Abstract
Studies consistently report that groups of individuals with major depressive disorder (MDD) demonstrate increased levels of a variety of peripheral inflammatory biomarkers when compared with groups of nondepressed individuals. These findings are often interpreted as meaning that MDD, even in medically healthy individuals, may be an inflammatory condition. In this article, we examine evidence for and against this idea by looking more closely into what the actual patterns of inflammatory findings indicate in terms of the relationship between MDD and the immune system. Data are presented in support of the idea that inflammation only contributes to depression in a subset of patients versus the possibility that the depressogenic effect of inflammatory activation is more widespread and varies depending on the degree of vulnerability any given individual evinces in interconnected physiologic systems known to be implicated in the etiology of MDD. Finally, the treatment implications of these various possibilities are discussed.
Keywords: Major depression, Fatigue, Immune, Inflammation, Cytokines, Interleukin-6, Tumor necrosis factor-α, p38 mitogen-activated kinase, Psychosocial stress, Glucocorticoids, Autonomic nervous system, Tryptophan, Kynurenine, Quinolinic acid
Introduction
Ten years ago, the idea that inflammatory processes might contribute to the development of major depressive disorder (MDD) was in itself so novel that it could (and did) support entire reviews. In contrast, a current Medline search revealed 37 review articles published since January 2009 that were specifically related to associations between depression, or the treatment of depression, and immune inflammatory factors [1–4].
Against such a burgeoning backdrop, another review linking depression to inflammation is hardly warranted. Rather, what seems to us to be worth doing, as an unmet need in the extant literature, is to address several questions of obvious scientific and clinical relevance that to our knowledge have not been adequately addressed in relation to inflammation and depression. The first question, which we believe to be deceptive in its apparent simplicity, comes from the title of this article: “Is depression an inflammatory disorder?” The second question is related to this and centers on whether inflammatory processes contribute to a majority or only a subset of depressive presentations. A final question is this: “Do the answers to questions 1 and 2 say anything important about potential treatment implications of the connection between inflammation and depression?”
Is Depression an Inflammatory Disorder?
By way of a thought experiment, suppose you are a rheumatologist seeing a middle-aged patient who complains of a painful right knee. To help diagnose the condition, you aspirate synovial fluid from the offending knee and submit it for analysis. Suspecting rheumatoid arthritis (RA), you are surprised when the results come back completely normal in terms of inflammatory markers in the synovial fluid. Had inflammatory measures been high, that would not have confirmed RA because other autoimmune and infectious causes for arthritis are also characterized by increased synovial inflammation. However, knowing that concentrations of an inflammatory cytokine such as interleukin (IL)-6 are typically 1,000 times higher in the synovial fluid of an affected RA joint than in the blood of a healthy adult, what would be your conclusion regarding the likelihood of RA in this patient? Most likely it would be: no joint inflammation, no RA. That is more or less what we mean when we say that RA is an inflammatory disorder.
Now compare this to an analogous clinical situation, but this time, you are a psychiatrist seeing a middle-aged patient complaining of severe depression. Hearing that MDD is an inflammatory condition, you measure plasma concentrations of inflammatory cytokines and the acute-phase reactant C-reactive protein (CRP). Believing MDD to be a brain disease, you also go beyond the call of duty and perform a lumbar puncture in your office to measure cerebrospinal fluid concentrations of the same inflammatory markers. A few days later, the patient returns, and you hold the laboratory results in your hands. There is no evidence of increased inflammation in the central nervous system or periphery. The patient is weeping and says he can think of nothing but killing himself. Would you decide that because the patient's inflammatory measures are normal that he cannot be depressed?
Based on these scenarios, we think the answer to the question of whether depression is an inflammatory disorder is a resounding “no.” Even with a nod of recognition toward the fact that all disease processes have an inherent “sloppiness” that precludes absolute one-to-one correspondences between putative causes and observed symptomatic outcomes, it is clear that inflammation is neither necessary nor sufficient to cause MDD. Desperately depressed people often have low levels of systemic inflammation, and individuals with ragingly high inflammatory activity are often—but less commonly—able to retain a good mood and hopeful stance toward their lives.
