Abstract
Methods for non-invasive imaging of specific disease-related molecular changes are being developed in order to expand and improve diagnostic capabilities, to enhance clinical care for patients. These new techniques are also now used in research programs in order to characterize pathophysiology and as a surrogate endpoint for therapeutic efficacy. Molecular imaging with contrast-enhanced ultrasound relies on the detection of microbubbles or other acoustically-active particulate agents that are targeted to and retained at sites of disease. This review describes the progress that has been made in the development and testing of methods for contrast ultrasound molecular imaging with a specific focus on cardiovascular disease. Specific topics addressed include probe development, detection methods, and specific biologic processes that are important in clinical cardiovascular medicine have been targeted with microbubble contrast agents.
The Spectrum of Molecular Imaging
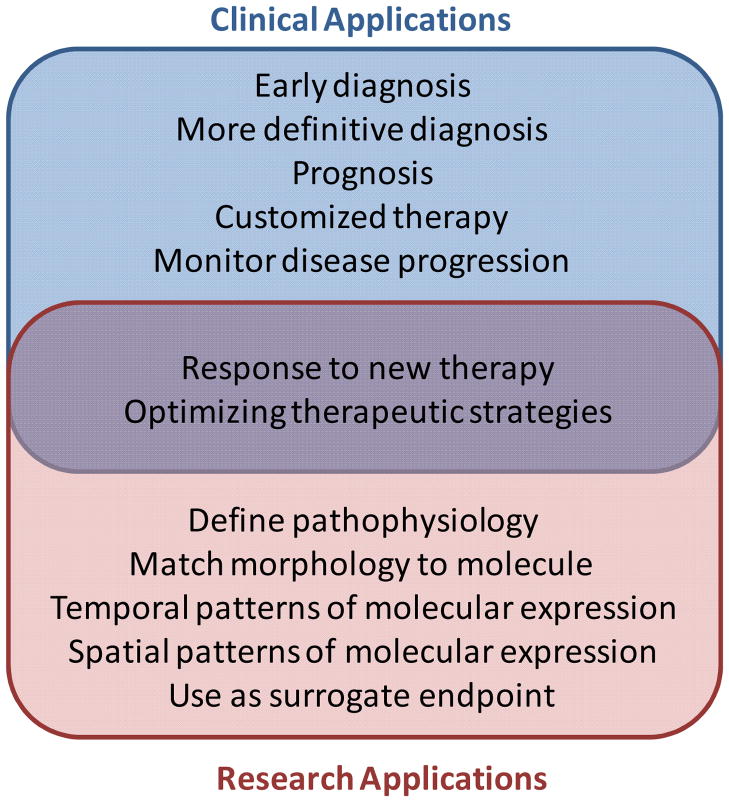
The term molecular imaging can be used in the broad sense to describe a very wide array of different imaging techniques that are used in both the research and clinical settings. For the biologist in the basic sciences, this term can be used to describe the myriad of techniques that have been developed to image cell properties such as protein synthesis and trafficking, metabolic status, gene expression, enzyme activity, etc. For the clinician or clinical researcher, molecular imaging is more often used to describe in vivo imaging methods that provide a non-invasive assessment of disease-related molecular or cellular phenotype that can be used in patients or in animal models of disease. For the most part, these techniques have been developed for specific clinical or research applications, partial list of which are represented in Figure 1.
Figure 1.
Examples of applications for molecular imaging that can be classified as clinical, research-oriented, or both.
For all major forms of non-invasive cardiovascular imaging which includes radionuclide/PET imaging; magnetic resonance imaging, ultrasound, CT, optical imaging; there have been major technologic achievements that have had to occur in order to perform molecular imaging. One general advance for all of forms of imaging has been the development of novel contrast agents that are either targeted to or activated by a disease process of interest. For the different imaging technologies, there are major differences between probes according to in vivo kinetics, distribution, sensitivity, specificity, signal-to-noise ratio, toxicity, and cost. In this review, we will review methods for ultrasound molecular and cellular imaging using targeted microbubble contrast agents.
