Summary
BCR-ABL tyrosine kinase inhibitors (TKI) fail to eliminate quiescent leukemia stem cells (LSC) in chronic myelogenous leukemia (CML). Thus strategies targeting LSC are required to achieve cure. We show that the NAD+ dependent deacetylase SIRT1 is overexpressed in human CML LSC. Pharmacological inhibition of SIRT1 or SIRT1 knockdown increased apoptosis in LSC of chronic phase and blast crisis CML and reduced their growth in vitro and in vivo. SIRT1 effects were enhanced in combination with the BCR-ABL TKI imatinib. SIRT1 inhibition increased p53 acetylation and transcriptional activity in CML progenitors, and the inhibitory effects of SIRT1 targeting on CML cells depended on p53 expression and acetylation. Activation of p53 via SIRT1 inhibition represents a potential approach to target CML LSC.
Introduction
Chronic myelogenous leukemia (CML) results from malignant transformation of a hematopoietic stem cell (HSC) by the BCR-ABL oncogene. CML usually presents in a chronic phase (CP), but progresses to an accelerated phase (AP) and a terminal blast crisis (BC) (Sawyers., 1999). The BCR-ABL tyrosine kinase inhibitors (TKI) imatinib (IM), nilotinib and dasatinib are effective in inducing remissions and prolonging survival of CP CML patients but is less effective against advanced phase CML (Eiring et al., 2011). However, even in CP CML, primitive leukemia stem cells (LSC) are retained in patients achieving remission with TKI treatment (Chu et al., 2011). Primitive, quiescent CML LSC are resistant to apoptosis following TKI treatment despite effective inhibition of BCR-ABL kinase activity (Holtz et al., 2005; Corbin et al., 2011), the mechanisms for which are not well understood. Disease recurrence is usually seen following cessation of drug treatment, even in patients with undetectable BCR-ABL expression by q-PCR (Mahon et al., 2010). These observations suggest that “cure” may be elusive for most CML patients with TKI alone. CML patients currently need to take TKI treatment indefinitely, with risks of toxicity, lack of compliance, drug resistance, relapse and associated expense.
Recent studies from our group have shown that pan-histone deacetylase (HDAC) inhibitors in combination with IM significantly increase apoptosis in quiescent CML stem cells (Zhang et al., 2010). However, toxicity of this approach to normal stem cells remains a potential concern. Sirtuins are NAD-dependent histone deacetylases that have been linked to longevity in lower organisms and to mammalian metabolism (Bordone et al., 2005; Liu et al., 2009). Sirtuin 1 (SIRT1) is a member of the sirtuin family that regulates numerous processes including aging, DNA repair, cell cycle, metabolism, and cell survival under stress conditions (Bordone et al., 2005; Liu et al.,2009). In contrast to Class I, II and IV HDACs, SIRT1 activity is not inhibited by pan-HDAC inhibitors (Liu et al., 2009). SIRT1 plays an important role in maintaining self-renewal and differentiation of murine embryonic stem cells (ESC) and HSC especially under conditions of stress (Han et al., 2008; Narala et al., 2008; Ou et al, 2011). Importantly, SIRT1 may have a pathogenetic role in solid tumors and leukemias (Brooks et al., 2009; Liu et al., 2009). SIRT1 can potentially regulate the acetylation of several transcription factors, including p53 (Luo et al., 2001), Ku70 and FoxOs (Brooks et al., 2009). Despite the clear inhibitory effect of increased SIRT1 expression on tumor suppressors like p53 and FoxOs, other studies suggest that SIRT1 may also have tumor-suppressive functions. In the Apc+/− mouse model of colon cancer, increased SIRT1 expression resulted in reduced cell proliferation and tumor formation (Firestein et al., 2008). Activation of SIRT1 by resveratrol can limit cell growth and reduce tumor formation in BRCA1-deficient tumor cells and in Trp53+/−;Sirt1+/− mice (Wang et al., 2008a; 2008b). The precise role of SIRT1 in cancer may depend on the specific cell or tumor type and the presence or absence of p53 (Brooks et al., 2009).
Previous studies have shown that SIRT1 expression is increased in CML blast crisis (BC) cell lines (Chen et al., 2005). Here we investigated the contribution of SIRT1 to survival and growth of CP and BC CML LSC and progenitor cells, and in LSC resistance to TKI treatment. We also investigated the role of p53 in mediating the effects of SIRT1 inhibition on CML progenitors.
Results
SIRT1 is overexpressed in CML CD34+ cells
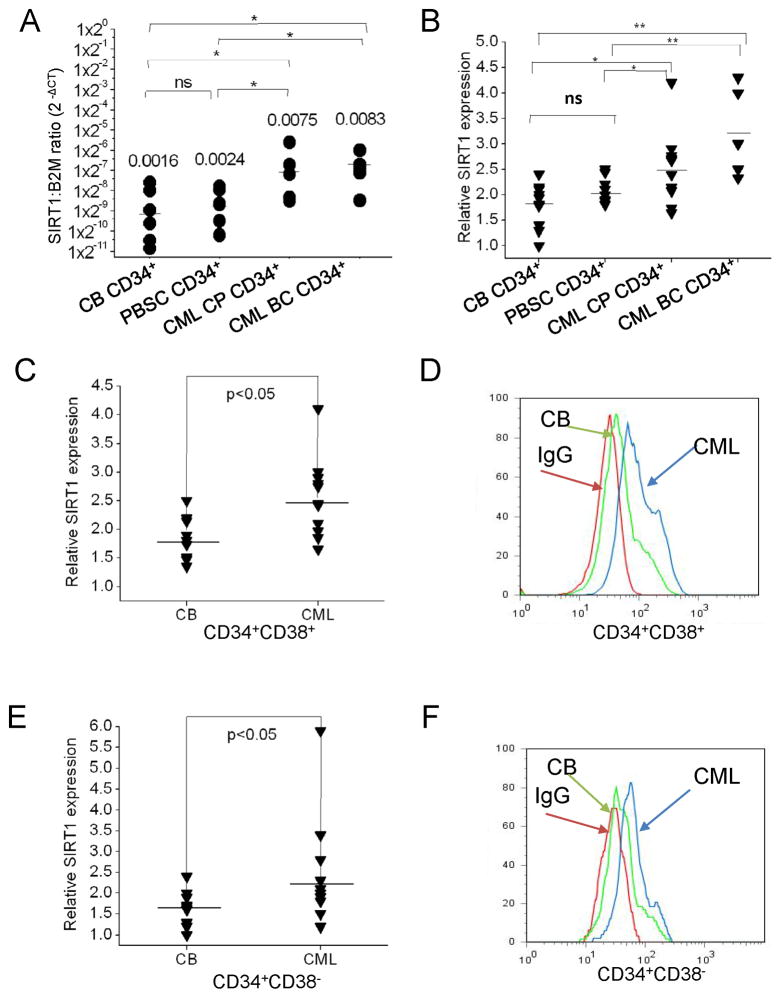
SIRT1 mRNA levels were significantly elevated in CP and BC CML CD34+ cells (Table S1) compared to CD34+ cells from cord blood (CB) or normal peripheral blood stem cell collections (PBSC) (Figure 1A). SIRT1 protein levels in CML and normal CD34+CD38+ committed progenitors and CD34+CD38− primitive progenitors were measured by intracellular labeling with anti-SIRT1 antibody and flow cytometry (Figure S1A). The ability of intracellular labeling to reliably measure SIRT1 expression was confirmed by Western blotting (Figure S1B). SIRT1 protein levels were significantly elevated in CML CP and BC CD34+ cells (Figure 1B), CML CP CD34+CD38+ (Figure 1C & 1D), and CD34+CD38− cells (Figure 1E & 1F) compared to their normal counterparts.
Figure 1. Increased SIRT1 expression in CML patients compared with normal stem/progenitor cells.
(A) Expression of SIRT1 mRNA in CP CML (n=5), BC CML (n=5), cord blood (CB) (n=6) and PBSC (n=6) CD34+ cells analyzed by Q-PCR. (B) Expression of SIRT1 protein in CP CML (n=11), BC CML (n=5) compared with CB CD34+ cells (n=10) and PBSC CD34+ cells (n=8) analyzed by intra-cellular labeling with anti-SIRT1 antibody. Median fluorescence intensity (MFI) of SIRT1 was expressed relative to IgG control. (C) Expression of SIRT1 in CML (n=11) and CB (n=10) CD34+CD38+ committed progenitors (right panel). Representative results are shown in panel (D): CML (blue), CB (green), IgG (red). (E) Expression of SIRT1 in CML (n=11) and CB (n=10) CD34+CD38− stem cells/primitive progenitors. Representative results are shown in panel (F): CML (blue), CB (green), IgG (red). Significance: *p<0.05, **p<0.01 for the indicated comparisons. See also Figure S1 and Table S1.