Do Inflammatory Processes Contribute to a Majority or Only a Subset of Depressive Presentations?
If depression is not an inflammatory disorder in the sense that RA and other autoimmune conditions are inflammatory disorders, how should we best understand the growing database (which now includes three meta-analyses [5–7]) indicating that depression is associated with increased inflammation? Three points are most important here.
First, what studies actually report is that on average, the mean value for an array of inflammatory mediators tends to be higher in depressed groups than in groups of nondepressed individuals. In many studies, this increase in the mean is independent of other factors associated with increased inflammation, such as body mass index and sex. In other studies, the difference between depressed and nondepressed groups goes away when these types of factors (many of which are overrepresented in depression) are taken into account [2].
Second, the elevations in proinflammatory cytokines and other inflammatory elements observed in groups of depressed individuals are far more modest than increases typically observed in autoimmune or infectious diseases, with mean values in depression typically not exceeding two to three times the values found in healthy control groups [5]. Consistent with these modest differences, mean values for inflammatory markers in groups of depressed individuals are typically in the “normal” range when such norms have been established [8•, 9–13], as is the case with CRP [14].
When put this way, the connection between depression and increased inflammation seems underwhelming, and one might be tempted to dismiss it as physiologically irrelevant. However, this would be a mistake of the first order, and a profound misunderstanding of the huge effect that small physiologic differences can have over time if they are consistently skewed in one direction. As it turns out, depression is far from alone in being a condition characterized by reliable—but often only mildly increased— inflammatory activity. Other modern illnesses with evidence of moderately increased inflammatory signaling include cardiovascular disease, stroke, cancer, diabetes, and dementia. Conversely, even minor increases in inflammation—such as are observed in depression—are enough to strongly predict the development over time of many of these modern disease states [15–20].
Finally, we come to a third point that is vital for understanding the nature of the association between inflammation and depression: the values for any given inflammatory marker always overlap between groups of depressed and nondepressed individuals, regardless of how much higher the marker's mean value may be in the depressed group. Thus, it is not unheard of for the highest value in any particular study to be in the nondepressed group and the lowest value to be in the depressed group. More importantly, it also means that a large proportion of the depressed group in any given study will have values similar to those in the nondepressed group, which—at least on first blush—makes it hard to envision these people as suffering from a disease state caused by increased inflammation. On the other hand, even though values tend to be fairly evenly distributed across the whole range in both depressed and nondepressed populations, typically about one third or so of the depressed group have values that are clearly higher than the majority of nondepressed comparison individuals. These are the people responsible for the finding that depression is associated with increased inflammation. It is a dirty little secret of sorts that they have been pulling all their noninflamed depressed colleagues along with them in publication after publication, giving the world a slightly misguided sense that depression—as a whole—is driven by increased inflammation.
Evidence Consistent With Increased Inflammation as a Depressive Subtype
How should we conceptualize this situation? On the one hand, the association between depression and increased inflammation can be conceived of as being probabilistic, such that any individual with depression has such-and-such an increased risk of having elevated inflammatory markers compared with someone of the same age and sex without depression. However, this way of thinking—while perfectly legitimate—seems somehow inadequate to the actual situation on the ground, in which some depressed people have increased measures of inflammation and others do not. From this perspective, it seems more logical to wonder if individuals with increased inflammation represent a biologically pertinent subtype of depression in which immune processes are especially relevant to disease development.
For this “inflammatory subtype” to be ontologically valid, inflammatory mediators—singly or in combination— must be shown capable of inducing depressive symptoms, and at the concentrations seen in actual depressed patients. That inflammatory activation reliably produces a depressive syndrome with strong homology to idiopathic MDD has been shown in many studies using treatment with interferon (IFN)-α as a source of chronic cytokine exposure [21, 22]. The power of IFN-α to induce psychic misery in patients taking it for hepatitis C virus infection or cancer is not subtle, as any clinician who has treated these patients can attest. At high doses, fully 50% of patients without pretreatment depression will meet criteria for MDD within 3 months of commencing therapy [23], and a far higher percentage—perhaps 90% or more—will endorse at least one or two significant depressive symptoms (with fatigue being the most common) [24]. Consistent with the centrality of inflammation in this model system, in separate studies, IFN-α–induced increases in IL-6 and tumor necrosis factor (TNF)-α signaling have been shown to correlate with increases in depressive symptoms [25, 26].
