Abstract
Background
Increased susceptibility for developing alcohol dependence (AD) may be related to structural and functional differences in brain circuits that influence social cognition and more specifically, theory of mind (ToM). Alcohol dependent individuals have a greater likelihood of having deficits in social skills and greater social alienation. These characteristics may be related to inherited differences in the neuroanatomical network that comprises the social brain.
Methods
Adolescent/young adult participants from multiplex AD families and controls (n = 16) were matched for gender, age, IQ, education, and handedness and administered the Eyes Task of Baron-Cohen during functional magnetic resonance imaging (fMRI).
Results
High-risk (HR) subjects showed significantly diminished blood oxygen level dependent (BOLD) response in comparison with low-risk control young adults in the right middle temporal gyrus (RMTG) and the left inferior frontal gyrus (LIFG), areas that have previously been implicated in ToM tasks.
Conclusions
Offspring from multiplex families for AD may manifest one aspect of their genetic susceptibility by having a diminished BOLD response in brain regions associated with performance of ToM tasks. These results suggest that those at risk for developing AD may have reduced ability to empathize with others’ state of mind, possibly resulting in diminished social skill.
Keywords: fMRI Eyes Task, Theory of Mind (ToM), Right Middle Temporal Gyrus (RMTG), High Risk Offspring, Alcohol Dependence
There is considerable evidence that social intelligence is independent of general intelligence and may be supported by a specific neural network that includes the orbitofrontal cortex, superior temporal gyrus, and amygdala (Brothers, 1990; Humphreys, 1984). A key aspect of social cognition is the ability to infer other people’s mental states, thoughts, and feelings based largely on reading of facial expression. This ability has been termed “theory of mind” (ToM). The temporal lobes of nonhuman primates contain a specific cell type known as “mirror neurons” which have the unique capacity to discharge during the execution of certain hand and mouth actions or with the mere observation of these behaviors in another individual (Gallese and Goldman, 1998). These mirror neurons have been identified in humans using transcranial magnetic stimulation through observations that goal-directed hand movement of another person results in enhanced motor evoked potentials in muscles the observer would use himself to carry out such an action (Fadiga et al., 1995). Williams et al. (2001) have suggested that this ability to imitate the actions of others could have evolved into the ability to simulate mental states of other individuals (ToM).
Tests have been developed to operationalize this trait including “Reading the Mind in the Eyes test” (Baron-Cohen et al., 1994, 1999a). This task consists of presentations of a set of eyes for which the subject is alternatively asked to determine the person’s gender or emotional state. Using this task, specific brain regions have been shown to be activated in control subjects, most notably the medial and frontal gyrus, the inferior frontal gyrus and both middle and superior temporal gyrus (Baron-Cohen et al., 1999a). In clinical populations, reduced activation of one or more of these regions has been reported. Schizophrenic patients have been reported to show reduced activation relative to controls in the left medial temporal gyrus (LMTG) (Johnston et al., 2005). Also, the right fusiform gyrus, middle occipital gyrus, and the right amygdala have been reported to show reduced activation in schizophrenic patients (Johnston et al., 2005) along with reduced activation in the left middle frontal gyrus (LMFG) and in the left inferior frontal gyrus (LIFG) (Russell et al., 2000). In comparison with controls, parents of children with Asperger syndrome show less activation in LMTG, LIFG, and right inferior frontal gyrus (RIFG) (Baron-Cohen et al., 2006).
The capacity for emotional learning has been shown to have important implications for the development of a number of clinical conditions including autism (Baron-Cohen et al., 1999a), Asperger’s Syndrome (Baron-Cohen et al., 2006), and schizophrenia (Johnston et al., 2005; Russell et al., 2000). There is evidence that emotional learning may also be deficient in patients with pediatric bipolar illness (McClure et al., 2005). To our knowledge, emotional learning has not been studied in offspring at high risk for developing alcohol dependence (AD).
