Summary
In delineating the molecular pathogenesis of hepatocellular carcinoma (HCC), recent experiments on mouse tumor models have revealed unexpected tumor-suppressing effect in genes previously identified as pro-oncogenic. This contradiction underscores the complexity of hepatocarcinogenesis and predicts uncertainty in targeting these molecules for HCC therapy. Deciphering the underlying mechanisms for these paradoxical functions will elucidate the complex molecular and cellular communications driving HCC development, and will also suggest more thoughtful therapeutic strategies for this deadly disease.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. However, the molecular and cellular mechanisms underlying HCC initiation and development are poorly understood. In recent studies, a number of groups have employed cell type-specific gene knockout (KO) mouse models to dissect HCC pathogenesis. This approach has no doubt provided mechanistic insights into hepatocarcinogenesis. However, opposing roles of promoting and suppressing HCC have been reported for the same molecules in different animal models (Figure 1). These conflicting results do not necessarily obscure the understanding of molecular pathogenesis in HCC. Instead, further experiments carefully designed to decipher the opposing roles will lead to better understanding of HCC development.
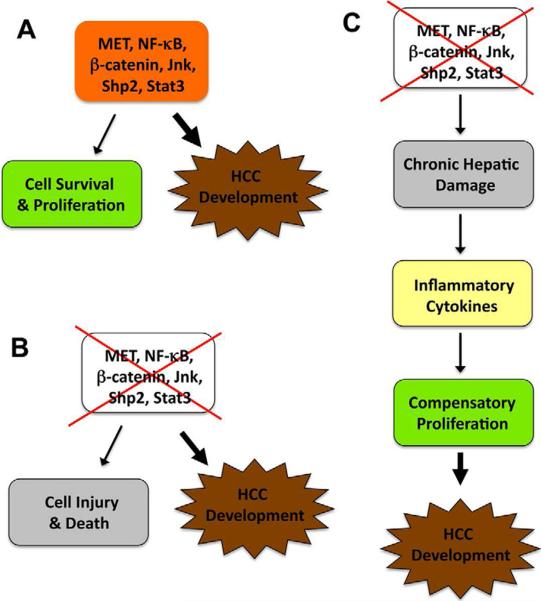
Figure 1. A simplified model for the pro- and anti-oncogenic activities of the same molecules in hepatocarcinogenesis.
A) MET, NF-κB, Stat3, Jnk, Shp2 and β-catenin constitute pathways that normally promote cell survival and proliferation. Aberrant activation or over-expression of these molecules enhances cell transformation and tumorigenesis.
B) Removal or inactivation of these pro-tumorigenic molecules, which normally causes hepatocyte damage and death, also enhances HCC development under certain conditions.
C) One possible explanation is that loss of a pro-survival signal enhances chronic hepatic damage, increases production of inflammatory cytokines, and triggers excessive compensatory proliferation of hepatocytes and/or progenitor cells, resulting in elevated hapatocarcinogenesis.
Contradictory Results from Different Animal Models
The NF-κB pathway that promotes cell survival and proliferation is constitutively activated in a variety of tumors. Consistently, several groups reported tumor-promoting effects of NF-κB in mouse models (Haybaeck et al., 2009; Pikarsky et al., 2004). Removal of Mdr-2 P-glycoprotein triggers spontaneous development of cholestatic hepatitis and consequently HCC in mice, and inactivation of the NF-κB pathway in hepatocytes suppressed HCC progression initiated by Mdr-2 deletion (Pikarsky et al., 2004). By controlling the timing of shutting down the NF-κB pathway, the authors demonstrated that NF-κB was required for the later stage of HCC progression, without significant impact on transformation or the early stage of tumor development. In contrast, Karin's group reported that hepatocyte-specific ablation of Ikkβ (IkkβΔhep), a kinase required for the activation of NF-κB, resulted in dramatic increase in HCC development induced by the chemical carcinogen diethylnitrosamine (DEN), pointing to an anti-tumor effect of NF-κB in the liver (Maeda et al., 2005). DEN, as a pro-carcinogen, can be metabolically activated in hepatocytes and forms bulky DNA adducts that induce genomic instability and gene mutations (Aleksic et al., 2011; Verna et al., 1996). The carcinogenic effect of DEN can be enhanced by other tumor promoters such as phenobarbital in adult mice. Consistent with the increased susceptibility of IkkβΔhep mice to DEN-induced tumorigenesis, hepatocyte-specific deletion of NEMO/Ikkγ, another component of the Ikk complex, leads to spontaneous development of HCC, following hepatic steatosis and chronic inflammation in mice (Luedde et al., 2007).
