–3 In fact, the most well-known amide isosteres in this class are represented by hydroxyethylamines, hydroxyethylenes (mono or dihydroxy), statines, hydroxymethylenes and norstatines 1.2–5,7 This laboratory has recently been actively involved in the generation of conformationally constrained analogs of the latter norstatines, enabling entry into feasibly unique biologically active aspartic protease space. On this theme, the rapid generation of libraries of cis-constrained norstatine analogs of general structure 2 using a TMSN3-modified Passerini/de-Boc/N-capping protocol (a slight modification of the PADAM strategy used to produce libraries of 1) was reported in 2002 (Figure 1). Such tetrazoles are well known isosteres for cis-amide bonds.8
Figure 1.
General structure of norstatines 1, cis-constrained norstatine analogs 2 and targeted novel benzimidazole analogs 3.
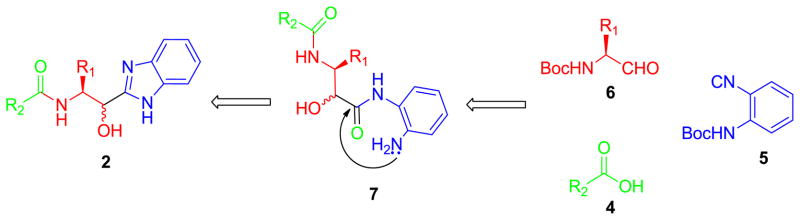
In a continuation of our studies, we herein report a novel synthetic protocol for the synthesis of unique norstatine analogs of general structure 3 (Figure 1). Partially driving the decision making process toward this new isostere of norstatine was the increased pKa ~ 5.2 of the benzimidazole which dramatically alters the physicochemical properties of the molecules under investigation relative to that of the tetrazole 2 or parent norstatine 1.9 Thus, synthetic methodology coined PADAM (Passerini reaction-Amine deprotection-Acyl migration) by Banfi, which is typically used to access norstatines 1, was thought potentially applicable to the synthesis of 3.10 However, in this example, utilizing ortho-N-Boc-phenylisonitrile 5, N-Boc-α-aminoaldehydes 6 and supporting carboxylic acids 4, two reagents contain protected ‘internal’ amines and it was envisioned that in addition to the PADAM sequence, amino-cyclodehydration onto the carbonyl in 7, derived from the isocyanide input, would simultaneously deliver a benzimidazole moiety, Scheme 1. Note that the PADAM sequence of reactions has the advantage of enabling assembly of complex peptidomimetics in a straightforward and rapid way and the methodology has been extensively applied to the synthesis of proteases inhibitors.11
Scheme 1.
Retrosynthetic analysis.
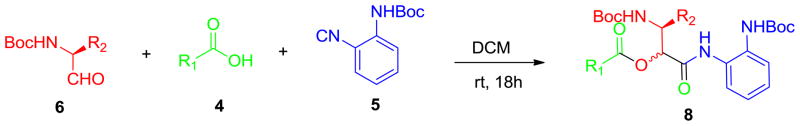
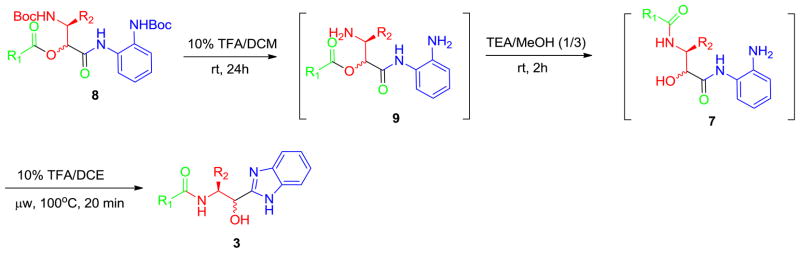
Thus, condensation between N-Boc-α-aminoaldehyde 6, carboxylic acid 4 and ortho-N-Boc phenylisonitrile 5 afforded the corresponding Passerini product 8 in good isolated yield, Scheme 2. Removal of the two Boc protecting groups of 8 occurred at room temperature upon treatment with 10% TFA in DCM, Scheme 3.
Scheme 2.
Passerini condensation (R1 = see Figure 2. R2 = iso-propyl, 6a; methyl, 6b; benzyl, 6c).
Scheme 3.
Synthesis of 3 from Passerini product 8.
