Abstract
We measured levels of 10 polybrominated diphenyl ether (PBDE) congeners in serum collected during pregnancy and at delivery from 416 pregnant, predominantly immigrant, women living in Monterey County, CA. The most frequently detected congeners were BDE-47, -99, -100, and -153, all components of the penta mixture, detected in >97% of samples. We used multivariable regression models to examine factors associated with exposure to individual PBDE congeners as well as their total summed concentration (ng/g lipid). Prenatal and delivery total PBDE levels were correlated between sampling times (n = 21; Pearson r = 0.99, p < 0.001). In multivariable models, total PBDE levels increased significantly with time residing in the U.S. (p < 0.001) and among women with ≥3 pieces of stuffed furniture in their homes (p < 0.05). Women’s total PBDE levels increased 4.0% (95% CI = 2.8, 5.3) for each additional year residing in the U.S., after adjustment for prepregnancy BMI, weight gain during pregnancy, and SES. Having ≥3 pieces of stuffed furniture in the home was associated with a 26.8% (95% CI = 2.0, 57.5) increase in women’s serum PBDE levels. Findings suggest PBDE indoor contamination in California homes is contributing to human exposures in a population of recent immigrants.
INTRODUCTION
Polybrominated diphenyl ethers (PBDE) are used as flame-retardants in the production of various consumer products, including foam, plastics, and textiles. These synthetic compounds have been produced for several decades primarily in the form of three technical mixtures (penta-, octa-, and decabromo diphenyl ethers (-BDEs)). Penta-BDE has been mixed most often into polyurethane foam used in upholstered furniture and carpet padding, whereas octa- and deca-BDE are used in electronics (e.g., computer casings), textiles and other plastic products.1
PBDEs are persistent, bioaccumulative and globally distributed in the environment.2 Congeners with six or fewer bromines are more persistent, with estimated half-lives ranging from 2 to 12 years in humans.3 Biological half-lives of the higher brominated PBDEs with 7–10 bromine substituents are lower, with half-lives estimated on the order of 15–90 days.4 Although penta- and octa-BDE have been banned for use since 2006 in at least nine U.S. states, including California, exposure continues because these compounds remain in older furniture and other products in the home.
BDE-47, -99, -100, and -153, which are the primary components of the penta mixture, are the most common congeners found in serum in the U.S. population.5 These compounds have also been measured in cord blood, placental tissue, and breast milk, demonstrating that PBDEs can be transferred pre- and postnatally from women to their children.6,7 Levels of PBDEs in human serum are approximately 20 times higher in the U.S population than in Europe,8 with California residents among the highest exposed.9 Higher historical use and subsequent continuing human exposure to PBDEs in California is likely due to use of these brominated flame retardants to comply with the State’s strict furniture flammability standards.9,10 Because PBDEs are not chemically bound to the materials in the products in which they are used, they are released into the environment over time. Important exposure pathways include household dust, food, and to a lesser extent, air.11,12 While PBDEs are frequently measured in U.S. foods and market-basket surveys,13 pharmacokinetic modeling suggests that PBDEs present in food may not substantially contribute to body burdens in the U.S. population.11 Multiple studies point to high levels of PBDEs measured in house dust as the primary route of exposure in North America, especially in children.11,14,15 Further, recent studies have reported significant correlations between levels of PBDE congeners in house dust and breast milk, and human serum.15
PBDEs are endocrine disrupting compounds and the congeners BDE-47 and BDE-99 have been shown to adversely affect reproductive and thyroid hormone function in young animals exposed in utero to environmentally relevant doses.2,16 Thyroid hormone mediated effects on neurobehavioral development have also been reported in animals.16 While few human health studies of PBDEs have been published, recent evidence suggests that higher exposure levels are related to reduced fecundability,17 decreased sperm count,18 altered thyroid hormone levels in adults19 and infants,20 and developmental neurotoxicity in prenatally exposed children.6
The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) is a longitudinal birth cohort study investigating environmental exposures and their health effects on children residing in Monterey County, California. In this paper we report serum PBDE levels in 416 pregnant, predominantly immigrant, women, and identify important determinants of exposure for this cohort. We also compare CHAMACOS PBDE serum levels to those measured in pregnant women participating in the National Health and Nutrition Examination Survey (NHANES) (2003–2004).
MATERIALS AND METHODS
Study Population
Between October 1999 and November 2000, 601 pregnant women were enrolled in the CHAMACOS birth cohort study from six local prenatal clinics. Eligible women were g18 years old, <20 weeks gestation, Spanish- or English-speaking, eligible for Medi-Cal, receiving prenatal care at local community clinics, and planning to deliver at the county hospital in Salinas, California.21 Women were excluded from the current analyses if they bore twins (n = 5), or provided a sample of insufficient volume for PBDE analysis (n = 168).