Site-targeted Ultrasound Contrast Agents
Ultrasound contrast agents that have been developed for clinical use in cardiology or radiology are composed of microbubbles that, in general, contain a high molecular weight gas core and are stabilized by encapsulation with protein (albumin), lipid/surfactants, or biocompatible polymers.(1) The mean diameter for most of these agents is several microns, although acoustically-active emulsion-based nanoparticles or submicron gas-containing liposomes have also been developed. The basis for signal generation from these agents is thought to be largely from the stable cavitation (vibration) or inertial cavitation (disruption with release free gas) of these particles within the pressure peaks and nadirs in the ultrasound field.(2,3) Cavitation occurs by virtue of the gas core which makes microbubbles much more compressible than water or cells and, because they are also smaller than the wavelength of conventional diagnostic ultrasound, these particles undergo volumetric vibration during the oscillatory pressures of ultrasound imaging.(2) With sufficient acoustic pressures near the resonant frequency for microbubbles (determined by their size and compressibility) either stable non-linear oscillation or inertial cavitation produce harmonic or even off-harmonic signals which can be used to specifically detect microbubbles signal relative to tissue signal. These algorithms have been reviewed elsewhere.(1) However, a key point is that ultrasound signal intensity is related to the integrity of the microbubble. Accordingly, both encapsulation and the use of inert high-molecular weight gases (perfluorocarbons, sulfur hexafluoride) that have low solubility and diffusivity in plasma are key aspects in designing agents that are stable in the circulation and are appropriate for perfusion imaging or for molecular imaging.
Like other many other forms of molecular imaging, targeted contrast ultrasound relies on the selective retention of the contrast agent at specific sites of disease and detection of the agent after clearance of the freely circulating non-attached population. Non-targeted microbubble contrast agents are, in general, pure intravascular tracers that remain entirely within the vascular space and behave similar to red blood cells within the microcirculation.(4,5) Because of this property, microbubbles designed for molecular imaging have been targeted to antigens that are expressed within the vascular compartment such as on endothelial cells, adherent leukocytes or platelets. Targeting of microbubbles or nanoparticle agents has been accomplished by one of two strategies.
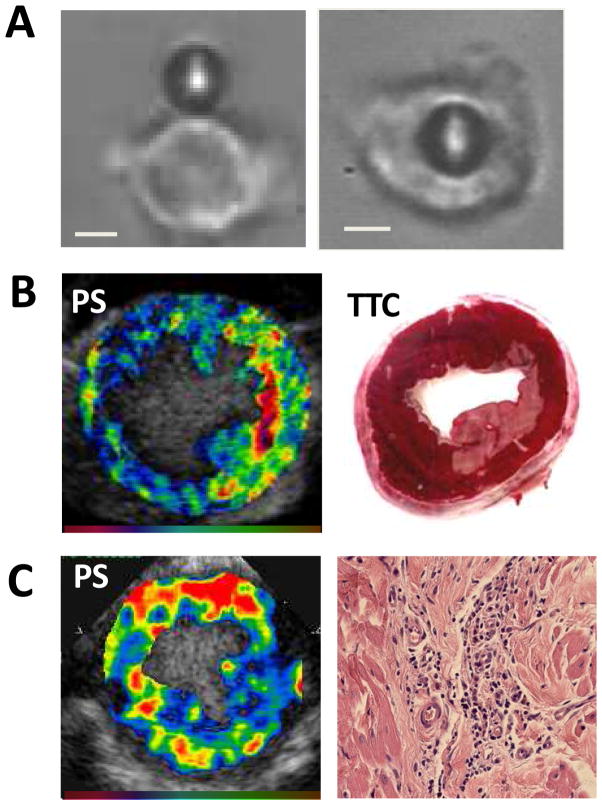
A simple approach for targeting has been to take advantage of the natural ability for certain microbubble shell constituents to bind directly or indirectly to cells that have undergone pathologic activation. Although it somewhat lacks specificity, it is the simplest of targeting approaches and relies on the ability of the albumin or lipid shell components of microbubbles to bind to disease related receptors.(6–9) Albumin and lipid microbubbles are able to bind via opsonization to leukocyte and endothelial complement receptors, and albumin microbubbles have the added ability to bind to leukocyte β2-integrins that recognize denatured albumin.(6) The proclivity to bind to leukocytes and activated endothelium provides a mechanism to detect severe acute inflammatory processes that occur during myocardial infarction, chronic ischemic left ventricular dysfunction, transplant rejection, and atherosclerosis (Figure 2).(10–12)
Figure 2.