Treatment with IFN-α has the unique advantage of offering a human model of inflammation that is chronic in nature, which is important because the physiologic effect of cytokines on systems central to depression (eg, monoamine neurotransmitters and the hypothalamic-pituitary-adrenal [HPA] axis) changes profoundly as an inflammatory stimulus transitions from acute to chronic [27–29]. On the other hand, a distinct limitation of IFN-α therapy is that when used for therapeutic purposes, the cytokine is administered at supraphysiologic doses that may or may not yield effects relevant to the far lower levels of inflammatory activation seen in medically healthy individuals with MDD. Given the conceivable validity of this concern, it is remarkable that far more modest short-term immune stimuli (eg, low-dose lipopolysaccharide, typhoid vaccine) have been repeatedly observed to yield the emotional symptoms of depression/anxiety, even while being insufficient to induce the symptoms of sickness [30–32, 33•]. These studies also provide convergent validity for the use of IFN-α as a model system for cytokine-induced depression, given that the modest immune stimuli they utilize yield changes in brain functional activity similar to those seen after chronic treatment with IFN-α [33•, 34, 35•, 36–38].
Two additional lines of evidence lend credence to the possibility that the increased levels of inflammatory biomarkers seen in some patients with MDD might contribute to disease pathophysiology in this depressive subgroup. First, recent studies have reported that even mildly elevated levels of CRP and IL-6 independently predict the subsequent development of depression over a decade or more, even in individuals with no current or past history of depression at the time of the immune assay [8•, 39]. In contrast, baseline levels of depression did not predict an increase in inflammatory biomarkers at follow-up [39], suggesting that the frequently observed association between inflammation and depression may be better explained by a causal arrow running from inflammation to depression than the reverse. A final line of evidence supporting the relevance of inflammation for depressive pathogenesis is more circumstantial but—we believe— fairly impressive when considered in toto. If inflammatory processes contribute to disease pathogenesis, one would predict that known risk factors for MDD also should be associated with increased levels of inflammatory biomarkers. In this regard, we cannot identify a single significant risk factor for MDD, ranging from psychosocial stress and medical illness to obesity, diet, poverty, and sleep loss, that has not been repeatedly associated with increased inflammation [2, 40–49]. That a single physiologic process—inflammation—links so divergent an assemblage of elements strongly suggests that it might be a shared pathway by which these factors confer risk. More direct evidence for this possibility comes from a study by Capuron and colleagues [50], who observed that the association between the metabolic syndrome and increased depression was partially accounted for by increased plasma concentrations of IL-6 and CRP.
Although the data presented in this section are certainly consistent with the possibility that inflammatory processes only contribute in a significant way to the pathogenesis of MDD in the subset of patients with evidence of increased inflammation, nothing we have presented directly confirms this hypothesis. Such confirmation would require a demonstration that only depressed individuals with increased activity in inflammatory pathways (however operationalized) showed a reduction in symptoms when these pathways were blocked. Even more convincing would be the demonstration of a linear relationship between pretreatment levels of inflammatory biomarkers and symptomatic response to an anti-inflammatory intervention, such as administration of a cytokine antagonist. To our knowledge, only one study has reported such data. Persoons et al. [51] found that individuals with Crohn's disease and MDD who experienced a remission in their depressive symptoms following an infusion of the TNF-α antagonist infliximab had higher pretreatment plasma concentrations of CRP than did individuals with Crohn's disease and MDD who failed to achieve remission. Whether such a relationship between increased inflammation and clinical response to cytokine antagonism will turn out to be relevant to medically healthy individuals with MDD (who typically have far lower levels of markers such as CRP) is an issue upon which the validity and usefulness of the concept of an “inflammatory depressive subtype” will ultimately rest.