Study Interviews and Home Visits
Participants’ demographic, health, diet and household information were collected through personal interviews conducted in English or Spanish by bicultural staff. The first prenatal interview occurred shortly after enrollment in the study, at approximately 13 weeks gestation (mean = 13.4 weeks, SD = 5.2). The second prenatal interview occurred at approximately 26 weeks gestation (mean = 25.9 weeks, SD = 2.6) and a postpartum interview occurred shortly after delivery (mean = 8.8 days, SD = 17.9). We administered a food frequency questionnaire to each participant during the second prenatal interview. Home visits involving extensive visual inspections were conducted between the first and second pre-natal interviews. Information obtained included quality of house-keeping, number of pieces of stuffed furniture present (chairs, couches, love seats), and percentage of floors covered with wall-to-wall carpeting.22 Medical records were abstracted by a registered nurse to obtain data on women’s weight gain during pregnancy and general health status.
Blood Collection and PBDE Analysis
We collected blood from participants by venipuncture at the time of the second prenatal interview and just before delivery. Samples were immediately processed and stored at −80 °C until they were shipped on dry ice for analysis. The Centers for Disease Control and Prevention (CDC) (Atlanta, GA) measured PBDEs in serum samples using gas chromatography isotope dilution high resolution mass spectrometry (GC-IDHRMS).23 Samples were analyzed for 10 tri- to heptabrominated congeners BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183. The chemical names and molecular weights of these PBDE congeners as well as a more detailed description of the analytical laboratory methods are provided in the Supporting Information (SI).
PBDE serum concentrations are expressed on a blood lipid basis. Total lipids were determined based on the measurement of triglyceride and total cholesterol in serum using standard enzymatic methods (Roche Chemicals, Indianapolis, IN).24
The limits of detection (LOD) for PBDE analyses ranged between 0.2 and 0.7 ng/g lipids for all congeners, except for BDE-47, which ranged from 0.8 to 2.6 ng/g lipids (Table 1) using on average 1.7 g (range: 0.9 – 3.5 g) of serum for analysis. Quality control samples (n = 3) and method blanks (n = 3) were included in each run. Data below the LOD but for which a signal was detected were coded with the concentration obtained. Data below the LOD for which no signal was detected were imputed from the log-normal probability distribution.25 To evaluate total PBDE levels, we summed all 10 congeners by weight; we present total PBDE concentrations converted to their molar equivalents and summed in the SI.
Table 1.
Serum PBDE Concentrations (ng/g lipid) among Women in the CHAMACOS Cohort (n = 416)
| LOD range | DF (%) | geo mean (95% Cl) | min | 25th | 50th | 75th | 90th | max | |
|---|---|---|---|---|---|---|---|---|---|
| BDE-17 | 0.2–0.7 | 1.4 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 2.8 |
| BDE-28 | 0.2–0.7 | 51.9 | 0.4 (0.3, 0.5) | <LOD | <LOD | 0.4 | 1.1 | 2.2 | 29.7 |
| BDE-47 | 0.8–2.6 | 99.5 | 15.8 (14.3, 17.3) | <LOD | 8.2 | 15.2 | 26.6 | 50.4 | 761 |
| BDE-66 | 0.2–0.7 | 15.0 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.4 | 10.1 |
| BDE-85 | 0.2–0.7 | 41.7 | <LOD | <LOD | <LOD | <LOD | 0.6 | 1.1 | 27.4 |
| BDE-99 | 0.2–0.7 | 99.0 | 4.4 (4.0, 4.9) | <LOD | 2.3 | 4.0 | 7.0 | 13.9 | 298 |
| BDE-100 | 0.2–0.7 | 97.8 | 2.8 (2.6, 3.1) | <LOD | 1.6 | 2.5 | 4.7 | 8.8 | 138 |
| BDE-153 | 0.2–0.7 | 97.8 | 2.4 (2.2, 2.7) | <LOD | 1.4 | 2.2 | 3.9 | 7.8 | 96.9 |
| BDE-154 | 0.2–0.7 | 40.8 | <LOD | <LOD | <LOD | <LOD | 0.6 | 1.2 | 20.6 |
| BDE-183 | 0.2–0.7 | 24.6 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.6 | 10.8 |
| Sum of 4 PBDE congenersa | |||||||||
| 100 | 26.3 (24.0, 28.9) | 2.6 | 14.6 | 24.6 | 42.0 | 78.1 | 1290 | ||
| Sum of 10 PBDE congenersb | |||||||||
| 100 | 28.7 (26.2, 31.4) | 4.2 | 15.9 | 26.9 | 44.8 | 83.8 | 1380 | ||
Sum of 4 PBDE congeners with DFs > 95%: BDE-47; BDE-99; BDE-100; BDE-153.
Sum of 10 PBDE congeners: BDE-17; BDE-28; BDE-47; BDE-66; BDE-85; BDE-99; BDE-100; BDE-153; BDE-154; BDE-183.