Examples of ultrasound imaging of inflammation by non-specific interactions between leukocytes and lipid-shelled microbubbles with enhanced complement-mediated binding capability provided by the phosphatidylserine (PS) in the shell. (A) Light microscopy image demonstrating neutrophil attachment (left) and phagocytosis (right) of a microbubble).(9) (B) Short axis images from a dog 2 hours after circumflex ischemia and reperfusion demonstrating myocardial enhancement several minutes after intravenous injection of PS-containing microbubbles and corresponding TTC histology demonstrating the corresponding region of infarction.5 (C) Contrast echocardiography in the short axis using PS-containing microbubbles demonstrating leukocyte recruitment in a model of progressive ischemic left ventricular dysfunction caused by ameroid constrictors (left) and corresponding hematoxylin and eosin staining from the anterior myocardium illustrating perivascular leukocyte infiltration (right). Color scales at bottom.
A more specific approach has been to attach disease-targeted ligands such as antibodies or small peptides to the surface of ultrasound contrast agents. This conjugation of these molecules to the shell surface almost generally relies on using a bifunctional molecule which has a polycarbon anchor for the lipid shell, and a molecular spacer that projects the ligand away from the shell surface at the end of which is a chemical moiety that can be used for ligand attachment. The resulting surface density of targeting ligands ranges from several hundred to several thousand per μm2 of shell surface area.
Contrast Agent Attachment and Detection
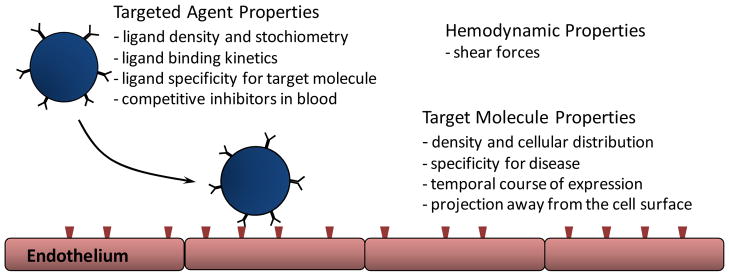
The firm attachment of a particle contrast agent to a disease target is dependent on multiple different factors (Figure 3). These factors include binding characteristics and density of the targeting ligand, properties of the target molecule, and vascular conditions that affect bubble distribution and shear. As mentioned previously, intravascular confinement of most ultrasound contrast agents is also an important consideration when deciding on appropriate targets for ultrasound contrast agents.
Figure 3.
Schematic illustrating some of the determinants of targeted microbubble retention within a vessel.
