SUMMARY
Tumor-initiating cells have been suggested to be rare in many cancers. We tested this in mouse malignant peripheral nerve sheath tumors (MPNSTs) and found that 18% of primary and 49% of passaged MPNSTs cells from Nf1+/−;Ink4a/Arf−/− mice formed tumors upon transplantation whereas only 1.8% to 2.6% of MPNSTs cells from Nf1+/−;p53+/− mice did. MPNST cells of both genotypes require laminin binding to β1-integrin for clonogenic growth. Most MPNST cells from Nf1+/−;Ink4a/Arf−/− mice expressed laminin whereas most MPNST cells from Nf1+/−;p53+/− mice did not. Exogenous laminin increased the percentage of MPNST cells from Nf1+/−;p53+/−, but not Nf1+/−;Ink4a/Arf−/−, mice that formed tumorigenic colonies. Tumor-forming potential is therefore common among MPNST cells but the assay conditions required to detect it vary with tumor genotype.
INTRODUCTION
The question of which cells contribute to cancer growth and progression has fundamental implications for therapy. If tumor growth and metastasis are driven primarily by rare, or at least infrequent, cancer stem cells then cancer might be more effectively treated by specifically targeting the cancer stem cells (Reya et al., 2001; Pardal et al., 2003; Dick, 2008). On the other hand, if many cancer cells are capable of driving tumor growth and metastasis it will be necessary to eliminate most or all cancer cells.
Evidence suggests that small populations of cancer stem cells drive the growth and progression of a number of cancers, including some germ lineage cancers (Kleinsmith and Pierce, 1964; Illmensee and Mintz, 1976), myeloid leukemias (Lapidot et al., 1994; Bonnet and Dick, 1997; Yilmaz et al., 2006; Oravecz-Wilson et al., 2009), breast cancers (Al-Hajj et al., 2003), gliomas (Singh et al., 2004) and colon cancers (Dalerba et al., 2007; O’Brien et al., 2007; Ricci-Vitiani et al., 2007; Merlos-Suarez et al., 2011). In each case, transplantation of cancer cells into highly immunocompromised mice revealed a small, phenotypically distinct subpopulation of cells that was uniquely capable of transferring disease and forming phenotypically diverse non-tumorigenic progeny. This suggests that some cancers are hierarchically organized, with small numbers of cancer stem cells that sustain tumor growth by forming large numbers of cancer cells with limited proliferative potential (Reya et al., 2001; Pardal et al., 2003; Dick, 2008).
In contrast to this model, human melanomas (Quintana et al., 2008; Quintana et al., 2010), mouse melanomas (Held et al., 2010) and some mouse leukemias (Kelly et al., 2007; Williams et al., 2007) have many cells with tumorigenic potential. For example, 30% of single cells obtained directly from human melanomas and over 50% of cells obtained from primary mouse melanomas can form tumors in highly immunocompromised mice (Quintana et al., 2008; Held et al., 2010; Quintana et al., 2010). These results raise three critical questions. Is melanoma unique among solid cancers in having common tumorigenic cells? Can high frequencies of tumorigenic cells be detected in immunocompetent recipients for some cancers or does the immune system play such a central role in the control of tumor-initiating cells that common tumorigenic cells can only be detected in immunocompromised recipients? To address this question it is necessary to examine mouse models of cancer, which can be transplanted into syngeneic recipients without a confounding xenogeneic immune barrier that rejects human cells by mechanisms that are very different from the autologous immune responses that can occur within patients. Finally, what other factors influence the frequency of cancer cells that can form tumors?
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive soft tissue sarcomas that arise within peripheral nerves. Most MPNSTs arise in the context of Neurofibromatosis type 1, a hereditary tumor syndrome caused by mutations in NF1 that encodes Neurofibromin, a GTPase activating protein that negatively regulates Ras signaling (Rubin and Gutmann, 2005). About 30% of NF1 patients develop benign plexiform neurofibromas, which can transform into MPNSTs. Plexiform neurofibromas and MPNSTs arise from Schwann cells in peripheral nerves (Zhu et al., 2002; Joseph et al., 2008; Zheng et al., 2008). Beyond the loss of NF1 function, p16Ink4a, p14Arf (the human ortholog of mouse p19Arf), and p53 are often inactivated in MPNSTs by deletion or promoter hypermethylation (Menon et al., 1990; Perrone et al., 2003; Agesen et al., 2005). Mutations of these genes in mice also lead to the development of MPNSTs. Thus, mice that are heterozygous for a deletion of both Nf1 and p53 (Nf1+/−;p53+/−) (Cichowski et al., 1999; Vogel et al., 1999) and mice heterozygous for Nf1 and deficient for Ink4a/Arf (Nf1+/−;Ink4a/Arf−/−) (Joseph et al., 2008) develop MPNSTs.
We developed in vitro and in vivo assays to determine the frequency of MPNST cells with tumorigenic potential in these mouse models of MPNST.
RESULTS
More tumorigenic cells were detected among primary MPNST cells from Nf1+/−;Ink4a/Arf−/− mice
Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice were backcrossed onto a C57BL/Ka background (at least 6 generations), then aged to monitor the development of MPNSTs. Approximately 25% to 50% of Nf1+/−; Ink4a/Arf−/− mice and Nf1+/−;p53+/− mice developed MPNSTs on the legs and abdomens, typically between 4 and 7 months of age, as previously reported (Cichowski et al., 1999; Joseph et al., 2008). Tumors of both genotypes grew rapidly and at comparable rates (data not shown). Three of four primary and four of four secondary MPNSTs from Nf1+/−;p53+/− mice exhibited p16Ink4a and p19Arf expression, while p16Ink4a and p19Arf were not detected in MPNSTs from Nf1+/−; Ink4a/Arf−/− mice, as expected (Fig. S1). p53 was not detected in most MPNSTs from Nf1+/−;p53+/− mice, as expected (Vogel et al., 1999), but was detected in most MPNSTs from Nf1+/−; Ink4a/Arf−/− mice (data not shown).
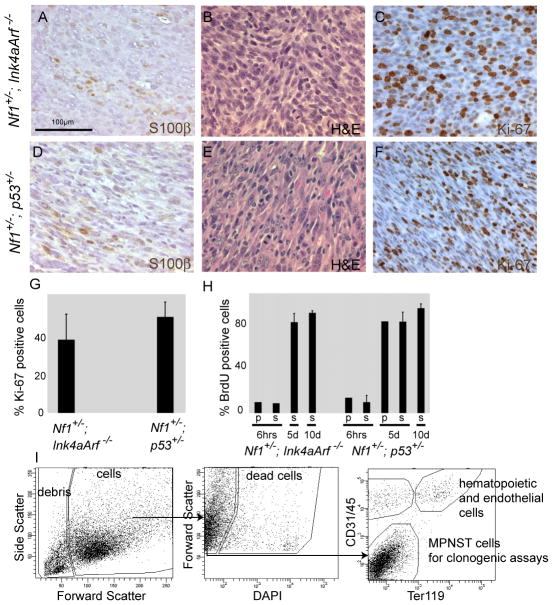
We sacrificed Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice at 6.1±0.9 or 5.3±1.4 (mean±SD) months of age, respectively, for analysis. Tumors of both genotypes contained tightly packed spindle cells with tapered nuclei, arranged in a fascicular pattern (Fig. 1A-F). Of 25 tumors analyzed, 24 were focally positive for the Schwann cell marker S100β (Fig. 1A, D). In tumors of both genotypes we observed frequent mitotic figures (an average of 1.1±0.7 in Nf1+/−; Ink4a/Arf−/− MPNSTs and 1.6±0.6 in Nf1+/−;p53+/− MPNSTs per high power field, objective x ocular magnification=400x) and a high frequency of Ki-67 positive cells (39±16% for Nf1+/−; Ink4a/Arf−/− and 52±9% for Nf1+/−;p53+/− MPNSTs) (Fig. 1C, F, G).
Figure 1. Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice develop MPNSTs that contain a high frequency of proliferating cells.