PBDE congeners were measured from 321 serum samples collected during pregnancy (at ~26 weeks gestation), and 116 samples collected just before delivery. Of the latter, 95 women provided only delivery serum samples. Twenty-one women provided samples at both time points, resulting in samples from 416 individuals. Total PBDE, BDE-47, BDE-99, BDE-100, and BDE-153 levels (n = 21) were very strongly correlated between the two sampling time points (Pearson r = 0.98 0.99, p-values <0.001). We used linear regression model parameters – derived from the 21 matched pairs to estimate PBDE levels at 26 weeks gestation for those women with only delivery samples. This approach ensured that all the PBDE values consistently reflected levels expected at the same gestational age for all women (i.e., ~26 weeks). The regression results are provided in the SI.
Data Analysis
Statistical analysis of the PBDE measurements included computation of descriptive statistics for individual congeners as well as a measure of total exposure to PBDEs (sum of all ten congeners). All values were log10-transformed. For individual PBDE congeners with detection frequencies (DFs) >95%, we computed within sample Pearson correlations (n = 416) (Table 2). We also calculated the ratio of BDE-99 to BDE-47. Differences in this ratio can provide insight about penta-BDE exposure patterns because the ratio of these congeners are known in dust and have been estimated in relevant human populations. We evaluated the BDE-99 to BDE-47 ratio to determine whether there was a difference between women who resided in the U.S. for shorter (≤5 years) compared to longer (>5 years) periods of time.
Table 2.
Pearson Correlations of Prenatal PBDE Congener and Total PBDE Levels (ng/g lipid) within Women’s Serum Samples (n = 416)
| BDE-47 | BDE-99 | BDE-100 | BDE-153 | total PBDE | |
|---|---|---|---|---|---|
| BDE-47 | 1 | ||||
| BDE-99 | 0.93a | 1 | |||
| BDE-100 | 0.95a | 0.90a | 1 | ||
| BDE-153 | 0.78a | 0.73a | 0.89a | 1 | |
| total PBDE | 0.99a | 0.95a | 0.97a | 0.85a | 1 |
p < 0.001.
We used Pearson correlations and ANOVA to assess univariate associations between the total PBDE levels (log10-transformed) and potential factors associated with exposure, including age, diet, parity, weight gain during pregnancy, time residing in the U.S. at enrollment, percentage of rooms with wall-to-wall carpeting, number of pieces of stuffed furniture in the home, and quality of housekeeping. We then constructed multi-variable linear regression models with log10-transformed total PBDE levels as the dependent variable and exposure determinant variables found to have significant (p < 0.1) univariate relationships, including women’s country of birth, time residing in the U.S., education level, and number of stuffed pieces of furniture in the home (Table 3). We included variables to adjust our final model for women’s socioeconomic status (SES) (at or below federal poverty threshold or above the federal poverty threshold), prepregnancy BMI (underweight/average or overweight/obese) and weight gain during pregnancy (kg). For comparison, multivariable linear regression models were also created with log10-transformed concentrations of the four most frequently detected individual congeners (BDE-47, BDE-99, BDE-100, BDE-153).