Previously, we reported finding reduced volume of the right amygdala in high-risk (HR) for AD minor offspring from multiplex AD families (Hill et al., 2001). We view these findings as intriguing in view of current knowledge concerning the role of the amygdala in emotional learning and capacity for making accurate social appraisals. To the extent that the amygdala is necessary in the retrieval of information on the basis of prior social experience with particular emotional expressions (e.g., fear, anger), those with overt damage (e.g., bilateral amygdalar damage) are clearly disadvantaged (Adolphs et al., 1994) and perform more poorly on ToM tasks (Stone et al., 2003). Whether those with more covert alteration (reduced amygdalar volume) are disadvantaged is open to question. With the amygdala serving an important role in tasks involving facial expression, we have speculated (Hill et al., 2001) that individuals with reduced amygdala volume may find it difficult to maintain friendships because of misunderstandings arising from misreading of visual social cues. Clinical characteristics of alcohol dependent persons suggest difficulties in emotional learning. It has been known for some time now that alcoholics show poorer social skills than nonalcoholics (Van Hasselt et al., 1978). Alcohol dependent individuals show impaired performance on emotional facial expression tasks which may be seen as long as 3 months after achieving abstinence (Foisy et al., 2007).
We hypothesize that judgment of facial expression is a neurobiologically based trait and that deficits observed in alcohol dependent individuals may have preceded the onset of AD. This hypothesis is based in part on the observation that adult alcoholics and their HR nonalcoholic adult relatives report higher levels of Alienation than do controls (Hill et al., 1990; McGue et al., 1997). Persons scoring high on this trait, as measured by the Multidimensional Personality Questionnaire (Tellegen, 1985; Tellegen et al., 1988), report believing they are mistreated, that others wish them harm, and often feel betrayed and used by “friends.”
Because there have been no functional magnetic resonance imaging (fMRI) studies of offspring from alcohol dependent families to test for emotion recognition, we viewed this as an important goal. We chose the “Reading the Mind in the Eyes task” to measure fMRI blood oxygen level dependent (BOLD) response to stimuli varying in emotional content. As this was an exploratory study of Baron-Cohen and colleagues’ ToM test, it was expected that differences in BOLD activation in regions previously identified by these investigators as showing enhanced activation in response to processing of the Eyes Task might be seen. These regions primarily include medial prefrontal cortex, the temporal lobe, particularly in middle and superior regions, and in the left inferior frontal cortex, though one study noted amygdala activation (Baron-Cohen et al., 1999a). Moreover, testing for possible differences in emotional learning in individuals from multiplex AD families can be especially informative because the recurrence risk across generations appears to be much higher than that seen in the general population. The present sample of individuals, who were in late adolescence or young adulthood, were mostly without alcohol or drug dependence at the time the fMRI scan was completed, though 1 control and 2 HR offspring did carry a lifetime diagnosis.
METHODS
Participants
Participants included 8 individuals with multiplex family history of AD (4 female and 4 male) and 8 normal controls without a personal or family history (at least first-degree relatives) of AD or other psychiatric illness (4 female and 4 male). Twelve (8 HR and 4 low-risk (LR) controls) of the 16 individuals were part of an ongoing longitudinal study that has followed youngsters from childhood through young-adulthood. Therefore, extensive clinical information was available for determining if any had developed an alcohol use disorder or other substance use disorder (SUD) by young adulthood, when the fMRI assessment was performed. For the 12 individuals, who were part of the longitudinal follow up, family history of AD and other psychiatric disorders were obtained through either direct interview or multiple family history reports (minimum of 2). The 8 HR offspring from multiplex AD families had an average of 4 first- and second-degree relatives with AD. The 4 LR control offspring from the longitudinal follow-up had no first- or second-degree relatives with alcohol or drug dependence. The other 4 controls, who had participated in another fMRI study, had been administered the identical fMRI protocol. These controls were assessed as adolescent/young adults using the Structural Clinical Interview for DSM-IV (SCID) and found to be free of all Axis I diagnoses. In addition, family history information collected for these 4 control participants indicated that their first and second-degree relatives were free of Axis I diagnoses including SUDs. An attempt was made to match the groups for age, gender, IQ, education, and handedness. As may be seen in Table 1, the groups did not differ in these characteristics. Although the control subjects had somewhat higher scores on the Peabody Picture Vocabulary Test (PPVT), their educational attainment was well matched. Twelve of the 16 subjects were part of a longitudinal study that spanned childhood into young-adulthood. These individuals had been assessed yearly by parent and child reports using the Schedule for Affective Disorders and Schizophrenia (child version; K-SADS) (Chambers et al., 1985) to determine the presence or absence of Axis I DSM-III diagnoses (American Psychiatric Association, 1980). (DSM-III was the methodology in place at the time the study began in 1989.) All 8 HR participants had been assessed as young adults using the CIDI diagnostic instrument (Robins et al., 1988) to determine presence or absence of a DSM-IV Axis I diagnosis, (American Psychiatric Association, 1984), and as children using the K-SADS. The 8 LR controls included 4 participants who had been followed in the longitudinal study (3 of 4 had both child and young adult evaluations; 1 followed through childhood is about to enter the young adult follow-up) and 4 who had completed the Eyes task as part of another study. Clinical information for the latter 4 adolescent/young adults was available from administration of the SCID diagnostic instrument (Spitzer et al., 1992) and through collection of family history information concerning first and second-degree relatives.