Targeted microbubble agents are generally detected after clearance by the reticuloendothelial system of the freely circulating agent from the blood pool. This generally requires 5–10 min for microbubble agents and somewhat longer for nanoscale contrast agents. Hence, encapsulation and use of high-molecular weight gases are generally necessary for molecular imaging probes. Another consideration is whether the signal from targeted contrast agents is dampened by their attachment to cells, similar to placing a finger gently on a ringing bell. It has been shown that signal for microbubbles is reduced by their phagocytosis by leukocytes.(9) However, this is a rather extreme situation and subsequent studies have demonstrated that simple cell surface attachment does not substantially affect bubble signal when using ultrasound in the typical diagnostic frequency range.(13,14)
Advantages and Disadvantages of Ultrasound-based Imaging
Since methods for molecular imaging of cardiovascular disease are being developed for essentially every form of molecular imaging, there must be a rationale for choosing any particular technology (i.e. matching the technology to the disease state). The specific properties of the detectors and tracers (Table 1) will govern these decisions. For ultrasound imaging, a limitation is that with the current targeted microbubble agents, only intravascular events can be accessed which reduces the array of potential molecular targets. Hence, most of the applications for targeted contrast ultrasound imaging listed below involve targeting vascular endothelial or blood cell events. It is also key to realize that with small molecule targeting agents such as those used with nuclear imaging, there is in theory more of a direct relationship between molecular expression and retention/activation of a targeted probe. In contrast particle-based contrast agents such as microbubbles are more likely to obey similar principles as cell attachment whereby a critical threshold amount of molecular expression may be needed to begin to see attachment. Contrast ultrasound does, however, have several major advantages. First, contrast ultrasound provides a good balance between spatial resolution and sensitivity for detecting contrast agent. From a clinical perspective, the portability and speed of the imaging algorithms, requiring only minutes to perform, are significant advantages as well. These properties make the technique well-suited to its potential application for either screening large populations or for detecting diseases such as acute coronary syndromes where time is of the essence.
Table 1.
Differentiating Features of Molecular Imaging Technologies
| Sensitivity | Signal-to-noise |
| Spatial resolution | Temporal resolution |
| Quantitative accuracy | Speed of acquisition |
| Cost | Availability |
| Availability | Ease of use |
| Safety |
Detection of Tissue Ischemia
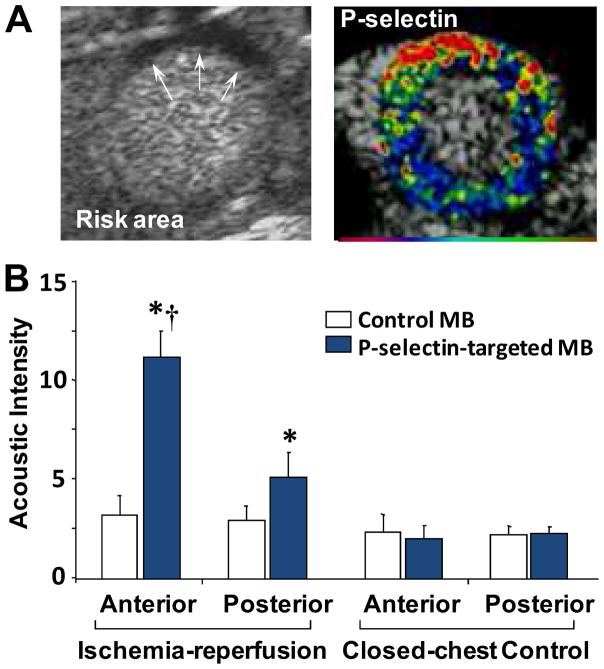
In the current evaluation of patients with suspected acute coronary syndromes (ACS) currently includes combining clinical information with diagnostic tests such as electrocardiography (ECG) and serologic markers (troponin, creatine kinase). It is widely recognized that there are limitations in both sensitivity of these markers, especially in non-STEMI and unstable angina.(15,16) In certain populations, abnormalities can be non-specific for both the ECG (e.g. left bundle branch block) and/or serologic markers (e.g. severe renal disease). Rapid point-of-care imaging is one possible solution. Evaluation of left ventricular function and myocardial perfusion by contrast echocardiography has been used to confirm or exclude ACS and is particularly useful in those with a non-diagnostic initial ECG.