Abstract
Background
Injecting drug use (IDU) is an important risk for viral hepatitis transmission. Detailed, transparent estimates of the scale of the problem at regional and global levels have never been made. We report national, regional and global prevalence and population size estimates for hepatitis C (HCV) and hepatitis B (HBV) among people who inject drugs.
Methods
Systematic search of peer-reviewed (Medline/Embase/PsycINFO) and grey literature databases, conference abstracts and online resources, with a widely distributed call for additional data. From 4386 peer-reviewed and 1019 grey literature sources, 1125 were reviewed in full. Studies were extracted to a customised database and graded according their methods. Serological reports of HCV antibodies/anti-HCV, HBV antibodies/anti-HBc, and/or HBV surface antigen/HBsAg among IDUs samples with n>40 participants, <100% HIV-positive, and sampling frames that did not exclude participants on the basis of age or sex were included. Using endorsed decision rules, prevalence estimates were calculated with anti-HCV and anti-HBV as proxies for exposure and HBsAg for current infection. These were combined with IDU population sizes to estimate the number of HBV and HCV positive IDUs.
Findings
Eligible reports of anti-HCV among IDUs were located for 77 countries. Prevalence was 60–80% in 26 countries and >80% in 12. We estimate worldwide about 10.0 million (range 6.0–15.2M) IDUs might be anti-HCV positive. China, (1.6M), the USA (1.5M) and the Russian Federation (1.3M) had by far the largest such populations. HBsAg reports were found for 59 countries, ranging from 5–10% in 21 countries and over 10% in 10. Worldwide, 6.4 million IDU might be anti-HBc positive (2.3–9.7M), and 1.2 million (0.3–2.7M) HBsAg positive.
Interpretation
The prevalence of anti-HCV among IDUs is far greater than HIV. Viral hepatitis clearly poses a challenge to public health. Variation in the coverage and quality of existing research creates uncertainty around estimates. Better and more complete data and reporting are required to estimate the scale of the problem, to inform efforts to prevent and treat HCV and HBV among IDUs.
Introduction
Injecting drug use (IDU) is an important public health issue across the globe: it was estimated that in 2007 16 million people worldwide injected drugs (range 11–21 million)1. Much of the estimated burden of disease attributable to the use of illicit drugs is likely due to blood-borne viral infections through unsafe drug injection2. Hepatitis B and C viruses (HBV and HCV) are even more efficiently spread by these means than HIV 3.
Around 80% of individuals exposed to HCV develop chronic infection4; 3–11% of those with chronic HCV infection will develop liver cirrhosis within 20 years5, with associated risks of liver failure and hepatocellular carcinoma (HCC)6. HCV transmission is increasingly driven by IDU7, but in many developing countries unsafe medical injections and transfusions are predominant sources of infection. The emergence of IDU in settings where the prevalence of HCV is high (Africa, the Middle East and South East Asia) presents an additional threat.
HBV is highly contagious through parenteral, sexual and vertical (perinatal transmission) routes. Around 5% of adults exposed to HBV develop chronic HBV infection (CHB)4; most of the 350 million chronically infected people worldwide were infected in childhood8. Cirrhosis and death due to HCC are important sequelae of CHB9.
Despite its higher prevalence and transmissibility, viral hepatitis has received far less global attention than HIV. The World Health Organization (WHO) called prevention and control efforts “successful but fragmented….[with no] comprehensive strategy for viral hepatitis”10. At the WHO’s 63rd World Health Assembly in May 2010, a resolution was passed to establish “goals and strategies for disease control, increasing education and promoting screening and treatment” of people infected with HBV and HCV (p.3)10. The WHO argues that IDUs are a particularly important group that need to be specifically targeted for prevention and treatment of HBV and HCV10. For such efforts to be appropriately scaled and targeted, policymakers and healthcare professionals need accurate and detailed data on the size of the population concerned, as has been undertaken for HIV1.
There have been no global systematic reviews of HBV prevalence among IDUs11. Previous reviews of HCV among IDUs have been selective in their geographic coverage12, have not provided sources or estimation methods13 or did not make population size estimates14. Here, we report a systematic search and critical review of the peer-reviewed and grey literature on anti-HCV, anti-HBV and HBsAg among IDUs, presenting the best available country-level data, and the first regional and global estimates of the number of IDUs living with HCV and HBV.
We do not present estimates of chronic hepatitis A, D or E viral infection (HAV, HDV, HEV). Chronic HAV infection does not occur, and in developing countries most adults are immune, making epidemics uncommon, although with increased sanitation this epidemiological pattern may be changing in some populations15. HDV has been associated with IDU; however, the extent of the literature on HDV (which requires concurrent HBV for infection to be established) is far more limited and the diversity in prevalence, even in high-HBV prevalence countries, makes extrapolation across countries problematic16. HEV is enterically transmitted and HEV data for IDUs is scarce.
Method
Our review was conducted in accordance with the methods outlined by the Global Burden of Disease (GBD) project (www.globalburden.org) and complied with PRISMA guidelines relevant to a descriptive review of this nature17. Our searches comprised multiple stages of searches of the peer-reviewed and grey literature, international consultations and expert critique and review, as undertaken in the review of HIV among IDUs1 (LD and BM). Data from the HCV Synthesis Project (led by HH) were also provided for review and inclusion. This was a systematic global review of published and unpublished sources containing reports of HCV infection and co-occurring HBV infection in IDUs until 2006; its methods have been described in detail18.
Search strategy
Peer-reviewed literature databases (Medline, EMBASE, and PsycINFO) were searched in November 2010 using search strings developed in consultation with specialist drug and alcohol librarians (webappendix 1). English language abstracts were included; translations were sought for promising non-English papers. Searches were updated in May 2011.
Grey literature and online databases were searched, including websites of drug surveillance systems, regional harm reduction networks, and country-specific ministries of health. Methods to identify and systematically search these sources have been described previously and utilised in earlier systematic reviews by us19, 20. Grey literature sources comprised 14% (18/127) of those ultimately used to generate regional estimates. Grey literature searching concluded in May 2011.
To identify further studies, an email was sent in December 2010 to GBD Illicit Drug Use Expert Group members (www.gbd.unsw.edu.au/gbdweb.nsf/page/ExpertGroups), UN agency staff, relevant international email lists, and other contacts of our team (n~300 initial recipients). It was redistributed by staff from the WHO, UNODC, US Centers for Disease Control (CDC), and the EMCDDA. The email requested information that might inform estimates of the prevalence of hepatitis among IDUs (webappendix 2 shows an example). By June 2011, replies were received from 61 experts regarding 52 countries in all 12 world regions. This included data on 14 countries across eight regions that were ultimately reported here. Data were received, and clarification sought, until June 2011.