(A–F) Paraffin sections of Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− MPNSTs were stained with antibodies against S100β (A,D), hematoxylin and eosin (H&E in B,E) and antibodies against Ki-67 (C,F). Sections were counterstained with hematoxylin (blue) in A, D, C and F. Each panel reflects a different section. (G) The percentage of MPNST cells that were Ki-67-positive was determined for 4 tumors of each genotype on at least 20 high power fields (400x magnification, ocular x objective) for each tumor. (H) The percentage of MPNST cells (that stained negatively for blood or endothelial markers) in mice bearing primary (p) or secondary (transplanted into wild-type mice; s) MPNSTs treated with BrdU for either 6 hours, 5 or 10 days that were BrdU-positive. The data reflect 1 primary and 8 secondary tumors from Nf1+/−; Ink4a/Arf−/− mice and 3 primary and 9 secondary tumors from Nf1+/−;p53+/− mice. All statistics represent mean±SD. (I) Isolation of MPNST cells from primary tumors using flow cytometry. See also Figure S1 and Tables S1 and S2.
We also administered bromo-deoxyuridine (BrdU) to tumor-bearing mice. After 6 hours of administration 10% and 14% of cells were BrdU+ in primary MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice, respectively. Similar results were obtained when secondary MPNSTs were analyzed: 9% and 10±6% of cells from MPNSTs obtained from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice incorporated BrdU, respectively. After 5 or 10 days of administration, over 80% of cells were BrdU+ in MPNSTs of both genotypes (Fig. 1H). MPNSTs from both Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice therefore contained similarly high frequencies of rapidly dividing cells.
To obtain MPNST cells for experiments, primary tumors of comparable size from mice of both genotypes were enzymatically dissociated and stained with antibodies. Only tumors that were confirmed to be MPNSTs by histopathology were included in our analysis. The cells were sorted by flow cytometry to exclude debris (based on light scatter), dead cells (4′,6-diamidino-2-phenylindole+ (DAPI+)), hematopoietic cells (Ter119+ or CD45+), and endothelial cells (CD31+, Fig. 1I). Viable cells that were negative for hematopoietic/endothelial markers were used in all experiments. These cells comprised an average of 48±17% and 44±14% (mean±SD) of nucleated cells in MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice, respectively.
We injected MPNST cells subcutaneously, into sciatic nerves, or under the kidney capsules of wild-type C57BL/Ka mice. The kidney capsule is a common site for heterologous cell grafting where many cell types are able to efficiently engraft. We rarely observed the formation of tumors after subcutaneous injection, even when we injected large numbers of cells, but transplanted cells readily formed tumors in sciatic nerve and under the kidney capsule at higher cell doses (Table S1). At lower cell doses the cells engrafted more efficiently under the kidney capsule, where the cells were retained after injection (Table S1). Injection of cells into the endoneurial space increased pressure within nerves and led to the ejection of the injected material through the needle track, rendering the assay non-quantitative in peripheral nerve. For this reason, subsequent experiments to quantify the frequency of cells with tumorigenic potential were conducted by transplanting cells under the kidney capsule.
We transplanted aliquots of 100 cells, 10 cells, or single cells from 10 primary MPNSTs obtained from Nf1+/−; Ink4a/Arf−/− mice and 8 primary MPNSTs from Nf1+/−;p53+/− mice under the kidney capsule of C57BL/Ka mice. We monitored the mice for at least 15 weeks after transplantation. When tumors became evident by palpation, or at the end of the experiment, the mice were sacrificed for gross and histological examination. Sorted events from the debris fraction (Fig. 1I) did not form tumors upon transplantation and cells from the fractions that stained positively for hematopoietic or endothelial markers rarely formed tumors (Table S2). When viable MPNST cells from the non-hematopoietic/non-endothelial fraction of Nf1+/−; Ink4a/Arf−/− mice were injected, 28 of 29 mice (97%) injected with 100 cells, 28 of 39 mice (72%) injected with 10 cells, and 16 of 76 mice (21%) injected with single cells formed MPNSTs (Table 1). Almost all of these tumors were observed within 10 weeks after transplantation and appeared histologically similar to the primary tumors from which they derived (Fig. S2A). Limit dilution analysis indicated that 18±14% (mean±SD; range=6% to 53%) of cells from primary MPNST formed tumors after transplantation. Tumorigenic cells were therefore common within primary MPNSTs obtained from Nf1+/−; Ink4a/Arf−/− mice.
Table 1.
Tumor formation by primary MPNST cells
| Tumor | Injected cell dose (# tumors/# injections) | Cells that formed tumors (%) | 95% CI (%) | ||
|---|---|---|---|---|---|
| 100 cells | 10 cells | 1 cell | |||
| Nf1+/−; Ink4a/Arf−/− mice | |||||
| 10 | 3/3 | nd | 1/8 | 13 | 1.9–95 |
| 11 | 1/2 | 1/2 | 2/6 | 16 | 4.3–61 |
| 12 | 5/5 | 4/6 | 6/8 | 27 | 11–65 |
| 14 | 3/3 | 4/4 | 0/8 | 18 | 6.6–48 |
| 15 | 3/3 | 3/6 | 0/7 | 6.0 | 2.1–18 |
| 16 | 4/4 | 6/6 | 4/10 | 53 | 22–100 |
| 17 | 4/4 | 4/5 | 1/9 | 15 | 5.9–37 |
| 23 | nd | nd | 1/10 | 10 | nd |
| 29 | nd | 2/5 | 1/10 | 6.2 | 1.9–20 |
| 35 | 5/5 | 4/5 | nd | 16 | 5.4–48 |
| TOTAL | 28/29 (97%) | 28/39 (72%) | 16/76 (21%) | 18 | 6.8–55 |
| Nf1+/−;p53+/− mice | |||||
| 1 | 1/3 | 0/4 | 1/10 | 0.7 | 0.1–3.0 |
| 3 | 4/5 | 0/5 | nd | 1.3 | 0.5–3.6 |
| 4 | 4/5 | 2/5 | 1/10 | 2.6 | 1.0–6.8 |
| 5 | 4/5 | 1/5 | 0/10 | 1.7 | 0.6–4.3 |
| 6 | 2/4 | 0/5 | 0/8 | 0.6 | 0.1–2.2 |
| 7 | 5/5 | 3/8 | nd | 5.0 | 1.8–13 |
| 8 | 4/6 | 0/5 | nd | 1.0 | 0.3–2.6 |
| 9 | 0/4 | 0/6 | nd | nd | nd |
| TOTAL | 24/37 (65%) | 6/43 (14%) | 2/38 (5%) | 1.8** | 0.5–4.0 |
Tumorigenic cell frequency significantly differed in this assay between MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice (**: p=0.008).
nd: not determined.
For tumor histology see Figure S2.
When non-hematopoietic/non-endothelial cells from primary tumors obtained from 8 Nf1+/−;p53+/− mice were injected, 24 of 37 mice (65%) injected with 100 cells, 6 of 43 mice (14%) injected with 10 cells, and 2 of 38 mice (5%) injected with single cells formed MPNSTs (Table 1). All of these tumors were observed within 10 weeks after transplantation and appeared histologically similar to the primary tumors from which they derived (Fig. S2B). Limit dilution analysis indicated that an average of 1.8±1.6% (mean±SD; range=0.6% to 5.0%) of cells from these MPNSTs formed tumors after transplantation. The frequency of tumorigenic cells in MPNSTs from Nf1+/−;p53+/− mice was therefore significantly lower (10-fold, p=0.008) in this assay than in MPNSTs from Nf1+/−; Ink4a/Arf−/− mice. This demonstrates that MPNST genetic background can affect tumorigenic cell frequency.