SIRT1 inhibition using shRNA reduces CML progenitor proliferation, survival and colony growth
To investigate the functional role of SIRT1 in CML and normal progenitors, CML and normal CD34+ cells were transduced with lentivirus vectors coexpressing SIRT1 or control shRNAs together with RFP. CD34+RFP+ cells were selected using flow cytometry. Western blotting confirmed effective inhibition of SIRT1 expression whereas the expression of the related SIRT2 was not affected (Figures 2A and 2B).
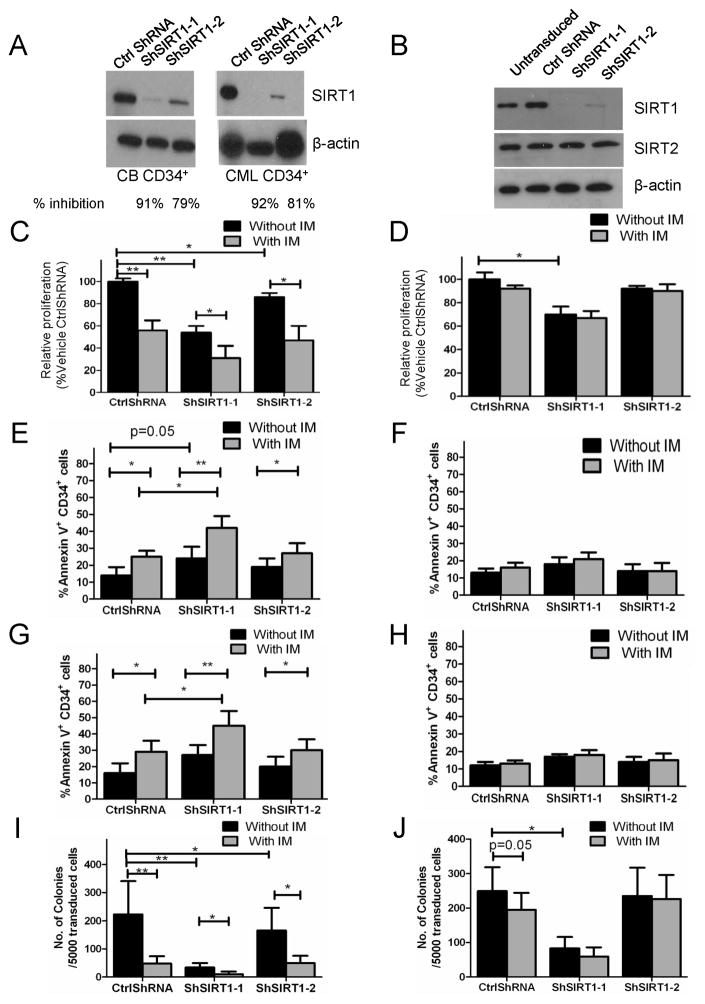
Figure 2. SIRT1 knockdown using specific anti-SIRT1 shRNA increases apoptosis and inhibits proliferation of CML progenitors.
(A) Western blotting of SIRT1 and β-actin in CB CD34+ and CML CD34+ cells transduced with SIRT1 shRNAs (ShSIRT1-1 and ShSIRT1-2) or with Ctrl shRNA. (B) Western blotting for SIRT1, SIRT2 and β-actin in ShSIRT1-1, ShSIRT1-2 or CtrlShRNA transduced TF-1 cells. Results are representative of 3 independent experiments. (C–J) CML (n=5) and normal CB (n=5) CD34+ cells transduced with Ctrl ShRNA, ShSIRT1-1 or ShSIRT1-2 vectors were cultured with or without IM (2.5 μM) for 72 hours. Division of CML (C) and normal CD34+ (D) cell was analyzed based on reduction in CFSE intensity, and a proliferation index was determined using ModFit software. Relative proliferation was calculated normalized to untreated controls. Apoptosis of CML (E) and normal (F) CD34+RFP+ cells was analyzed by Annexin V-Cy5 labeling. Apoptosis of undivided (CFSEhigh) CML (G) and CB CD34+ (H) cells was analyzed by Annexin V-Cy5 labeling. CFC assays were performed on CML (I) and normal CD34+ (J) RFP+ cells after culture with or without IM (2.5 μM) for 72 hours. Erythrocytic and granulocytic colonies were enumerated after 14 days. Results represent mean ± SEM of separate experiments. Significance: *p<0.05, **p<0.01, ***p<0.001, compared with untreated cells. See also Figure S2.
CD34+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) followed by culture for 72 hours in low growth factor concentrations. SIRT1 knockdown inhibited CML progenitor proliferation as measured by reduction in CFSE fluorescence. Treatment with IM resulted in further reduction of proliferation (Figure 2C). SIRT1 knockdown inhibited proliferation of CB CD34+ cells to a lesser extent than CML CD34+ cells (Figure 2D). Expression of ShSIRT1-1, which results in near complete inhibition of SIRT1 expression, resulted in reduced survival of CML CD34+ cells (Figure 2E). IM treatment significantly increased apoptosis of SIRT1 knockdown cells, indicating that SIRT1 inhibition enhanced sensitivity of CML progenitors to IM-induced apoptosis (Figure 2E). Enhanced apoptosis of CML CD34+ cells following SIRT1 knockdown, and further increase in apoptosis with IM treatment, was confirmed by Wright-Giemsa staining (Figure S2A), Trypan Blue staining (Figure S2B), and activated Caspase-3 labeling (Figure S2C). Interestingly, SIRT1 knockdown did not affect survival of normal progenitors, with or without IM treatment (Figure 2F). Primitive, quiescent CML CD34+ cells are especially resistant to IM-induced apoptosis (Holtz et al., 2005). Importantly the combination of SIRT1 inhibition and IM enhanced apoptosis of quiescent CML progenitors identified on the basis of high CFSE fluorescence (Figure 2G). In contrast, SIRT1 inhibition did not affect survival of quiescent normal progenitors (Figure 2H). Expression of both ShSIRT1-1 and ShSIRT1-2 significantly reduced CML colony forming cell (CFC) frequency in methylcellulose progenitor assays, which was enhanced by IM (Figure 2I). Inhibition of normal CFC growth was also seen, but was significantly less than for CML progenitors (Figure 2J).
The increased effects of ShSIRT1-1 compared to ShSIRT1-2, suggest that partial inhibition of SIRT1 expression is sufficient to inhibit CML progenitor proliferation, but that near complete knockdown is required to inhibit survival. To exclude the possibility that these results were related to off-target effects, we designed a SIRT1 construct resistant to ShSIRT1-1 (SIRT1-R) (Figure S2D). Lentivirus-mediated expression of wild type (WT) or SIRT1-R resulted in enhanced SIRT1 protein levels in TF-1-BCR-ABL cells transduced with ShSIRT1-1 (Figure S2E) and abrogated the ability of ShSIRT1-1 to induce apoptosis and inhibit growth (Figure S2F & S2G). These results indicate that ShSIRT1-1 shRNA effects are related to SIRT1 knockdown rather than off-target effects, and confirm that near complete SIRT1 suppression is required to induce apoptosis in CML cells.