Data extraction and selection
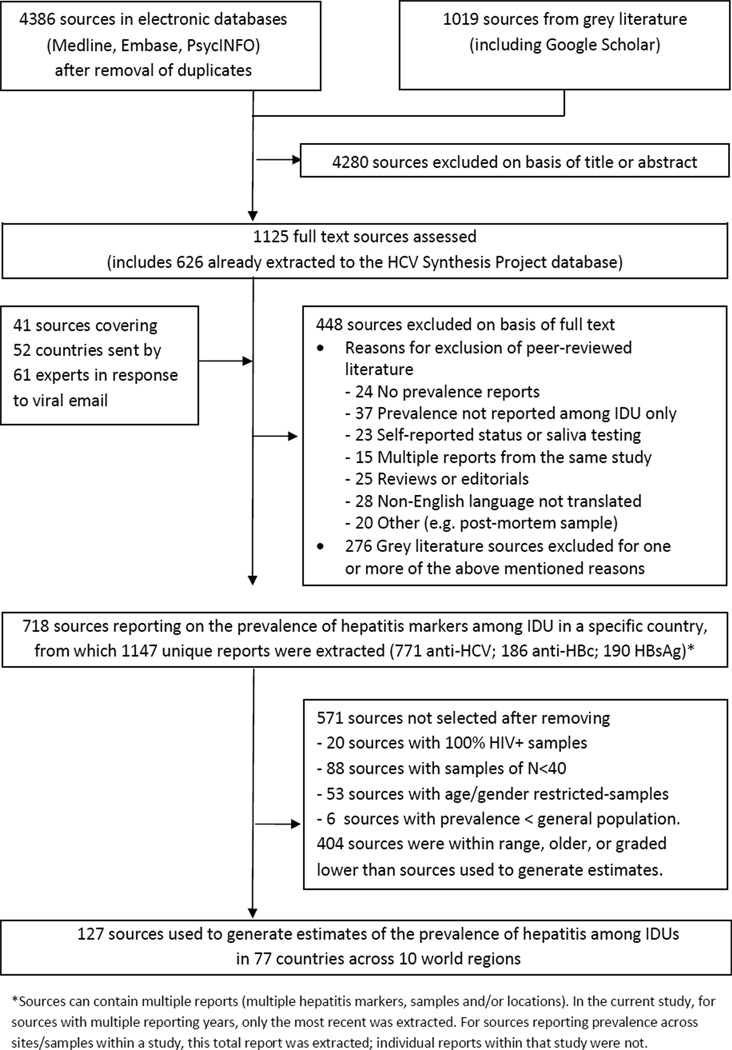
Documents were catalogued using Endnote X4. Figure 1 shows the search process and flow chart. Search results were systematically screened by two authors. Studies in the HCV Synthesis Project database were automatically included. Other references were reviewed if the title or abstract indicated the document contained relevant information on the prevalence of HCV or HBV among IDUs. 1125 documents (peer-reviewed and grey literature) were reviewed in full. Data were considered eligible where mention was made of the number or prevalence of hepatitis-infected IDUs in a country or sub-national area.
Figure 1.
Outline of systematic review process
Information was extracted on: (1) study methods (specimen type, eligibility criteria, recruitment and enrolment dates, and recruitment methods and locations); (2) Participant characteristics (age range, gender breakdown); and (3) hepatitis reports (number of participants tested, number and proportion testing positive for anti-HCV, anti-HBV and HBsAg, and reports broken down by age and gender). Detailed methodological information was used to grade and select studies for inclusion given that study methodology is thought to strongly influence descriptive methodology18. Extracted information was initially reviewed by two authors, and valid reports included in a Microsoft Access database, and reviewed by another author. Data were graded as outlined in Table 1.
Table 1.
Classification system used in the evaluation of study methodologies
| Grade | Hepatitis prevalence data |
|---|---|
| A | Multi-site seroprevalence study with >1 sample types (e.g. needle-syringe programmes, drug treatment centres, incarcerated IDUs) |
| B | B1 Seroprevalence study, single sample type and multiple sites |
| B2 Seroprevalence study, multiple sample types and a single site | |
| C | Seroprevalence study, single sample type |
| D | Registration or notification of cases of hepatitis infection |
| E | Prevalence study using self-reported hepatitis status, saliva or RNA testing only |
| Ungraded | Report with methodology unknown |
No hierarchical relationship was assumed between B1 & B2. ‘D’, ‘E’ and ungraded data have not been included in the estimates presented here.
For this paper, high and low reports of each sero-marker were selected for each country in accordance with the decision rules described in Text box 1. These were entered into Microsoft Excel by one author and independently reviewed by two others. Provisional reports were circulated to all authors for review and comment. External checks were made with specific requests to experts in countries where additional data or clarification were required.
Text box: Decision rules for data selection and extraction processes.
Selection, grading and clarification of hepatitis reports
Hepatitis reports were restricted to serological test results for anti-HCV, anti-HBc and HBsAg.
Hepatitis B reports that did not specify a specific serological marker were reviewed by BC (infectious disease physician) and assigned a serological maker or excluded, as appropriate.
If hepatitis reports were available from the same sample(s) and same site(s) in multiple years, only the most recent report was selected.
Hepatitis reports from one city were assumed to be from a single site unless otherwise stated.
Hepatitis reports were assumed to be single site and single sample type unless otherwise stated.
If calculation or typographical errors were detected in source documents, reports were recalculated and clarified with authors where possible.
Grade and date-based selection of reports
If grade A (multi-sample multi-site) reports were available, we selected the range of these and did not select lower graded reports. If recent reports (2000 onwards) were available, older (pre-2000) reports were not selected.
If grade A reports were unavailable, we selected the range of recent reports of the next highest grade. Older reports were selected if no recent reports were available.
Recent grade B (B1/multi-site single sample, or B2/single sample multi-site) reports were selected in preference to older grade B reports. Recent grade C reports were selected in preference to older grade B reports. Older grade C reports were selected if no grade B reports were available.
Pre-1990 reports were selected only if later reports were unavailable.
Additional selection and exclusion criteria
Reports from case notifications (grade D), self-report studies (grade E) or unspecified methodologies (ungraded) were excluded.
Reports of genetic or saliva testing, or residue from syringes were excluded.
Reports from 100% HIV-positive IDU samples were excluded.
Reports from studies restricted to ‘young’ IDUs, and baseline descriptions of studies of primarily HCV or HBV-negative IDUs (some seroincidence studies) were excluded.
Reports from studies excluding IDUs of either gender were excluded if mixed gender reports were available.
Reports of any hepatitis marker among IDUs that were lower than the general population prevalence for that marker were excluded.
Reports based on test results of fewer than 40 IDUs were excluded.
IDU prevalence reports and estimates
Mathers et al1 detail the selection of IDU prevalence reports and generation of IDU estimates.
Viral hepatitis prevalence data
Existing reports on HCV prevalence among IDU are based predominantly on serological testing for hepatitis C antibodies (anti-HCV). A positive anti-HCV test result can indicate acute, chronic or resolved HCV infection. A polymerase chain reaction (PCR) test is used to test for HCV viraemia, indicating current infection, however PCR test results are rarely reported in epidemiological studies. This review focuses exclusively on reports of anti-HCV prevalence.
HBV studies were included if they reported serological testing for HBsAg or anti-HBc. HBsAg indicates active (either acute or chronic) infection, but ~95% of adults with acute HBV infection will clear the virus, lose HBsAg and develop anti-HBc and hepatitis B surface antibodies (anti-HBs). Clearance rates for HBV may be lower for IDUs however, as more may go on to be chronically infected than the general population; this may relate to repeated exposure, or lower immunity due to poorer health and other viral infections21. The presence of anti-HBc indicates previous exposure; it is also a more durable marker than HBsAg. To clearly determine whether HBV infection was resolved or resulted in immunity, or to determine vaccination-related immunity, the results of multiple tests in combination would be assessed but this is rarely available in population-level or other large-scale epidemiological studies.
Injecting drug use prevalence
IDU and HIV prevalence data were drawn from a systematic review previously undertaken on behalf of the Reference Group to the UN on HIV and Injecting Drug Use1 (the ‘Reference Group’), adhering to international guidelines for systematic reviews22, with decision rules and estimates approved by all Reference Group members. During the course of a subsequent review of HIV prevention, treatment and care for people who inject drugs, updated prevalence data for some countries were submitted to the Reference Group23. These data were included in our current analysis, along with more recent data on IDU and HIV prevalence reported by EMCDDA and UNAIDS. Overall we included updated estimates for the following countries: IDU population size (Belarus, Brazil, Croatia, Cyprus, Czech Republic, Greece, Nepal, Philippines, and Ukraine) and HIV prevalence (Croatia, Cyprus, Mauritius, Moldova, Norway, Pakistan, Portugal, Sierra Leone, Somalia, Swaziland, Togo, Ukraine, the UK and Zimbabwe).
Statistical analysis
Data were also entered into MapInfo 10 to generate maps of prevalence estimates for IDU and hepatitis among IDUs. Following the collation of country-specific estimates, regional and global estimates for 2010 were derived. All authors reviewed the resulting estimates; regional or country-specific queries were made with experts where clarification was required. Prevalence of IDU was assumed to be the same in 2010 as in the year of the estimate. UN Population Division estimates of 2010 population size 15–64 years were used24. Regional estimates were derived through estimation of regional-specific, weighted estimates of the prevalence of IDU and hepatitis infection and uncertainty bounds, using methods previously endorsed by the Reference Group1; webappendix 3 provides further detail. Regional groupings were based on previous UNAIDS categorisations to facilitate comparability with the Reference Group HIV review1.