MPNSTs from Nf1+/−; Ink4a/Arf−/− mice retain higher frequencies of tumorigenic cells upon secondary transplantation
We wondered whether the reduced frequency of tumorigenic cells within primary MPNSTs from Nf1+/−;p53+/− mice reflected increased infiltration of normal cells into these tumors as compared to MPNSTs from Nf1+/−; Ink4a/Arf−/− mice. To test this, we performed secondary transplants of dissociated cells from tumors that arose under the kidney capsule of primary recipient mice. Transplanted MPNST cells form focal masses that are distinct from normal kidney tissue. We reasoned that only cancer cells would proliferate extensively in primary recipient mice, reducing or eliminating normal cells from the passaged tumors. Therefore, if normal cells within primary MPNSTs from Nf1+/−;p53+/− mice diluted the cancer cells, then secondary transplantation of these MPNSTs should eliminate the difference in tumorigenic cell frequency among MPNSTs of different genotypes. When MPNST cells from 11 first generation grafts from 5 different primary MPNSTs from Nf1+/−; Ink4a/Arf−/− mice were injected into secondary recipients, 14 of 15 (93%) 10 cell injections and 44 of 99 (44%) single cell injections gave rise to tumors (Table 2). Virtually all tumors became palpable within 10 weeks of transplantation. Limit dilution analysis indicated that 49±8.6% (mean±SD; range=40% to 58%) of cells from each of these serially transplanted MPNST formed tumors after transplantation. Thus, approximately half of the cells within MPNSTs are capable of forming tumors by the time the MPNSTs have been passaged once.
Table 2.
Tumor formation after secondary transplantation of MPNST cells
| Tumor | Injected cell dose | Cells that formed tumors (%) | 95% CI (%) | ||
|---|---|---|---|---|---|
| 100 cells | 10 cells | 1 cell | |||
| Nf1+/−; Ink4a/Arf−/− mice | |||||
| 9 (N=1) | nd | nd | 4/8 | 50 | nd |
| 10 (N=1) | nd | nd | 5/9 | 56 | nd |
| 11 (N=3) | nd | 3/3 | 8/25 | 40 | 21–76 |
| 12 (N=4) | nd | 11/12 | 15/38 | 40 | 24–65 |
| 17 (N=2) | nd | nd | 12/19 | 58 | nd |
| TOTAL | nd | 14/15 (93%) | 44/99 (44%) | 49 | 22–71 |
| Nf1+/−;p53+/− mice | |||||
| 3 (N=2) | 7/9 | 2/11 | 1/10 | 1.8 | 0.9–3.6 |
| 4 (N=2) | 3/10 | 0/10 | 0/10 | 0.3 | 0.1–1.0 |
| 5 (N=1) | 4/4 | 1/5 | 2/10 | 5.7 | 1.9–17 |
| 8 (N=1) | 3/4 | 3/6 | nd | 2.5 | 0.9–7.1 |
| TOTAL | 17/27 (63%) | 6/32 (19%) | 3/30 (10%) | 2.6** | 0.9–7.3 |
Tumorigenic cell frequency significantly differed in this assay between MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice (**: p=2×10−5).
nd: not determined.
When MPNST cells from 6 first generation grafts from 4 primary MPNSTs from Nf1+/−;p53+/− mice were injected into secondary recipients, 6 of 32 (19%) injections of 10 cells and 3 of 30 (10%) injections of single cells gave rise to tumors (Table 2). All tumors became palpable within 10 weeks of transplantation. Limit dilution analysis indicated that an average of 2.6±2.3% (mean±SD; range=0.3 to 5.7%) of cells from each of these serially transplanted MPNSTs formed tumors after transplantation. Thus, MPNSTs from Nf1+/−; Ink4a/Arf−/− mice retained a significantly (19-fold; p=2×10−5) higher frequency of tumorigenic cells than MPNSTs from Nf1+/−;p53+/− mice, even upon secondary transplantation. This suggests that the reduced frequency of tumorigenic cells within primary MPNSTs from Nf1+/−;p53+/− mice did not reflect the presence of normal cells in these tumors.
MPNSTs from Nf1+/−; Ink4a/Arf−/− mice retain higher frequencies of tumorigenic cells upon transplantation into NOD/SCID ILR2γnull mice
To test whether MPNST cells from Nf1+/−;p53+/− mice were more immunogenic than MPNST cells from Nf1+/−; Ink4a/Arf−/− mice we transplanted dissociated cells from primary MPNSTs into immunocompetent C57BL/Ka recipients or into highly immunocompromised NOD/SCID ILR2γnull mice, which lack B, T and natural killer cells (Shultz et al., 2005). We found that 16–20% of cells from two primary MPNSTs from Nf1+/−; Ink4a/Arf−/− mice formed tumors irrespective of whether they were transplanted into C57BL/Ka or NOD/SCID ILR2γnull mice. Similarly, 1–5% of MPNST cells from Nf1+/−;p53+/− mice formed tumors in C57BL/Ka recipients and 0.6–2.5% formed tumors in NOD/SCID ILR2γnull recipients (Table 3). These results indicate that the recipient immune system has little effect on the frequency of MPNST cells that form tumors after transplantation, irrespective of genotype, and that the reduced frequency of tumorigenic MPNST cells from Nf1+/−;p53+/− mice cannot be explained by increased immunogenicity.
Table 3.
Tumor formation by primary MPNST cells in immunocompetent C57BL/Ka mice and immunocompromised NOD/SCID ILR2γnull mice
| Tumor | Recipient mice | Injected cell dose | Tumorigenic cells (%) | 95% CI | ||
|---|---|---|---|---|---|---|
| 100 | 10 | 5 | ||||
| Nf1+/−;Ink4a/Arf−/− (35) | C57Bl6 (BA) | 5/5 | 4/5 | 16 | 5.4–48 | |
| Nf1+/−;Ink4a/Arf−/− (35) | NOD/SCID IL2Rγnull | 5/5 | 4/5 | 16 | 5.4–48 | |
| Nf1+/−;Ink4a/Arf−/− (40) | C57Bl6 (BA) | 4/7 | 17 | 6.2–46 | ||
| Nf1+/−;Ink4a/Arf−/− (40) | NOD/SCID IL2Rγnull | 5/8 | 20 | 7.9–49 | ||
| Nf1+/−;p53+/− (7) | C57Bl6 (BA) | 5/5 | 3/8 | 5.0 | 1.8–13 | |
| Nf1+/−;p53+/− (7) | NOD/SCID IL2Rγnull | 4/5 | 3/7 | 2.5 | 1.0–6.5 | |
| Nf1+/−;p53+/− (28) | C57Bl6 (BA) | 3/5 | 1/8 | 1.0 | 0.4–2.8 | |
| Nf1+/−;p53+/− (28) | NOD/SCID IL2Rγnull | 2/5 | 1/8 | 0.6 | 0.2–2.1 | |
A higher percentage of MPNST cells from Nf1+/−; Ink4a/Arf−/− mice formed colonies in non-adherent culture as compared to Nf1+/−;p53+/− mice
We cultured primary mouse MPNST cells, 1 cell/well in 96 well plates for 10–14 days in non-adherent conditions (Molofsky et al., 2003). 35±20% (mean±SD) of MPNST cells from 12 different Nf1+/−; Ink4a/Arf−/− mice formed colonies, and 62% of these colonies (62 of 100) formed tumors after transplantation under the kidney capsule of C57BL/Ka mice. In contrast, only 4.1±3.4% of MPNST cells from 12 Nf1+/−;p53+/− mice formed colonies, and 32% of these colonies (13 of 40) formed tumors after transplantation into C57BL/Ka mice (Table 4). Averaging across tumors, 23±21% of single MPNST cells from Nf1+/−; Ink4a/Arf−/− mice and 2.0±2.9% of MPNST cells from Nf1+/−;p53+/− mice formed tumorigenic colonies. This indicates that tumorigenic cells were also significantly less common (11-fold, p=0.02) in MPNSTs from Nf1+/−;p53+/− mice as compared to Nf1+/−; Ink4a/Arf−/− mice, even when these cells were initially propagated in culture instead of transplanting uncultured cells directly in vivo (as in Tables 1–3).
Table 4.