Table 1.
Demographic Characteristics of High- and Low-Risk Young Adults
| High risk (n = 8)
|
Low risk (n = 8)
|
F | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Agea | 22.62 | 3.34 | 23.63 | 4.20 | 0.282 | NS |
| SESb | 45.62 | 7.69 | 49.06 | 7.45 | 0.825 | NS |
| Education | 14.38 | 1.41 | 14.00 | 2.2 | 0.41 | NS |
| IQd | 113.44 | 10.42 | 120.50 | 10.43 | 4.368 | NS |
| Right handedc | 87.5% | 87.5% | NS | |||
Four participants were adolescents ages 18 to 19; the remainder were young adults over 20 years of age.
Hollingshead Four Factor Index (Hollingshead, 1975). These values were based on the education and occupation of the participants’ parents. Because parental values were considered more appropriate, socioeconomic status (SES) based on the adolescent/young adults’ occupation and education was not calculated. Parental SES appeared more descriptive because most of the participants were in the process of continuing their education, and relying on part time and temporary jobs. These jobs appeared to be codable by Hollingshead criteria at a lower level (e.g., waiter) than would be expected, once they finished college or professional education.
One high-risk female was left handed; 1 low-risk male was ambidextrous.
IQ was determined by the administration of the PPVT.
As may be seen in Table 2, among the 12 participants seen during childhood, 2 met criteria for depression, 1 for Attention Deficit Hyperactivity Disorder (ADHD) and 1 for anxiety disorder. During young-adulthood, 3 participants met criteria for a SUD. One LR control met criteria for marihuana dependence and 2 HR participants met criteria for alcohol or drug dependence. Because these participants were followed from childhood through young adulthood, it was possible to determine the period of exposure. All 3 had become dependent after age 19.
Table 2.
Cases With a DSM-III Diagnosis by K-SADS and/or Young Adult DSM-IV CIDI Interviews
| Diagnosis | K-SADS evaluationa |
CIDI evaluationa |
Positive clinical evaluation at either time point
|
Low risk | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High risk
|
Low risk
|
High risk
|
Low risk
|
|||||||
| n | With diagnosis (n) | n | With diagnosis (n) | n | With diagnosis (n) | nc | With Diagnosis (n) | High risk | ||
| Alcohol or drug dependence | 8 | 0 | 4 | 0 | 8 | 2 | 7 | 1 | 2 | 1 |
| Anxiety disorders | 8 | 0 | 4 | 1 | 8 | 0 | 7 | 0 | 0 | 1 |
| Depression | 8 | 2 | 4 | 0 | 8 | 2 | 7 | 1 | 2 | 1 |
| ADHDb | 8 | 1 | 4 | 0 | 1 | 0 | ||||
| Oppositional/conduct disorderb | 8 | 0 | 4 | 0 | 0 | 0 | ||||
All K-SADS evaluations were completed prior to age 19 years, and all CIDI evaluations were completed at age 19 years or older.
These diagnoses are determined with the K-SADS evaluation for all youngsters between the ages of 8 to 18 years and are not diagnosed by CIDI evaluation.
Four low-risk controls were diagnosed as young adults only for participation in another study. A SCID interview was administered and participants found to be free of any Axis I disorder. The total n evaluated in young adulthood is 7 rather than 8 because 1 control had not reached the age of 19 years and begun young adult follow-up that includes CIDI administration.
At the time the subjects were scheduled for their visit, they were asked to refrain from using alcohol or psychoactive street drugs for 48 hours before the scan. As most of the subjects had participated in yearly longitudinal assessments since childhood, they were accustomed to being asked to refrain from such use 48 hours before their scheduled visit and generally were compliant in this regard. (Event-related potentials were part of the annual evaluation and might be influenced by drug or alcohol use.)