Highlighting a similar paradox, several groups demonstrated either tumor-promoting or tumor-suppressing actions of Jnk kinases in HCC. Hui et al. detected high levels of p-Jnk1 in human HCC lesions and found that the incidence of DEN/phenobarbital-induced HCC was significantly reduced in Jnk1−/− but not in Jnk2−/− mice (Hui et al., 2008). Consistently, siRNA-mediated Jnk1 or Jnk1 together with Jnk2 knockdown in Huh7 human HCC cells suppressed in vitro proliferation and tumor formation as subcutaneous xenografts in nude mice, with no effect observed for Jnk2 knockdown. In contrast, Davis and colleagues presented data suggesting a tumor-suppressing effect of Jnk1 and Jnk2 in hepatocytes (Das et al., 2011). The authors generated mutant mice (HΔJnk) with Jnk1 and Jnk2 deficiency in hepatocytes, by deleting Jnk1 specifically in hepatocytes in the Jnk2−/− background. Their results indicate that combined deficiency of Jnk1 and Jnk2 lead to increased tumor sizes of DEN-treated mice.
Stat3 clearly has a pro-oncogenic function, based on the detection of activating mutations or enhanced p-Stat3 signals in different types of cancer (Calvisi, 2011; Yu et al., 2009). However, both pro- and anti-oncogenic activities of Stat3 in HCC development were observed in hepatocyte-specific Stat3 knockouts, transgenic mice expressing dominant active or negative mutant of Stat3, or tumor-bearing animals treated with Stat3 inhibitors (Lin et al., 2009; Schneller et al., 2011; Wang et al., 2011b). Interestingly, hepatocyte-specific deletion of tyrosine phosphatase Shp2 resulted in a marked increase in DEN-induced HCC incidences, which was compromised by additional removal of Stat3 (Bard-Chapeau et al., 2011). However, deleting Stat3 alone also led to enhanced tumorigenesis induced by DEN (Bard-Chapeau et al., 2011) or CCl4 (Wang et al., 2011b) as compared to wild-type mice.
Contrasting roles in HCC have also been observed for β-catenin, a chief effector in the Wnt pathway. Consistent with the detection of activating mutations in the β-catenin gene (CTNNB1) in HCCs (de La Coste et al., 1998), Monga and colleagues reported that overexpression of a dominant active β-catenin mutant in hepatocytes accelerated DEN-induced hepatocarcinogenesis (Nejak-Bowen et al., 2010). Surprisingly, the same group found that hepatocyte-specific Ctnnb1 KO mice also displayed higher susceptibility to DEN-induced tumorigenesis, due to excessive oxidative stress, hepatic injury and inflammation (Zhang et al., 2010). Therefore, HCC is promoted by both gain and loss of β-catenin function in hepatocytes (Nejak-Bowen and Monga, 2011).
The opposing roles in promoting and suppressing HCC are not restricted to intracellular signaling molecules. The HGF receptor (MET) tyrosine kinase system represents a classic pro-oncogenic signaling pathway, and dominant active mutations of MET have been found in human HCC and other tumors (Birchmeier et al., 2003). As expected, overexpression of MET in hepatocytes promoted HCC development in transgenic mice (Wang et al., 2001). Turning off the transgene expression in tumor-bearing mice led to increased cell apoptosis and impaired proliferation resulting in tumor regression, suggesting that MET is required in genesis and maintenance of HCC. Surprisingly, Thorgeirsson's group showed that hepatocyte-specific Met KO mice exhibited higher susceptibility to DEN-induced tumorigenesis and developed larger and more tumors than control animals (Takami et al., 2007).
Possible Explanations and Unanswered Questions
How should one interpret these conflicting findings? One can easily explain the tumor-promoting effect of these molecules based on their known pro-proliferation and anti-apoptosis functions. It is certainly more challenging to understand the unanticipated anti-tumor effect of these molecules. In dissecting the underlying mechanisms, several groups have shown that the outcome depends on the cell types in the liver from which a target gene is removed (Das et al., 2011; Maeda et al., 2005). Deleting Ikkβ or Jnk1 and Jnk2 in hepatocytes only by Alb-Cre enhanced DEN-induced HCC whereas inactivating the same gene in both hepatocytes and non-parenchymal cells using Mx1-Cre suppressed tumorigenesis. Based on this and other experimental data, one emerging theory is that the loss of a pro-survival molecule triggers chronic hepatic injury, which in turn enhances compensatory proliferation through elevated production of cytokines from infiltrated inflammatory cells including Kupffer cells. Excessive and continuous proliferation of hepatocytes or progenitor cells eventually leads to spontaneous or DEN-induced neoplastic growth in the liver (Figure 1).