Pharmacological inhibition of SIRT1 induces apoptosis and inhibits proliferation of CML stem/primitive progenitor cells
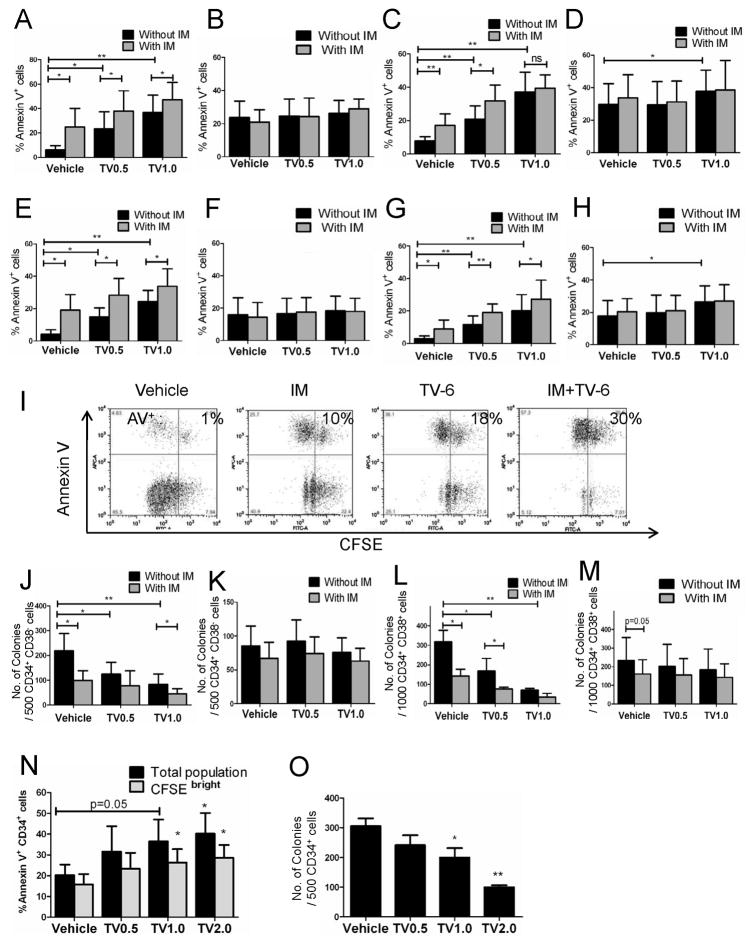
We tested the effects of Tenovin-6 (TV-6), a small molecule inhibitor of SIRT1, on CML and normal stem/progenitor cells (Lain et al., 2008). CFSE labeled CML and normal CD34+CD38− and CD34+CD38+ cells were cultured for 72 hours with TV-6, IM, or the combination. TV-6 significantly increased apoptosis of CML CD34+CD38− cells and CD34+CD38+ cells, but not normal cells (Figure 3A–3D). A small increase in apoptosis of normal CD34+CD38+ cells was seen with higher doses of TV-6 (Figure 3D). Normal progenitor apoptosis was significantly less than for CML progenitors (p <0.05). The combination of IM and TV-6 increased apoptosis in CML progenitors compared to either agent alone, and to a significantly greater extent than normal progenitors (Figure 3A–3D). Importantly, TV-6 also resulted in increased apoptosis of CFSEhigh undivided CML CD34+CD38− and CD34+CD38+ cells, but did not increase apoptosis of undivided normal cells (Figure 3E–3H). A small increase in apoptosis of undivided normal cells was seen with higher doses of TV-6 (Figure 3H), but was significantly less than for CML cells (p<0.05). Pretreatment with TV-6 for 72 hours inhibited CFC production from CML CD34+CD38− and CD34+CD38+ cells but not normal CD34+CD38− and CD34+CD38+ cells (Figure 3J–3M). The combination of TV-6 with IM enhanced inhibition of CML CFC growth compared to either agent alone, but did not enhance inhibition of normal CFC. Similar results were seen for cells exposed to TV-6 in methylcellulose for the 14 day duration of the CFC assay (Figure S3A & S3B). Exposure to IM for 14 days markedly inhibited CML and normal CFC growth (p<0.01) compared to 14 days exposure to TV-6 (Figure S3A & S3B). TV-6 also increased apoptosis in total and undivided CD34+ cells from CML BC patients that failed IM treatment, and inhibited growth of CD34+ cells in CFC assays (Figure 3N & 3O). In addition TV-6 inhibited BaF3 cells expressing the IM-resistant T315I BCR-ABL mutant (BaF3/T315I) to a similar extent as BaF3 cells expressing wild-type BCR-ABL (BaF3/BA) (Figure S3C).
Figure 3. Pharmacological inhibition of SIRT1 induces apoptosis and inhibits proliferation of CML stem/primitive progenitor cells.
(A) CML CD34+CD38− (n = 6), (B) normal CD34+CD38− (n=4, 2 CB and 2 PBSC), (C) CML CD34+CD38+ (n=6), and (D) normal CD34+CD38+ cells (n=4, 2 CB and 2 PBSC) cells were exposed to TV-6 (0.5 μM, TV0.5, or 1 μM, TV1.0), IM (2.5μM), or the combination for 72 hours. Apoptosis was analyzed by Annexin V-Cy5 labeling. Apoptosis of undivided (E) CML CD34+CD38−CFSEhigh, (F) normal CD34+CD38−CFSEhigh, (G) CML CD34+CD38+CFSEhigh, and (H) normal CD34+CD38+CFSEhigh cells analyzed by Annexin V labeling. (I) Representative flow cytometry plots showing Annexin V+ versus CFSE expression. (J) CML or (K) normal CD34+ CD38− cells, and (L) CML or (M) normal CD34+CD38− cells were cultured with TV-6 (0.5 or 1μM), IM (2.5μM), or the combination for 72 hours then plated in methylcellulose progenitor culture and colonies enumerated after 14 days. (N) Apoptosis of total or undivided CFSEhigh CML BC (n = 4) CD34+ cells exposed to TV-6 (0.5, 1, or 2μM) for 72 hrs analyzed by Annexin V-Cy5 labeling. (O) CML BC (n = 4) CD34+ cells exposed to TV-6 (0.5, 1, or 2μM) were plated in methylcellulose progenitor culture and erythrocytic and granulocytic colonies enumerated after 14 days. Results represent the mean ± SEM of separate experiments. Significance: *p<0.05, **p<0.01, ***p<0.001, compared with untreated controls. See also Figure S3.
The effects of SIRT1 deletion on BM progenitors from young mice are only apparent when cells are grown at low O2 tensions (Ou et al., 2011). We investigated whether selective effects of SIRT1 inhibition on CML compared to normal cells were maintained at low O2 tensions. TV-6 (2μM) induced apoptosis of total (Figure S3D-S3K) or undivided normal CD34+CD38+ cells was increased in hypoxic compared to normoxic conditions (Figure S3G & S3K). Inhibition of normal CD34+CD38+ cell proliferation and CFC formation was also increased in hypoxic conditions (Figure S3O & S3S). On the other hand, the effects of TV-6 on survival, proliferation and colony formation of normal CD34+CD38− cells (Figure S3E, S3I, S3M & S3Q) or CML CD34+CD38− cells (Figure S3D, S3H, S3L, & S3P) and CD34+CD38+ cells were not significantly different in hypoxic versus normoxic conditions (Figure S3F, S3J, S3N & S3R).
TV-6 did not result in additional inhibition of proliferation of SIRT1 knockdown TF-1/BCR-ABL cells (Figure S3T). In addition MEF from SIRT1 knockout mice (SIRT1-KO) cells were resistant to TV-6 (data not shown). Finally ectopic expression of SIRT1 decreased the sensitivity of TF-1/BCR-ABL cells to TV-6 (IC50 2μM in parental TF-1/BCR-ABL and 4.3μM in ectopic SIRT1 expressing TF-1/BCR-ABL cells). These results suggest that TV-6 effects are indeed SIRT1 mediated.
SIRT1 inhibition impairs CML LSC engraftment in immunodeficient Mice
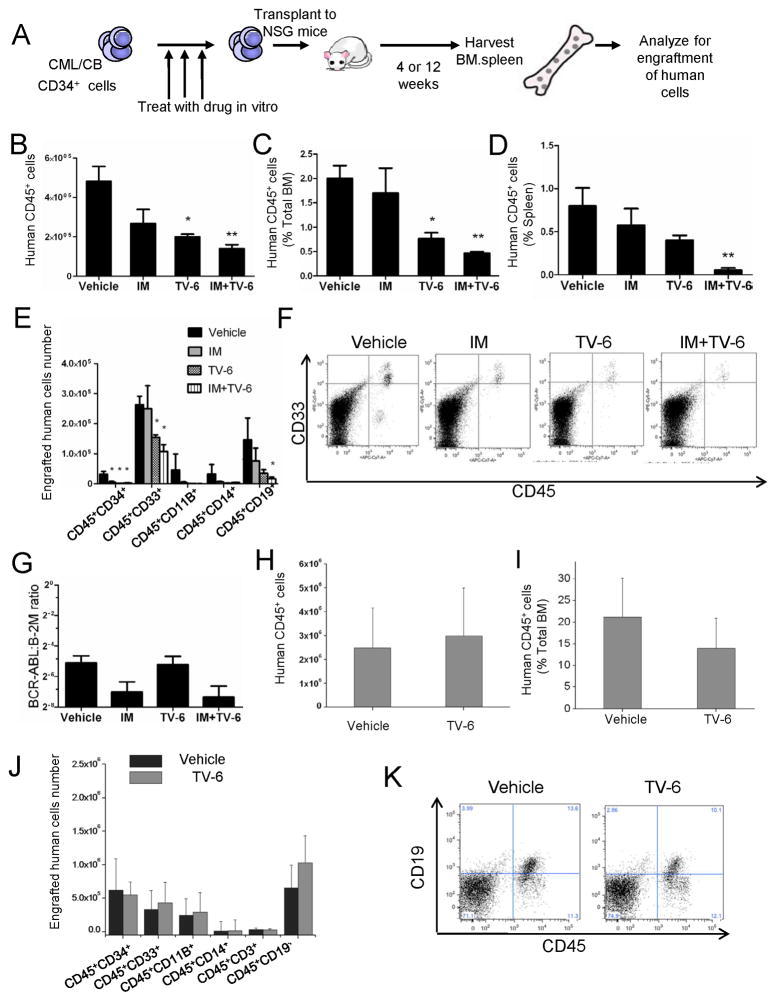
We evaluated the effect of ex vivo treatment with TV-6 on CML and normal CD34+ stem cells capable of engraftment in NOD/SCID interleukin-2 receptor-γ chain-deficient (NSG) mice (Shultz et al., 2005) (Figure 4A). We observed reduced engraftment of CML CD34+ cells treated with TV-6 (1μM) for 72 hours at 4 weeks (short-term engraftment) (p<0.05) (Figure S4A) and 12 weeks post-transplantation (longer-term engraftment) (Figure S4C & S4D). Engraftment of CD33+ and CD14+ myeloid cells was decreased (Figure S4E & S4F). Q-PCR analysis confirmed that engrafted human cells expressed BCR-ABL (Figure S4G), and FISH analysis showed that 90% of engrafted human cells were BCR-ABL+. These results show that SIRT1 inhibition by TV-6 selectively targets primitive human CML cells with in vivo engraftment capacity. We also compared the effect of IM (2.5μM), TV-6 (1μM), or the combination on CML CD34+ cell engraftment in NSG mice (Figure 4B–4G). CML cells treated with IM alone (p=0.06) and TV-6 alone (p<0.05) demonstrated reduced engraftment in BM at 12 weeks post-transplant compared to untreated controls (Figure 4B). The combination of IM and TV-6 resulted in further inhibition of CML CD34+ cell engraftment compared to IM or TV-6 alone (Figure 4B–4D), including reduced engraftment of myeloid cells (Figure 4E & 4F). Q-PCR analysis confirmed that engrafted cells expressed BCR-ABL, and that BCR-ABL expression was reduced in cells treated with IM and IM plus TV-6 (Figure 4G). Interestingly, engraftment of CB CD34+ cells at both 4 weeks (Figure S4B) and 12 weeks (Figure 4H–4K) was not reduced after treatment with TV-6.