Table 3.
Exposure Characteristics of Pregnant Women in the CHAMACOS Cohort (n = 416) and PBDE Concentrations (ng/g Lipid)
| total PBDE |
BDE-47 |
BDE-99 |
BDE-100 |
BDE-153 |
||
|---|---|---|---|---|---|---|
| n (%) | GM (95% CI)c | GM (95% CI)c | GM (95% CI)c | GM (95%CI)c | GM (95% CI)c | |
| Demographic Information | ||||||
| Age Categories | ||||||
| ≤25 years | 243 (58.4) | 28.3 (25.2, 31.8) | 15.4 (13.6, 17.5) | 4.5 (4.0, 5.1) | 2.7 (2.4, 3.1) | 2.3 (2.1, 2.6) |
| >25 years | 173 (41.6) | 29.2 (25.4, 33.6) | 16.2 (14.0, 18.8) | 4.4 (3.7, 5.1) | 2.9 (2.5, 3.4) | 2.6 (2.3, 3.0) |
| Country of Birth | ||||||
| U.S. born | 57 (13.7) | 51.8b (39.9, 67.3) | 28.9b (22.1, 37.8) | 7.2b (5.3, 9.7) | 5.3b (4.1, 6.9) | 5.0b (3.9, 6.5) |
| born in Mexico/other | 359 (86.3) | 26.1 (23.8, 28.6) | 14.3 (12.9, 15.8) | 4.1 (3.7, 4.5) | 2.5 (2.3, 2.8) | 2.2 (2.0, 2.4) |
| Time Residing in the U.S. | ||||||
| ≤5 years | 224 (53.9) | 23.3 (20.6, 26.3) | 12.7 (11.1, 14.5) | 3.9 (3.4, 4.5) | 2.1 (1.9, 2.4) | 1.8 (1.5, 2.0) |
| 6–10 years | 83 (19.9) | 30.9 (26.6, 36.1) | 17.3 (14.5, 20.5) | 4.5 (3.8, 5.3) | 3.3 (2.8, 3.9) | 2.9 (2.5, 3.3) |
| 11+ years | 62 (14.9) | 32.6 (26.3, 40.5) | 18.0 (14.3, 22.7) | 4.4 (3.6, 5.6) | 3.5 (2.7, 4.4) | 3.4 (2.8, 4.2) |
| entire life | 47 (11.3) | 57.2b (42.3, 77.3) | 31.7b (23.2, 43.4) | 8.0b (5.6, 11.5) | 5.8b (4.4, 7.8) | 5.7b (4.3, 7.4) |
| Years Residing in the U.S. | ||||||
| 416 (100) | 0.34b | 0.33b | 0.23b | 0.37b | 0.44b | |
| Education | ||||||
| ≤6 years school | 179 (43.0) | 24.4 (21.6, 27.6) | 13.4 (11.7, 15.3) | 3.9 (3.4, 4.4) | 2.4 (2.1, 2.8) | 2.0 (1.8, 2.3) |
| 7–12 years school | 151 (36.3) | 29.9 (25.6, 34.9) | 16.6 (14.1, 19.5) | 4.6 (3.9, 5.4) | 2.9 (2.5, 3.4) | 2.6 (2.2, 3.0) |
| high school grad + | 86 (20.7) | 37.3a (30.0, 46.3) | 20.2a (15.9, 25.7) | 5.6a (4.4, 7.1) | 3.6b (2.9, 4.5) | 3.3b (2.7, 4.0) |
| Poverty | ||||||
| at or below poverty threshold | 247 (63.3) | 27.9 (25.1, 31.0) | 15.2 (13.6, 17.1) | 4.3 (3.8, 4.8) | 2.8 (2.5, 3.1) | 2.4 (2.2, 2.7) |
| within 200% of poverty threshold | 129 (33.1) | 32.2 (26.7, 38.7) | 18.0 (14.8, 21.9) | 5.0 (4.1, 6.2) | 3.2 (2.6, 3.8) | 2.6 (2.2, 3.1) |
| above 200% of poverty threshold | 14 (3.6) | 21.4 (13.1, 34.8) | 10.9 (6.3, 18.8) | 2.5a (1.4, 4.4) | 1.9 (1.0, 3.6) | 2.6 (1.5, 4.6) |
| Prepregnancy BMI Categories | ||||||
| under/normal weight (<25) | 164 (39.5) | 25.0 (21.8, 28.6) | 13.4 (11.6, 15.6) | 3.8 (3.3, 4.4) | 2.5 (2.1, 2.9) | 2.3 (2.0, 2.7) |
| overweight/obese (≥25) | 251 (60.5) | 31.4a (27.9, 35.4) | 17.5a (15.4, 19.9) | 4.9a (4.3, 5.6) | 3.1a (2.7, 3.5) | 2.5 (2.2, 2.8) |
| Prepregnancy BMI | ||||||
| Pearson r | 415 (99.8) | 0.14a | 0.16b | 0.12a | 0.13a | 0.05 |
| Weight Gain during Pregnancy (kgs) | ||||||
| Pearson r | 409 (98.3) | −0.10 | −0.09 | −0.08 | −0.10a | −0.13a |
| Parity | ||||||
| 0 children | 151 (36.3) | 25.9 (22.1, 30.3) | 14.1 (11.9, 16.7) | 4.2 (3.5, 5.0) | 2.5 (2.1, 2.9) | 2.1 (1.8, 2.4) |
| 1 child | 128 (30.8) | 29.5 (24.9, 34.9) | 16.0 (13.3, 19.1) | 4.6 (3.8, 5.5) | 2.9 (2.4, 3.5) | 2.6 (2.2, 3.1) |
| ≥2 children | 137 (32.9) | 31.3 (27.2, 36.0) | 17.6 (15.2, 20.4) | 4.6 (4.0, 5.4) | 3.2 (2.7, 3.7) | 2.7a (2.4, 3.2) |
| Breastfeeding History Previous Births | ||||||