In the multistage pathogenic process, elevated hepatocyte death is the first step in promoting hepatocarcinogenesis. Indeed, deletion of Ikkβ or β-catenin in hepatocytes caused dramatically increased accumulation of reactive oxygen species, and feeding the mice with antioxidants reduced HCC incidence (Maeda et al., 2005; Zhang et al., 2010). Additional deletion of FADD, an essential adaptor molecule downstream of death receptors, alleviated excessive apoptosis, inflammation and steatohepatitis in NemoLPC-KO mice (Luedde et al., 2007), resulting in decreased tumorigenesis in Fadd and Nemo compound mutant mice (Pasparakis, personal communication).
The enhanced cell death may increase compensatory proliferation of tumor initiating cells (TICs) originating from mature hepatocytes or progenitor cells, and several lines of evidence support this notion. For example, proliferating cells were frequently located around apoptotic cells in centrilobular lesions after DEN exposure (Maeda et al., 2005). Additional deletion of Jnk1 in IkkβΔhep mice suppressed compensatory proliferation following DEN injection and consequently reduced tumor burden (Sakurai et al., 2006). Severe steatosis induced by loss of PTEN enhanced hepatocyte death and compensatory proliferation of TICs (Galicia et al., 2010). Although loss of either Shp2 or Stat3 in hepatocytes led to increased tumorigenesis, combined deletion of both did not show an additive or synergistic effect, but interestingly a neutralizing result (Bard-Chapeau et al., 2011), which is likely due to impaired compensatory proliferation in the absence of both Shp2 and Stat3 (our unpublished data). A similar compromising effect of Tak1 and NEMO ablation was observed (Bettermann et al., 2010), although it was unclear whether removal of the two molecules suppressed hepatocyte proliferation potential.
Pro-inflammatory cytokines play important roles in these HCC animal models. Hepatic expression of TNFα, IL-6 and HGF were increased in IkkβΔhep mice (Maeda et al., 2005). Higher circulating levels of IL-1α and IL-6 were detected in IkkβΔhep and p38αΔhep mice following DEN injection, which was alleviated by antioxidant treatment that prevented hepatocyte death (Sakurai et al., 2008). Blocking IL-1α action by injection of IL-1R antagonist (IL-1Ra) or ablation of the IL-1R suppressed DEN-induced IL-6 production, compensatory proliferation and tumorigenesis. These results suggest that IL-1α released from dying hepatocytes stimulates production of IL-6, which in turn induces surviving hepatocytes to proliferate (Sakurai et al., 2008). A similar mechanism involving paracrine action of cytokines was identified in mice with Jnk or Stat3 deficiency in hepatocytes only or in hepatocytes plus non-parenchymal cells (Das et al., 2011; Horiguchi et al., 2008). In aggregate, these results highlight the importance of hepatic cell communications in liver tumorigenesis.
It remains to be elucidated at which stage(s) the paracrine cytokines and the infiltrated inflammatory cells contribute to hepatocarcinogenesis. The ability to isolate TICs at early stages of HCC development following DEN exposure (He et al., 2010) will help clarify this issue. Opposite effects on tumorigenesis were observed when deleting the same gene using Alb-Cre and Mx1-Cre lines, respectively (Das et al., 2011; Maeda et al., 2005). It will be interesting to isolate and compare TICs from the two mutant mouse lines. If mutant TICs of the two origins display opposite growth properties in vitro and in allografts in recipient animals, the result would suggest that the initial transformation event is already influenced by the hepatic microenvironment. However, if the TICs of the two origins display similar phenotypes, one may conclude that the inflammatory cytokines or the hepatic microenvironment influences tumor progression without effect on TIC genesis.