Figure 4. SIRT1 inhibition reduces longer term engraftment of CML stem cells in immunodeficient mice.
(A) CML or normal CD34+ cells were treated with TV-6, IM, or combination in vitro and injected into sublethally irradiated (300cGy) NSG mice. After 4 or 12 weeks, human cell engraftment was analyzed by flow cytometric assessment of human CD45+ cells. (B) The number and (C) the percent of human CD45+ cells engrafted in the BM 12 weeks after transplantation of CML CD34+ cells (1×106 cells per mouse). (D) The percent of human CD45+ cells engrafted in the spleen at 12 weeks. Cells from two patients were injected. The figure shows representative results from one patient (n=3 for untreated control and TV-6 treated cells, n=4 for IM and combination treatment). (E) Engraftment of human CD34, CD33, CD11b, CD14, and CD19 subsets (no CD3 positive cells were seen). (F) Representative results for CD45 and CD33 expression. (G) BCR-ABL mRNA levels in CD45+ cells engrafted in BM at 12 weeks were measured by Q-PCR. (H) The total number and(I) percent of human CD45+ cells engrafted in the BM of NSG mice receiving CB CD34+ cells at 12 weeks (1×105 cells injected per mouse; n=4 for untreated control; n=7 for TV-6 treated cells). (J) Engraftment of human CD34, CD33, CD11b, CD14, CD3 and CD19 cell subsets. (K) Representative results for CD45 and CD19 expression. Results represent the mean ± SEM of separate experiments. Significance: *p<0.05, **p<0.01 compared with untreated cells. See also Figure S4.
SIRT1 inhibitor treatment reduces CML stem and progenitor cell growth in vivo
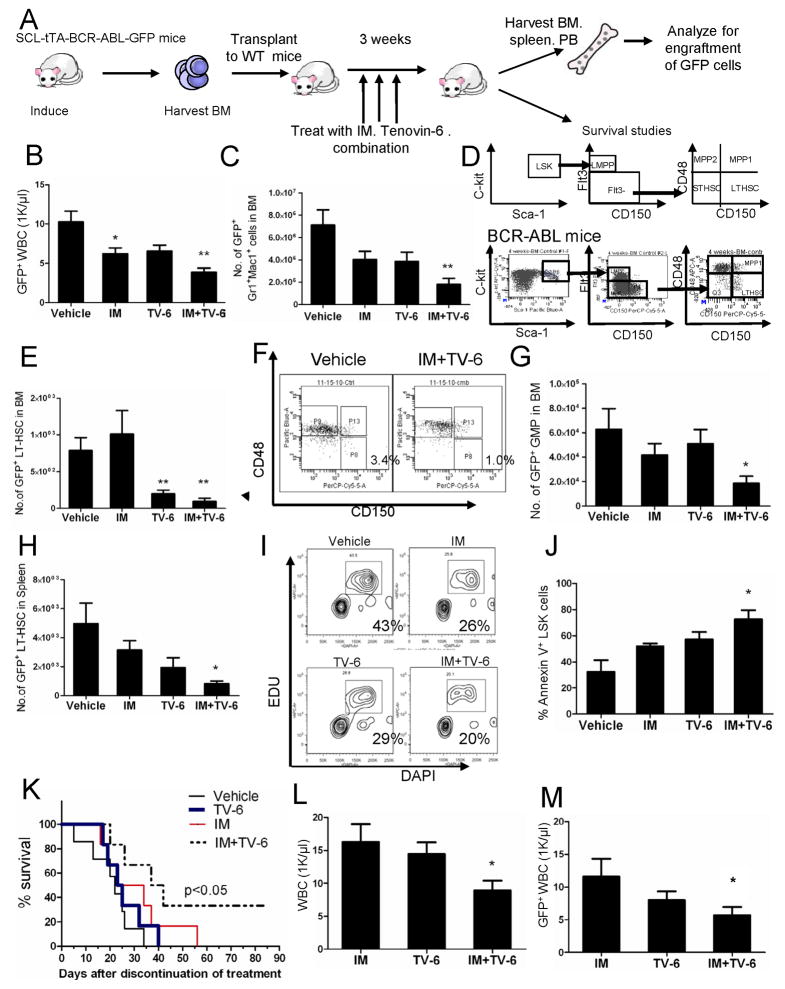
The low levels of longer-term engraftment of CML CP cells in NSG mice limits the use of this model to evaluate effects of treatments administered in vivo. An inducible BCR-ABL transgenic mouse model of CML provides a representative model of CP CML that can be used for in vivo therapeutic studies (Zhang et al., 2010). These BCR-ABL mice were crossed with GFP transgenic mice and BM cells were obtained 4 weeks after BCR-ABL induction. GFP-expressing cells selected using flow cytometry were transplanted into wild-type FVB/N mice irradiated at 900 cGy. Following engraftment, mice were treated for 3 weeks with IM (200mg/kg/day by gavage), TV-6 (50mg/kg/day intraperitoneally), the combination of TV-6 and IM, or vehicle (controls) (Figure 5A). TV-6 treated mice demonstrated loss of weight (data not shown) compared to control or IM-treated mice. Leukemic GFP+ WBC and BM myeloid cells were reduced in TV-6 or IM treated mice, with further reduction with combination treatment (Figure 5B, 5C, & S5A). Flow cytometry analysis (Figure 5D) showed that combined TV-6 and IM treatment inhibited primitive GFP+Lin−Sca-1+Kit+ cells (LSK cells) (Figure S5B), LTHSC (LSK Flt3−CD150+CD48− cells) (Figure 5E & 5F), common myeloid progenitors (CMP) and granulocytic-macrophage progenitors (GMP) in the BM of CML mice to a greater extent than IM or TV-6 alone (Figure 5G & S5C). Similar results were observed for splenic cells (Figure 5H, S5D, and data not shown). In vivo administration of IM and TV-6 resulted in inhibition of LSK cell proliferation measured by EdU and DAPI labeling (Figure 5I) and enhanced LSK cells apoptosis (Figure 5J). Mice treated with the combination demonstrated significantly improved survival and maintained normal WBC counts with only small number of residual GFP+ WBCs after discontinuation of treatment, compared to control, IM and TV-6 treated mice (Figure 5K, 5L & 5M).
Figure 5. SIRT1 inhibition reduces in vivo growth of CML stem cells.
(A) BCR-ABL mice were crossed with GFP transgenic mice and BM cells obtained 4 weeks after induction of BCR-ABL expression. GFP+ cells selected by flow cytometry were transplanted into wild-type FVB/N mice irradiated at 900 cGy. Following engraftment, mice were treated for 3 weeks with IM (200mg/kg/day by gavage), TV-6 (50mg/kg/day intraperitoneally), the combination of TV-6 and IM, or vehicle (controls) (n=6 mice each). (B) GFP+ WBC counts 3 weeks after start of treatment. (C) GFP+ myeloid cells (Gr-1+Mac-1+) cells in the BM. (D) Schema for analysis of LTHSC (E) GFP+ LTHSC in BM. (F) Representative plot for BM LTHSC for vehicle control and combination. (G) GFP+ GMP in BM. (H) GFP+ LTHSC in spleen. Apoptosis and cell cycling in BM LSK cells was evaluated after 5 days of treatment. (I) Mice were injected intraperitoneally with EdU and euthanized 2 hours later. The percentage of stem cells in S-phase was determined based of EdU incorporation in BM LSK cells. Representative results from one of two experiments are shown. (J) Apoptosis was evaluated by Annexin-V and DAPI labeling (n = 3 mice per group). Significance: *p < 0.05, **p < 0.01, compared with vehicle control. (K) Mice were followed for survival for 85 days after discontinuation of treatment (n=6 per group). Survival of mice receiving combination treatment was significantly longer than mice receiving IM or TV-6 alone or vehicle-treated cohorts (p < 0.05, Wilcoxon test). Two mice receiving combination treatment remained alive till day 85 when the experiment was terminated. (L) The total WBC count and (M) GFP+ WBC count in PB of mice 4 weeks after discontinuation of treatment. Significance: *p < 0.05, **p < 0.01, compared with IM. Results represent the mean ± SEM of separate experiments. See also Figure S5.