| none | 192 (46.2) | 27.8 (24.2, 32.0) | 15.2 (13.0, 17.6) | 4.4 (3.8, 5.2) | 2.7 (2.3, 3.1) | 2.3 (2.0, 2.7) |
| <12 months | 118 (28.4) | 30.1 (25.9, 35.0) | 16.8 (14.3, 19.7) | 4.4 (3.8, 5.2) | 3.0 (2.6, 3.6) | 2.7 (2.3, 3.1) |
| ≥12 months | 106 (25.5) | 28.8 (24.0, 34.5) | 15.7 (13.0, 19.1) | 4.5 (3.7, 5.4) | 2.8 (2.3, 3.5) | 2.4 (2.0, 2.9) |
| Maternal Smoking during Pregnancy | ||||||
| no | 393 (94.5) | 28.3 (25.8, 31.1) | 15.6 (14.1, 17.2) | 4.4 (4.0, 4.8) | 2.8 (2.5, 3.1) | 2.4 (2.2, 2.6) |
| yes | 23 (5.5) | 35.3 (23.0, 54.0) | 18.8 (12.0, 29.5) | 5.3 (3.4, 8.5) | 3.8 (2.4, 5.9) | 3.6a (2.3, 5.5) |
| Housing Characteristics | ||||||
| No. Pieces of Stuffed Furniture in Home | ||||||
| 0 | 48 (12.7) | 23.9 (17.5, 32.5) | 13.0 (9.3, 18.2) | 3.7 (2.7, 5.2) | 2.3 (1.7, 3.2) | 1.9 (1.4, 2.5) |
| 1–2 | 239 (63.1) | 27.5 (24.6, 30.7) | 15.2 (13.5, 17.2) | 4.1 (3.6, 4.6) | 2.7 (2.4, 3.1) | 2.4 (2.1, 2.6) |
| 3 + | 92 (24.3) | 35.4a (28.6, 43.9) | 19.1 (15.3, 23.8) | 5.7a (4.5, 7.1) | 3.5a (2.8, 4.4) | 3.1 (2.5, 3.9) |
| Any Carpet in the Home | ||||||
| no | 29 (7.7) | 28.8 (19.3, 42.9) | 15.5 (10.2, 23.5) | 4.5 (2.8, 7.0) | 2.7 (1.8, 4.2) | 2.7 (1.8, 4.1) |
| yes | 350 (92.4) | 28.7 (26.0, 31.7) | 15.8 (14.2, 17.6) | 4.4 (3.9, 4.9) | 2.8 (2.6, 3.2) | 2.4 (2.2, 2.7) |
| % of Rooms with Carpet | ||||||
| Pearson r | 379 (91.1) | 0.04 | 0.05 | 0.04 | 0.07 | 0.03 |
| Resident Density | ||||||
| <1 person per room | 39 (10.4) | 34.2 (23.8, 49.0) | 18.6 (12.8, 27.0) | 4.7 (3.1, 7.2) | 3.3 (2.3, 4.8) | 3.3 (2.3, 4.6) |
| ≥1 person per room | 337 (89.6) | 28.2 (25.5, 31.1) | 15.5 (14.0, 17.3) | 4.4 (3.9, 4.8) | 2.8 (2.5, 3.1) | 2.4 (2.2, 2.6) |
| Quality of Housekeeping | ||||||
| less clean | 21 (5.6) | 25.6 (18.3, 36.0) | 14.5 (10.0, 30.0) | 4.2 (3.0, 5.9) | 2.6 (1.8, 3.6) | 2.0 (1.5, 2.7) |
| average | 206 (54.5) | 30.4 (26.3, 35.1) | 16.5 (14.1, 19.3) | 4.7 (4.0, 5.5) | 3.0 (2.6, 3.5) | 2.6 (2.3, 3.0) |
| excellent | 151 (39.9) | 27.2 (23.9, 31.0) | 15.2 (13.2, 17.4) | 4.1 (3.5, 4.7) | 2.6 (2.3, 3.1) | 2.3 (2.0, 2.6) |
| Diet | ||||||
| Daily Servings of Dairy | ||||||
| Pearson r | 416 (100) | −0.06 | −0.06 | −0.05 | −0.05 | −0.04 |
| Daily Servings of Meat | ||||||
| Pearson r | 416 (100) | 0.05 | 0.04 | 0.03 | 0.03 | 0.05 |
| Total Daily Fat Intake | ||||||
| Pearson r | 393 (94.5) | −0.04 | −0.07 | −0.009 | −0.04 | −0.02 |
| Consumption of Fish | ||||||
| ≤1 per month | 226 (56.2) | 28.9 (25.6, 32.7) | 15.9 (13.9, 18.1) | 4.5 (3.9, 5.1) | 2.9 (2.5, 3.3) | 2.5 (2.2, 2.8) |
| >1 per month | 176 (43.8) | 28.3 (24.6, 32.5) | 15.5 (13.4, 18.0) | 4.4 (3.8, 5.1) | 2.8 (2.4, 3.2) | 2.4 (2.1, 2.7) |
ANOVA or Pearson’s correlation p < 0.05.
p < 0.01.
GMs and 95% confidence interval except when Pearson coefficient (r) is presented.
Finally, we compared adjusted geometric mean (GM) PBDE congener concentrations among pregnant women in NHANES (n = 71) and CHAMACOS using linear regression. When comparing NHANES and CHAMACOS women’s PBDE levels, we adjusted for age (continuous), ethnicity (Mexican or Mexican American; other Hispanic; non-Hispanic white; non-Hispanic black or other race, including multiracial) and SES. All analyses were conducted using Stata software, version 10 (StataCorp LP, College Station, TX).