(17) However, this approach is less useful in those with prior ischemic events who have pre-existing abnormalities, or in patients who are seen several hours after resolution of ischemia in whom perfusion and wall motion have returned to normal. Molecular imaging of the molecular consequences of ischemia that can persist for hours can be used to correctly identify this category of patients and can also potentially be used to further risk stratify according to the area at jeopardy. This approach is often referred to as “ischemic memory” imaging. Ischemic memory radionuclide imaging is currently being tested in patients with possible ACS using the 125I-labeled fatty acid analogue 123I-beta-methyl-iodophenylpentadecanoic acid (BMIPP) which detects the switch from fatty acid to glucose metabolism.(18) Ischemic memory imaging with echocardiography has been achieved using microbubbles targeted to the endothelial cell adhesion molecule P-selectin by surface conjugation of antibodies or glycoproteins on their surface. Selectins are endothelial cell adhesion molecules that are expressed in response to inflammatory stimuli and are involved in the initial rolling of leukocytes along the endothelial surface. These molecules are ideal for molecular imaging with ultrasound since selectin expression occurs on the microvascular endothelial surface which is readily available to microbubble targeting, and requires only a mild ischemic stimulus for rapid exteriorization from its pre-formed stores.(19) P-selectin-targeted imaging has been shown to detect brief myocardial and renal ischemic injury even after the ischemic insult has resolved and in the absence of infarction (Figure 4).(20–22) Agents targeting both P- and E-selectin have been developed for potential application in humans. By targeting E-selectin expression which occurs late after ischemic injury and persists for up to 24 hours, it may be possible to extend the time window that molecular imaging is effective for detecting ischemia that has resolved. An ultrasound approach to ischemic memory molecular imaging may have distinct advantages in a population with suspected ACS because of speed, low cost, convenience, and ability to be performed at the bedside. For this reason, molecular imaging of ischemic memory is likely to be the first application for molecular imaging of cardiovascular disease that is tested in man.
Figure 4.
Contrast echocardiographic imaging of recent myocardial ischemia with P-selectin targeted microbubbles.(21) (A) Short axis contrast echocardiographic images from a mouse illustrating hypoperfusion (arrows) of the anterior wall on perfusion imaging during brief occlusion of the LAD (left) and enhancement within the previously ischemic anterior territory with P-selectin-targeted microbubbles injected intravenously 45 minutes after reflow. Color scale at bottom. (B) Qauntitative data of signal enhancement from P-selectin microbubbles in the LAD (anterior) territory in control mice and in mice 45 min after ischemia-reperfusion injury.
Molecular Imaging of Atherosclerosis
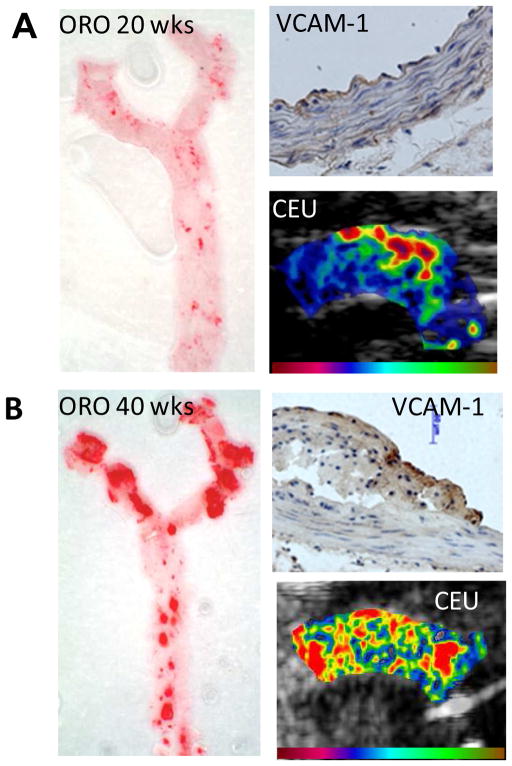
There are many potential targets for molecular imaging in atherosclerosis that could be useful in diagnosing or managing atherosclerotic disease. In this disease process, the molecules that one chooses to target must be governed by the intended clinical use. In a patient with known atherosclerotic disease, evaluation for risk for acute atherothrombotic complication is best accomplished by targeting disease processes that lead to plaque rupture or thrombotic occlusion. These might include include macrophage accumulation, protease activity, platelet adhesion, plaque angiogenesis, microthrombosis, and apoptosis. On the other hand, assessing future risk of aggressive disease in an individual without known disease may instead rely on the detection of early inciting events