Consistent with previous reports, BC CML cells demonstrate robust engraftment in NSG mice with primitive CD34+ cells (Figure S5E–S5I), allowing evaluation of in vivo treatment with TV-6. NSG mice engrafted with cells from an IM-resistant CML BC patient were treated with TV-6 (50mg/kg/day intraperitoneally) or vehicle control for 3 weeks. TV-6 significantly reduced total human cells (p<0.01) (Figure S5F & S5G), and human CD34+ cells and myeloid cells in the BM, spleen and peripheral blood (Figure S5H–S5K). TV-6 treatment also enhanced survival of mice transplanted with BaF3/T315I cells that are resistant to treatment with IM (Figure S5L), further demonstrating its in vivo activity against IM-resistant cells.
SIRT1 inhibition enhances p53 acetylation in CML Progenitors
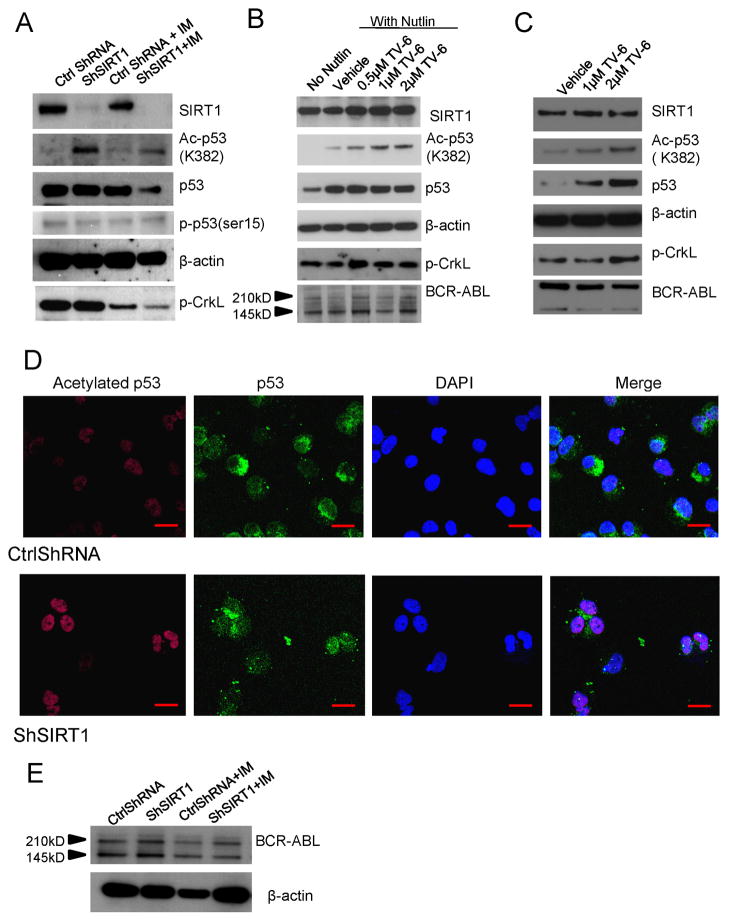
SIRT1 activity can be assessed by examining acetylation of p53 at K382, a known SIRT1 deacetylation site. SIRT1 knockdown using shRNA resulted in an increase in acetylated p53 protein levels in CP CML CD34+ cells (Figure 6A, S6A). Although increased p53 acetylation was detectable in SIRT1 knockdown cells in the absence of TSA (Figure S6A), it was more clearly seen in cells in which classic HDAC activity was inhibited by TSA treatment (Luo et al., 2001; Cheng et al., 2003). Exposure of CML CP or BC CD34+ cells to TV-6 also significantly enhanced acetylated p53 levels in both hypoxic and normoxic conditions (Figure 6B, 6C, S6B, S6C). TV-6 treatment also increased total p53 levels, possibly by reducing degradation (Lain et al., 2008). Cells pretreated with the MDM2 antagonist Nutlin-3 to stabilize p53 levels (Vassilev et al., 2004) before addition of TV-6 showed a rapid and marked increase in p53 acetylation without change in total p53 levels (Figure 6B). In contrast TV-6 did not increase acetylated p53 or total p53 levels in normal CD34+ cells (Figure S6D). Acetylated p53 signals were increased and showed a nuclear distribution in SIRT1 knockdown cells (Figure 6D). A modest increase in acetylation of the SIRT2 substrate α-tubulin (Lain et al., 2008) was also seen after 48 hours, suggesting that prolonged TV-6 exposure may also modestly inhibit SIRT2 activity (Figure S6E). However, the more rapid and pronounced effect on p53 acetylation indicates more efficient inhibition of SIRT1 compared to SIRT2 activity. Ectopic expression of BCR-ABL in CB CD34+ cells also resulted in increased expression of SIRT1, which was associated with reduced levels of acetylated p53 despite increased levels of total p53 (Figure S6F).
Figure 6. Increased p53 acetylation and nuclear localization in SIRT1 inhibited CML CD34+ cells.
(A) Western blotting for acetylated p53 (K382), total p53, p-p53 (ser 15), β-actin, SIRT1, p-CrkL in SIRT1 knockdown in CML CD34+ cells cultured with TSA (0.1μM) for 2 hours and exposed to IM 2.5μM for 8 hours. (B) Western blotting for acetylated p53 (K382), total p53, β-actin, SIRT1, p-CrkL, and BCR-ABL in CML CD34+ cells cultured with Nutlin-3 (10μM) for 2 hours, and exposed to TV-6 for 6 hours. (C) Western blotting for acetylated p53 (K382), total p53, β-actin, SIRT1, p-CrkL, and BCR-ABL in CML BC CD34+ cells cultured with Tenovin-6 (1 or 2μM) for 16 hours. (D) Immunofluorescence analysis of Ac-p53 and p53 in CD34+ cells. All scale bars represent a size of 10μm. Results are representative of 3 independent experiments. (E) Western blotting for BCR-ABL and β-actin in SIRT1 knockdown CML CD34+ cells exposed to IM 2.5μM for 24 hours. See also Figure S6.
Treatment with classical HDACis can reduce BCR-ABL expression in cell lines and BC CML cells (Fiskus et al., 2006). BCR-ABL protein levels were not reduced in CP or BC CML CD34+ cells after SIRT1 inhibition using either shRNA or TV-6 (Figure 6B, 6C & 6E; Figure S6B & S6C). Treatment with IM reduced tyrosine phosphorylation of the BCR-ABL substrate CrkL in CML CD34+ cells, confirming inhibition of BCR-ABL kinase activity (Figure 6A, Figure S6B). In contrast SIRT1 inhibition did not reduce phosphorylation in CML CD34+ cells (Figure 6A & 6C, Figure S6B & S6C). Therefore the effects of SIRT1 inhibition on CML CD34+ cells cannot be explained by inhibition of BCR-ABL expression or activity. Treatment with IM resulted in modest reduction in SIRT1 levels and in total p53 but not acetylated p53 levels (Figure 6A, Figure S6B).