Evidence Consistent with a More Inclusive Role for Inflammation in the Pathogenesis of Major Depressive Disorder
Thus far, we have discussed inflammatory pathways as if they exist in functional isolation from other physiologic systems in the body that have been implicated in the pathogenesis of MDD. In fact, nothing could be further from the truth. Increasing data indicate that inflammation may be relevant to MDD precisely because inflammatory pathways interact so extensively with every other system in the brain and body believed to be involved in depressive pathophysiology [52–54, 55•]. Viewing inflammation from this larger perspective immediately invites us to envision a more complex scenario for the relationship between inflammatory processes and the pathogenesis of MDD, one that assigns a far grander role to immune processes in the overall disease state than is allowed by any type of inflammatory subtype model. From this larger perspective, it is apparent that as a result of response differences in physiologic pathways interconnected with the immune system, individuals might vary widely in their sensitivity to the behavioral effects of inflammatory signaling. Put differently, some individuals’ overall physiology might protect them from developing depression in response to all but the highest levels of inflammatory stimulation, whereas the physiology of others might make them vulnerable to developing depressive symptoms in response to even low levels of inflammatory stimulation. Note that from this perspective, inflammation plays a role similar to the one ascribed to environmental stress in many currently popular gene–environment interaction models of depressive vulnerability [56]. In fact, allelic variants such as the short form of the serotonin transporter that have been repeatedly implicated in gene–environment interactions may also confer vulnerability to depression in response to inflammation—which, after all, can be conceived of as a type of environmental stressor [57].
Two approaches may be employed to test the theory that even low levels of inflammatory activation may be depressogenic in individuals with vulnerabilities in other segments of the larger physiologic “super-network” that evolved to respond to danger, a theory we might label as immune response element amplification (IREA). A first approach might involve identifying people with resilient versus vulnerable activity patterns in various nonimmune segments of this danger-related super-network, and then demonstrating that those with vulnerable activity patterns become depressed in response to lower levels of induced inflammatory activity than those with resilient activity patterns. Any number of vulnerabilities might be selected for this purpose, including—but not limited to—insensitivity to glucocorticoid inhibitory feedback [58], reduced parasympathetic signaling [59], reduced production of brain-derived neurotrophic factor (BDNF) [60], increased activity in anterior cingulated cortex or amygdala in response to social threat [61, 62], and reduced hippocampal volume [63], all of which have been associated with MDD and all of which would be predicted to make an individual more sensitive than others to the depressogenic effects of inflammatory stimuli. To our knowledge, this approach has never been attempted.
On the other hand, there is a second, albeit less direct, approach to testing whether differential sensitivity to inflammation might explain how immune processes could promote depression even when apparently operating at nonproblematic levels. One could deliver a fixed inflammatory stimulus and examine whether the degree to which depression ensued was associated with vulnerability-like changes in any of the nonimmune variables described above. Fortunately, the many studies that have used various short-term immune stimuli or longer-term treatment with IFN-α to examine the effect of immune activation on mood have availed themselves of just this strategy.
Results from these studies provide very strong support for the IREA theory by demonstrating that vulnerability in physiologic systems “downstream” from inflammation is as relevant as inflammatory pathways themselves in the production of depression in response to stimuli that activate innate immunity (in essence, all the depression risk factors described above [2, 40–49]). Figure 1 demonstrates this idea based on data from our group and others that have worked with IFN-α as a model system. As with any other environmental stressor known to be depressogenic, the chronic inflammatory stimulation that results from a standardized dosage regimen of IFN-α yields a wide array of behavioral outcomes. Except for the rare induction of mania, no one feels better during IFN-α treatment. However, some people slog through with only malaise and fatigue to report, whereas others develop suicidal major depressive episodes that require IFN-α discontinuation and psychiatric hospitalization [64–66]. Between these two extremes, one observes every possible level of depressive outcome.