All participants signed informed consent documents after having the study explained to them. All subjects were screened by clinical staff and MRI Center staff to insure absence of ferromagnetic metal in or on their body. All subjects were administered a preprotocol checklist that covered drug and alcohol use in the past 48 hours, use of caffeinated beverages on the day of the fMRI procedure, time of the last meal, and number of hours of sleep the previous night. All female subjects were asked to provide information on use of birth control pills and the number of days since the onset of last menstrual cycle. Also, they were screened for the possibility of an early and unknown pregnancy using Icon® 25hCG pregnancy kits, a test with high sensitivity (25 mIU/ ml) and good accuracy (>99%), and determined to not be pregnant. (Human chorionic gonadotropin [hCG] can be detected in urine 7 to 10 days after conception, frequently exceeding 100 mIU/ml by the first missed menstrual period [2 to 4 days]).
Procedures
Acquisition Methods
All fMRI subjects were scanned on a GE 3.0 Tesla scanner (GE Medical Systems, Milwaukee, WI) located in the Department of Radiology MR Research Center. The fMRI procedure included 283 axial images collected over 26 slices using a T2* spiral sequence (echo time [TE] = 18 ms; repetition time [TR] = 1500 ms, flip angle = 70°, acquisition matrix = 64 × 64, field of view [FOV] = 20) at a slice thickness of 3.2 mm (0 gap). The fMRI scans are obtained following a scout series. Coronal and axial scouts are used to prescribe the midsagittal slice for defining the mid-line. Total brain volume was measured with T1 weighted coronal images through the anterior commissures (124 slices) using a 3-dimensional spoiled gradient recalled echo in the steady state (SPGR) pulse sequence (TE = 5 ms, TR = 25 ms, flip angle = 40°) obtained at a thickness of 1.5 mm, acquisition matrix = 256 × 192; FOV = 24 × 18. To provide co-registration, a structural series is then obtained in the axial plane with the same prescription as the functional data using 26 slices (TE = minimum full [MF]; TR = 350 ms, 2 acquisitions; FOV = 256 × 192, FOV = 20 cm).
Two tasks involving extracting visual information that is socially relevant, either gender or inferred mental state from photographs (24 faces of men and women) were presented following the method described by Baron-Cohen et al. (1999a). A blocked periodic ABA… design was used which was expected to induce maximal signal in regions specialized for mental state, or ToM recognition (Task B) using behavioral data for both Task A and B to evaluate accuracy of performance. The experiment alternated between conditions of Task A (gender) or Task B (emotion) and rest. Each epoch (A or B) was presented for 36 seconds with 6 stimuli, each presented for 5 seconds with a 1-second interstimulus interval. The rest epochs were 30 seconds. Each image was seen in a given condition (gender or emotion) only once. Subjects were instructed to indicate their selection for the alternative on the left or on the right by indicating his/her choice using a “glove” (right = second finger; left = third finger, respectively). For task A, the subject was instructed to decide for each stimulus which of 2 simultaneously presented words, “male” or “female”, best described the eyes presented. Response for the emotion condition was a 2-alternative forced choice (modal response versus a randomly picked alternative) based on the original Baron-Cohen et al. (1999a) norms. For this task, the subject was required to make a forced choice decision between 2 simultaneously presented words.
Statistical Analysis
BOLD fMRI analysis of the high risk (n = 8) versus low-risk comparison subjects (n = 8) were conducted at a preset threshold of uncorrected p=0.005 and with no extent threshold. An activation difference between the BOLD response to Task B (emotion task) and the rest epoch defined the individual subject contrast. These contrast images were then used to perform a between group analysis. Results were analyzed using SPM5 running on Matlab version 7.0.4. (The Mathworks, Inc., Natick, MA). Small volume correction analysis was applied to the regions showing overall uncorrected values of 0.001 or less and for which an a priori reason could be justified. This was achieved by creating masks using the Wake Forest Pick Atlas (Maldjian et al., 2003). The following regions met these criteria: right middle temporal gyrus (RMTG), right superior frontal gyrus (RSFG) and left inferior frontal gyrus (LIFG). This a priori justification was based on reports of significant BOLD activation in controls relative to clinical populations. Using a facial emotion recognition task, greater BOLD activation of the LMTG in controls relative to schizophrenic patients has been reported (Johnston et al., 2005). Also, greater activation of LMTG has been reported during the Eyes task in controls relative to first-degree relatives of Asperger patients (Baron-Cohen et al., 2006). Across all subjects, controls and schizophrenics, the RSFG showed greater activation during an emotion discrimination task than during a gender discrimination task (Johnston et al., 2005). A prominent activation in LIFG with a large cluster size (1356) has been reported for healthy control subjects in response to the facial emotion discrimination task (Johnston et al., 2005). Similarly, Baron-Cohen et al. (2006) report that 3 areas show greater bold activation in controls relative to parents of Asperger children, namely, left MTG and both right and left inferior frontal gyri.