Subsequent treatment of the crude intermediate 9 with triethylamine (TEA) in methanol at room temperature delivered the expected intermediate 7, the product of acyl transfer. Due to the excellent purity of the crude reaction mixtures of 7 and 9, column chromatography was unnecessary for the final two steps, greatly facilitating the overall production process. Encouragingly, the formation of the expected benzimidazole scaffold 3 was achieved upon treatment of crude 7 with trifluoroacetic acid in dichloroethane, promoted by microwave irradiation, Scheme 3.
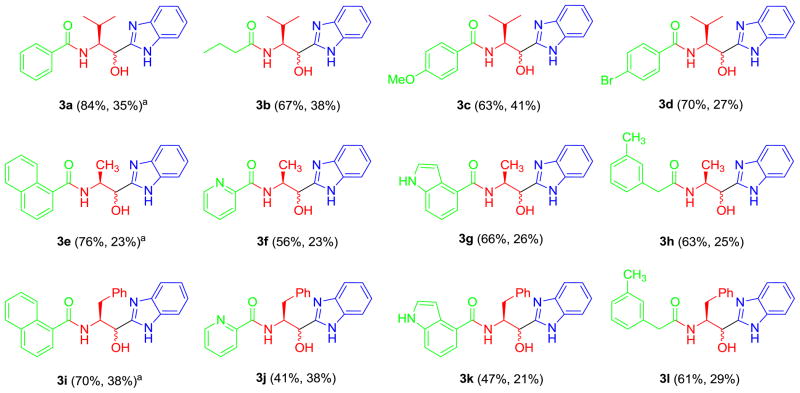
With satisfactory conditions in hand, the reaction scope in terms of substrate tolerance was explored. A small collection of twelve examples was prepared according to the same synthetic protocol to demonstrate the generality of the reaction, Figure 2. Diversity was generated through the employment of eight different carboxylic acids and three N-Boc protected α-amino aldehydes. Whilst the carboxylic acids and isonitriles were commercially available, known α-aminoaldehydes 6 were prepared through LiAlH4 reduction of the corresponding Weinreb hydroxamates, in line with reported methodology.12,13 The synthesis of the Passerini products proceeded smoothly with isolated yields ranging from 41 to 84%, Figure 2.14 Noteworthy, a slight decrease in the yields of the Passerini product was observed when 2-pyridine carboxylic acid and 1H-indole-6-carboxylic acid were employed. In all the examples, purification of intermediates 7 and 9 was avoided, thus significantly simplifying the synthetic protocol. The final products were obtained after silica-gel column chromatography in satisfying overall yields (23–38%), spanning 4 functional transformations in one pot.14 As expected, the observed average stereoselectivity for the final products was ca. 1:1, as judged by 1H NMR spectra of the pure compounds. In view of the potential applicability of this methodology to combinatorial synthesis, the lack of stereoselectivity and production of diastereomers is not considered as a drawback.
Figure 2.
Example analogs (x% = Passerini yield, x% = yield from 8 to 3).
In summary, a series of novel conformationally constrained norstatine isosteres were synthesized in four steps by means of PADAM methodology, combined with benzimidazole formation. The methodology also represents the first example of an application of the Passerini reaction utilizing two internal amine nucleophiles. With final products characterized by two points of diversity and a facile and practical production protocol, access to large libraries of diverse analogs is now possible. Being amenable to high-throughput synthesis, it is expected that this methodology will be embraced by the lead generation community.