SIRT1 inhibition increases p53 transcriptional activity
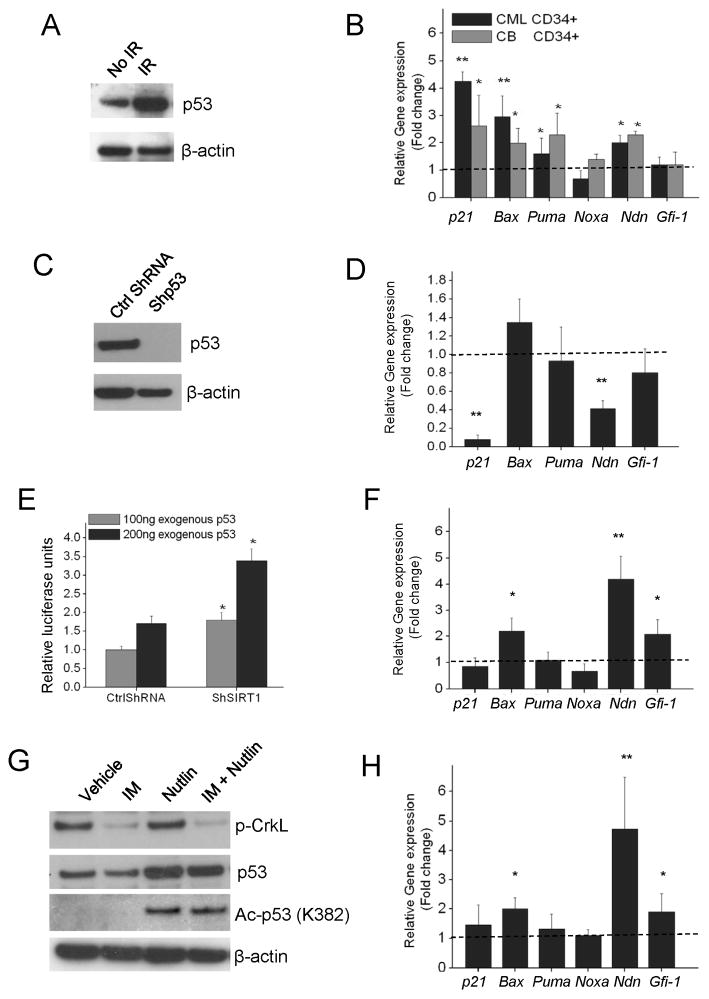
Irradiation of CML CD34+ progenitors resulted in increased p53 levels (Figure 7A) and increased expression of p53 target genes including p21, Necdin (Ndn), Puma and Bax (Figure 7B). Knockdown of p53 in CML CD34+ cells using lentivirus vectors expressing anti-p53 shRNA (Figure 7C) resulted in reduced expression of p21 and Ndn (Figure 7D). These results indicate that p53 signaling remains subject to activation in CP CML CD34+ cells. To examine the effect of SIRT1 knockdown on p53 transcriptional activity in BCR-ABL expressing cells, we co-transfected a p53 expression vector and the BP-100 p53 reporter plasmid (MDM2 promoter cloned upstream of the luciferase gene) (Dai et al., 2004) into the p53-null CML cell line K562 (Bi et al., 1992). SIRT1 knockdown resulted in increased p53 activity (p<0.05) compared with controls (Figure 7E). SIRT1 knockdown also increased the activity of endogenous p53 in 293 cells (Figure S7A). Importantly, SIRT1 knockdown increased expression of p53 target genes in CML CD34+ cells on Q-PCR analysis including Gfi-1, Ndn, and Bax (p<0.05) (Figure 7F). Although p21 is also a p53 regulated gene in CML CD34+ cells, it was not further induced by SIRT1 knock-down.
Figure 7. Signaling through p53 is intact in CML progenitors, is enhanced by SIRT1 knockdown, and is not affected by IM.
(A) Western blotting for p53 in CML CD34+ cells 6 hours after exposure to irradiation (3Gy). Results are representative of 3 independent experiments. (B) Q-PCR analysis of p53 target genes in CP CML and normal CD34+ cells 6 hours after irradiation (3Gy) compared with non-irradiated control cells. β-2M was used as an internal control. (C) Western blotting for p53 expression in CML CD34+ cells transduced with an anti-p53 shRNA vector. Results are representative of 3 independent experiments. (D) Q-PCR analysis of p53 target genes in p53 knockdown and control cells. (E) SIRT1 knockdown or control K562 cells (n=3) were co-transfected with plasmids expressing p53, a luciferase reporter for p53 transcription (BP100) and β-galactosidase. Relative luciferase units are normalized to β-gal expression. (F) Q-PCR analysis of p53 target genes in shSIRT1 compared with ctrl shRNA expressing CML CD34+ cells (n=3). (G) Western blotting for acetylated p53 (K382), total p53, p-CrkL, and β-actin in CML CD34+ cells treated with IM (2.5μM), Nutlin-3 or the combination for 8 hours. (H) Q-PCR analysis for p53 target genes in ShSIRT1 compared with ctrl shRNA transduced CML CD34+ cells treated with IM (2.5μM) for 24 hours (n=4). Significance: *p<0.05, **p<0.01, compared with controls. Results shown represent the mean ± SEM of separate experiments. See also Figure S7.
Treatment with IM resulted in a modest reduction in p53 levels in CML CD34+ progenitors (Figure 6A & 7G), but did not significantly affect p53 acetylation or expression of p53 target genes (Figure S7B). Knockdown of p53 in CML CD34+ cells using anti-p53 shRNA did not affect the ability of IM to induce apoptosis (Figure S7C) or inhibit proliferation (Figure S7D), indicating that IM–mediated inhibition of CML progenitors is independent of p53 expression. The combination of IM and Nutlin-3 induced significantly more apoptosis than either agent alone (data not shown), indicating that p53 signaling can be activated in IM-treated CML CD34+ cells. SIRT1 inhibition in IM-treated CML CD34+ progenitors by anti-SIRT1 shRNA expression resulted in increased expression of acetylated p53 (Figure 6A). Upregulation of p53 target genes was also seen following SIRT1 knockdown in IM-treated cells (Figure 7H). These observations indicate that SIRT1 knockdown can activate p53 signaling in IM-treated CML cells.
Inhibition of CML progenitor survival by SIRT1 knockdown requires p53 expression and acetylation
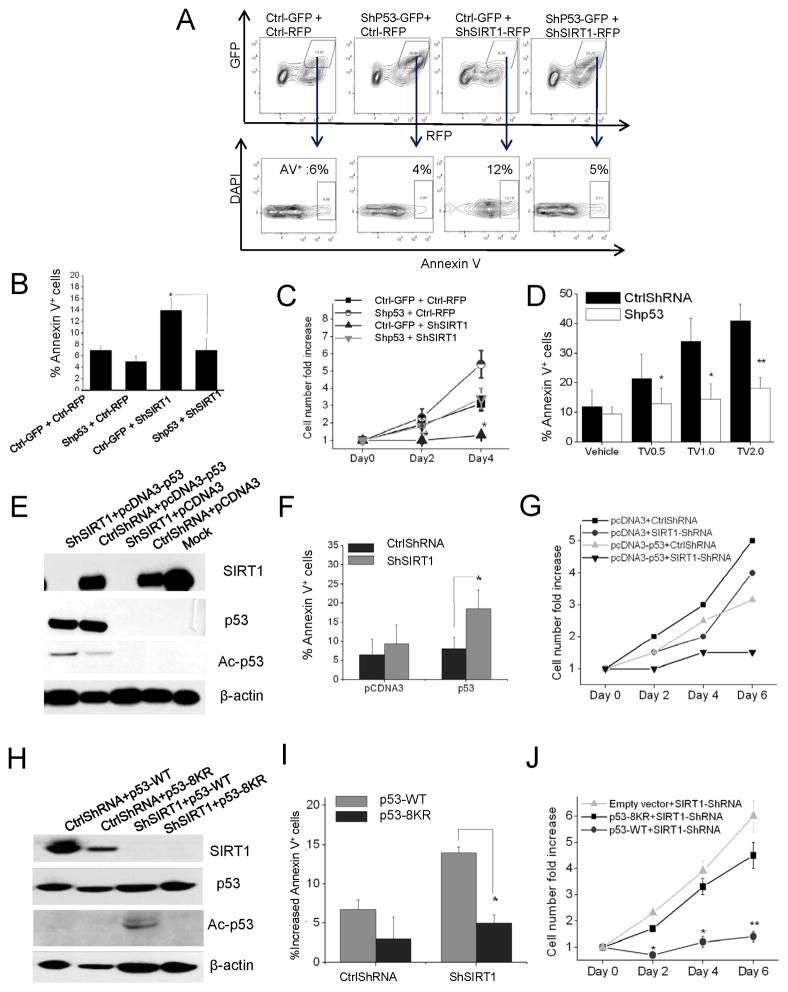
To determine the role of p53 in mediating the effects of SIRT1 inhibition on CML progenitors we evaluated whether shRNA-mediated p53 knockdown could mitigate the effects of SIRT1 inhibition on CML progenitors. CML CD34+ cells were co-transduced with a lentivirus vector coexpressing SIRT1 shRNA and RFP and a second vector coexpressing p53 shRNA and GFP, followed by selection of cells expressing both RFP and GFP (Figure 8A). Inhibition of p53 expression significantly reduced apoptosis (Figure 8A & 8B) and enhanced growth (Figure 8C) of SIRT1-knockdown CML CD34+ cells. In addition p53 knockdown CML progenitors exposed to TV-6 showed reduced apoptosis compared with control cells exposed to TV-6 (Figure 8D). These results confirm an important role for p53 in SIRT1-mediated signaling in CML progenitors.
Figure 8. Effect of SIRT1 inhibition in CML progenitors is dependent on p53 expression and acetylation.