Colony formation by single primary MPNST cells cultured under non-adherent conditions and tumor formation by these colonies after transplantation in vivo
| Tumor | Single cells that formed colonies (%) | Tumors/colonies injected | Single cells that formed tumorigenic colonies (%) |
|---|---|---|---|
| Nf1+/−; Ink4a/Arf−/− mice | |||
| 6 | 26 | 2/9 (22%) | 5.8 |
| 7 | 47 | 4/5 (80%) | 38 |
| 8 | 39 | 2/7 (29%) | 11 |
| 9 | 14 | 5/11 (45%) | 6.2 |
| 10 | 22 | 5/10 (50%) | 11 |
| 11 | 34 | 8/13 (62%) | 21 |
| 12 | 66 | 12/13 (92%) | 60 |
| 14 | 11 | 4/5 (80%) | 8.7 |
| 15 | 7.6 | 2/7 (29%) | 2.2 |
| 16 | 38 | 9/11 (82%) | 31 |
| 17 | 66 | 9/10 (90%) | 59 |
| 23 | 55 | nd | nd |
| Mean±SD | 35±20 | 62/100 (62%) | 23±21 |
| Nf1+/−;p53+/− mice | |||
| 1 | 10 | nd | nd |
| 2 | 3.1 | nd | nd |
| 3 | 6.1 | 0/10 (0%) | 0.0 |
| 4 | 2.4 | 1/5 (20%) | 0.5 |
| 5 | 0.5 | nd | nd |
| 6 | 8.3 | 4/4 (100%) | 8.3 |
| 7 | 0.5 | nd | nd |
| 8 | 5.6 | 4/10 (40%) | 2.2 |
| 9 | 1.6 | nd | nd |
| 12 | 0.5 | 1/1 (100%) | 0.5 |
| 13 | 8.1 | 1/6 (17%) | 1.4 |
| 14 | 2.9 | 2/4 (50%) | 1.5 |
| Mean±SD | 4.1±3.4** | 13/40 (32%) | 2.0±2.9* |
For each tumor, we determined the percentage of cells that formed colonies in culture and the percentage of these colonies that formed tumors upon transplantation into C57BL/Ka mice. The last column represents the product of these statistics: the percentage of MPNST cells that formed tumorigenic colonies.
p=3×10−5;
p=0.02. All statistics represent mean ± SD. For data on cell death in culture see Figure S3.
The reduced colony formation by MPNST cells from Nf1+/−;p53+/− mice was largely attributable to increased cell death as compared to MPNST cells from Nf1+/−; Ink4a/Arf−/− mice. Ten to fourteen days after adding MPNST cells to culture we carefully examined the wells. We found that 43±2.4% of wells in which we had plated single MPNST cells from Nf1+/−; Ink4a/Arf−/− mice did not contain any cells, indicating that these cells must have undergone cell death. In contrast, 69±13% of wells in which we had plated single MPNST cells from Nf1+/−;p53+/− mice did not contain any cells. This suggests that a significantly (p=0.02) higher percentage of single MPNST cells from Nf1+/−;p53+/− mice underwent cell death after being added to culture. The remaining wells that did not contain colonies, but were not empty, contained small numbers of cells (<10), indicating that these cells underwent a limited number of divisions.
We examined the number of apoptotic cells in MPNSTs in vivo and in MPNST cells cultured under non-adherent conditions (Fig. S3). MPNST tumors in Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice in vivo contained similar frequencies of cleaved caspase-3 positive cells. In culture, we found that a significantly higher percentage of cells from Nf1+/−;p53+/− mice compared to Nf1+/−; Ink4a/Arf−/− mice expressed cleaved caspase-3 after 12 days of culture (5.8±3.4% vs 2.2±2.4; mean±SD; p=0.03). MPNST cells from Nf1+/−; Ink4a/Arf−/− mice are therefore more likely to survive non-adherent culture than MPNST cells from Nf1+/−;p53+/− mice even though no difference in the frequency of apoptotic cells was observed in tumors in vivo. This suggests that differences in the rate of cell death between MPNSTs from different genetic backgrounds are context dependent. We therefore decided to explore whether changes in assay conditions could increase the frequency of clonogenic MPNST cells.
Laminin is expressed by more MPNST cells in Nf1+/−; Ink4a/Arf−/− mice than in Nf1+/−;p53+/− mice
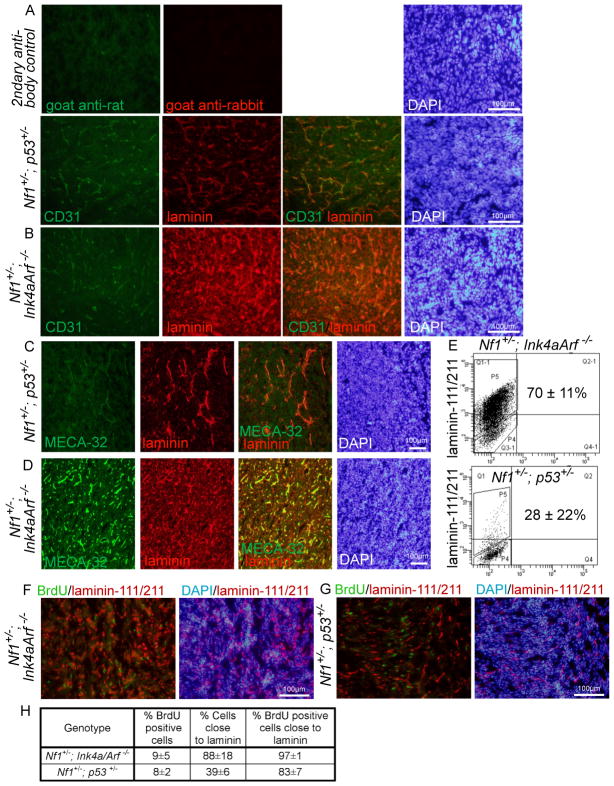
One common way of improving the growth and survival of cells in culture is by growing cells adherently on extracellular matrix, rather than non-adherently as described above. Adherent culture on laminin, in particular, enhances the growth and survival of neural stem/progenitor cells and glioma cells (Sun et al., 2008; Pollard et al., 2009; Lathia et al., 2010). Since MPNST cells from Nf1+/−;p53+/− mice did not exhibit increased cell death in vivo, in contrast to what was observed in culture, we wondered whether MPNST cells were exposed to laminin in the tumor environment in vivo. We examined the expression of laminin-111/−211 (laminin α1/β1/γ1 and α2/β1/γ1) on sections through MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice (Fig 2A–D). In MPNSTs from Nf1+/−; Ink4a/Arf−/− mice laminin-111/−211 was widely expressed by most tumor cells, including cancer cells and vascular/perivascular stromal cells (Fig. 2B, D). In contrast, in MPNSTs from Nf1+/−;p53+/− mice laminin appeared to be expressed by many fewer cells and to be mainly vascular/perivascular (Fig. 2A, C).
Figure 2. Laminin is expressed by most MPNST cells from Nf1+/−; Ink4a/Arf −/− mice, but only by a subset of MPNST cells from Nf1+/−;p53+/− mice.
(A–D) MPNSTs from Nf1+/−;p53+/− (A, C) and Nf1+/−; Ink4a/Arf−/− (B, D) mice were stained with antibodies against laminin-111/211, CD31 and MECA-32 (to label endothelial cells) as well as DAPI to identify nuclei.. (E) Dissociated MPNST cells, after exclusion of blood and endothelial cells, were stained with antibodies against laminin-111/211 and analyzed by flow cytometry. Numbers reflect the percentage of cells that stained more strongly than isotype control (3–5 MPNSTs/genotype, p=0.02 between the two genotypes). (F–H) Mice bearing MPNSTs were treated with BrdU for 6 hours and cryosections were stained with DAPI as well as antibodies against laminin-111/211 and BrdU. In MPNSTs from Nf1+/−;p53+/− mice, BrdU+ cells were 2.1-fold more likely (p=0.04) than the average MPNST cell to localize adjacent to laminin (the data reflect 3 tumors/genotype and 2200–3200 cells/tumor). All statistics are mean±SD.