RESULTS
Table 1 presents the GM and distribution of 10 individual and summed PBDE congeners, and the sum of the four most frequently detected congeners. Total PBDE levels ranged from 4.2 to 1380 ng/g lipid. The congener with the highest serum concentration was BDE-47 (GM =15.8 ng/g lipid), followed by BDE-99 (GM = 4.4 ng/g lipid), BDE-100 (GM = 2.8 ng/g lipid) and BDE-153 (GM = 2.4 ng/g lipid). BDE-47 was the dominant congener representing an average of 61.2% of the total PBDE molar concentration. BDE-99 represented an average of 15.8%, BDE-100 represented an average of 9.5%, and BDE-153 represented an average of 8.0% of the total. The DFs for BDE-47, BDE-99, BDE-100, and BDE-153 ranged from 97.8% to 99.5%, and for the other six less frequently detected congeners (BDE-17, BDE-28, BDE-66, BDE-85, BDE-154, and BDE-183) ranged from 1.4% to 51.9% (Table 1). The four most frequently detected PBDE congeners were strongly correlated with each other (Pearson r = 0.73–0.95, p-values <0.001) (Table 2). As noted in the SI, total PBDE levels were highly associated (β = 0.96; p < 0.001) between paired samples collected at 26 weeks gestation and delivery, but slightly lower when collected at delivery (GM (95% CI): 29.5 ng/g (17.1, 51.0) and 21.4 ng/g (12.5, 36.6), respectively).
Table 3 presents demographic, occupational, housing and diet information by level of women’s total PBDE, and BDE-47, BDE-99, BDE-100, and BDE-153 congener concentrations (n = 416).
Demographic Information
Women were on average ((SD) 25.3 ± 5.0 years of age, overweight and multiparous, with a previous history of lactation. Ninety-six percent were of Mexican descent. Eighty-six percent were born in Mexico or another Latin American country, with 53.9% having lived in the U.S. ≤5 years (mean= 7.7 ± 7.8 years). Total PBDE levels were significantly higher among women who were born in the U.S. (GM = 51.8 vs 26.1 ng/g lipid), attained more education (high school graduate or higher) (37.3 vs 26.4 ng/g lipid), and were overweight/obese before becoming pregnant (BMI g 25) (31.4 vs 25.0 ng/g lipid) (ANOVA p-values <0.05). PBDE levels increased with the amount of time women resided in the U.S. (Pearson r = 0.34; p < 0.001), with women who had lived their entire lives in the U.S. having significantly higher PBDE levels compared to women who had not lived their entire lives in the U.S. (GM = 57.2 vs 26.3 ng/g lipid). Within the CHAMACOS cohort, adjusted GM BDE-47, BDE-99, BDE-100, and BDE-153 levels among women born in the U.S. (n = 57) were significantly higher compared to the rest of the cohort born outside the U.S. (n = 359) (p < 0.001).
Total PBDE, BDE-47, BDE-99, and BDE-100 levels were higher among overweight/obese women (prepregnancy BMI g 25) (ANOVA p < 0.05), and the negative correlations of BDE-100 and BDE-153 with weight gain during pregnancy were statistically significant (Pearson r, p < 0.05). A small percentage (5.5%) of women reported smoking during pregnancy. BDE-153 levels were significantly higher among women who smoked (ANOVA p < 0.05), and had ≥2 children (Cuzick trend test p < 0.05)26 (Table 3).
Housing Characteristics
Most women had <3 stuffed pieces of furniture (75.7%) and at least some wall-to-wall carpeting (92.4%) in their homes. Home resident density was relatively high with 89.6% having ≥1 person(s) per room (average 1.5 ± 0.7 people per room).
Total PBDE levels were significantly higher among women with ≥3 pieces of stuffed furniture in the home (GM = 35.4 vs 26.8 ng/g lipid). Levels of BDE-47, -99, -100, and -153 were each higher among women who had ≥3 pieces of stuffed furniture in the home. No significant relationships were found between total PBDEs or the four individual congeners and resident density or carpeting in the home.
Diet
The majority of CHAMACOS women consumed ≤1 serving of meat per day (66%) and ≤1 serving of fish per month (56.3%). Average fat intake in this population was 102 g per day. We found no significant relationships between women’s PBDE levels and daily servings of diary or meat, total daily fat intake or fish consumption (Table 3).
The time women resided in the U.S. appears to confound the relationship between PBDE levels and several potential determinants of exposure described above. The following variables were significantly and positively associated with length of women’s residency in the U.S. (years): prepregnancy BMI, level of education, parity, and smoking during pregnancy (Kruskal–Wallis, p < 0.01).