Results
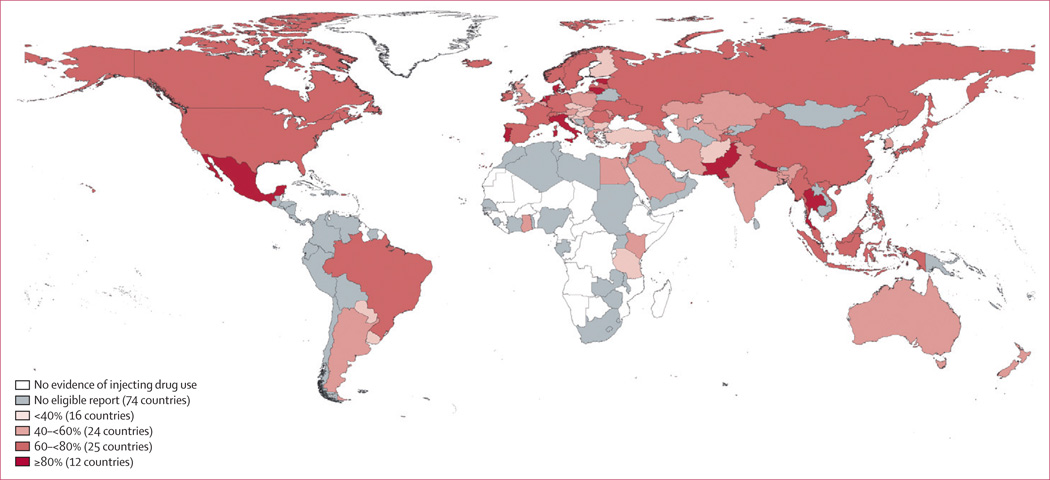
Anti-HCV reports among IDUs meeting inclusion criteria were found for 77 of the 152 countries/territories where IDU has been reported (Figure 2; Tables 2–6; see webappendix 4 for sources); these 77 countries collectively hold 82% of the world’s estimated IDU population. Anti-HCV prevalence varied greatly: midpoint reports ranged from 9.8% to 97% (Tables 2–6). Anti-HCV prevalence was estimated at 60–80% among IDUs in 26 countries, and 80% or higher in a further 12. The countries with the largest estimated populations of IDUs (China, Russia, and the USA) had midpoint estimates of anti-HCV prevalence amongst IDUs of 67.0%, 72.5% and 73.4% respectively (Tables 2, 3, 4). No studies were located for Caribbean countries or Pacific Island States and Territories (Tables 4, 5).
Figure 2.
Prevalence of hepatitis C antibodies in injecting drug users
Table 2.
Prevalence of hepatitis C antibodies (anti-HCV), hepatitis B core antibodies (anti-HBc) and surface antigen (HBsAg) among IDUs in Europe
| Prevalence of anti-HCV among IDUs (%) | Prevalence of anti-HBc among IDUs (%) | Prevalence of HBsAg among IDUs (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | |
| Eastern Europe | |||||||||||||||
| Armenia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Azerbaijan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Belarus | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Bosnia & Herzegovina | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Bulgaria | 2006, 2008 | 17.9 | 37.7 | 57.5 | B1,B2 | 2003 | -- | 6 | -- | B2 | 2008, 2006 | 5.5 | 8.6 | 11.6 | B2, B1 |
| Croatia | 2008, 2007 | 27.1 | 36.6 | 46 | A | 2008, 2007 | 7.5 | 13.8 | 20 | A | 2008, 2007 | 0.0 | 0.4 | 0.8 | A |
| Czech Republic | 2001*, 2002–03 | 20.8 | 25.3 | 29.7 | A | -- | NK | -- | 2010 | -- | 15.1 | -- | C | ||
| Estonia | 2002 | -- | 90.5 | -- | C | 2004, 2007 | 76.8 | 81.0 | 85.1 | C | 2004 | -- | 21.3 | -- | C |
| Georgia | 1997–98 | -- | 58.2 | -- | B1 | 1997–98 | -- | 51.3 | -- | A | 2002–03 | -- | 7.2 | -- | A |
| Hungary | 2008 | -- | 22.6 | -- | A | -- | -- | NK | -- | 2008 | -- | 0.5 | -- | A | |
| Latvia | 2007 | -- | 74.4 | -- | C | 2007 | -- | 55.8 | -- | C | -- | -- | NK | -- | |
| Lithuania | 2005 | 85 | 89.4 | 93.7 | B1,B2 | -- | NK | -- | 2005 | 9.5 | 11.2 | 12.9 | B2,B1 | ||
| Moldova | 2007 | -- | 42.7 | -- | B1 | -- | NK | -- | -- | NK | -- | ||||
| Poland | 2005 | 43.7 | 53.9 | 64.0 | A | 2005 | 24.4 | 40.1 | 55.7 | A | 2005 | 1.2 | 4.9 | 8.5 | A |
| Romania | 2007, 2009 | 65.6 | 74.3 | 83 | B2, B1 | -- | NK | -- | 2009, 2006 | 5 | 6.9 | 8.8 | B1 | ||
| Russian Federation | 2008 | 49 | 72.5 | 96 | B1 | 2002 | -- | 38 | -- | C | 2002 | -- | 9 | -- | C |
| Slovakia | 2002 | -- | 32.5 | -- | C | 2002 | -- | 6.3 | -- | C | -- | NK | -- | ||
| Ukraine | 2005 | 60.9 | 67 | 73 | C | 2005 | -- | 46.7 | -- | C | 2005 | -- | 6.7 | -- | C |
| Western Europe | |||||||||||||||
| Albania | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Andorra | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Austria | 2008 | -- | 47.1 | -- | A | 2008 | -- | 19.0 | -- | A | -- | -- | NK | -- | |
| Belgium | 2008 | 27 | 55.0 | 82.7 | B1, B2 | 2004, 2008 | 16.7 | 37 | 57.3 | C | 2008 | 1.9 | 3.0 | 4.0 | B1 |
| Denmark | 1996 | -- | 85 | -- | B2 | 2007 | -- | 65 | -- | C | 2007 | -- | 1.3 | -- | C |
| Finland | 2007 | 20.7 | 21.1 | 21.4 | B1 | -- | NK | -- | -- | NK | -- | ||||
| FYR of Macedonia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| France | 2006 | -- | 73.8 | -- | A | 1995 | 26.9 | 41.6 | 56.2 | C | 1995, 1992 | 3.4 | 4.8 | 6.2 | C |
| Germany | 2001–03 | -- | 75.0 | -- | C | 2001–03 | -- | 53.0 | -- | C | 1994, 92–93 | 6 | 7.2 | 8.4 | B2 |
| Greece | 2008 | 44.9 | 50.2 | 55.5 | A | 2008 | 14.6 | 20.5 | 26.3 | B1 | 2008 | 2.3 | 2.5 | 2.7 | B2 |
| Iceland | 1990–93 | -- | 63 | -- | C | -- | NK | -- | -- | NK | -- | ||||
| Ireland | 2003, 2001 | 72.3 | 74.6 | 76.9 | C | 2003 | -- | 17.5 | -- | B1 | 2003 | -- | 0.0 | -- | C |
| Italy | 2000, 05–07 | 72.9 | 81.1 | 89.3 | B1 | 2000, 2005 | 39.8 | 55.1 | 70.4 | B1 | 1992–93, 90–91 | 0.9 | 5.1 | 9.3 | C |
| Luxembourg | 2005 | -- | 81.3 | -- | A | 2005 | -- | 24.7 | -- | A | 2005 | -- | 3.9 | -- | A |
| Malta | 2006 | -- | 33.1 | -- | B2 | -- | NK | -- | -- | -- | NK | -- | |||
| Monaco | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Montenegro | 2008, 2005 | 22 | 37.8 | 53.6 | C | -- | NK | -- | 2008 | -- | 0 | -- | C | ||
| Netherlands | 2008 | -- | 86.2 | -- | A | 1999 | -- | 67.5 | -- | A | 2000 | -- | 3.0 | -- | A |
| Norway | 2008 | 68.4 | 71.3 | 74.1 | A | 2008 | -- | 41.0 | -- | A | 2008 | -- | 1.2 | -- | A |
| Portugal | 2009 | -- | 83.1 | -- | B1 | 2000 | -- | 53.7 | -- | C | 2009 | -- | 2.9 | -- | B1 |
| San Marino | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Serbia | 2008 | 45 | 57 | 69 | C | -- | NK | -- | -- | NK | -- | ||||
| Slovenia | 2002, 2008 | 21.0 | 21.7 | 22.3 | B1 | 2008 | -- | 4.2 | -- | B1 | 2002 | -- | 3.4 | -- | B1 |
| Spain | 2003, 1999–01 | 73.3 | 79.6 | 85.9 | B1 | 2003 | -- | 22.5 | B1 | 2006 | 1.8 | 3.6 | 5.3 | C | |
| Sweden | 2007 | 62.0 | 75.1 | 88.2 | A | 2006 | -- | 52.1 | -- | C | 2006 | -- | 2.3 | -- | C |
| Switzerland | 2002 | -- | 78.3 | -- | B1 | 2000–02 | -- | 83.3 | -- | C | 1996 | -- | 4 | -- | C |
| United Kingdom | 2004, 2009 | 47 | 50.5 | 54 | A | 2003–05 | -- | 32 | -- | A | 1996–00 | 0^ | 8.9 | 17.8 | C |
Countries in Western Europe for which no reports of IDU identified: Liechtenstein. Notes: For source documents for all figures listed in tables see webappendix 4. NK – although IDU has been identified or IDU prevalence estimated, no eligible studies of HCV or HBV among IDUs were located. When more than one year or grade is given, these are listed in order of the estimate they refer to, i.e. lower followed by upper.