RESULTS
Preprotocol Screen
Preprotocol screens were available for 12 participants. They were found to be mostly compliant with our request to refrain from use of alcohol or street drugs before the fMRI scan (1 participant had a beer 18 hours before performing the scan and 1 had smoked marihuana the day before). All but 1 had abstained from use of caffeinated beverages the day of the scan. Because there has been a report that caffeine induces decreases in cerebral perfusion that enhances BOLD response to visual and auditory stimuli (Laurienti et al. 2003), information on caffeine use had been recorded in this study. All but 1 had slept a minimum of 6 hours the night before the scan. Participants were scanned between the hours of 9 AM and 1 PM to minimize any diurnal effects on performance or activation. Of the 6 female participants, 3 were on birth control pills and 3 were not. All were in the days 4 to 14 of their menstrual cycle. None tested positive for pregnancy.
Behavioral Findings
Behavioral data showed that the groups had identical performance on the gender task (94% accuracy). For task B, the ToM procedure gave 86% and 90% performance accuracy for the HR and LR groups.
BOLD Response
Differences by group may be seen in Table 3. As may be seen, diminished BOLD response was seen in high-risk relative to control participants in the RMTG, RSFG, and the LIFG (uncorrected significance). Small volume correction analysis was performed for the 3 regions showing diminished BOLD response in the uncorrected analysis and for which there was a priori evidence to suggest ToM-associated activation. This analysis revealed significant reduction in BOLD response in the RMTG and a trend for reduction in the RSFG and the LIFG. Figure 1 depicts the control minus high-risk comparison. Reduced activations may be seen in HR subjects in left inferior frontal and right temporal regions.
Table 3.
Significant BOLD Response Differences Between High-Risk and Low-Risk Control Young Adults on the Eyes Task
| Region | Brodman area | Coordinates x, y, z | Volume (Voxels) | Overall statistical analysis (p-value uncorrected) | Small volume correction (p-value uncorrected) | Volume (Voxels) | Small volume correction (p-value FDR corrected) |
|---|---|---|---|---|---|---|---|
| Low-risk controls > high-risk offspring | |||||||
| Right middle temporal gyrus | 21 | 63, −36, −6 | 50 | 4.69, 0.000 | 4.69, 0.000 | 43 | 0.057 |
| Right superior frontal gyrus | 10 | 21, 57, 9 | 27 | 4.14, 0.001 | 4.14, 0.001 | 18 | 0.09 |
| Left inferior frontal gyrus | 46 | −48, 30, 9 | 23 | 3.88, 0.001 | 3.88, 0.001 | 23 | 0.10 |
| High-risk offspring > low-risk controls | |||||||
| None with voxels > 10; none significant | |||||||
Degrees of freedom = (1, 12); height threshold T = 3.05, p = 0.005; expected voxels per cluster = 19.66 (small volume correction); voxel size: 3.0, 3.0, 3.0 mm. FDR, false discovery rate.
Fig. 1.

An example of eyes stimuli used during task A. Stimuli were presented with the choice of words “male” or “female” and the participant asked to choose the correct gender of eyes portrayed. These stimuli are used to reference the activation seen in Task B which requires judgment of the emotional state of the person whose eyes are portrayed.
DISCUSSION
This pilot fMRI study suggests that adolescent /young adult individuals at high genetic risk for developing AD (members of a multiplex for AD families) may inherit deficits in social intelligence that are reflected in reduced BOLD response in brain regions previously associated with performance of ToM tasks. We found significant differences in the RMTG, RSFG, and in the LIFG, though only the RMTG survived the small volume correction and the correction for multiple comparisons. Because of the small sample used for this pilot investigation, it is unknown whether all 3 regions might prove to be significant if greater power were available. At any rate, these results showing reduced activation in areas previously associated with performance of ToM tasks suggest that HR offspring may experience deficiencies in emotion recognition that may make it more difficult for them to interpret the actions of others in terms of mental states, such as their thoughts or beliefs, to empathize with them, and to predict how others will react or feel. These deficits may have important implications for life satisfaction as it relates to successful social skill and absence of an alienated personality style.