MULTIPLE LINEAR REGRESSION MODEL
Table 4 presents results from the multivariable linear regression model for total PBDE levels as a function of exposure determinants. Total PBDE levels increased 4.0% (95% CI = 2.8, 5.3; p < 0.001) for each additional year residing in the U.S., after adjustment for prepregnancy BMI, weight gain during pregnancy, and SES. Having ≥3 pieces of stuffed furniture in the home was associated with a 26.8% (95% CI = 2.0, 57.5; p < 0.05) increase in women’s PBDE serum levels. Similarly, results from multivariable models using BDE-47, -99, -100, and -153 as the dependent variables indicate that women’s length of residency in the U.S. and having ≥3 pieces of stuffed furniture in the home were significant determinants for each of these congeners in this cohort. We observed inverse associations between PBDE congeners and maternal weight gain during pregnancy that reached statistical significance for BDE-153. These individual PBDE congener results are presented in the SI.
Table 4.
Results from Multiple Regression Analyses of Log-Transformed Total PBDE Levels (ng/g lipid) and Exposure Factors in Pregnant Women (n = 352)a
| % change (95% CI) |
p-value | |
|---|---|---|
| Years residing in the U.S. (continuous) | 4.0 (2.8, 5.3) | <0.001 |
| Prepregnancy BMI (overweight/obese (≥25)) |
16.1 (−4.9, 41.7) | 0.14 |
| Weight gained during pregnancy (kg) | −1.1 (−2.7, 0.32) | 0.20 |
| No. pieces of stuffed furniture in home (≥3) |
26.8 (2.0, 57.5) | 0.03 |
| SES (at or below poverty threshold) | −1.3 (−18.6, 19.8) | 0.87 |
Model R-squared = 0.15.
Ratio of BDE-99 to BDE-47
The median (95% CI) BDE-99/ BDE-47 ratio in our sample was 0.28 (0.27, 0.29), with a maximum of 1.9, which is consistent with previous human studies.27–30 The ratio of BDE-99 to BDE-47 by years women resided in the U.S are presented in the SI. We found statistically higher ratios among CHAMACOS women who resided ≤5 years in the U.S. compared to women who resided ≤5 years in the U.S. (median (95% CI) ratio = 0.31 (0.30, 0.32) (range = 0.13 1.9) vs 0.25 (0.24, 0.26) (range = 0.12 0.75), respectively; Kruskal – Wallis, p < 0.001).
Comparison of CHAMACOS and NHANES PBDE Levels
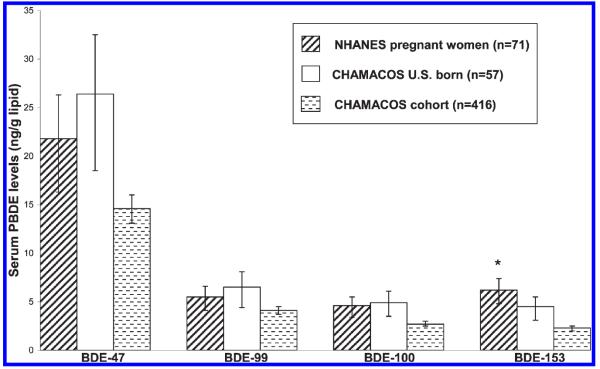
Figure 1 presents a comparison of adjusted GM serum levels of the four most frequently detected PBDE congeners in the CHAMACOS cohort (n = 416) and 71 pregnant women in NHANES. The NHANES women were on average 24 (SD = 9.4) weeks pregnant at the time of sample collection versus 26 (SD = 2.9) weeks among CHAMACOS women. CHAMACOS adjusted GM values for BDE-47, BDE-99, BDE-100, and BDE-153 were lower compared to NHANES. With the exception of BDE-153, these differences were not statistically significant after adjustment for age, race and SES (multivariable regression, p > 0.05). Among U.S. born CHAMACOS participants (n = 57), adjusted GM values of BDE-47, BDE-99, and BDE-100 were higher compared to NHANES, but these differences were not statistically significant (Figure 1).
Figure 1.
Comparison of adjusted GM PBDE congener levels among pregnant women in NHANES and the CHAMACOS cohort. Asterisk indicates significant comparison between entire CHAMACOS cohort (n = 416) and pregnant women in NHANES (from multivariable regression models adjusting for age, race and SES; p < 0.05). The error bars represent the 95% confidence interval.
DISCUSSION
This study reports serum PBDE levels from a largely Mexican immigrant population of pregnant women living in California. We found that total PBDE concentrations increased significantly with increasing years residing in the U.S., suggesting that the California environment is contributing to their PBDE exposures. CHAMACOS women who were born in the United States, however, had adjusted GM levels only slightly higher than pregnant women in NHANES. We would have expected these women to have significantly higher PBDE levels compared to a U.S. reference population based on previous findings of high levels in California residents.9 It is possible that we did not find significant differences due to the small number of U.S. born women in our study. We also were not able to assess what proportion of the NHANES pregnant women live in California.
For many decades PBDEs have been used in polyurethane foam in furniture and we found that women having ≥3pieces of stuffed furniture in their home had higher serum PBDE levels. A strength of our study is that this information was not obtained from questionnaire but rather we performed home visits where we recorded the type and number of pieces of furniture present.