100% HAV+ sample.
Publication year minus three; year of estimate not stated. Estimates received for Scotland and Wales are not reported within
Table 6.
Prevalence of hepatitis C antibodies (anti-HCV), hepatitis B core antibodies (anti-HBc) & surface antigen (HBsAg) among IDUs in the Middle East & Africa
| Country | Prevalence of Anti-HCV among IDUs (%) | Prevalence of Anti-HBc among IDUs (%) | Prevalence of HBsAg among IDUs (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | |
| Middle East and North Africa | |||||||||||||||
| Algeria | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Bahrain | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Cyprus | 2008 | 29.2 | 39.6 | 50.0 | C | -- | NK | -- | 2008 | -- | 0.0 | -- | C | ||
| Egypt | 1989–91, 1995 | 35.8 | 49.4 | 63.0 | C | 1989–91, 1995 | 53.6 | 57.8 | 62.0 | C | 1989–91, 1995 | 10.9 | 13.5 | 16.0 | C |
| Iraq | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Israel | 2001–03* | -- | 67.6 | -- | C | 1988–89, 1986 | 26.0 | 39.0 | 52.0 | C | 1988–89, 1986 | 0.0 | 2.8 | 5.5 | C |
| Jordan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Kuwait | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Lebanon | 2000–02, 07–08 | 5.0 | 28.9 | 52.8 | C | -- | NK | -- | 2000–02, 07–08 | 0 | 2.5 | 5 | C | ||
| Libyan Arab Jamahiriya | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Morocco | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Occ. Palestinian Terr. | 2007* | -- | 45.3 | -- | C | -- | NK | -- | 2007* | -- | 6.4 | -- | C | ||
| Oman | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Qatar | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Saudi Arabia | 2002, 03–06 | 14.1 | 49.8 | 85.4 | C | -- | -- | NK | -- | 1992–93 | -- | 18.5 | -- | C | |
| Sudan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Syrian Arab Republic | 1999* | -- | 60.5 | -- | C | 1999* | -- | 28.9 | -- | C | -- | NK | -- | ||
| Tunisia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Turkey | 2009 | -- | 28.9 | -- | B1 | -- | NK | -- | 2009 | -- | 5.2 | -- | B1 | ||
| United Arab Emirates | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Yemen | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Sub Saharan Africa | |||||||||||||||
| Cote d'Ivoire | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Djibouti | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Gabon | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Ghana | 2004–05 | -- | 40.1 | -- | B1 | -- | NK | -- | -- | NK | -- | ||||
| Kenya | 2000 | 42.2 | 51.4 | 60.6 | B1, B2 | -- | -- | NK | -- | 2000 | -- | 6.4 | -- | B2 | |
| Malawi | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Mauritius | 2009 | -- | 97.3 | -- | B1 | -- | NK | -- | 2009 | -- | 9.0 | -- | B1 | ||
| Nigeria | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Senegal | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Sierra Leone | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| South Africa | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Swaziland | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Tanzania, United Republic | 2007 | -- | 22.2 | -- | C | -- | NK | -- | 2007 | -- | 3.8 | -- | C | ||
| Togo | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Uganda | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Zambia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Zimbabwe | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
Countries in Africa for which no reports of IDU identified: Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo (Democratic Republic), Equatorial Guinea, Eritrea, Ethiopia, Gambia, Guinea, Guinea-Bissau, Lesotho, Liberia, Madagascar, Mali, Mauritania, Mozambique, Namibia, Niger, Republic of the Congo, Rwanda, Sao Tome & Principe, Seychelles, Somalia. Notes: For source documents for all figures listed in tables see webappendix 4. NK – although IDU has been identified or IDU prevalence estimated, no eligible studies of HCV or HBV among IDUs were located. When more than one year or grade is given, these are listed in order of the estimate they refer to, i.e. lower followed by upper.
Publication year minus three; year of estimate not stated.
Table 3.
Prevalence of hepatitis C antibodies (anti-HCV), hepatitis B core antibodies (anti-HBc) and surface antigen (HBsAg) among IDUs in Asia
| Country | Prevalence of anti-HCV among IDUs (%) | Prevalence of anti-HBc among IDUs (%) | Prevalence of HBsAg among IDUs (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | |
| East & SouthEast Asia | |||||||||||||||
| Brunei | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Cambodia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| China# | 2010 | 60.9 | 67 | 73.1 | B1 | 2002–03 | -- | 36.5 | -- | C | 1999–2000 | 3.8 | 9.6 | 15.4 | C |
| Indonesia | 2007–09 | -- | 77.3 | -- | C | 2007–09 | -- | 57.6 | -- | C | 2007–09 | -- | 2.9 | -- | C |
| Japan | 1993–94, 93 | 55.0 | 64.8 | 74.5 | C | -- | NK | -- | 1993–94, 93 | 2.0 | 3.2 | 4.3 | C | ||
| Lao PDR | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Malaysia | 2006–07 | -- | 67.1 | -- | B1 | -- | NK | -- | -- | NK | -- | ||||
| Mongolia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Myanmar | 2009 | -- | 79.2 | -- | B1 | -- | NK | -- | 2009 | -- | 9.1 | -- | B1 | ||
| Philippines | 2002 | -- | 70 | -- | C | -- | NK | -- | -- | NK | -- | ||||
| Republic of Korea | 2005 | -- | 57 | -- | C | 2005 | -- | 51 | -- | C | 1994–95 | -- | 4.0 | -- | C |
| Singapore | 2005–06 | -- | 42.5 | -- | C | -- | NK | -- | 2005–06 | -- | 8.5 | -- | C | ||
| Taiwan | 2001 | -- | 41 | -- | B2 | 1984, 1986 | 11.3 | 50.7 | 90 | C | 2005 | -- | 16.7 | -- | C |
| Thailand | 2000 | -- | 89.8 | -- | B2 | 1996 | -- | 76.5 | -- | C | -- | -- | NK | -- | |
| Timor Leste | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Viet Nam | 2003 | -- | 74.1 | -- | B1 | -- | NK | -- | 1993 | -- | 19.5 | -- | B1 | ||
| South Asia: | |||||||||||||||
| Afghanistan | 2008 | -- | 36.0 | -- | A | -- | NK | -- | 2008 | -- | 5.8 | -- | A | ||
| Bangladesh | 1999–2005 | -- | 48.2 | -- | A | 1996–97 | -- | 31.8 | -- | C | 2002 | -- | 9.4 | -- | C |
| Bhutan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| India | 2006 | -- | 41.0 | -- | B1 | -- | NK | -- | 2006 | 2.7 | 10.2 | 17.8 | C | ||
| Iran (Islamic Republic) | 2007, 2001 | 34.5 | 50.2 | 65.9 | B2 | 2001–02 | -- | 61.2 | -- | B2 | 2001, 06–07 | 3.7 | 17.3 | 30.9 | B2 |
| Maldives | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Nepal | 1997–2002, 1997 | 80.5 | 87.3 | 94.0 | C | 1993* | -- | 82.0 | -- | C | 1996–97 | 5.5 | 5.8 | 6.0 | C |
| Pakistan | 2003–04 | 75.0 | 84.0 | 92.9 | B1 | -- | NK | -- | 2004, 2003 | 6.0 | 6.8 | 7.5 | C | ||
| Sri Lanka | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Central Asia: | |||||||||||||||
| Kazakhstan | 2005 | -- | 58.8 | -- | C | 2002 | -- | 79.5 | -- | A | 2002 | -- | 7.9 | -- | A |
| Kyrgyzstan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Tajikistan | 2004 | -- | 61.3 | -- | C | -- | NK | -- | -- | NK | -- | ||||
| Turkmenistan | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Uzbekistan | 2001 | -- | 51.7 | -- | A | -- | NK | -- | -- | NK | -- | ||||
Countries in East and South East Asia for which no reports of IDU identified: Democratic People’s Republic of Korea (North Korea). Notes: For source documents for all figures listed in tables see webappendix 4. NK – although IDU has been identified or IDU prevalence estimated, no eligible studies of HCV or HBV among IDUs were located. When more than one year or grade is given, these are listed in order of the estimate they refer to, i.e. lower followed by upper.