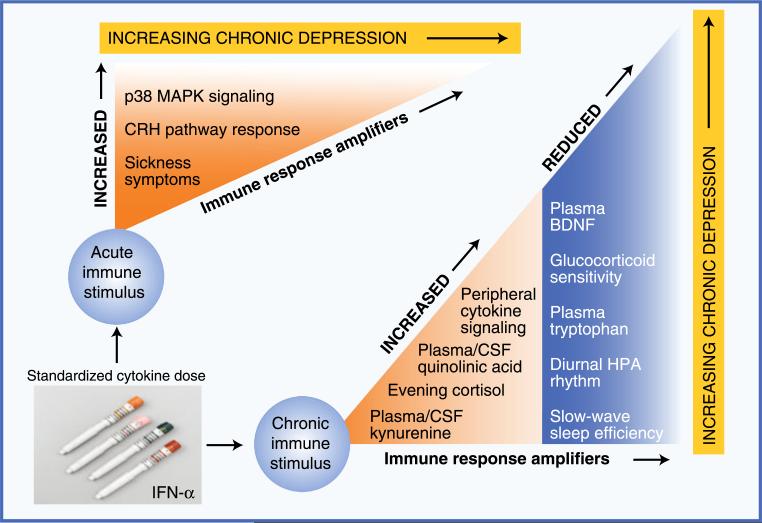
Fig. 1.
Evidence supporting the importance of immune response amplifiers in the etiology of immune-based depression. Studies using exposure to a constant dosage of inflammatory input have identified multiple pathways by which immune activation produces depressive symptoms. This figure illustrates patterns of physiologic vulnerability in such pathways, using treatment with interferon (IFN)-α as a model system for chronic cytokine exposure. Immune response amplifiers identified in response to an initial dose of IFN-α include increased activation of p38 mitogen-activated protein kinase (MAPK), an important intracellular proinflammatory signaling cascade, and increased response of corticotropin-releasing hormone (CRH) pathways. Although not a physiologic pathway per se, increased sickness-type symptoms in response to a first dose of IFN-α predict the later development of full major depressive disorder. In terms of patterns of functional disability in response to chronic cytokine exposure, evidence suggests that individuals with any of the following changes are at significantly increased risk of developing significant depressive symptoms during IFN-α treatment: 1) increased peripheral proinflammatory cytokine concentrations (eg, tumor necrosis factor-α or interleukin-6), 2) flattening of the diurnal cortisol rhythm and increased evening cortisol, 3) increased peripheral and central nervous system concentrations of kynurenine and its metabolite quinolinic acid, and 4) disrupted sleep as reflected by reduced slow-wave sleep and diminished sleep efficiency. Importantly, all these changes have been observed in the context of idiopathic major depressive disorder in medically healthy individuals. BDNF—brain-derived neurotrophic factor; CSF—cerebrospinal fluid; HPA—hypothalamic-pituitary-adrenal (axis)
That the same dosing schedule of IFN-α can yield such widely various outcomes strongly implicates factors over and above the initial inflammatory stimulus as essential to depressogenesis in this most “immune based” of depressive states. As shown in Fig. 1, this phenomenon can be seen within hours of receiving a first dose of IFN-α. Remarkably, individuals who respond with enhanced corticotropin-releasing hormone and p38 mitogen-activated protein kinase responses within 12 h of a first infusion or injection are significantly more likely to develop clinically relevant depressive symptoms 1 or 2 months later in the midst of IFN-α treatment [67•, 68]. On a symptomatic level, people who respond to a first dose with increased sickness-type symptoms are far more likely to meet full criteria for MDD (including profound emotional distress) by 8 weeks of IFN-α therapy [69, 70]. Also as shown in Fig. 1, several vulnerability factors, or immune response amplifiers, have been identified that are temporally associated with the development of depression during chronic treatment, including the following: (1) increased TNF-α and/or IL-6 signaling [25, 26, 71], (2) reduced HPA axis diurnal variation (ie, flatter cortisol slope) [26], (3) increased evening cortisol [26], (4) reduced sensitivity to glucocorticoid inhibitory feedback (unpublished data), (5) reduced plasma concentrations of BDNF [72], (6) reduced plasma concentrations of tryptophan [73–75], (7) increased plasma and cerebrospinal fluid concentrations of kynurenine and quinolinic acid [73, 75, 76••], and (8) reduced slow-wave sleep and sleep continuity [77]
Thus far, we have conceptualized immune element response amplifiers as existing downstream of inflammatory pathways, but significant data suggest that these elements also exist “upstream” of the immune system in a type of bidirectional round-robin dance. Intriguing evidence for this comes from data from our group showing that increased inflammatory responses to a laboratory psychosocial stress test administered prior to commencing IFN-α therapy predict increased depression several months later during IFN-α therapy (Raison et al., unpublished data). The oft-repeated observation that psychosocial stress—whether acute or chronic—activates proinflammatory cytokine release in the periphery of the body may also explain why increased resting state plasma cytokine concentrations measured prior to IFN-α therapy predict increased levels of depression during treatment [78]. Consistent with the possibility that baseline cytokine levels are (at least to some degree) a reflection of pretreatment psychosocial stress, many studies have found that even mild increases in depressive and anxiety symptoms (which are commonly induced by stress) just prior to treatment strongly predict the development of clinically relevant depression during IFN-α therapy [65].