such oxidative stress, lipid accumulation, or upregulation of endothelial cell adhesion molecules that participate in leukocyte recruitment. For ultrasound molecular imaging of these processes, it has been necessary to focus on the events that occur within the vascular compartment. Accordingly, targeting of endothelial cell adhesion molecules which play a critical role in progression of disease has been a popular approach. In animal models of disease, ultrasound molecular imaging of ICAM-1, VCAM-1, and P-selectin has been shown to produce signal enhancement preferentially at sites of plaque formation and to reveal the degree of underlying plaque inflammatory activity.(23–25) Imaging of VCAM-1 and P-selectin has been used to detect the earliest stages of atherosclerotic disease even prior to the development of fatty streakes (Figure 5), supporting the notion that molecular imaging could potentially be used to guide the use of the next generation of preventive therapies.(25)
Figure 5.
Molecular imaging of VCAM-1 to detect endothelial activation in early atherosclerotic disease.(25) Images are from a model of age-dependent atherosclerosis in mice (A) at 10 wks an (B) at 40 weeks of age. En face images of the thoracic aorta with Oil Red-O (ORO) staining demonstrates age-related increase in spatial extent of plaque formation. Immunohistochemistry shows VCAM-1 expression despite only mild intimal thickening at 10 wks whereas there is dense VCAM-1 expression and large lesion formation at 40 wks. Contrast-enhanced ultrasound (CEU) molecular imaging of VCAM-1 shows enhancement in the aortic arch at both stages, although signal at the early time point was mostly localized to the region where future plaque formation is most severe which is at the origin of the brachiocephalic artery.
There is evidence that complex targeting strategies may not be necessary for imaging high-risk atherosclerotic disease and that non-specific attachment may provide prognostic information. Albumin-shelled microbubbles have been shown to bind to atherosclerotic vessels, putatively through complement deposition on the microbubble shell and attachment to complement receptors that are upregulated by the activated endothelium.(7,12) Hence, it is possible that a more simple approach could be used to identify a endothelial activation in atherosclerotic disease, although sensitivity of this approach is likely to be less than for ligand-mediated molecular targeting.
Molecular imaging of Thrombus
Molecular imaging of intravascular or intracavitary thrombus, thrombotic complications of plaque rupture or even pro-thrombotic phenotype has been attempted with a wide variety of targeted ultrasound contrast agents. Again, the targeting moiety employed is influenced by the intended role of molecular imaging. Microbubble targeting of the thrombus proper has relied on the surface conjugation of ligands that recognize the platelet GPIIb/IIIa receptor, fibrin, and tissue factor.(23,26–28) Fibrin, tissue factor, and activated GPIIb/IIIa can also potentially be used to detect high risk atherosclerotic complications that involve disruption of the normal endothelial barrier and/or microthrombus formation.(26,28,29) Targeting of dysregulated von Willebrand factor (the major receptor for the platelet GP1bα) on the endothelium has similarly been used to detect high-risk pro-thrombotic and pro-inflammatory phenotype.(27)