Publication year minus three; year of estimate not stated.
A systematic review and meta-analysis by Xia and colleagues45 was not included here as the source documents were in Chinese and could not be verified. In that review, the pooled prevalence was 61.4% (55.7–67.2) across 53 Chinese and two English language multi-region studies of HCV among IDUs in China.
Table 4.
Prevalence of hepatitis C antibodies (anti-HCV), hepatitis B core antibodies (anti-HBc) and surface antigen (HBsAg) among IDUs in the Americas
| Country | Prevalence of anti-HCV among IDUs (%) | Prevalence of anti-HBc among IDUs (%) | Prevalence of HBsAg among IDUs (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | |
| Caribbean# | |||||||||||||||
| Bahamas | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Bermuda | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Dominican Republic | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Haiti | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Jamaica | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Latin America | |||||||||||||||
| Argentina | 2000–01 | -- | 54.6 | -- | B1 | -- | NK | -- | 2000–01 | -- | 8.6 | -- | B1 | ||
| Bolivia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Brazil | 2000–01 | -- | 63.9 | -- | B1 | 1994–96 | -- | 55.8 | -- | B2 | 2000 | -- | 2.3 | -- | C |
| Chile | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Colombia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Costa Rica | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Ecuador | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| El Salvador | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Guatemala | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Honduras | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Mexico | 2005 | 96 | 97.4 | 98.7 | B1 | 2005 | -- | 85.0 | -- | B1 | -- | NK | -- | ||
| Nicaragua | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Panama | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Paraguay | 2006 | -- | 9.8 | -- | C | -- | NK | -- | -- | NK | -- | ||||
| Peru | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Suriname | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Uruguay | 2003 | -- | 21.9 | -- | C | 2003 | -- | 19.6 | -- | C | 2003 | -- | 4.5 | -- | C |
| Venezuela | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| North America | |||||||||||||||
| Canada | 2005–08 | 51 | 64 | 77 | A | -- | NK | -- | -- | NK | -- | ||||
| USA | 2002–04, 2001 | 69.7 | 73.4 | 77 | B2 | 2002–04 | -- | 22.6 | -- | A | 1992 | 3.5 | 11.8 | 20 | B1, B2 |
Countries in Latin America for which no reports of IDU identified: Belize, Falkland Islands, Guyana. Countries in the Caribbean for which no reports of IDU identified: Anguilla, Antigua & Barbuda, Aruba, Barbados, British Virgin Islands, Cayman Islands, Cuba, Dominica, Grenada, Guadaloupe, Martinique, Montserrat, Netherlands Antilles, Saint Kitts & Nevis, Saint Lucia, Saint Vincent & Grenadines, Trinidad & Tobago Turks and Caicos Islands. Notes: For source documents for all figures listed in tables see webappendix 4. NK – although IDU has been identified or IDU prevalence estimated, no eligible studies of HCV or HBV among IDUs were located. When more than one year or grade is given, these are listed in order of the estimate they refer to, i.e. lower followed by upper.
A study in San Juan, Puerto Rico found 89% HCV prevalence46.
Table 5.
Prevalence of hepatitis C antibodies (Anti-HCV), hepatitis B core antibodies (anti-HBc) and surface antigen (HBsAg) among IDUs in Oceania
| Country | Prevalence of anti-HCV among IDUs (%) | Prevalence of anti-HBc among IDUs (%) | Prevalence of HBsAg among IDUs (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | Estimate year | Lower | Midpt | Upper | Grade | |
| Australasia | |||||||||||||||
| Australia | 1991–95, 90–91 | 41.2 | 54.6 | 68 | A | 1994, 90–91 | 18.9 | 33 | 47.0 | A | 1999–02, 05–08 | 2.9 | 4.0 | 5 | B2, B1 |
| New Zealand | 2009 | -- | 51.9 | -- | B1 | -- | NK | -- | 1994, 1991 | 1.2 | 2.8 | 4.4 | C | ||
| Pacific Islands | |||||||||||||||
| Fiji | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| French Polynesia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Guam | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Kiribati | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Micronesia (Fed. states) | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| New Caledonia | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Papua New Guinea | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Samoa | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Solomon Islands | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Tonga | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
| Vanuatu | -- | NK | -- | -- | NK | -- | -- | NK | -- | ||||||
Countries in the Pacific region for which no reports of IDU identified: American Samoa, Cook Islands, Marshall Islands, Nauru, Niue, Palau, Pitcairn, Tokelau, Tuvalu. Notes: For source documents for all figures listed in tables see webappendix 4. NK – although IDU has been identified or IDU prevalence estimated, no eligible studies of HCV or HBV among IDUs were located. When more than one year or grade is given, these are listed in order of the estimate they refer to, i.e. lower followed by upper.
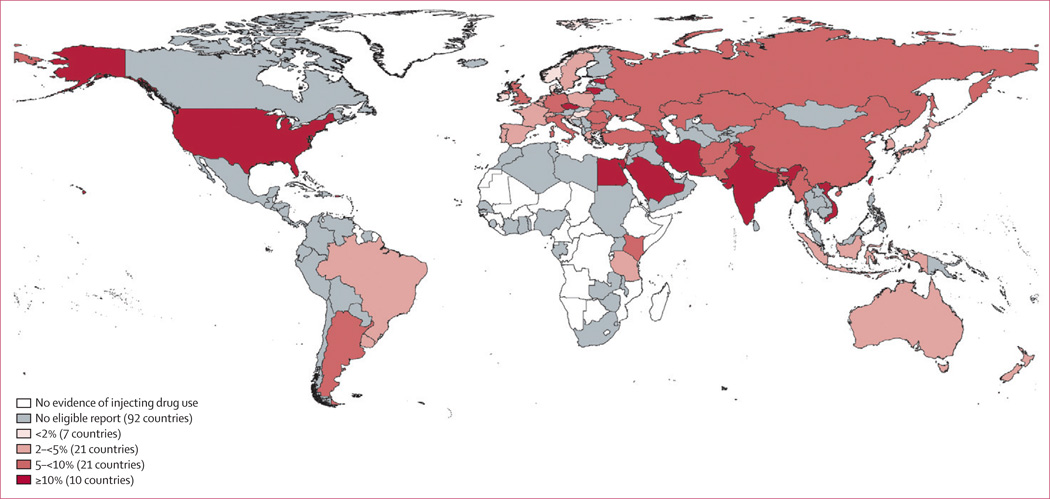
Reports of HBV exposure (anti-HBc positive) had been made in 43 countries comprising 65% of the world’s IDU population (see webappendix 5 for map). Levels varied widely across countries, from 4.2% (Slovenia) to 85% (Mexico) (Tables 2–6). Prevalence of HBsAg had been measured in 59 countries accounting for 73% of the world’s IDU population (Tables 2–6; Figure 3). The highest levels of HBsAg were in countries (mostly in Asia) known to have endemic HBV in the general population. HBsAg prevalence reports among IDUs varied within countries quite markedly (for example, in the USA, HBsAg reports ranged from 3.5% to 20%; in Iran, from 3.7% to 30.9%; Tables 4, 3).