Importantly, the reduction in activation seen in regions associated with performance of the ToM task occurred in individuals who showed equivalent IQ and educational attainment to the controls. Also, a comment is in order regarding the dissociation between the behavioral performance of the task and the brain response (BOLD activation) to the stimuli. Clearly the high and low-risk subjects performed equally well on the task administered. This further suggests that the HR individuals were processing emotional material differently than controls, though clearly unimpaired in their cognitive performance. This dissociation between cognitive performance and social intelligence has been addressed by Baron-Cohen et al. (1999a, 2001) noting that diminished social intelligence, including capacity for ToM tasks, can often be seen among individuals with higher levels of general intelligence such as mathematicians and scientists.
Also, dissociation can be expected because event related brain activity may be more sensitive to salient environmental cues than is overt behavioral performance or even overt cognitive appraisal of what one has seen. Some event-related brain activity may be outside an individual’s conscious awareness. The presence of “mirror” neurons in primates, including humans, appears to make it possible for an observer to respond to the actions of others through the imitation of the behavior, sometimes without conscious awareness of a specific intent. This illustrates the sophisticated nature of neurons specialized for comprehending social interaction. Also, individuals may discriminate between stimuli on the basis of their acquired significance. This acquisition can be beyond the subjects’ conscious awareness, though clearly registered by specific brain regions.
In a novel study designed to test this notion, Morris (1998) conducted a PET study in which activity in the right and left amygdala were monitored in response to angry faces. In this experiment, characteristics of the stimulus (both were angry faces) were not responsible for the observed activation, but rather activation was based on the associative history of the stimulus. To demonstrate this, stimuli were masked or unmasked by presentation of a neutral face that was either a target (CS+ = pairing with unconditioned stimulus [UCS] of 100 dB white noise) or nontarget (CS− = no pairing with UCS). Following conditioning, the CS+ and CS− faces were presented sequentially in either a masked (seen 0% of the time) or unmasked (seen 100% of the time) condition. Activation in the right amygdala occurred even when subjects could not state what they had seen. Specifically, a significant response in the region of the right amygdala was seen to the presentation of the masked CS+ faces with no response seen in the left amygdala to masked CS+ stimuli. In contrast, there was a left sided activation by unmasked CS+ and CS− faces. These results illustrate the dissociation between the conscious report of what one sees and what the brain “sees” (Fig. 2).
Fig. 2.

An example of eyes stimuli used in task B. Participants were presented with a choice of mental state words that best described the mental state of the person whose eyes are depicted. The correct response in the upper figure is “worried” and “fantasizing” in the lower figure. Brain regions typically activated by the theory of mind task include middle temporal gyrus, superior frontal gyrus, and inferior frontal gyrus (see Baron-Cohen et al., 1999a, 2006).
In the present study, it appears that the control subjects not only correctly inferred the mental state of the eyes they were asked to judge, but also the mental state of the eyes invoked a greater emotional response in them. This response in turn generated greater activation in regions specialized for emotional appraisal. The HR subjects also correctly inferred the mental state of the eyes they were asked to judge, but showed reduced activation in regions previously shown to be activated in ToM paradigms (Fig. 3).
Fig. 3.