There are many potential diagnostic roles for imaging either thrombus (e.g. deep vein thrombosis, left atrial thrombus, microvascular thrombus in post-ischemic no-reflow) or pro-thrombotic phenotype (e.g. atherosclerotic diseases, critically ill patients at risk for thrombotic thrombocytopenic purpura). There are also potential therapeutic applications. When microbubbles are exposed to high pressure ultrasound, they undergo inertial cavitation whereby they are rapidly destroyed from exaggerated oscillation. The energy released by inertial cavitation can produce bioeffects that can be entrained for therapeutic purposes such as for accelerating endogenous or therapeutic thrombolysis.(30,31) This approach has been used to enhance thrombolysis in animal models of acute MI and in humans with stroke, although in the latter the safety of the approach has been called into question.(32,33) The use of thrombus-targeted microbubbles has been shown to slightly improve efficacy of sonothrombolysis, probably through direct apposition of the bubbles to the clot surface, although the real benefit of this approach may be its ability to improve the safety of the technique by reducing the acoustic pressures required to achieve clinical endpoints.(30)
Imaging Angiogenesis
Neovascularization is a broad term that encompasses angiogenesis (formation of new vessels that generally lack a tunica media), arteriogenesis (growth and remodeling of the arteriolar network), and development of large collaterals. Ultrasound molecular imaging of all of these forms of vascular remodeling has been achieved by targeting the complex processes involved. For these purposes, microbubbles have been targeted to specific endothelial integrins expressed in neovascularization, endothelial adhesion molecules that are a marker for activation and remodeling, growth factor receptors, and specific markers for inflammatory cells that play a central role in vascular remodeling.(34–36) Although molecular imaging of angiogenesis is unlikely to lead to any new diagnostic algorithm in cardiovascular disease, the technique is beginning now to be used to investigate mechanisms responsible for impaired angiogenesis in response to ischemia in certain patient populations such as diabetes, and as a method for evaluating pro-angiogenic therapies. It is worth noting that in the field of cancer medicine, molecular imaging of angiogenesis with ultrasound represents a promising approach for early diagnosis of solid tumors or metastasis, and for choosing appropriate adjuvant anti-angiogenesis therapy based and tumor microvascular phenotype.
Conclusions and Current Directions
At the current time, it is safe to say that molecular imaging with ultrasound and targeted microbubble agents is starting to emerge beyond the stage where simply feasibility is tested in predictable animal models of disease. The techniques pioneered in the early studies are now being applied in animal models to gain insight into pathophysiology and to assess response to new therapies for atherosclerosis and ischemic diseases. There is still hope that that molecular imaging will evolve into a clinical technique that can either improve diagnostic performance or for provide a means for individualizing therapy based on disease phenotype. At the current time there are efforts underway to develop agents for human use for some of the most clinically promising applications. This step will of course involve the development of surface conjugation methods that have a proven safety profile in humans, and the use of high-affinity small molecules as targeting ligands. The majority of the targeted microbubble research has involved using monoclonal antibodies as targeting ligands. The current trend has been to use of small molecular ligands such as peptides and glycoproteins will be less immunogenic in man, cheaper to produce, and may produce more favorable binding kinetics of microbubbles in vessels with high shear forces. It should be noted that the most important hurdle for clinical translation of this technology are trials that clearly demonstrated that molecular imaging provides information that is useful and incremental in value to established diagnostic methods.