Figure 3.
Prevalence of hepatitis B surface antigen in injecting drug users
Data quality varied across all three hepatitis indicators, with only 19 countries having eligible ‘A’ grade reports for at least one marker, and few of those being truly national (Tables 2–6, “Grade” columns). In many countries, prevalence reports came from samples from different sites (‘B1’ grade). For most countries, we were able to use reports produced since 2000, however one in four countries had only older HBV reports (Tables 2–6, “Year of report” columns).
Extrapolating to all countries, we estimate that in 2010 about 10.0 million IDUs (range 6.0–15.2 million) were anti-HCV positive (table 7; a midpoint prevalence of 67.0% among all IDUs globally). This was around 3.5 times larger than the 2.8 million IDUs (range 0.8–6.2 million) estimated to be living with HIV (see webappendix6).
Table 7.
Regional and global estimates of the numbers of IDUs who are anti-HCV, anti-HBc and HBsAg positive, 2010
| Region | Estimated number of IDUs who are anti-HCV positive | Estimated number of IDUs who are anti-HBc positive | Estimated number of IDUs who are HBsAg positive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Countries with eligible reports |
% ERIP with eligible reports |
Lower | Mid | Upper | No. Countries with eligible reports |
% ERIP with eligible reports |
Lower | Mid | Upper | No. Countries with eligible reports |
% ERIP with eligible reports |
Lower | Mid | Upper | |
| Eastern Europe | 14 | 87 | 1,244,500 | 2,346,000 | 3,918,000 | 9 | 80 | 610,500 | 1,362,000 | 2,426,000 | 11 | 86 | 100,000 | 280,000 | 543,000 |
| Western Europe | 22 | 99 | 497,000 | 727,500 | 1,018,000 | 17 | 94 | 209,000 | 447,000 | 660,000 | 17 | 93 | 13,500 | 54,000 | 108,500 |
| East and South East Asia | 11 | 99 | 1,820,000 | 2,642,000 | 3,576,500 | 5 | 77 | 678,000 | 1,592,500 | 2,374,500 | 8 | 88 | 112,000 | 340,000 | 696,000 |
| South Asia | 6 | 99 | 233,000 | 354,500 | 532 000 | 3 | 45 | 158,000 | 370,500 | 565,000 | 6 | 99 | 20,000 | 71,500 | 154,500 |
| Central Asia | 3 | 81 | 91,500 | 146,000 | 213,000 | 1 | 40 | 59,500 | 145,500 | 226,500 | 1 | 40 | 6,000 | 21,500 | 46,000 |
| Caribbean | 0 | 0 | --# | -- | -- | 0 | 0 | --# | -- | -- | 0 | 0 | --# | -- | -- |
| Latin America | 5 | 67 | 83,500 | 132,500 | 194,500 | 3 | 60 | 386,500 | 926,000 | 1,425,000 | 3 | 45 | 12,500 | 43,500 | 90,500 |
| Canada and United States | 2 | 100 | 675,500 | 1,022,000 | 1,441,000 | 1 | 87 | 206,500 | 524,500 | 864,000 | 1 | 87 | 57,500 | 272,500 | 642,000 |
| Pacific Island States & Terr. | 0 | 0 | --# | -- | -- | 0 | 0 | --# | -- | -- | 0 | 0 | --# | -- | -- |
| Australia and New Zealand | 2 | 100 | 44,500 | 97,000 | 165,000 | 1 | 88 | 20,500 | 60,500 | 115,000 | 2 | 100 | 3,000 | 7,000 | 12,000 |
| Middle East & North Africa | 8 | 54 | 29,000 | 63,500 | 115,500 | 3 | 26 | 50,000 | 74,500 | 106,000 | 7 | 49 | 7,500 | 14,000 | 26,500 |
| Sub-Saharan Africa* | 4 | 25 | 206,500 | 800,000 | 1,524,000 | 0 | 0 | 136,000 | 821,500 | 1,666,500 | 3 | 19 | 11,500 | 106,500 | 296,500 |
| Extrapolated global | 77 | 82 | 6,031,000 | 10,018,000 | 15,186,500 | 43 | 65 | 2,550,000 | 6,411,500 | 10,564,000 | 59 | 73 | 346,500 | 1,229,000 | 2,654,500 |
Notes: ERIP: estimated regional IDU population. All figures rounded to nearest 500; global figure totalled from regional estimates prior to rounding. 2010 UN population division estimates were used to derive 2010 estimates of IDU population size.
Sub-Saharan African IDU numbers and derived population estimates should be viewed with caution as IDU prevalence estimates were derived from three countries in the region (South Africa, Mauritius, Kenya). The estimated range of IDU was derived by applying the regional observed error; this large error band reflects the uncertainty around these estimates.
Insufficient data to produce a region-specific estimate for IDU populations in this region; countries in this region were still included in global estimates.
The largest HCV-positive IDU populations were estimated to be living in Eastern Europe (2.3 million; range 1.2–3.9 million) and East and South-East Asia (2.6 million; range 1.8–3.6 million). The three countries with the largest IDU populations, China, the Russian Federation and the USA, were estimated to have 1.6, 1.3 and 1.5 million IDUs living with HCV, respectively.
We estimate that globally in 2010, 1.2 million IDUs were HBsAg-positive (range 0.3–2.7 million), with an IDU population-weighted, global prevalence of 8.4%. The largest populations by region are estimated to be East and South-East Asia (0.3 million; range 0.1–0.7 million) and Eastern Europe (0.3 million; range 0.1–0.5 million). The large range around all these estimates reflects the uncertainty resulting from prevalence varying among different sub-populations of IDUs, across different recruitment settings, and geographically.
Discussion
This is the first global systematic review of HCV and HBV infection among people who inject drugs and the first to produce regional and global population estimates. We estimate midpoint populations of IDUs who may be HCV-positive of around 10.0 million, and those who are HBsAg-positive of around 1.3 million. Clear geographic differences exist in prevalence. Eastern Europe, and East and South East Asia are estimated to hold the largest populations of IDUs infected with viral hepatitis.
It is important to note that the population size estimates reported here refer to the estimated number of anti-HCV, anti-HBc or HBsAg-positive people who are current or recent injecting drug users not people who have ever injected drugs. This is the same methodology undertaken for HIV previously1. Many people who inject drugs cease injecting at some point25, so the current estimates cannot be interpreted as the total number of cases of HCV or HBV attributable to IDU. Given the limitations in current knowledge of the natural history of IDU (such as the range in duration of injecting, and the likelihood and timing of resuming IDU following cessation), particularly in low and middle-income countries, it is not possible to make defensible regional and global estimates of the number of former IDUs, and the numbers of whom may be anti-HCV, anti-HBc and HBsAg-positive. An estimate of the burden of chronic viral hepatitis among current IDUs is essential for assessing secular trends in the risk of infection, the impact of control strategies, and the importance of implementing these, as well as implications for future burden of disease and health care requirements.