We found that dissociated MPNST cells from Nf1+/−; Ink4a/Arf−/− mice exhibited much stronger laminin-111/211 staining than MPNST cells from Nf1+/−;p53+/− mice (Fig. 2E). Indeed, 70±11% of MPNST cells from Nf1+/−; Ink4a/Arf−/− mice and 28±22% of MPNST cells from Nf1+/−;p53+/− mice stained with laminin by flow cytometry. This demonstrates that most MPNST cells from Nf1+/−; Ink4a/Arf−/− mice carry laminin with them after dissociation, whereas only a minority of MPNST cells from Nf1+/−;p53+/− mice do so. If laminin promotes the survival or proliferation of MPNST cells, then MPNST cells from Nf1+/−;p53+/− mice would be expected to depend more upon exogenous laminin, as compared to MPNST cells from Nf1+/−; Ink4a/Arf−/− mice.
We administered a 6 hour pulse of BrdU to mice then stained sections through their MPNSTs with antibodies against BrdU and laminin-111/211 to assess the localization of dividing cells relative to laminin-expressing cells. BrdU+ cells tended to localize adjacent to laminin expressing cells and regions of the tumor that lacked laminin staining also had fewer dividing cells. MPNSTs from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice included 9±5 and 8±2% (mean±SD) BrdU+ cells, respectively (Fig. 2F–H). In MPNSTs from Nf1+/−; Ink4a/Arf−/− mice, laminin was widely expressed such that almost all cells (88±18%), including nearly all BrdU+ cells (97±1%) were adjacent to laminin-stained cells (Fig. 2F, H). However, in MPNSTs from Nf1+/−;p53+/− mice, laminin was more restricted in its expression such that only 39±6% of MPNST cells were adjacent to laminin-stained cells. Yet most BrdU+ cells (83±7%) remained adjacent to laminin-stained cells in these tumors (Fig. 2G, H). Therefore, BrdU+ cells were more than twice as likely as all MPNST cells to localize adjacent to laminin-expressing cells in MPNSTs from Nf1+/−;p53+/− mice. This is consistent with the possibility that laminin may promote the proliferation of MPNST cells, though laminin staining may also be associated with vasculature that promotes proliferation through other mechanisms.
MPNSTs from Nf1+/−;p53+/−, but not Nf1+/−; Ink4a/Arf−/−, mice form more colonies on laminin
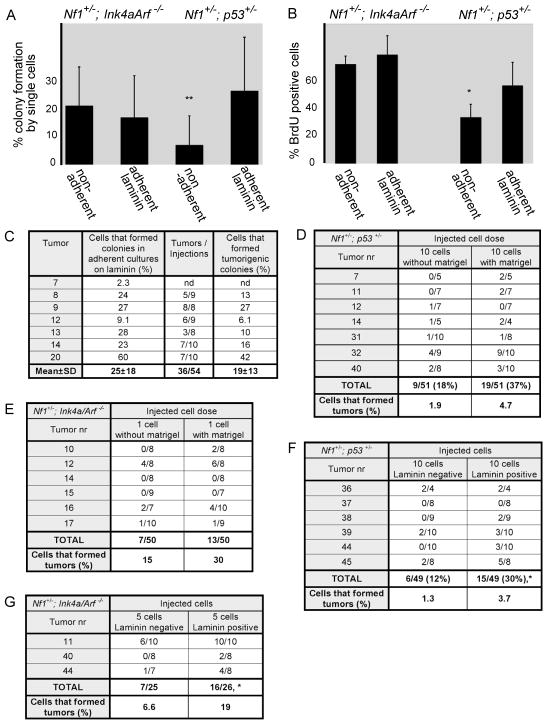
If laminin promotes clonogenic growth by MPNST cells and MPNST cells from Nf1+/−;p53+/− mice are less likely to carry laminin after dissociation (Fig. 2E), then exogenous laminin might stimulate clonogenic growth by MPNST cells from Nf1+/−;p53+/− mice to a greater extent than MPNST cells from Nf1+/−; Ink4a/Arf−/− mice. To test this we compared colony formation by single MPNST cells from both genetic backgrounds in non-adherent cultures and in adherent cultures on laminin-111 (Fig. 3A). Adherent culture on laminin did not affect the percentage of MPNST cells from Nf1+/−; Ink4a/Arf−/− mice that formed colonies compared to non-adherent cultures (Fig. 3A); however, it significantly increased the percentage of MPNST cells from Nf1+/−;p53+/− mice that formed colonies, from 6.7±9.8% to 25±18% (p=0.003; Fig. 3A). Although the percentage of cells that formed colonies varied between tumors, the rate of colony formation on laminin was always at least as high as in non-adherent cultures and the effect was statistically significant in a paired t-test (p=0.003). Adherent culture on fibronectin or collagen may have enhanced the rate of tumor formation to some extent, but the effects were variable among tumors and not statistically significant (Table S3). Exposure to laminin thus increased colony-formation by MPNST cells from Nf1+/−;p53+/− mice, but not Nf1+/−; Ink4a/Arf−/− mice, consistent with the difference in endogenous laminin expression between these genetic backgrounds.
Figure 3. MPNST cells from Nf1+/−;p53+/− mice formed more colonies when plated adherently on laminin.
(A) Single MPNST cells from Nf1+/−;Ink4a/Arf−/− (N=6) and Nf1+/−;p53+/− mice (N=7) were sorted into individual wells of 96-well plates and cultured either non-adherently or adherently on laminin-111. **: p=0.003 by paired t-test between adherent and non-adherent cultures of cells from Nf1+/−;p53+/− mice. (B) 10000 MPNST cells from Nf1+/−; Ink4a/Arf−/− or Nf1+/−;p53+/− mice (3 tumors each) were cultured non-adherently or adherently on laminin-111 for 1–3 days, then a 3 hour pulse of BrdU was administered (*, p<0.05). Error bars in A and B represent SD. (C) Adherent colonies from single MPNST cells from Nf1+/−;p53+/− mice grown on laminin-111 were trypsinized and injected under the kidney capsules of C57BL/Ka mice, then monitored for the ability to form tumors for 15 weeks. The last column represents the percentage of MPNST cells that formed adherent colonies and that went on to form tumors upon transplantation of the colonies in vivo (the product of columns 1 and 2). nd means not determined.(D, E) Freshly isolated MPNST cells from Nf1+/−;p53+/− (D) or Nf1+/−;Ink4a/Arf−/− (E) mice were transplanted under the kidney capsule of C57BL/Ka mice, with or without Matrigel, then monitored for tumor formation for at least 15 weeks. (F, G) Freshly dissociated laminin-positive or laminin-negative MPNST cells from Nf1+/−;p53+/− (F) or Nf1+/−;Ink4a/Arf−/− (G) mice were transplanted under the kidney capsule of C57BL/Ka mice. Cells that stained positively for laminin were more likely to form tumors in both genetic backgrounds (p=0.02 for Nf1+/−; Ink4a/Arf−/− mice and p=0.049 for Nf1+/−;p53+/− mice, paired T-test). See also Table S3 and Figure S4.
Adherent colonies grown on laminin by MPNST cells from Nf1+/−;p53+/− mice, but not Nf1+/−; Ink4a/Arf−/− mice, also tended to be larger than those that grew in non-adherent cultures (data not shown). Consistent with this, a significantly higher frequency of cells within colonies from Nf1+/−;p53+/− mice incorporated a pulse of BrdU when cultured on laminin-111 compared to non-adherent culture conditions whereas, adherent culture on laminin did not significantly affect the rate of BrdU incorporation into colonies cultured from Nf1+/−; Ink4a/Arf−/− mice (Fig. 3B).
To assess the proliferative potential of MPNST cells that formed colonies on laminin we dissociated the colonies that arose from single cells and replated them into secondary cultures. 23 of 24 (96%) colonies from three primary MPNSTs obtained from Nf1+/−;p53+/− mice gave rise to secondary colonies, yielding an average of 2573±626 secondary colonies per primary colony. An average of 30±10% of cells within the primary colonies formed secondary colonies upon replating. These results suggest that many MPNST cells from Nf1+/−;p53+/− mice have the potential to proliferate extensively if assayed under permissive conditions.