(A–C) CML CD34+ cells were co-transduced with PLKO-GFP vectors expressing anti-p53 or control shRNA and PHIV7-RFP vectors expressing anti-SIRT1 or control shRNA. (A) CD34+GFP+RFP+ cells were analyzed for apoptosis by Annexin V labeling. A representative plot is shown. (B) Cumulative results for apoptosis (n=3). (C) The total number of CD34+GFP+RFP+ cells normalized to ctrl ShRNA expressing cells (n=3). (D) p53 knockdown or control CML progenitors were exposed to TV-6 for 48 hours and apoptosis analyzed by Annexin V labeling (n=3). Significance values: *p<0.05, **p<0.01, ***p<0.001, compared with untreated cells. (E–G) K562 cells transduced with HIV7-RFP vectors expressing anti-SIRT1 or control shRNA were transfected with p53 expressing plasmids. (E) Western blotting for acetylated p53 (K382), total p53, SIRT1 and β-actin. (F) Apoptosis was assessed after 48 hours by Annexin V labeling. (G) The fold change in cell numbers was calculated at day 2, day 4, and day 6 relative to day 0. (H–J) K562 cells expressing a tet transactivator gene (K562-TTA) were generated and transduced with HIV7-RFP vectors expressing ShSIRT1 or Ctrl ShRNA. RFP+ cells were selected and transfected with acetylation-defective (p53-8KR) and wild type p53 constructs (n=3). Similar transfection efficiency was confirmed by co-transfection with a β-gal plasmid. (H) Western blotting for acetylated p53 (K382), total p53, SIRT1 and β-actin. (I) Apoptosis evaluated after 48hrs by Annexin V labeling. Results are normalized to K562-RFP cells transfected with empty vector (J) The fold change in cell numbers at day 2, day 4, and day 6 was calculated relative to day 0. Results represent mean±SEM for separate experiments. Significance: *p<0.05, **p<0.01, compared with controls. (See also Figure S8)
To determine the role of p53 acetylation in mediating SIRT1 effects, we expressed an acetylation-defective p53 mutant (p53-8KR, with all eight potential acetylation sites mutated) in p53-null K562 cells. Inhibition of SIRT1 using shRNA or TV-6 did not inhibit growth or induce apoptosis in parental K562 cells (p>0.05) (Figure S8). However SIRT1 knockdown in K562 cells that ectopically expressed WT p53 protein led to increased p53 acetylation (Figure 8E) and significant growth inhibition and apoptosis (p<0.05) (Figure 8F & 8G). In contrast, ectopically expressed p53-8KR was not acetylated following SIRT1 knockdown (Figure 8H), and K562 cells transfected with p53-8KR did not show significant growth inhibition or apoptosis following SIRT1 knockdown (Figure 8I & 8J). These results indicate that p53 acetylation is required for growth inhibition and apoptosis following SIRT1 inhibition in BCR-ABL expressing cells.
Discussion
Our results show that inhibition of SIRT1 deacetylase enhances targeting of LSC from CML patients by TKI treatment via activation of p53 signaling, indicating an important role for SIRT1 in maintaining LSC growth and survival. Both BCR-ABL kinase-dependent and kinase-independent mechanisms contribute to increased SIRT1 activity in CML cells, with the latter potentially including epigenetic silencing of HIC1, a negative regulator of SIRT1, through methylation (Chen et al., 2005), or altered miRNA regulation of SIRT1 expression (Strum et al., 2009). The selectivity of SIRT1 inhibition towards CML stem/progenitor cells is maintained in hypoxic conditions, where SIRT1 plays an important role in supporting normal hematopoiesis (Ou et al., 2011).
SIRT1 can deacetylate several lysine residues in the tumor suppressor p53 (Luo et al., 2001; Brooks et al., 2009). A variety of posttranslational modifications that can regulate p53 activity, including phosphorylation, acetylation, methylation and sumoylation have been described (Vousden et al., 2007). Acetylation is reported to play an important role in stabilization, nuclear localization and transcriptional activation of p53 (Prives et al., 2001), and can lead to p53 activation independent of phosphorylation status (Tang et al., 2008). Although p53 mutations may occur on progression to BC CML, they are rare in CP CML (Prokocimer et al., 1994). Our results indicate that p53 remains responsive to stress-induced activation in CML progenitors. SIRT1 inhibition increased p53 acetylation and expression of several p53 target genes, including Bax, Necdin and Gfi-1 in CML CD34+ cells. Bax is an important pro-apoptotic gene, and Necdin and Gfi-1 may be important for p53 regulated quiescence of HSC (Liu et al., 2009). Additional p53 target genes besides those identified here may also contribute to the effects of SIRT1 inhibition. Although p21 expression was reduced in CML progenitors after p53 knockdown, SIRT1 knockdown did not increase expression of p21 in CML progenitors, suggesting that other SIRT1-regulated pathways may counteract the effects of p53 acetylation on p21 induction (Cheng et al., 2003). BC CML cells also demonstrated increased p53 acetylation following SIRT1 inhibition consistent with recent reports that p53 can be activated in CML BC cells (Peterson et al., 2011). Of note, the CML BC samples studied here did not have p53 mutations (Table S1).
Although previous studies indicated that the p53 inactivation by SIRT1 promotes cell survival during stress (Luo et al., 2001), other studies have suggested that small-molecule SIRT1 inhibitors do not affect cell survival (Solomon et al., 2006), and that developmental defects in a SIRT1 knockout mouse strain are not rescued by crossing to p53 null mice (Kamel et al., 2006). The importance of p53 in mediating SIRT1 effects may depend on the cellular context. SIRT1 deacetylates several other proteins that regulate cell growth and survival besides p53. The importance of individual SIRT1 targets may depend on the cell process and cell type studied. Recent studies within our group indicate an important role for SIRT1 regulation of Ku70 in DNA repair and mutagenicity of CML cells (unpublished data). The role of other SIRT1 targets such as the FoxOs and E2F1 transcription factors in regulating quiescence and survival of CML stem and progenitor cells requires further evaluation.
There is considerable interest in restoring p53 function in cancer cells as a means of inhibiting their proliferation, or inducing senescence or apoptosis. Deacetylation of p53 via SIRT1 may play an important role in preventing p53 activation in TKI-treated CML progenitors. BCR-ABL kinase activity could also modulate p53 in CML cells by upregulation of ARF (Williams et al., 2006), phosphorylation and inactivation of MDM2 and MDMX (Zuckerman et al., 2009), or increased translation of MDM2 (Trotta et al., 2003). Despite the complex regulation of p53 in CML cells, our studies show that enhanced p53 acetylation following SIRT1 inhibition is sufficient to increase p53 transcriptional activity in CML progenitor cells, that p53 deacetylation is an important protective mechanism for CML LSC following TKI treatment. Therefore, p53 activation is a potential strategy to enhance targeting of CML LSC, especially in combination with TKI.
SIRT1 inhibitors are being investigated as potential anti-cancer treatments. We observed weight loss in mice during the course of three-week TV-6 treatment, but it is unclear whether this was related to SIRT1 inhibition or an off-target effect of this agent. Although Tenovin-6 itself may not be a candidate for drug development, our observations support further investigation of SIRT1 inhibition as an approach for targeting of CML stem/progenitor cells in combination with TKI treatment. The potential tumor suppressive effects of SIRT1 need to be kept in mind when considering SIRT1 inhibitors for cancer treatment. Improved understanding of mechanisms underlying the anti-cancer versus tumor promoting effects of SIRT1 inhibition in specific cell types will aid the development of more selective, nontoxic approaches for targeting LSCs in future. The results of the current studies have broader implication to other leukemias, such as AML, where SIRT1 over-expression is also observed and p53 mutations are rare (Kojima et al. 2005).
Experimental Procedures
Samples and materials
CB samples were provided by StemCyte (Arcadia, CA). CP CML samples were obtained from previously untreated patients at the City of Hope (COH) and the University of Glasgow. CML BC samples were obtained from patients at COH (Table S1). CD34+ cell isolation and CD3+ cell depletion were performed using magnetic beads (StemCell Technologies, Vancouver, BC, Canada). Leukopheresis samples were processed for CD34+ cell selection with CliniMACS (Miltenyi Biotech, Germany). CD34+CD38− and CD34+CD38+ cells were obtained by flow cytometry sorting. All subjects signed an informed consent form. Sample acquisition was approved by the Institutional Review Boards at the City of Hope, in accordance with an assurance filed with and approved by the Department of Health and Human Services, and the North Glasgow University Hospital Division of NHS Greater Glasgow and Clyde, and met all requirements of the Declaration of Helsinki. Details of cell lines, drugs and DNA constructs are provided in the Supplemental Experimental procedures.
Cell transduction and transfection
CD34+ cells were transduced with lentivirus vectors expressing SIRT1 shRNA or p53 shRNA. TF-1/BCR-ABL (TF-1/BA) cells were transduced with PITA-SIRT1-R, PITA-SIRT1-WT and vector controls (PITA). Details of the transduction procedure are provided in the supplemental experimental procedures.
Intracellular staining for SIRT1
CD34+ cells were labeled with antibodies to CD34-PeCy7, Lin-APC-Cy7 (including CD2, CD7, CD10, CD11B, CD19, CD33 and CD235a) and CD38-APC (e-Bioscience), followed by fixation and permeabilization (Cytofix/Cytoperm Kit, Beckman Coulter, Fullerton, CA). Cells were then labeled with rabbit anti-human SIRT1 (Epitomics) followed by Alexa 488-conjugated goat anti-rabbit antibodies (Molecular Probes) and analyzed by flow cytometry. Data were analyzed using FlowJo software (version 8.5.2; TreeStar, Ashland, OR). For immunofluorescence analysis, cells were labeled with anti-p53-FITC (DO-7, BD) and anti-acetylated p53-K382-Alex647 (BD), as described in the Supplemental Experimental Methods.