Efforts to prevent, treat and reduce harms related to liver disease among IDUs are essential, particularly in situations where HIV has successfully been prevented or managed, since the larger numbers of IDUs infected with HCV, and significant morbidity arising from this infection, mean that the health and economic costs of HCV transmitted by IDU may be as high as (or higher than) those of HIV. HCV treatment remains under-utilised, however10. Part of the reason for this is the high cost, which will remain a significant barrier to increasing treatment coverage in resource poor settings until this is reduced. There is increasing attention on this issue among international groups who are advocating for cost reductions, generic manufacturing, and changes to licensing conditions10, 26. Not long ago, the high cost of HIV antiretrovirals similarly prevented access in high prevalence, low-income countries: in recognition of this barrier, there are growing efforts to bring viral hepatitis treatments into the same (lower cost) access framework as HIV antiretrovirals10. Another barrier is the toxicity of HCV treatment, although a large number of new HCV drugs are being developed, which will revolutionise HCV treatment in the next few years27.
Greater attention needs to be paid to reducing the impact of other causes of liver disease progression in people chronically infected with viral hepatitis. This includes addressing alcohol use problems, and providing HAV and HBV vaccination, particularly given evidence that liver related disease will become a major cause of mortality as IDUs age28.
Evidence regarding the impact of needle and syringe programs29 and provision of other injecting paraphernalia upon prevention of HCV infection is limited, yet reduction of risk remains paramount, particularly during the period of initiation to injecting, when HCV incidence is highest6, 14. The potential for HCV treatment to reduce HCV prevalence among IDU populations and in this way reduce the force of infection acting on susceptible members of these populations has been supported by mathematical modelling30. This potential role of HCV treatment in the prevention of HCV transmission among IDU populations warrants further investigation.
Although significant variability in HBsAg prevalence reports was observed, prevalence typically reflected the differences in the prevalence of HBV infection in the general population. In low-intermediate prevalence countries, the prevalence of HBsAg in IDU was typically <10%, whereas in high-HBV prevalence countries, prevalence of HBsAg among IDUs was in the order of 10–20% (e.g. East and South-East Asia). Given the high prevalence of chronic HCV infection in IDUs, HBV infection is particularly likely to represent HBV/HCV co-infection, which is associated with more rapid progression of liver disease and attendant mortality31; this is similarly the case for coinfection between HIV and viral hepatitis32.
Effective treatments for CHB are available, which reduce progression of liver disease and complications such as HCC33. However, antiviral therapy for CHB is often of indefinite duration, and access to modern, potent drugs with high resistance barriers is limited in many high prevalence, resource-poor settings. Barriers to accessing treatment and care for CHB result in poor outcomes for those affected, and ongoing transmission to susceptible contacts.
Vaccination against HBV must be prioritised for all susceptible IDU, especially those already infected with HCV. However, selective vaccination programs against HBV in this group have often been characterised by low uptake and difficulty reaching most at-risk individuals34. A significant reduction in the burden of HBV infection amongst IDU is expected in countries with universal infant vaccination programs once these individuals reach the age at which acquisition through IDU is most common. Correctional facilities provide one opportunity to vaccinate, treat and reduce the transmission of viral hepatitis in a population with high levels of IDU, HBV, and HCV, many of whom cycle in and out of the community35.
Limitations of existing data
There are a number of important limitations of existing data. One issue concerns the way in which HCV and HBV infection are measured and reported across studies: reporting was typically on only one (or perhaps two) markers, making estimates of the true prevalence of chronic HBV and HCV difficult. Without the measurement and reporting of multiple markers (anti-HCV plus HCV RNA-PCR, or HBsAg plus anti-HBc and ideally anti-HBs) it is not possible to make more accurate estimates of chronic infection, past infection, susceptibility or immunity. For HCV, we have estimated the number of current IDUs who are anti-HCV positive: this is not a measure of total chronic HCV infection, but rather HCV exposure among IDUs, given that a minority (perhaps 20%) of those infected with HCV (who would test positive for anti-HCV) are likely to have cleared the virus4.
For HBsAg, wide ranges in reports that met inclusion criteria were observed. In addition, where anti-HBc was not reported it was not possible to determine what proportion of HBsAg positive individuals were acutely infected and within the window period prior to anti-HBc seroconversion. Future studies should include both markers to allow a more accurate understanding of study results. Additional sample details including country of birth and ethnicity would also assist interpretation36. An additional limitation of existing data concerns a lack of data on the age range of samples and individuals duration of drug injecting history and hence period of elevated exposure to viral hepatitis, which would permit more accurate understanding of varying prevalence of both HCV and HBV among samples. The reliance upon older studies, with less accurate serological testing modalities, small sample sizes, and those conducted in countries where laboratory capacity is limited, increases uncertainty about the validity of both HCV and HBV reports.
A final issue concerns the representativeness of IDU samples. Some studies of HBV and HCV recruited “lifetime” IDUs, whereas others recruited “current” or “past year” IDUs. They also were recruited from a variety of locations: prisons, drug treatment centres, outpatient clinics and other medical settings, where IDUs may differ in their risk behaviour and exposure to viral hepatitis. Further, convenience sampling is most often used, so it is possible that the samples do not represent the IDU population from which they are drawn. Data were also typically sub-national, from a limited number of locations that may or may not be representative of the epidemic nationally, particularly in larger countries where there may be considerable geographic variation, potentially limiting national representativeness.
Limitations of this review
We have used the same methods as in our previous reviews, which have been detailed elsewhere1. As those previous investigations also revealed, limitations include the current concentration of peer-reviewed literature from high-income countries, the review being conducted by a small team, and the greater potential for papers in languages other than English to be overlooked. As we did for the earlier reviews we attempted to address these limitations by consulting widely with experts and stakeholders, seeking unpublished reports and verification of the data and reports included and enlisting the support of UN and other agencies, who facilitated access to data and contact with relevant in-country personnel.
Conclusions
The response to blood borne virus transmission among IDUs has primarily focussed on HIV. Maintaining and strengthening the response to HIV among IDUs remains crucial, but the significance of viral hepatitis must receive greater recognition than is currently the case. Investment in, and development of, comprehensive and effective strategies to prevent the transmission of viral hepatitis, and reduce resultant morbidity and mortality among IDUs, are urgently required. The Viral Hepatitis resolution of the 63rd World Health Assembly10 requested that the Director-General collaborate with all relevant stakeholders in supporting surveillance, prevention, and treatment goals, especially in developing countries. It is essential that policies and strategies for addressing viral hepatitis include IDUs, who are at elevated risk and often have poorer access to services. The current review has provided estimates of the scale of this problem at country, regional and global levels. These findings should inform efforts to efforts to accurately scale and appropriately target the response.
Acknowledgements
This study was completed as part of the work of the 2010 Global Burden of Disease (GBD) Illicit Drugs expert group. Thanks to the GBD team from the National Drug and Alcohol Research Centre (NDARC), University of New South Wales (UNSW), in particular Bianca Calabria, Chiara Bucello, and Anna Roberts, who contributed to earlier phases of the work. Thanks to Mary Kumvaj and Eva Congreve, who assisted with the compilation of the literature. Thanks to Paul McElwee (Burnet Institute) for assistance with data extraction.
The work of the Reference Group to the UN on HIV and Injecting Drug Use in 2007 was conducted by the Secretariat, based at NDARC, UNSW (see www.idurefgroup.unsw.edu.au//IDURGWeb.nsf/page/Secretariat). In particular, we thank Benjamin Phillips for his work on the systematic review of HIV and injecting drug use.
Thank you to the many local and international experts who assisted with compilation of data, provided further data, commented on estimates, or who provided support during the data collection process. In particular, we acknowledge the assistance of Lucas Wiessing (EMCDDA) in disseminating and endorsing our call for additional data within Europe, Maria Elena Medina-Mora (National Institute on Psychiatry Ramón de la Fuente Muñiz, Mexico) for sourcing and translation efforts, Khayriyyah Mohd Hanafiah (Johns Hopkins Bloomberg School of Public Health) and Steven Wiersma (World Health Organization) for advice on hepatitis epidemiology and early results from the GBD hepatitis expert group, and Annette Verster (WHO) for financial support of part of this review. Contributors of data and advice on this review are listed fully at http://www.gbd.unsw.edu.au/gbdweb.nsf/page/Contribution of Data
Funding: This review received some funding support from the World Health Organization’s HIV Department, and the Australian National Drug and Alcohol Research Centre, which receives funding from the Australian Department of Health and Ageing. The HCV Synthesis Project was funded by NIDA Grant R01 DA018609. Don Des Jarlais is supported by NIH grant DA003574. Louisa Degenhardt is supported by an Australian NHMRC Senior Research Fellowship. Paul Nelson and Danielle Horyniak are both supported by Australian Postgraduate Awards.