To assess whether colony-forming MPNST cells from Nf1+/−;p53+/− mice had the potential to form tumors in vivo, we dissociated individual colonies and injected the cells under the kidney capsule of C57BL/Ka mice. 25±18% of the MPNST cells formed adherent colonies on laminin-111 in these experiments and 36 of 54 colonies formed tumors in vivo (Fig. 3C). Thus, 19±13% of single MPNST cells from Nf1+/−;p53+/− mice formed tumorigenic colonies in the presence of laminin-111. The histological appearance of the tumors that arose from transplanted colonies was similar to primary MPNSTs (Fig. S4).
Given the increased colony formation by MPNST cells on laminin we tested whether the tumorigenicity of uncultured primary MPNST cells in vivo was increased by mixing the cells with Matrigel (of which laminin is the main component (Kleinman and Martin, 2005)). Suspension of primary MPNST cells in Matrigel prior to injection approximately doubled the frequency MPNST cells from both genetic backgrounds that formed tumors (Fig. 3D, E). Limit dilution analysis indicated that 4.7% of primary MPNST cells from Nf1+/−;p53+/− mice and 30% of primary MPNST cells from Nf1+/−; Ink4a/Arf−/− mice formed tumors when injected under the kidney capsule with Matrigel. These results further emphasize that the frequency of tumorigenic MPNST cells varies with tumor genotype and assay conditions but that many primary MPNST cells are capable of forming tumors in immunocompetent mice.
To test whether endogenous laminin expression distinguishes intrinsically tumorigenic from intrinsically non-tumorigenic subpopulations of MPNST cells, we sorted laminin-positive and laminin-negative MPNST cells from both genetic backgrounds and transplanted them under the kidney capsule of C57BL/Ka mice. Both laminin-positive and laminin-negative MPNST cells formed tumors, though the rate of tumor formation by the laminin-positive cells was significantly higher in both genetic backgrounds (2.2-fold difference, p=0.02 for cells from Nf1+/−; Ink4a/Arf−/− mice; 2.5-fold difference, p=0.049 for Nf1+/−;p53+/− mice; Fig. 3F, G). Since 6.6% and 1.3% of laminin negative MPNST cells from Nf1+/−; Ink4a/Arf−/− mice and Nf1+/−;p53+/− mice, respectively, formed tumors in vivo, our data do not support the idea that laminin negative cells intrinsically lack tumor-forming capacity. Rather, the data suggest that laminin increases the clonogenic potential of MPNST cells but that it does not matter whether the laminin is intrinsic or extrinsic to the cells. It remains possible there is a hierarchy of tumorigenic and non-tumorigenic MPNST cells that can be identified with other markers. Thus, our data demonstrate that many MPNST cells are capable of forming tumors, depending on assay conditions and genetic background, but the formal question of whether MPNSTs exhibit a hierarchical organization consistent with the cancer stem cell model is not addressed by our study.
Laminin promotes the survival and proliferation of MPNST cells via β1 integrin
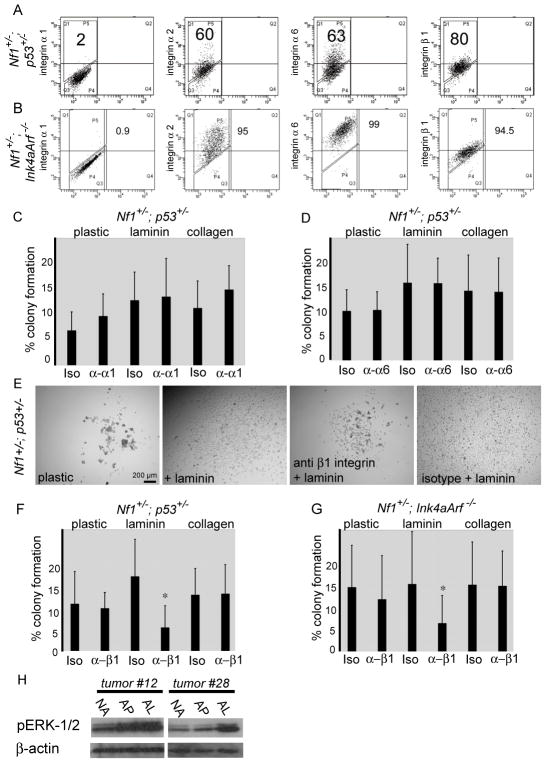
α1β1, α2β1, α3β1, α6β1, α7β1 and α6β4 integrins are laminin receptors (Miner and Yurchenco, 2004). By flow cytometry we found integrins α2, α6, and β1 were widely expressed on the surface of MPNST cells from Nf1+/−; Ink4a/Arf−/− and Nf1+/−;p53+/− mice (Fig. 4A). Integrins α1 (Fig. 4A) and β4 (data not shown) were rarely expressed at detectable levels. Thus, MPNST cells appear to express multiple laminin receptors, including α2β1 and α6β1. Laminin-111/211 is also strongly expressed by basement membranes under the kidney capsule (Fig. S5), just as in MPNSTs (Fig. 2) potentially explaining why the kidney capsule creates a permissive environment for tumor formation by MPNST cells.
Figure 4. MPNST cells expressed laminin receptors and blocking antibodies against β1 integrin decreased colony formation on laminin.
(A, B) Expression of indicated integrins by MPNST cells from Nf1+/−;p53+/− (A) and Nf1+/−; Ink4a/Arf−/− (B) mice. Numbers reflect the percentages of cells that stained more strongly than isotype control. (C, D) Colony formation by MPNST cells from Nf1+/−;p53+/− mice on the indicated substrate treated with function-blocking antibodies against α1 (C) or α6 (D) integrin (Iso means isotype control antibody). (E) Representative pictures of colonies formed by MPNST cells from Nf1+/−;p53+/− mice in the presence of function blocking antibodies against β1 integrin or isotype control. (F, G) The effect of blocking antibody against β1 integrin on the percentage of cells that formed colonies. *: p=0.02 for Nf1+/−;p53+/− mice (F) and p=0.05 for Nf1+/−; Ink4a/Arf−/− (G) on laminin. No significant difference on plastic or collagen. (H) Western blots of extracts from MPNSTs cells obtained from Nf1+/−;p53+/− mice using antibodies against phospho-ERK1/2. Cells were cultured for 3 days under non-adherent conditions (NA), adherently on plastic (AP) or adherently on laminin-111 (AL). Error bars represent SD. See also Figure S5.
We examined whether antibodies that block the binding of laminin-111 to integrin receptors would impede colony-formation. We were unable to find a preservative-free antibody against α2-integrin suitable for use in these experiments. Addition of blocking antibodies against α1-integrin (Fig. 4C) or α6-integrin (Fig. 4D) to culture did not affect the percentage of MPNST cells from Nf1+/−;p53+/− mice that formed colonies or the size of colonies. In contrast, addition of blocking antibodies against β1-integrin (Milner and Campbell, 2002), but not isotype control antibody, significantly decreased the percentage of MPNST cells from Nf1+/−;p53+/− mice (Fig. 4F) and Nf1+/−; Ink4a/Arf−/− mice (Fig. 4G) that formed colonies on laminin-111 as well as the size of these colonies (Fig. 4E) but did not affect the percentage of cells that formed adherent colonies on uncoated plastic or collagen 1 (Fig. 4F, G). These data demonstrate that laminin binding to β1-integrin-containing receptors increases the clonogenicity of MPNST cells from both genetic backgrounds.
Laminin promotes the survival of Schwann cells (Yu et al., 2005) and MPNSTs arise from Schwann cells (Zhu et al., 2002; Joseph et al., 2008; Zheng et al., 2008). Integrin receptors signal through the ERK pathway (Guo and Giancotti, 2004) and ERK provides a survival and proliferation signal to Schwann lineage cells (Newbern et al., 2011) and MPNST cells (Ambrosini et al., 2008). We found that adherent culture on laminin-111 increased the levels of phosphorylated ERK1/2 in MPNST cells from some Nf1+/−;p53+/− mice compared to non-adherent culture or adherent culture on plastic (Fig. 4H). Laminin binding to β1-integrin may therefore promote the survival and proliferation of MPNST cells by increasing ERK signaling.