What Does the Relationship Between Inflammation and Depression Suggest About Novel Treatment Strategies for Major Depressive Disorder?
That groups of depressed individuals demonstrate multiple indices of increased inflammation when compared with nondepressed populations immediately suggests that anti-inflammatory strategies, whether pharmacologic or behavioral, might hold promise for the treatment of depression in those who are otherwise medically healthy. Increasing evidence suggests this is the case. For example, large-molecule inhibitors of TNF-α signaling (eg, etanercept and infliximab) have been shown to reduce depressive symptoms in patients with psoriasis and Crohn's disease [51, 79], and are currently being examined for potential antidepressant efficacy in medically healthy individuals with treatment-resistant depression. Two randomized, placebo-controlled studies have reported that the addition of an inhibitor of the cyclooxygenase enzyme to a standard antidepressant enhances symptomatic improvement in medically healthy individuals with depression [80, 81]. Similarly, preclinical and clinical data suggest that acetylsalicylic acid (aspirin) may hold promise as an augmenting strategy in patients who fail to respond to monotherapy with a serotonin reuptake inhibitor [82–84]. In terms of behavioral strategies, recent research suggests that interventions known to benefit depression—such as physical exercise or psychotherapy—also reduce peripheral inflammatory biomarkers [85–88], although the degree to which this effect is essential for antidepressant efficacy is an open question. Other more experimental interventions for depression, such as meditation, also have been reported to reduce peripheral inflammatory responses to psychosocial stress [89, 90].
Let us assume—for the sake of argument—that the next few years witness the emergence of robust data supporting the efficacy of various classes of anti-inflammatory medications for treating MDD in medically healthy individuals. If this turns out to be the case, we likely will also have sufficient evidence to answer the questions around which this article has been constructed. Because several inflammatory measures—especially CRP—are widely available, it will be a fairly straightforward enterprise to evaluate whether the relationship between pretreatment inflammatory biomarkers and subsequent clinical response is strong enough to justify the view that depressed patients with elevated inflammation (however defined) comprise a physiologically defensible depressive subtype. If this happens, and inflammatory biomarkers become routinely employed to predict which patients will respond to which intervention, one of psychiatry's great grail quests—the search for clinically useful predictive biomarkers—will have been accomplished.
However, this accomplishment will come at a cost because it will have arisen from the fact that only a circumscribed subset of all depressed patients will have been shown to benefit from any future revolution in immune-based treatments for MDD. On the other hand, if inflammatory pathways behave like every other system implicated in MDD to date, the complexity of interactions between these pathways and the wider physiologic nexus to which they contribute will assure that any associations between pretreatment inflammatory biomarkers and subsequent clinical response will be too weak to provide useful clinical guidance. Some depressed patients with high levels of inflammation will fail to respond to anti-inflammatory treatments, and some patients with low levels of inflammation will respond robustly to anti-inflammatory interventions because even these low levels were driving depressive symptomatology as a result of interactions with extremely sensitive (ie, vulnerable) immune response amplifiers. A precedent for this possibility can be seen in studies examining the association between CRP concentrations and cardioprotection in patients with dyslipidemia, in which it turns out that even individuals with relatively low CRP levels achieve cardioprotection if statin therapy further lowers these levels [91].