Analysis of proliferation, apoptosis and colony growth
CD34+ cells were labeled with CFSE (Molecular Probes, Eugene, OR), labeled with CD34-PE-Cy7 and CD38-APC, and CD34+CD38− and CD34+CD38+ cells with uniform CFSE labeling selected by flow-cytometry (MoFlo; Cytomation, Fort Collins, CO). Cells were cultured with low concentrations of growth factors at 37°C for up to 72 hours in normoxic conditions (21% O2). For specific experiments cells were cultured hypoxic conditions (5% O2) as specifically indicated in the results. Cells were analyzed by flow cytometry for apoptosis by Annexin V labeling and for proliferation by reduction in CFSE labeling. Committed progenitors or colony forming cells (CFC) were evaluated in methylcellulose progenitor assays as previously described. Details are provided in Supplemental Experimental Methods.
Engraftment of human cells in immunodeficient mice
CML CD34+ cells (1× 106 cells/mouse) or CB CD34+ cells (1 × 105 cells/mouse) were cultured for 72 hr with TV-6 (1μM), IM (2.5μM), the combination or without drug (control), and transplanted via tail vein injection into sublethally irradiated (300 cGy) 8-week-old NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ mice (NSG mice, Jackson Laboratory, Bar Harbor, ME). Mice were euthanized after 4 or 12 weeks, and marrow contents of femurs, spleen cells, and blood cells obtained. For CML BC samples, MNC depleted of CD3+ cells were transplanted via tail vein injection (5 × 106 cells/mouse). Blood samples were obtained 4 weeks after transplantation to confirm human CD45+ cells engraftment. Mice were treated with TV-6 (50 mg/kg intraperitoneally daily for 21 days) (Lain et al., 2008) or vehicle (control)) for 3 weeks, euthanized, and marrow and spleen cells were obtained and analyzed as described in Supplemental Experimental Methods. Mouse care and experimental procedures were performed in accordance with established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee at COHNMC.
In Vivo Treatment of Transgenic BCR-ABL Mice
These experiments were performed using inducible, transgenic GFP-Scl-tTa-BCR-ABL mice in the FVB/N background crossed with transgenic GFP-expressing mice (FVB.Cg-Tg [ACTB-EGFP] B5Nagy/J, Jackson Laboratories) (Zhang et al., 2010). Mice were treated with IM (200 mg/kg daily by gavage for 21 days), TV-6 (50 mg/kg body weight intraperitoneally daily for 21 days), the combination, or vehicle alone (control). After 3 weeks of treatment, animals were euthanized, and marrow and spleen cells obtained. The number of total nucleated cells, GFP-expressing cells, and GFP+ myeloid, progenitor, and stem cell populations were measured by flow cytometry. The effect of drug administration on apoptosis and cycling of stem cells in vivo was evaluated. Another subset of mice was followed after discontinuation of treatment, and survival was monitored for 85 days, PB counts were monitored for 28 days. Details are provided in the Supplemental Experimental Procedures.
Luciferase reporter assays
K562 or 293 cells were transfected with reporter and internal control (β-gal or Renilla-CMV) plasmids. Luciferase assays were performed after 48 hours in triplicate using the luciferase reporter assay system (Promega).
Real-time Q-PCR analysis
Q-PCR analysis performed with primers and probes for p21, Bax, Puma, Noxa, Necdin, Gfi-1, and Sirt1, p210Bcr-Abl transcripts were measured using a real-time TaqMan assay as previously described (Chu et al., 2011). Details are provided in the Supplemental Experimental Procedures.
Western blotting
Western blotting was performed for p53, acetylated p53, phospho-p53, SIRT1, CrkL, phopho-CrkL, SIRT1, Bax, ABL, tubulin and actin. Details are provided in the Supplemental Experimental Procedures.
Statistics
Data from independent experiments were reported as the mean ± SEM. Student’s t test analysis was performed to determine statistical significance.
Supplementary Material
Significance.
BCR-ABL kinase inhibitors (TKI) are effective in the treatment of CML but do not eliminate leukemia stem cells (LSC), which remain a potential source of recurrence. The NAD-dependent deacetylase SIRT1 is reported to protect stem cells from stress, and functions as a tumor suppressor or tumor promoter depending on cellular context. Our studies show that SIRT1 is overexpressed in CML LSC and that SIRT1 inhibition selectively reduces CML LSC survival and growth through acetylation and activation of the p53 tumor suppressor. These results are important because they show that SIRT1-mediated p53 deacetylation contributes to CML LSC survival and resistance to TKI treatment. SIRT1 inhibition is an attractive approach to selectively target LSC that resist elimination by current treatments.
Acknowledgments
This work was supported by NIH grants R01 HL77847 and R01 CA95684, a research grant from the Samuel Waxman Cancer Research Foundation, and a Translational Research grant from the Leukemia and Lymphoma Society to Ravi Bhatia, and a V foundation translational grant to WenYong Chen and Ravi Bhatia. WYC is supported by NIH R01 CA143421. We acknowledge the excellent technical support of the COHNMC Analytical Cytometry, Synthetic Chemistry, and Cytogenetics cores, and the Animal Resources Center. We thank StemCyte for their generous gift of CB samples, and Allen Lin for collection of patient samples. We thank Dr. MS Dai (Oregon Health and Sciences University) for the generous gift of the pcDNA3-p53 and mdm2-luc (BP100) plasmids, and Dr. Wei Gu (Columbia University) for the generous gift of the PTRE2-hyg-p53 and pTRE2-hyg-p53-8KR plasmids.
Footnotes
Author contributions:
Ling Li: Designed and performed research, analyzed and interpreted data, wrote manuscript
Lisheng Wang: Designed and performed experiments, reviewed manuscript
Liang Li: Designed and performed experiments, reviewed manuscript
Zhiqiang Wang: Designed and performed experiments, reviewed manuscript
Yinwei Ho: Performed experiments, reviewed manuscript
Tinisha McDonald: Performed experiments, reviewed manuscript
Tessa Holyoake: Provided material, interpreted data, reviewed manuscript
Wenyong Chen: Designed study, analyzed and interpreted data, wrote manuscript
Ravi Bhatia: Designed study, analyzed and interpreted data, wrote manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bi S, Hughes T, Bungey J, Chase A, de Fabritiis P, Goldman JM. p53 in chronic myeloid leukemia cell lines. Leukemia. 1992;6:839–842. [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Wang DHX, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chu S, McDonald T, Lin A, Chakraborty S, Huang Q, Snyder DS, Bhatia R. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118:5565–5572. doi: 10.1182/blood-2010-12-327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24:7654–7668. doi: 10.1128/MCB.24.17.7654-7668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiring AM, Khorashad JS, Morley K, Deininger MW. Advances in the treatment of chronic myeloid leukemia. BMC Med. 2011;9:99. doi: 10.1186/1741-7015-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskus W, Pranpat M, Balasis M, Bali P, Estrella V, Kumaraswamy S, Rao R, Rocha K, Herger B, Lee F, et al. Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin Cancer Res. 2006;12:5869–5878. doi: 10.1158/1078-0432.CCR-06-0980. [DOI] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;4:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2:241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- Kamel C, Abrol M, Jardine K, He X, McBurney MW. SirT1 fails to affect p53-mediated biological functions. Aging cell. 2006;5:81–8. doi: 10.1111/j.1474-9726.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, McCarthy A, Appleyard V, Murray KE, Baker L, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13:454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- Narala SR, Allsopp RC, Wells TB, Zhang G, Prasad P, Coussens MJ, Rossi DJ, Weissman IL, Vaziri H. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell. 2008;19:1210–1219. doi: 10.1091/mbc.E07-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Chae HD, Wang RH, Shelley WC, Cooper S, Taylor T, Kim YJ, Deng CX, Yoder MC, Broxmeyer HE. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440–450. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LF, Mitrikeska E, Giannola D, Lui Y, Sun H, Bixby D, Malek SN, Donato NJ, Wang S, Talpaz M. p53 stabilization induces apoptosis in chronic myeloid leukemia blast crisis cells. Leukemia. 2011;25:761–769. doi: 10.1038/leu.2011.7. [DOI] [PubMed] [Google Scholar]
- Prives C, Manley JL. Why is p53 acetylated? Cell. 2001;107:815–818. doi: 10.1016/s0092-8674(01)00619-5. [DOI] [PubMed] [Google Scholar]
- Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84:2391–2411. [PubMed] [Google Scholar]
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSzscid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, Huber LJ. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, field J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Molecular Endocrinology. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R, Vignudelli T, Candini O, Intine RV, Pecorari L, Guerzoni C, Santilli G, Byrom MW, Goldoni S, Ford LP, et al. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003;3:145–160. doi: 10.1016/s1535-6108(03)00020-5. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, Snyder DS, Huettner CS, Shultz L, Holyoake T, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2001;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman V, Lenos K, Popowicz GM, Silberman I, Grossman T, Marine JC, Holak TA, Jochemsen AG, Haupt Y. c-Abl phosphorylates Hdmx and regulates its interaction with p53. J Biol Chem. 2009;284:4031–4039. doi: 10.1074/jbc.M809211200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.