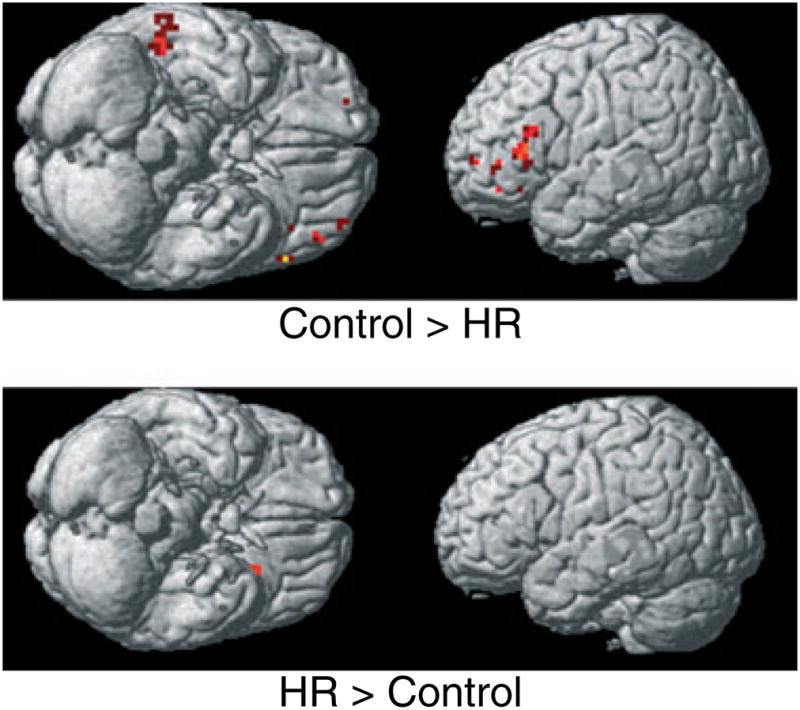
Blood oxygen level dependent functional magnetic resonance (BOLD) imaging analysis of high-risk for alcohol dependence participants from multiplex families (n = 8) versus low-risk control comparison subjects (n = 8) conducted at a preset threshold of uncorrected p = 0.005 and no extent threshold. Note reduced activations in high-risk subjects in the right middle temporal gyrus, left middle frontal and left inferior frontal gyri.
It has been suggested that ToM evolved as an adaptive response to increasingly complex primate social interaction, but at some cost to those without this ability (Brune and Brune-Cohrs, 2006). Specifically, evidence is reviewed showing that ToM task performance may be impaired in a broad range of psychopathological conditions. This deficit in social perception has been noted in autism, Asperger syndrome, schizophrenia, and pediatric bipolar disorder. While it has been often suggested that deficiencies in ToM appraisal may underlie the callousness and diminished capacity for remorse seen in antisocial personality disorder, direct test of this hypothesis using the Reading the Mind in Eyes’ ToM Test failed to reveal a connection (Richell et al., 2003). It appears that deficits in ToM are not characteristic of all psychiatric conditions, though quite notable in some.
Commonalities in response on the Eyes’ task in Asperger Syndrome, schizophrenia, and autism may be expected based on recognition that individuals suffering from these disorders have marked problems in social interaction and find such interaction unrewarding. In contrast, alcohol dependent individuals are thought of as highly social, sometimes described as the “life of the party”. How might we resolve this seeming incongruity? Possibly the sober AD individual is highly asocial, finding use of alcohol necessary to become prosocial. Moreover, one who does not read others well will not easily make friends, and may well carry grudges toward those he/she misunderstands. This may then lead to attributions measured on the Alienation scale of the MPQ that suggest that AD individuals believe that “People cannot be trusted”, and that “Good things happen because of luck and not by negotiation with one’s social environment”. Moreover, intoxication may provide the excuse for lashing out at those who have “wronged” them.
Structural differences have been observed in this sample of offspring from multiplex for AD families. In addition to smaller volume of the amygdala in the right hemisphere (Hill et al., 2001), we have also reported age-related differences in grey matter volume of the cerebellum (Hill et al., 2007). Differences between the HR participants and controls in BOLD activation in the amygdala were not seen in the present study. There are 2 reasons this may have occurred. First, our sample size was relatively small. Also, previous studies using ToM tasks have reported amygdalar activation in a relatively small number (n=9) of voxels (Baron-Cohen et al., 1999a). Also, while the Eyes’ task has been reported to elicit a BOLD signal in the cerebellum of autistic children and in the amygdala of normal controls (Baron-Cohen et al., 1999a), reviews of many investigations using ToM tasks (Brune and Brune-Cohrs, 2006; Frith and Frith, 1999) have emphasized the prominence of the medial prefrontal cortex, the temporal lobe, particularly in middle and superior regions, and in the left inferior frontal cortex, without mention of BOLD activation of amygdala. Moreover, other tasks that utilize presentation of angry faces appear to be much more evocative of amygdalar activation (Hariri et al., 2002, 2003). As our goal was to investigate ToM and not to test amygdala activation per se, the results found in this pilot study cannot be thought of testing our previous observations concerning structural differences in the amygdala in HR offspring. Similarly, activation of the cerebellum could not be analyzed in the present study because only a small portion of cerebellum was included in the field of view. Future studies will be directed at both of these inquiries using tests that are more appropriate for activation of these regions.