DISCUSSION
Our data demonstrate that cells with tumorigenic potential are common in MPNSTs of both genotypes but that tumors with different genotypes sometimes require different assay conditions to read-out the full spectrum of cells with tumorigenic potential. If tumor genotype commonly influences the assay conditions that must be used to detect tumorigenic cells then assay conditions that detect the full spectrum of tumorigenic cells in one cancer patient may not detect the full spectrum of tumorigenic cells in other patients with the same cancer type. Studies of mouse lung cancers (Curtis et al., 2010), mouse mammary cancers (Vaillant et al., 2008) and mouse myeloid leukemias (Somervaille and Cleary, 2006; Kennedy et al., 2007), have shown that cancers from different genetic backgrounds have different frequencies of tumorigenic cells, differences in the extent to which they follow a cancer stem cell model, or tumorigenic cells that express different markers. Human acute lymphoblastic leukemia samples that differ with respect to CDKN2A/B genotype also display differences in leukemogenic cell frequencies in immunocompromised mice (Notta et al., 2011). We demonstrate here that tumors of different genotypes can have similarly high frequencies of cells with tumorigenic potential but can require different assay conditions to detect these tumorigenic cells. Furthermore, we show that sarcomas sometimes contain common tumorigenic cells.
Our data suggest that the binding of laminin to β1-integrin-containing receptors promotes the survival and proliferation of MPNST cells. MPNST cells from Nf1+/−;p53+/− and Nf1+/−; Ink4a/Arf−/− mice depended upon the binding of laminin to β1-integrin for proliferation and survival (Fig. 4) but MPNST cells from Nf1+/−; Ink4a/Arf−/− mice did not require the addition of exogenous laminin to culture (Fig. 3A, B), presumably because these cells were much more likely to carry endogenous laminin as compared to MPNST cells from Nf1+/−;p53+/− mice. Suspension of MPNST cells from either genetic background in Matrigel doubled the frequency of cells that formed tumors in vivo (Fig. 3D, E) and dividing cells in primary MPNSTs in vivo almost always had contact with laminin-expressing cells (Fig. 2F–H). Thus laminin appears to be one important determinant of clonogenicity in MPNST cells but it does not appear to matter whether the laminin is intrinsic or extrinsic to the MPNST cells.
Our results raise the possibility that the failure to restore critical extracellular matrix cues after tumor dissociation may lead to underestimates of the frequency of tumor-initiating cells. The common use of sphere-formation assays in non-adherent cultures to identify tumor-initiating cells may exacerbate this problem by depriving dissociated cancer cells of extracellular matrix.
Our data demonstrate that melanoma is not unique among solid tumors in containing common cells with tumorigenic potential. We have demonstrated that at least 30% of human melanoma cells obtained directly from patients have the potential to form tumors upon transplantation into NOD/SCID ILR2γnull mice (Quintana et al., 2008; Quintana et al., 2010). An even higher percentage of single cells obtained from primary mouse melanomas can form tumors upon transplantation (Held et al., 2010). While some cancers are likely to have only rare cells with the potential to proliferate extensively, other cancers are likely to contain common tumorigenic cells and it remains uncertain what fraction of cancers fall in each category. Some mouse leukemias contain common leukemogenic cells (Somervaille and Cleary, 2006; Kelly et al., 2007; Williams et al., 2007). However, other hematopoietic malignancies contain only rare leukemogenic cells (Lapidot et al., 1994; Kennedy et al., 2007) even when mouse leukemias are transplanted into histocompatible mice (Yilmaz et al., 2006; Oravecz-Wilson et al., 2009). A recent study discovered that human hematopoietic stem cells engraft more efficiently in female as compared to male mice (Notta et al., 2010), a variable that has rarely been taken into account in xenotransplantation experiments. Thus, while many solid cancers have appeared to contain only rare tumorigenic cells (Ishizawa et al., 2010) an open question concerns the extent to which existing assays underestimate the frequency of cells with tumorigenic potential.
Our observation that approximately 20% of MPNST cells from Nf1+/−;p53+/− and Nf1+/−; Ink4a/Arf−/− mice have the potential to form tumors is likely an underestimate because there are likely to be important features of the tumor environment that are not adequately modeled in the tumorigenesis assays we used. For example, it is possible that tumor-associated fibroblasts or tumor-infiltrating inflammatory cells promote the survival or proliferation of MPNST cells (Le et al., 2009; Staser et al., 2010) in a way that is not initially replicated when we place small numbers of dissociated MPNST cells in culture or under the kidney capsule in vivo. More sophisticated models of this tumor microenvironment could reveal even higher frequencies of MPNST cells that have the potential to form tumors.
Although we found that cells with tumorigenic potential were common in mouse MPNSTs, a separate question concerns the spectrum of cells that are actually fated to contribute to disease progression in vivo. Our data demonstrating that many cells divide rapidly within MPNSTs (Fig. 1H) suggests that many cells contribute to tumor growth. However, it is also possible that many of these cells divide only a limited number of times and that only a small fraction of the cells with the potential to proliferate extensively actually does so in vivo. Fate-mapping studies will be required to assess whether many or few MPNST cells actually contribute extensively to tumor growth or disease progression in vivo.
The question of whether MPNSTs follow a cancer stem cell model in which cancer cells are hierarchically organized into intrinsically different subpopulations of tumorigenic and non-tumorigenic cells also remains an open question. One possibility is that MPNSTs contain common tumorigenic cells with no hierarchical organization. Another possibility is that MPNSTs contain a shallow hierarchy in which common tumorigenic cells differentiate into non-tumorigenic MPNST cells. Even if the latter is the case, MPNST would be quite different from other cancers suggested to follow the cancer stem cell model.
Our data demonstrate that cells with tumorigenic potential are common in MPNSTs and that tumors from different genetic backgrounds can require different assay conditions to readout the full spectrum of cells with tumorigenic potential. This suggests that efforts to quantify the frequency of tumorigenic cells in human cancers may be complicated by confounding effects of tumor genotype on assay conditions. As assays improve it is likely that estimates of tumorigenic cell frequencies will continue to increase in many cancers. It remains to be determined what fraction of cancers have only rare tumorigenic cells and what fraction of cancers have common tumorigenic cells. Determining which cancers fall in each category has fundamental implications for therapy as attempts to target small subpopulations of cancer cells are not likely to improve the treatment of cancers in which many cells can drive disease progression.
EXPERIMENTAL PROCEDURES
Mice
Nf1+/− (Jacks et al., 1994) mice were bred with Ink4aArf−/− mice (Serrano et al., 1996) to yield compound mutant Nf1+/−; Ink4a/Arf−/− mice (Joseph et al., 2008). Nf1+/−; Ink4a/Arf−/− mice and Nf1+/−;p53+/− mice (Cichowski et al., 1999) were backcrossed on a C57BL/Ka background for at least 6 generations. Genotyping was performed as described by Joseph et al. (2008). All animal experiments were performed in accordance with protocols approved by the University of Michigan Committee on the Use and Care of Animals.
Dissociation of MPNSTs and flow cytometry
Tumors were harvested from mice and minced using a scalpel. Minced tissue was washed in Ca2+ and Mg2+ -free Hanks Buffered Salt Solution (HBSS, Invitrogen product # 14175-103), centrifuged, and treated for 20 min at 37°C with a mixture of 25% Ca2+ and Mg2+ -free HBSS, 25% Collagenase IV (Worthington, product # 4189, resuspended to 2000 U/mL in HBSS containing Ca2+ and Mg2+, Invitrogen # 14025-134) and 50% Trypsin-EDTA solution (0.05%, Invitrogen # 25300) with constant agitation. The reaction was quenched with staining medium consisting of L15 medium (Invitrogen # 21083-027), 1 mg/ml BSA (Sigma, # A-3912), 10 mM HEPES (BioWhittaker # 17-738E), 1% penicillin/streptomycin (Invitrogen # 15140), supplemented with DNAse 1 (100 U/ml, Sigma D-4527) and filtered through 70μm nylon filter (Fisher # 22363548). The cells were centrifuged and resuspended in staining medium, then counted on a hemocytometer. Cells were stained with antibodies against CD31 (BioLegend # 102410), CD45 (e-bioscience # 17-0451-83) and Ter119 (BioLegend # 116206) in 200μl of staining medium for 20 min at 4°C. The cells were then washed and resuspended in staining medium supplemented with DAPI (Sigma # D-8417, 10μg/mL). To evaluate the expression of laminin receptors, cells were stained with antibodies against integrin α1 (BD Pharmingen # 555001), α2 (BD Pharmingen # 554998), α6 (e-bioscience # 12-0495-83) and integrin β1 (BD Pharmingen # 553837) and appropriate secondary antibodies prior to staining with blood lineage markers. Flow cytometry was performed with a FACSVantage SE-dual laser, three-line flow cytometer or with a FACSAria II (Becton-Dickinson).