Conclusions
While believing that the association between inflammation and MDD has a great deal to teach us about depressive pathophysiology, in this review, we have highlighted that activation of inflammatory processes is neither necessary nor sufficient to reliably produce the syndrome we currently define as depression. Rather, data show that groups of depressed individuals demonstrate elevated levels of multiple inflammatory biomarkers because there are more individuals with these elevations within depressed populations than comparison groups. We have provided data that support two opposing explanations for this phenomenon: first that individuals with increased inflammation comprise a physiologically discrete depressive subtype, and second that because inflammatory pathways are fully integrated into larger mind– body systems evolved to cope with environmental danger, it may be the case that even low levels of inflammatory stimulation may be depressogenic in individuals with vulnerability patterns in nonimmune elements of these larger systems. We conclude by noting that studies under way to determine the efficacy of anti-inflammatory interventions for depression will at one and the same time confirm or refute a role for inflammatory signaling pathways in MDD, and help resolve the important question of whether inflammation contributes to depression on a broad scale or in a far more circumscribed manner relevant only to individuals with measurably increased indices of inflammatory activation.
Acknowledgments
Dr. Miller has received grant support and support for travel to meetings from the National Institute of Mental Health.
Footnotes
Disclosure Dr. Raison has served as a consultant for Biolex Therapeutics and Pamlab.
Dr. Miller has served as a consultant for Schering-Plough Corp., GlaxoSmithKline, Abbott Laboratories, AstraZeneca, Roche, Pfizer, H. Lundbeck A/S, Wyeth, and Johnson & Johnson and has received grant support from Schering-Plough Corp., GlaxoSmithKline, and Centocor.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–9. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Berardis D, Conti CM, Serroni N, et al. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: a review of the current literature. Int J Immunopathol Pharmacol. 2010;23:417–22. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- 4.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 7.Zorilla E, Luborsky L, McKay J, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 8•.Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–7. doi: 10.1192/bjp.bp.109.076430. [This study was among the first to demonstrate that inflammatory biomarkers predict future depressive status, which is consistent with a causal role for inflammatory processes in the development of depression, even in the absence of documented medical illness.] [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 10.Huang TL, Lin FC. High-sensitivity C-reactive protein levels in patients with major depressive disorder and bipolar mania. Progr Neuropsychopharmacol Biol Psychiatry. 2007;31:370–2. doi: 10.1016/j.pnpbp.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Liukkonen T, Silvennoinen-Kassinen S, Jokelainen J, et al. The association between C-reactive protein levels and depression: results from the northern Finland 1966 birth cohort study. Biol Psychiatry. 2006;60:825–30. doi: 10.1016/j.biopsych.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur Heart J. 2004;25:492–9. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–56. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 15.Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65:S253–9. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 18.Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–33. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 20.Laurin D, David Curb J, Masaki KH, et al. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol Aging. 2009;30:1724–7. doi: 10.1016/j.neurobiolaging.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capuron L, Fornwalt FB, Knight BT, et al. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–5. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric side effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:1–19. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 24.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacol. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 25.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109–16. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raison CL, Borisov AS, Woolwine BJ, et al. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–47. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn AJ, Wang J. Cytokine effects on CNS biogenic amines. Neuroimmunomodulation. 1995;2:319–28. doi: 10.1159/000097211. [DOI] [PubMed] [Google Scholar]
- 28.Schafer A, Scheurlen M, Seufert J, et al. Platelet serotonin (5-HT) levels in interferon-treated patients with hepatitis C and its possible association with interferon-induced depression. J Hepatol. 2010;52:10–5. doi: 10.1016/j.jhep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–5. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 30.Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–50. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–54. doi: 10.1016/j.biopsych.2010.06.010. [This study demonstrated that acute exposure to lipopolysaccharide reduces ventral striatal responses to rewarding stimuli, which associates with induction of anhedonia in otherwise nondepressed individuals. This finding collaborates with other data demonstrating a tropism of proinflammatory cytokines for the basal ganglia, and demonstrates that cytokines are capable of producing brain changes observed frequently in MDD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison NA, Brydon L, Walker C, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–22. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Harrison NA, Brydon L, Walker C, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. [This was a seminal paper showing that typhoid vaccine induces depressed mood independent of changes in the cingulate cortex that are also commonly observed in MDD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brydon L, Harrison NA, Walker C, et al. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–9. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacol. 2007;32:2384–92. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 38.Capuron L, Pagnoni G, Demetrashvili M, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–6. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gimeno D, Kivimaki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2008;39:413–23. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller GE, Freedland KE, Carney RM, et al. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–85. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 42.Ranjit N, Diez-Roux AV, Shea S, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 43.Dai J, Miller AH, Bremner JD, et al. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–75. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zampelas A, Panagiotakos DB, Pitsavos C, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiology. 2005;46:120–4. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 45.Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 46.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago Health, Aging, and Social Relations study. Psychosom Medicine. 2006;68:376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 47.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 48.Bailey MT, Kinsey SG, Padgett DA, et al. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav. 2009;98:351–8. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 50.Capuron L, Su S, Miller AH, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn's disease treatment with infliximab. Aliment Pharm Ther. 2005;22:101–10. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 52.Anisman H, Gibb J, Hayley S. Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacol (Berl) 2008;199:231–44. doi: 10.1007/s00213-008-1166-z. [DOI] [PubMed] [Google Scholar]
- 53.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 54.Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.205. Epub. [This is a comprehensive review of known data supporting a role for inflammation in the pathophysiology and treatment of MDD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspi A, Hariri AR, Holmes A, et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bull SJ, Huezo-Diaz P, Binder EB, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008;14:1095–104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 59.Vaccarino V, Lampert R, Bremner JD, et al. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70:628–36. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–32. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masten CL, Eisenberger NI, Borofsky LA, et al. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol. 2011;23:283–92. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 64.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–7. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 65.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18:153–60. doi: 10.1111/j.1365-2893.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 67•.Felger JC, Alagbe O, Pace TW, et al. Early activation of p38 mitogen activated protein kinase is associated with interferon-alpha-induced depression and fatigue. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.015. Epub. [This study demonstrated that activity in inflammation-related intracellular signaling cascades in response to an initial inflammatory stimulus (IFN-α) strongly predicts the later development of depressive symptoms during chronic cytokine exposure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–5. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 69.Robaeys G, De Bie J, Wichers MC, et al. Early prediction of major depression in chronic hepatitis C patients during peginterferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J Gastroenterol. 2007;13:5736–40. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wichers MC, Koek GH, Robaeys G, et al. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med. 2005;35:433–41. doi: 10.1017/s0033291704003526. [DOI] [PubMed] [Google Scholar]
- 71.Wichers MC, Kenis G, Koek GH, et al. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–14. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Kenis G, Prickaerts J, van Os J, et al. Depressive symptoms following interferon-alpha therapy: mediated by immune-induced reductions in brain-derived neurotrophic factor? Int J Neuropsychopharmacol. 2011;14:247–53. doi: 10.1017/S1461145710000830. [DOI] [PubMed] [Google Scholar]
- 73.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 74.Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 75.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 76••.Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [Data in this article support a recent conceptual shift in the role of tryptophan metabolism in the etiology of depression from a focus on reduced serotonin to increased catabolites along the kynurenine pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raison CL, Rye DB, Woolwine BJ, et al. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–9. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wichers MC, Kenis G, Leue C, et al. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60:77–9. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 79.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 80.Muller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 81.Akhondzadeh S, Jafari S, Raisi F, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–11. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Yang F, Liu YF, et al. Acetylsalicylic acid as an augmentation agent in fluoxetine treatment resistant depressive rats. Neurosci Lett. 2011 doi: 10.1016/j.neulet.2011.05.035. Epub. [DOI] [PubMed] [Google Scholar]
- 83.Brunello N, Alboni S, Capone G, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21:219–25. doi: 10.1097/00004850-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Mendlewicz J, Kriwin P, Oswald P, et al. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21:227–31. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Zautra AJ, Davis MC, Reich JW, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76:408–21. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- 86.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2009;20:608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 87.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–9. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293:1626–34. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 89.Pace TW, Negi LT, Sivilli TI, et al. Innate immune, neuroendocrine and behavioral responses to psychosocial stress do not predict subsequent compassion meditation practice time. Psycho-neuroendocrinol. 2010;35:310–5. doi: 10.1016/j.psyneuen.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pace TW, Negi LT, Adame DD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinol. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]