One issue that could affect the interpretation of data from hepatocyte-specific KO mice is the timing and efficiency of Cre expression. It remains to be determined the nature of the few surviving hepatocytes that become TICs and eventually develop into HCCs. It is possible that a few hepatocytes have escaped Cre-mediated DNA excision and thus gain survival and proliferative advantages over neighboring cells with a pro-survival gene ablated. Arguing against this theory is the data that the targeted genes were efficiently deleted in tumors (Bard-Chapeau et al., 2011; Das et al., 2011; Maeda et al., 2005; Sakurai et al., 2008). However, the possibility that a target gene was intact in TICs but was deleted later during cancer progression, which no longer requires the gene product, has not been excluded. Indeed, one report on HCC induced by Ddb1 deletion suggests that HCC is originated from hepatocytes that escaped Alb-Cre-mediated gene ablation (Yamaji et al., 2010). In another study, Chen and colleagues found that liver tumors developed from β-catenin-positive hepatic progenitor cells in Alb-Cre mediated Ctnnb1 deletion (Wang et al., 2011a). To unequivocally determine the gene deletion status during HCC initiation and progression, it is important to use inducible and tightly-controlled Cre expression strategies that are yet to be optimized.
Thus far, most studies have largely focused on the final outcome of HCC development after a long period of DEN treatment. More rigorous analyses of the kinetics of hepatic injury and disorders associated with early stages of tumorigenesis will provide mechanistic insights into the pathogenic process. With regard to the hepatic microenvironment in tumorigenesis, relative contributions of Kupffer cells versus other infiltrating cell types will need to be clarified. Possible roles of oval cells and hepatic stellate cells (HSCs) in the HCC development need to be carefully examined, which may help interpreting the seemingly contradictory findings.
There are many open questions for the anti-tumor effect of pro-tumorigenic molecules. The cell death-compensatory proliferation-transformation-hepatocarcinogenesis scheme (Figure 1) is likely an over-simplified model, and other mechanisms may also be involved in promoting HCC development. Inactivation of one pro-tumorigenic pathway in hepatocytes may lead to aberrant activation of another. For example, over-expression of PDGFRα and c-Myc was detected in β-catenin-deficient livers (Zhang et al., 2010); up-regulated Stat3 activity was observed in the liver of IkkβΔhep or Shp2Δhep mice (Bard-Chapeau et al., 2011; He et al., 2010). Rather than showing increased apoptosis, Ikkβ-deficient TICs were found to exhibit higher survival and proliferative capacity in vitro, although the molecular basis is not fully understood (He et al., 2010). Thus, excessive compensatory reaction to loss of a proliferative signal may also contribute to the unexpected tumor-promoting effect observed upon removal of a pro-tumorigenic molecule, at least in some cases.
Implications in Human HCC Pathogenesis and Therapy
As described above, either aberrant gain or loss of function of the same molecule can lead to malignant growth, reinforcing the concept that homeostasis is critical for preventing tumorigenesis in the liver. A critical question is whether the results from these animal experiments are relevant to human HCCs. Constitutive activation of MET, β-catenin, NF-κB and Stat3 have been detected in human HCC samples or cell lines (Birchmeier et al., 2003; Calvisi, 2011; He et al., 2010; Nejak-Bowen and Monga, 2011; Yu et al., 2009). In contrast, there has been almost no definitive report on loss of function mutations in these genes in human HCCs, and thus the paucity of evidence to support their tumor suppressor role. Although deficient expression of Shp2 was detected in primary human HCCs (Bard-Chapeau et al., 2011), it was unclear whether Shp2-deficiency plays a causative role in their pathogenesis. Genome-wide sequencing of human HCC samples worldwide may identify inactivating mutations in these proto-oncogenes, most likely at low frequencies.
Despite the rare detection of corresponding mutations in humans, these mouse mutants recapitulate many aspects of pathological features in human patients, including chronic hepatocyte damage, elevated oxidative stress, inflammation, fibrosis, steatohepatitis or cholestasis. Therefore, these animal models are useful for understanding the molecular and cellular mechanisms of liver cancer in humans. In particular, the mouse models provide access to the early events in tumorigenesis, particularly the dynamic interplay of TICs with the hepatic microenvironment. Given the 100% penetrance in DEN-induced carcinogenesis and the high degree of similarity in pathogenesis, the mutant mice can serve as platforms in the search of biomarkers for early diagnosis of HCC, the significance of which cannot be over-stated given the current poor prognosis of patients with late stages of HCC.