Cell culture
Cells were typically cultured in 96-well tissue culture plates (Corning # 3595) at a density of 1 cell/well. For non-adherent cultures, ultra-low binding plates (Corning # 3474) were used. For adherent cultures, plates were coated with fibronectin (Biomedical Technologies # BT225), laminin-111 (Invitrogen # 23017-015) or collagen-1 (BD Bioscience # 354236), all at 50μg/ml. The culture medium for both adherent and non-adherent cultures was based on a 5:3 mixture of DMEM-low:Neurobasal medium (Invitrogen # 11885-092 and 21103-049). The medium was supplemented with 15% chick embryo extract (CEE, prepared as described, (Stemple and Anderson, 1992)), 20 ng/ml recombinant human bFGF (R&D Systems #233-FB-025), 20 ng/ml IGF1 (R&D Systems #291-G1-050), 1% N2 supplement (Invitrogen # 17502-048), 2% B27 supplement (Invitrogen # 17504-044), 50 mM 2-mercaptoethanol (Acros Organics), 35 ng/ml retinoic acid (Sigma # R2625), 1% penicillin/streptomycin (Invitrogen # 15140). In some cases the medium was supplemented with laminin-111 or fibronectin, both at 50 μg/ml. All cultures were maintained at 37°C in 6% CO2/balance air. For experiments using blocking antibodies, cells were cultured at a density of 50-100 cells/well on 24-well plates on plastic or laminin-111 (50μg/ml) in the presence or absence of antibodies against integrin α1 (BD Bioscience #555000), α6 (clone GoH3, RD Systems #MAB13501) or β1 (BD Bioscience #555002) or corresponding isotype controls (all at 10 μg/ml). Colony formation was assessed after 5–8 days.
Kidney capsule injections
MPNST cells were resuspended in 30μl of sterile staining medium. Mice were anesthetized by intra-peritoneal injection of ketamine/xylazine. After shaving the skin and prepping with betadine (Fisher # 19-027136), a 1 cm longitudinal incision was made in the skin of the abdomen and in the body wall above the kidney. Cell suspensions were injected under the capsule of the exposed kidney using an insulin syringe with a 31G needle (BD Bioscience # 305937). In some cases 50% Matrigel (BD Bioscience # 354262) was added to the cell suspension. The incisions were closed using absorbable suture (Tevdek, Genzyme Surgical #7-734). Animals were given antibiotic water (1.1g/l neomycin sulfate and 106 U/l polymixin B sulfate) and monitored closely. Tumor formation was assessed by weekly palpation of the abdomen or by magnetic resonance imaging for at least 15 weeks. The frequency of tumorigenic cells and the 95%-confidence interval (CI) were calculated using extreme limiting dilution (ELDA) software (http://bioinf.wehi.edu.au/software/elda/index.html).
Supplementary Material
Highlights.
Many MPNST cells have the potential to form tumors
Some sarcomas contain high frequencies of tumor-initiating cells
Tumors of different genotypes require different assays to detect tumorigenic cells
Laminin promotes clonogenic growth but can be intrinsic or extrinsic to MPNST cells
SIGNIFICANCE.
Our results demonstrate that mouse MPNSTs contain high frequencies of tumorigenic cells, which were similar upon transplantation into immunocompetent or immunocompromised mice. However, tumor genotype influenced the laminin expression pattern, and therefore the dependence of MPNST cells on exogenous laminin and the frequency of tumorigenic cells that could be detected in some assays. Although laminin binding to β1-integrin was important for clonogenic growth, the laminin could either be intrinsic or extrinsic to MPNST cells. Tumors of different genotypes, even with the same malignant phenotype, can therefore require different assay conditions to detect the full range of cells with tumorigenic potential.
Acknowledgments
This work was supported by the National Institute of Neurological Disorder and Stroke (NS-040750), the Howard Hughes Medical Institute, and the Liddy Shriver Sarcoma Initiative. JB was supported by postdoctoral fellowships from the Swiss National Foundation of Science and the Children’s Tumor Foundation. Thanks to Kristina Harter for technical assistance. Thanks to David Adams and Martin White for assistance with flow cytometry, which was partially supported by the University of Michigan (UM) Comprehensive Cancer Center, NIH CA46592. Thanks to Maria Ripberger, Alan Burgess and Nancy McAnsh for paraffin embedding, sectioning and immunohistochemistry. Thanks to Amanda Welton in the UM Center for Molecular Imaging for magnetic resonance imaging. Thanks to Tyler Jacks for providing Nf1 germline knockout mice, to Tyler Jacks and Karlyne Reilly for providing Nf1+/−;p53+/− mice and to Ron DePinho for providing Ink4a/Arf mutant mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agesen TH, Florenes VA, Molenaar WM, Lind GE, Berner JM, Plaat BE, Komdeur R, Myklebost O, van den Berg E, Lothe RA. Expression patterns of cell cycle components in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2005;64:74–81. doi: 10.1093/jnen/64.1.74. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Cheema HS, Seelman S, Teed A, Sambol EB, Singer S, Schwartz GK. Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther. 2008;7:890–896. doi: 10.1158/1535-7163.MCT-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R, Woolfenden AE, Kirsch DG, Wong KK, Kim CF. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010;7:127–133. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Held MA, Curley DP, Dankort D, McMahon M, Muthusamy V, Bosenberg MW. Characterization of melanoma cells capable of propagating tumors from a single cell. Cancer Research. 2010;70:388–397. doi: 10.1158/0008-5472.CAN-09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumor predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genetics. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13:129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. doi: 10.1126/science.1149590. author reply 1722. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Pierce GB. Multipotentiality of single embryonal carcinoma cells. Cancer Research. 1964;24:1544–1551. [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature. 1994;17:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP, et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Munoz P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the alpha6beta1 integrin. J Neurosci. 2002;22:1562–1572. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Ann Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgcnull recipients. Blood. 2010 doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- Notta F, Mullighan CG, Wang JC, Poeppl A, Doulatov S, Phillips LA, Ma J, Minden MD, Downing JR, Dick JE. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469:362–367. doi: 10.1038/nature09733. [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Oravecz-Wilson KI, Philips ST, Yilmaz OH, Ames HM, Li L, Crawford BD, Gauvin AM, Lucas PC, Sitwala K, Downing JR, et al. Persistence of leukemia-initiating cells in a conditional knockin model of an imatinib-responsive myeloproliferative disorder. Cancer Cell. 2009;16:137–148. doi: 10.1016/j.ccr.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nature Cancer Reviews. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Perrone F, Tabano S, Colombo F, Dagrada G, Birindelli S, Gronchi A, Colecchia M, Pierotti MA, Pilotti S. p15INK4b, p14ARF, and p16INK4a inactivation in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. Clin Cancer Res. 2003;9:4132–4138. [PubMed] [Google Scholar]
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic Heterogeneity among Tumorigenic Melanoma Cells from Patients that Is Reversible and Not Hierarchically Organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rubin JB, Gutmann DH. Neurofibromatosis type 1 - a model for nervous system tumour formation? Nature Reviews. 2005;5:557–564. doi: 10.1038/nrc1653. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116:157–164. doi: 10.1182/blood-2009-09-242875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Research. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes & Development. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by non-myelinating Schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.