Role of funding source
The US NIH supported the work of the HCV Synthesis project. The HIV department of WHO provided some funds to support this review. Staff from WHO assisted with data collection by circulating requests for data to WHO and other UN agency staff, and facilitating access to reports that may have had data of use in this review. Decisions on analysis, write up, interpretation of results and submission of the manuscript for publication were all made by the authors. The funders had no role in the design, conduct, analysis, write up or interpretation of this study.
Footnotes
Contributions
PN & LD developed the overall methodology for use in the reviews. HH and DDJ developed the methodology and oversaw data extraction for the HCV Synthesis Project, and provided this for use in this review. DH maintained the customised database. PN & DH conducted literature searches, extracted data and provisionally selected reports for use in generating estimates. PN & LD decided on the final set of reports, with advice from BC; these were reviewed by HH, DDJ, DH & BM. BM developed the methodology and generated regional and global estimates; these were reviewed by PN & LD. PN & LD led the writing of the manuscript; HH, DDJ, DH, BC & BM commented and contributed text. PN generated the maps. All authors had access to all data used in this review. All authors gave approval for the manuscript to be submitted.
Conflicts of interest
LD and BM have received grant money and have acted as independent consultants to the World Health Organization, UNAIDS and the United Nations Office on Drugs and Crime. DDJ has been funded by, and acted as a consultant to, the WHO. LD received an untied educational grant (2006–2008) from Reckitt Benckiser to conduct a post-marketing surveillance study of buprenorphine-naloxone in the treatment of heroin dependence in Australia.
References
- 1.Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee S, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Hall W. What we know about the extent of illicit drug use, dependence, and their contribution to the global burden of disease. Lancet. doi: 10.1016/S0140-6736(11)61138-0. in press. [DOI] [PubMed] [Google Scholar]
- 3.Donoghoe M, Wodak A. Health and social consequences of injecting drug use. In: Stimson G, Des Jarlais D, Ball A, editors. Drug injecting and HIV infection: Global dimensions and local responses. London: UCL Press; 1998. pp. 42–57. [Google Scholar]
- 4.Te HS, Jensen DM. Epidemiology of hepatitis B and C virus: a global overview. Clinics in Liver Disease. 2010;14:1–21. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Dore GJ, Freeman AJ, Law M, Kaldor JM. Is severe liver disease a common outcome for people with chronic hepatitis C? Journal of Gastroenterology and Hepatology. 2002;17:423–430. doi: 10.1046/j.1440-1746.2002.02730.x. [DOI] [PubMed] [Google Scholar]
- 6.Limberg W. Natural history, treatment and prevention of hepatitis C in injecting drug users: an overview. In: Jager J, Limburg W, Kretzschmar M, Postma M, Wiessing L, editors. Hepatitis C and injecting drug user: impact, costs and policy options. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2004. pp. 21–38. [Google Scholar]
- 7.Alter MJ. Epidemiology of hepatitis C virus infection. World Journal of Gastroenterology. 2007;13(17):2436. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 63rd World Health Assembly. Geneva: World Health Organization; 2010. [Accessed on May 24th 2011]. Resolution A63/15: Viral Hepatitis. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_15-en.pdf. [Google Scholar]
- 11.Levine OS, Vlahov D, Nelson KE. Epidemiology of hepatitis B virus infections among injecting drug users: seroprevalence, risk factors, and viral interactions. Epidemiologic Reviews. 1994;16(2):418–436. doi: 10.1093/oxfordjournals.epirev.a036161. [DOI] [PubMed] [Google Scholar]
- 12.Walsh N, Verster A, Doupe A, Vitoria M, Lo Y-R, Wiersma ST. The silent epidemic: Responding to viral hepatitis among people who inject drugs. In: Cook C, editor. The Global State of Harm Reduction 2010: Key issues for broadening the response. London: International Harm Reduction Association; 2010. pp. 71–80. [Google Scholar]
- 13.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18(5):352–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. American Journal of Epidemiology. 2008;168(10):1099–1109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011 doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Stern R, Hagan H, Lelutiu-Weinberger C, Des Jarlais D, Scheinmann R, Strauss S, et al. The HCV synthesis project: Scope, methodology and preliminary results. BMC Medical Reseacrh Methodology. 2008;18:341–351. doi: 10.1186/1471-2288-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali H, Calabria B, Phillips B, Singleton J, Sigmundsdottir L, Roberts A, et al. NDARC Technical Report No. 314. Sydney: National Drug and Alcohol Research Centre, University of NSW; 2010. [Accessed on May 27th 2011]. Searching the grey literature to access information on drug, alcohol and HIV/AIDS research: An update. http://ndarc.med.unsw.edu.au/NDARCWeb.nsf/resources/TR313-317/$file/TR+314.pdf. [Google Scholar]
- 20.Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, et al. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug & Alcohol Dependence. doi: 10.1016/j.drugalcdep.2010.11.032. in press; [DOI] [PubMed] [Google Scholar]
- 21.Matthews G, Dore GJ. The epidemiology of HIV and viral hepatitis coinfection. In: Dore GJ, Sasadeusz J, editors. Coinfection – HIV and viral hepatitis: a guide for clinical management. Sydney: Australasian Society for HIV Medicine Inc.; 2003. [Accessed on May 27th 2011]. http://www.hep.org.au/documents/Coinfection-680KB.pdf. [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23.Mathers B, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick R, et al. HIV prevention, treatment and care for people who inject drugs: A systematic review of global, regional and country level coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 24.UN Population Division. World population prospects: the 2008 revision. New York: United Nations; 2009. [Google Scholar]
- 25.Kimber J, Copeland L, Hickman M, Macleod J, McKenzie J, De Angelis D, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. British Medical Journal. 2010;341:c3172. doi: 10.1136/bmj.c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Harm Reduction Association. A briefing note to the UNITAID Board. London: International Harm Reduction Association; 2009. [Accessed on 14th May 2011]. Poor access to HCV treatment is undermining Universal Access. http://www.ihra.net/files/2010/06/17/HCVBriefingPaperEnglish.pdf. [Google Scholar]
- 27.Swan T. The hepatitis C treatment pipeline report. New York City: Treatment Action Group; 2011. [Accessed on June 18th 2011]. http://www.treatmentactiongroup.org/uploadedFiles/About/Publications/TAG_Publications/2011/HCV%20pipeline%202011%20final.pdf. [Google Scholar]
- 28.Gibson A, Randall D, Degenhardt L. The increasing mortality burden of liver disease among opioid dependent people: Cohort study. Addiction. doi: 10.1111/j.1360-0443.2011.03575.x. in press. [DOI] [PubMed] [Google Scholar]
- 29.Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105(5):844–859. doi: 10.1111/j.1360-0443.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin NK, Vickerman P, Hickman M. Mathematical modelling of Hepatitis C treatment for injecting drug users. Journal of Theoretical Biology. 2011;274(1):58–66. doi: 10.1016/j.jtbi.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 31.Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368(9539):938–945. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 32.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. Journal of Hepatology. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 53(2):348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 34.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Morbidty and Mortality Weekly Reports. 2005;54(RR16):1–25. [PubMed] [Google Scholar]
- 35.Hunt DR, Saab S. Viral Hepatitis in Incarcerated Adults: A Medical and Public Health Concern. Am J Gastroenterol. 2009;104(4):1024–1031. doi: 10.1038/ajg.2008.143. [DOI] [PubMed] [Google Scholar]
- 36.Cowie B. The linguistic demography of Australians living with chronic hepatitis B. Australian and New Zealand Journal of Public Health. 2011;35(1):12–15. doi: 10.1111/j.1753-6405.2010.00634.x. [DOI] [PubMed] [Google Scholar]