Acknowledgments
We would like to thank the Office of the Director, NIH, and the National Institute of Mental Health for funding (1RC2MH090878-01). Particular thanks to N. Schechter PSM for copy editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.(a) Dunn BM. Structure and Function of the Aspartic Proteases: Genetics, Structures and Mechanisms. Vol. 306. Plenum Press; New York: 1991. p. xviii.p. 585. [Google Scholar]; (b) Takahashi K. Aspartic Proteinases: Structure, Function, Biology and Biomedical Implications. Plenum Press; New York: 1995. [Google Scholar]
- 2.Ghosh AK. J Med Chem. 2009;52(8):2163. doi: 10.1021/jm900064c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder J, Hommel U, Cumin F, Martoglio B, Gerhartz B. Current Pharmaceutical design. 2007;13:271. doi: 10.2174/138161207779313560. [DOI] [PubMed] [Google Scholar]
- 4.Lee CE, Kick EK, Ellman JA. J Am Chem Soc. 1998;120:9735. and references cited herein. [Google Scholar]
- 5.(a) Spatola AF. In: Chemistry and Biochemistry of Amino Acids, Peptides and Proteins. Weinstein B, editor. Vol. 7. Dekker; New York: 1983. p. 267. [Google Scholar]; (b) West ML, Fairlie DP. Trends Pharmacol Sci. 1995;16:67. doi: 10.1016/s0165-6147(00)88980-4. [DOI] [PubMed] [Google Scholar]; (c) Huff JR. J Med Chem. 1991;34:2305. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zabrocki J, Smith GD, Dunbar JB, Iijima H, Marshall GR. J Am Chem Soc. 1998;110:5875. [Google Scholar]; (b) Yu KL, Johnson RL. J Org Chem. 1987;52:2051. [Google Scholar]; (c) Marshall GR, Humblet C, Van Opdenbosch N, Zabrocki J. Peptide Synthesis-Structure-Function. [Google Scholar]
- 7.Rich DH, Gross E, editors. Proceedings of the Seventh American peptide Symposium. Pierce Chemical; Rockford, IL: 1981. p. 669. [Google Scholar]
- 8.Nixey T, Hulme C. Tetrahedron Lett. 2002;43:6833. [Google Scholar]
- 9.(a) Wells JI. Pharmaceutical Preformulation. Ellis Horwood Ltd; London: 1998. p. 25. [Google Scholar]; (b) Clark DE. Drug Discov Today. 2003;8:927. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]; (c) Upthagrove AL, Nelson WL. Drug Metab Dispos. 2001;29:1377. [PubMed] [Google Scholar]; (d) Wan H, Ulander J. Expert Opin Drug Metab Toxicol. 2006;2:139. doi: 10.1517/17425255.2.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Banfi L, Guanti G, Riva R. Chem Commun. 2000:985. [Google Scholar]
- 11.(a) Banfi L, Guanti G, Riva R, Basso A, Calcagno E. Tetrahedron Lett. 2002;43:4067. [Google Scholar]; (b) Banfi L, Basso A, Guanti G, Riva R. Mol Div. 2003;6:227. doi: 10.1023/b:modi.0000006778.42751.7f. [DOI] [PubMed] [Google Scholar]; (c) Faure S, Hjelmgaard T, Roche SP, Aitken DJ. Org Lett. 2009;11:1167. doi: 10.1021/ol900048r. [DOI] [PubMed] [Google Scholar]; (d) Basso A, Banfi L, Piaggio P, Riva R, Guanti G. Tetrahedron Lett. 2003;44:2367. [Google Scholar]
- 12.(a) Nahm S, Weinreb SM. Tetrahedron Lett. 1981;22:3815. [Google Scholar]; (b) Hulme C, Ma Liang, Romano J, Morrissette M. Tetrahedron Lett. 1999;40:7925. [Google Scholar]; (c) Goel OP, Krolls U, Stier M, Kesten S. Org Synth. 1988;67:69. [Google Scholar]
- 13.General procedure for the preparation of 6b: To a stirring solution of N-Boc-L-α-alanine (200 mg, 1.05 mmol, 1.0 eq) in DCM (5mL), 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (402 mg, 2.10 mmol, 2.0 eq), hydroxybenzotriazole (170 mg, 1.26 mmol, 1.25 eq), 4-dimethylaminopyridine (528 μL, 1.05 mmol, 1.0 eq) and N,O-dimethylhydroxylamine hydrochloride (123 mg, 1.26 mmol, 1.2 eq) were added and the reaction stirred for 18h at rt. After removal of the solvent under reduced pressure, the residue was dissolved in EtOAc (20 mL), washed with HCl (0.1 M, 3 x 20 mL), sat. NaHCO3 (3 x 20 mL), and brine (30 mL). The organic phase was collected, dried (MgSO4) and the solvent removed under reduced pressure. The crude product was used in the following step without further purification. The Weinreb amide (170 mg, 0.72 mmol, 1.0 eq) was dissolved in dry THF (5mL) and LiAlH4 (1.0 M in THF, 870 μL, 0.87 mmol, 1.2 eq) was added dropwise at 0°C over 20 min. The reaction was allowed to warm to room temperature and stirred for 5h. After completion, EtOAc (20 mL) was added, followed by HCl (0.1 M, 20 mL). The resulting mixture was extracted with EtOAc (20 mL x 3), the organic layers collected, washed with brine (50 mL), dried (MgSO4) and concentrated under reduced pressure. Purification by column chromatography (ISCO™ purification system, EtOAc-hexane, 30%) afforded the final product 6b as colorless oil (76 mg, 0.43 mmol, 61% over two steps). 14) General procedure for the preparation of3c: A mixture of (S)-tert-butyl (3-methyl-1-oxobutan-2-yl)carbamate (0.403g, 2.0 mmol, 1.0 eq), butyric acid (0.176g, 2.0 mmol, 1.0 eq) and tert-butyl (2-isocyanophenyl)carbamate 5 (0.437, 2.0 mmol, 1.0 eq) in DCM (2 mL, 1.0 M) was stirred at rt overnight. After removal of the solvent under reduced pressure, the residue was purified by silica-gel column chromatography (EtOAc-hexane, 0 to 30%) using an ISCO™ purification system to afford (3S)-3-((tert-butoxycarbonyl)amino)-1-((2-((tert-butoxycarbonyl)amino)phenyl)amino)-4-methyl-1-oxopentan-2-yl butyrate (0.640 g, 1.260 mmol, 63% yield). 8c (0.609 g, 1.2 mmol) in 10% TFA/DCM (5 mL) was stirred at room temperature for 48h. The reaction was diluted in DCM (10mL), washed with sat. NaHCO3 (10 mL) and brine (10 mL). The organic layers were collected, dried over MgSO4 and the solvent removed under reduced pressure to give intermediate (3S)-3-amino-1-((2-aminophenyl)amino)-4-methyl-1-oxopentan-2-yl butyrate 9c, which was used in the following reaction without further purification. 9c was dissolved in TEA/MeOH (1/3, 10 mL) and the reaction stirred at rt for 3h. After completion, the mixture was diluted with ethyl acetate (30 mL) and washed with HCl (0.1M, 3 x 10 mL). The organic layer was collected, dried over MgSO4 and the solvent was evaporated under in vacuo to afford (3S)-N-(2-aminophenyl)-3-butyramido-2-hydroxy-4-methylpentanamide 7c, which was used in the following reaction without further purification. 7c was dissolved in 10% TFA/DCE (3 mL), the resulting solution was placed in a 5 mL microwave tube and subjected to microwave irradiation in a Biotage Initiator (10 min, 100°C). The mixture was diluted in DCM (10 mL), washed with sat. NaHCO3 (10 mL) and the aqueous layer extracted with DCM (3 x 10 mL). The organic layers were collected, washed with further sat. NaHCO3 (10 mL), brine (20 mL), dried over MgSO4 and the solvent was removed under reduced pressure to get the crude product 2c. The crude product 3c were further purified by silica-gel column chromatography (EtOAc-hexane, 0 to 80%) using a ISCO™ purification system to afford N-((2R)-1-(1H-benzo[d]imidazol-2-yl)-1-hydroxy-3-methylbutan-2-yl)butyramide 3c (0.142 g, 0.492 mmol, 41% yield in three steps). LCMS [M+1]+ 290.1; diastereomeric ratio ~1:1; 1H NMR (300 MHz, DMSO-d6, mixture of 2 diastereoisomers): δ 0.58–0.71 (m, 3H), 0.84–0.88 (m, 2H), 0.90–0.93 (m, 3H), 1.05 (d, J = 6.0 Hz, 2 H), 1.22–1.27 (m, 3H), 1.88–1.97 (m, 3H), 2.35 (m, 0.5 H), 2.51 (s, 0.5H), 3.98 (t, J = 9.0 Hz, 0.5H), 4.17 (t, J = 9.0 Hz, 0.5H), 4.97 (d, J = 9.0 Hz, 0.5 Hz), 5.35 (s, 0.5H), 7.33–7.35 (m, 2 H), 7.61–7.64 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δ 13.14, 13.28, 15.66, 18.92, 18.94, 19.38, 19.77, 27.68, 29.31, 29.66, 37.91, 38.02, 57.63, 58.79, 67.40, 113.91, 114.02, 115.19, 118.09, 125.22, 125.36, 131.95, 132.13, 155.28, 155.50, 163.03, 163.38, 175.24, 175.35 ppm.