Personalized therapeutic strategies can be developed, based on dominantly activating mutations detected in tumors. A potent MET inhibitor might be effective in treating a liver cancer patient with an activating MET mutation (You et al., 2011). However, the pro- and anti-oncogenic roles of signaling molecules and the cell type-specific effects of gene deletion described above bring uncertainty to using inhibitors of these molecules as anticancer drugs, as reported for Stat3 inhibitors (Yue and Turkson, 2009). One interesting idea is development of anti-HCC therapeutics by sorting beneficial from harmful functions of a signaling molecule, such as β-catenin (Nejak-Bowen and Monga, 2011). Due to the compensatory up-regulation of one pathway in reaction to the loss of another, simultaneous blockade of several pathways by a cocktail of inhibitors may be a more effective approach. Additional removal of Stat3 reversed the tumor-promoting effect of Shp2 deletion (Bard-Chapeau et al., 2011), and inhibition of PDGFRα suppressed tumorigenesis induced by ablation of β-catenin (Zhang et al., 2010).
The debate over the relevance of mouse data to human HCCs will likely continue. However, in-depth molecular analyses in animal models cannot be replaced by examination of human HCC specimens. The opposing roles of signaling molecules revealed by these mouse models indicate a need for re-assessment of current approaches in drug targeting and screening. Finally, although the discussion in this article focuses on HCC, contrasting roles of NF-κB have been observed in other types of cancer, such as skin cancer (van Hogerlinden et al., 2002). Therefore, it is crucial to elucidate factors that regulate various functions of each of these oncogenic pathways.
Acknowledgment
The author apologizes for not citing many exciting papers in this short article, and thanks colleagues for critical reading of the manuscript. Work in the author's laboratory has been supported by NIH R01DK075916 and HL096125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aleksic K, Lackner C, Geigl JB, Schwarz M, Auer M, Ulz P, Fischer M, Trajanoski Z, Otte M, Speicher MR. Evolution of genomic instability in diethylnitrosamine-induced hepatocarcinogenesis in mice. Hepatology. 2011;53:895–904. doi: 10.1002/hep.24133. [DOI] [PubMed] [Google Scholar]
- Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V, et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19:629–639. doi: 10.1016/j.ccr.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Calvisi DF. Dr. Jekyll and Mr. Hyde: a paradoxical oncogenic and tumor suppressive role of signal transducer and activator of transcription 3 in liver cancer. Hepatology. 2011;54:9–12. doi: 10.1002/hep.24435. [DOI] [PubMed] [Google Scholar]
- Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, Zeng N, Bayan JA, Ding W, Wang KS, et al. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139:2170–2182. doi: 10.1053/j.gastro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr., Dar MJ, Khillan J, Dai C, Monga SP. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller D, Machat G, Sousek A, Proell V, van Zijl F, Zulehner G, Huber H, Mair M, Muellner MK, Nijman SM, et al. p19(ARF) /p14(ARF) controls oncogenic functions of signal transducer and activator of transcription 3 in hepatocellular carcinoma. Hepatology. 2011;54:164–172. doi: 10.1002/hep.24329. [DOI] [PubMed] [Google Scholar]
- Takami T, Kaposi-Novak P, Uchida K, Gomez-Quiroz LE, Conner EA, Factor VM, Thorgeirsson SS. Loss of hepatocyte growth factor/c-Met signaling pathway accelerates early stages of N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Res. 2007;67:9844–9851. doi: 10.1158/0008-5472.CAN-07-1905. [DOI] [PubMed] [Google Scholar]
- van Hogerlinden M, Auer G, Toftgard R. Inhibition of Rel/Nuclear Factor-kappaB signaling in skin results in defective DNA damage-induced cell cycle arrest and Ha-ras-and p53-independent tumor development. Oncogene. 2002;21:4969–4977. doi: 10.1038/sj.onc.1205620. [DOI] [PubMed] [Google Scholar]
- Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Wang EY, Yeh SH, Tsai TF, Huang HP, Jeng YM, Lin WH, Chen WC, Yeh KH, Chen PJ, Chen DS. Depletion of beta-catenin from mature hepatocytes of mice promotes expansion of hepatic progenitor cells and tumor development. Proc Natl Acad Sci U S A. 2011a;108:18384–18389. doi: 10.1073/pnas.1116386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lafdil F, Wang L, Park O, Yin S, Niu J, Miller AM, Sun Z, Gao B. Hepatoprotective versus Oncogenic Functions of STAT3 in Liver Tumorigenesis. Am J Pathol. 2011b;179:714–724. doi: 10.1016/j.ajpath.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji S, Zhang M, Zhang J, Endo Y, Bibikova E, Goff SP, Cang Y. Hepatocyte-specific deletion of DDB1 induces liver regeneration and tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:22237–22242. doi: 10.1073/pnas.1015793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–889. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Tan X, Zeng G, Misse A, Singh S, Kim Y, Klaunig JE, Monga SP. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–965. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]