In summary, the present results suggest a neurobiological basis for diminished social skills in alcohol dependent individuals. Brain regions previously identified as showing diminished activation in clinical populations who were administered ToM tests also appear to show diminished activation in young adult offspring from multiplex AD families. Because the participants in this study were in late adolescence or young adulthood at the time the fMRIs were obtained, it cannot be stated unequivocally that the risk group differences observed were the result of genetically mediated differences in brain regions specialized for social interaction as some social learning would be expected to have occurred. Nevertheless, recognition that HR offspring may have this disability suggests the need for cognitive interventions designed to ameliorate deficits in social intelligence. This strategy may hold promise for children from HR families in preventing perpetuation of AD across generations.
Acknowledgments
We would like to express our appreciation to the research participants and their families who have so generously given their time, many over the past 15 years, in support of our longitudinal research program. We would also like to thank Jeffrey Nutche for help training technicians, Debra Montrose Ph.D for providing information on the control participants from the Essence study, and Vaibhav Diwadkar, Ph.D. for providing the paradigm for use in this study. Finally, this manuscript is dedicated to the memory of a good friend and colleague, the late Professor Gerard Hogarty (former PI of the Essence Study—MH 60902), with whom many conversations took place regarding ToM and its possible application to addiction. Supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA05909 and AA08082 (SYH) andMH 60902 (MSK).
References
- Adolphs R, Tranel D, Damasio H, Damasio AR. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, D.C.: 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Baron-Cohen S, Ring H, Chitnis X, Wheelwright S, Gregory L, Williams S, Brammer M, Bullmore E. fMRI of parents of children with Asperger Syndrome: A pilot study. Brain Cogn. 2006;61:122–130. doi: 10.1016/j.bandc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Shmitz P, Costa D, Ell P. Recognition of mental state terms: a clinical study of autism, and a functional neuroimaging study of normal adults. Br J Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism spectrum quotient (AQ): evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autistic brain: An fMRI study. Eur J Neurosci. 1999a;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Stone V, et al. A mathematician, a physicist, and a computer scientist with Asperger syndrome: performance on folk psychology and folk physics test. Neurocase. 1999b;5:475–483. [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neuorsci. 1990;1:27–51. [Google Scholar]
- Brune M, Brune-Cohrs U. Theory of mind-evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev. 2006;30:437–455. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini P, Tabrizi MA, Davies M. Assessments of affective disorders in children and adolescents by semi-structured interview: Test-retest reliability of the K-SADS-P. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: A magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Foisy M-L, Kornreich C, Fobe A, D”Hondt L, Pelc I, Hanak C, Verbanck P, Phillippot P. Impaired emotional facial expression recognition in alcohol dependence: do these deficits persist in abstinence. Alcohol Clin Exp Res. 2007;31:404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—A biological basis. Science. 1999;286:1692–1694. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cog Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hill SY, DeBellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent/young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Steinhauer S, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Zubin J, Steinhauer SR. Personality resemblance in relatives of male alcoholics: A comparison with families of male control cases. Biol Psychiatry. 1990;27:1305–1322. doi: 10.1016/0006-3223(90)90501-r. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- Humphreys N. The social function of the intellect. In: Humphrey N, editor. Consciousness Regained. Oxford University Press; Oxford: 1984. pp. 14–28. [Google Scholar]
- Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Relationship between caffeine-induced changes in resting cerebral perfusion and blood oxygenation level-dependent signal. Am J Neuroradiol. 2003;24:1607–1611. [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchectonic atlas based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use diosrders: I. Effects of gender and alcoholism subtype. Alcohol Clin Exp Res. 1997;21:513–520. [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Richell RA, Mitchell DGV, Newman C, Leonard A, Baron-Cohen S, Blair RJR. Theory of mind and psychopathy: can psychopathic individuals read the “language of the eyes”? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle LH. The composite international diagnostic interview. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SCR, Sharma T. Exploring the social brain in schizophrenia: Left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157:2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia. 2003;41:209–220. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Multidimensional Personality Questionnaire. 1985. unpublished manual. [Google Scholar]
- Tellegen A, Lykken DT, Bouchyard TJ, Jr, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Van Hasselt VB, Hersen M, Milliones J. Social skills training for alcoholics and drug addicts: A review. Addict Behav. 1978;3:221–233. doi: 10.1016/0306-4603(78)90023-0. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]