Acknowledgments
Dr. Lindner is supported by grants R01-HL078610, and R01-DK063508, and RC01-100659 from the National Institutes of Health. All authors have read the journal’s policy on disclosure of potential conflicts of interest. There are no conflicts of interest that relate to the content of this article; Dr. Lindner is the recipient of an investigator-initiated grant from GE Medical Imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32:51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Dayton PA, Morgan KE, Klibanov AL, Brandenburger GH, Ferrara KW. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46:220–32. doi: 10.1109/58.741536. [DOI] [PubMed] [Google Scholar]
- 3.Church CC, Carstensen EL. “Stable” inertial cavitation. Ultrasound Med Biol. 2001;27:1435–7. doi: 10.1016/s0301-5629(01)00441-0. [DOI] [PubMed] [Google Scholar]
- 4.Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994;74:1157–65. doi: 10.1161/01.res.74.6.1157. [DOI] [PubMed] [Google Scholar]
- 5.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 6.Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101:668–75. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui JM, Xie F, Cano M, et al. Detection of retained microbubbles in carotid arteries with real-time low mechanical index imaging in the setting of endothelial dysfunction. J Am Coll Cardiol. 2004;44:1036–46. doi: 10.1016/j.jacc.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102:2745–50. doi: 10.1161/01.cir.102.22.2745. [DOI] [PubMed] [Google Scholar]
- 9.Lindner JR, Dayton PA, Coggins MP, et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000;102:531–8. doi: 10.1161/01.cir.102.5.531. [DOI] [PubMed] [Google Scholar]
- 10.Kondo I, Ohmori K, Oshita A, et al. Leukocyte-targeted myocardial contrast echocardiography can assess the degree of acute allograft rejection in a rat cardiac transplantation model. Circulation. 2004;109:1056–61. doi: 10.1161/01.CIR.0000115586.25803.D5. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen JP, Leong-Poi H, Klibanov AL, Kaul S, Lindner JR. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002;105:1764–7. doi: 10.1161/01.cir.0000015466.89771.e2. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DR, Tsutsui JM, Xie F, Radio SJ, Porter TR. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc Res. 2007;73:597–606. doi: 10.1016/j.cardiores.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Lankford M, Behm CZ, Yeh J, Klibanov AL, Robinson P, Lindner JR. Effect of microbubble ligation to cells on ultrasound signal enhancement: implications for targeted imaging. Invest Radiol. 2006;41:721–8. doi: 10.1097/01.rli.0000236825.72344.a9. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann BA, Carr CL, Belcik T, et al. Effect of acoustic power on in vivo molecular imaging with targeted microbubbles: implications for low-mechanical index real-time imaging. J Am Soc Echocardiogr. 2010;23:79–85. doi: 10.1016/j.echo.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337:1648–53. doi: 10.1056/NEJM199712043372302. [DOI] [PubMed] [Google Scholar]
- 16.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 17.Rinkevich D, Kaul S, Wang XQ, et al. Regional left ventricular perfusion and function in patients presenting to the emergency department with chest pain and no ST-segment elevation. Eur Heart J. 2005;26:1606–11. doi: 10.1093/eurheartj/ehi335. [DOI] [PubMed] [Google Scholar]
- 18.Dilsizian V. Metabolic imaging for identifying antecedent myocardial ischemia and acute coronary syndrome in the emergency department. Curr Cardiol Rep. 13:96–9. doi: 10.1007/s11886-010-0160-3. [DOI] [PubMed] [Google Scholar]
- 19.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–42. [PubMed] [Google Scholar]
- 20.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–12. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28:2011–7. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115:345–52. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton AJ, Huang SL, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–60. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–9. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000;13:608–14. doi: 10.1067/mje.2000.105840. [DOI] [PubMed] [Google Scholar]
- 27.McCarty OJ, Conley RB, Shentu W, et al. Molecular imaging of activated von Willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc Imaging. 3:947–55. doi: 10.1016/j.jcmg.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007;38:1508–14. doi: 10.1161/STROKEAHA.106.471391. [DOI] [PubMed] [Google Scholar]
- 29.Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87–94. doi: 10.1016/0002-9149(74)90743-7. [DOI] [PubMed] [Google Scholar]
- 30.Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation. 2009;119:1378–85. doi: 10.1161/CIRCULATIONAHA.108.825067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birnbaum Y, Luo H, Nagai T, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug: recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–4. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 32.Molina CA, Barreto AD, Tsivgoulis G, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66:28–38. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- 33.Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–9. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 34.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455–60. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 35.Behm CZ, Kaufmann BA, Carr C, et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–11. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 36.Palmowski M, Huppert J, Ladewig G, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008;7:101–9. doi: 10.1158/1535-7163.MCT-07-0409. [DOI] [PubMed] [Google Scholar]