Although PBDEs are chemically similar to PCBs, human exposure pathways for these two classes of compounds differ. Currently in the U.S., PCB exposures occur mostly from diet, while recent studies indicate that PBDE exposure likely comes from the indoor environment. We found no correlation between women’s PCB and the PBDE congener levels in this population (not shown), which is suggestive of nondietary routes of exposure to PBDEs. We also did not find significant associations of PBDE concentrations with dairy, meat or fish consumption, or with total fat intake. Our findings support research pointing to dust as a potentially dominant source of PBDE exposure.11,14,15
Recent studies have reported PBDE levels in California house dust that are several times higher than other regions of the U.S. and the world.9,31,32 The GM serum BDE-47 levels among U.S. born CHAMACOS participants were comparable to those reported by Zota et al.9 for California residents (36.2 ng/g lipid). Similar to other findings reported by these researchers,9 foreignborn Mexican American CHAMACOS participants had significantly lower serum PBDE levels compared to U.S.-born Mexican Americans and non-Hispanic whites.
The median BDE-99/BDE-47 ratio of 0.28 that we found in this study is considerably lower than that found in the commercial penta-BDE mixture or in indoor dust, which has been reported to have a ratio of BDE-99/BDE-47 greater than one.31,32 The lower level of BDE-99 compared to BDE-47 in biological samples suggests a shorter biological half-life of BDE-99 and/or differences in bioavailability. We also report that the ratio of BDE-99/BDE-47 was significantly higher in women who recently immigrated to the United States. This could have been caused by the women experiencing increased exposure to dust or other environmental sources that reflect the penta mixture. Women who lived in the United States for >5 years had a more metabolized pattern of these congeners as illustrated by their significantly lower ratio of BDE-99/BDE-47.
This study had several limitations. First, we did not have measurements of PBDEs in indoor air or house or car dust, so we cannot assess how well women’s serum and dust PBDE levels may be correlated. Additionally, we did not have direct measurements of PBDE levels in food (e.g., duplicate diet samples). Therefore, we cannot directly assess the importance of different pathways of exposure. Finally, complete information on the type and condition of all furniture and electrical devices present in the homes was lacking. Future studies without these limitations are needed to fully assess the sources and pathways of PBDE exposure among California residents.
In summary, we found that CHAMACOS women’s PBDE serum levels increased significantly with length of residence in the U.S and having ≥3 pieces of stuffed furniture in the home. These findings underscore the importance of the indoor environment in PBDE exposure. Additional research is needed to assess the health impacts of these exposures.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by PO1 ES009605 and RO1 ES015572 from NIEHS and RD 826709-01-1 from EPA. This paper has not been formally reviewed by the EPA or NIH. The views, findings and conclusions expressed in this document are solely those of the authors and do not necessarily represent the views of the EPA, NIH, or CDC. We gratefully acknowledge the CHAMACOS staff, students, community partners, and, especially, the CHAMACOS participants and their families, without whom this study would not be possible. Finally, we thank Christopher Salemme for designing our cover graphic.
Footnotes
ASSOCIATED CONTENT  Supporting Information. Molecular weights of 10 PBDE congeners, detailed analytical laboratory methods, and total PBDE molar concentrations in CHAMACOS maternal prenatal and delivery serum samples (pmol/g lipid). A summary of results from multiple regression analyses of log-transformed PBDE congener levels and determinants of exposure. Multiple plots representing total maternal PBDE levels by years residing in the U.S., matched maternal prenatal and delivery serum PBDE levels (n = 21), and the ratio of BDE-99 to BDE-47 by years women have resided in the U.S. This material is available free of charge via the Internet at http://pubs.acs.org.
Supporting Information. Molecular weights of 10 PBDE congeners, detailed analytical laboratory methods, and total PBDE molar concentrations in CHAMACOS maternal prenatal and delivery serum samples (pmol/g lipid). A summary of results from multiple regression analyses of log-transformed PBDE congener levels and determinants of exposure. Multiple plots representing total maternal PBDE levels by years residing in the U.S., matched maternal prenatal and delivery serum PBDE levels (n = 21), and the ratio of BDE-99 to BDE-47 by years women have resided in the U.S. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003;29(6):771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- (2).Birnbaum LS, Staskal DF. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004;112(1):9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht A, Fried KW. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66:3867–3872. [Google Scholar]
- (4).Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 2006;114(2):176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sjödin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol. 2008;42(4):1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- (6).Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wu N, McClean MD, Brown P, Aschengrau A, Webster TF. Participant experiences in a breastmilk biomonitoring study. Environ. Health. 2009;8(4) doi: 10.1186/1476-069X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hites RA. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ. Sci. Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- (9).Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ. Sci. Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- (10).Requirements, Test Procedure, and Apparatus for Testing the Flame Retardance of Resilient Filling Materials Used in Upholstered Furniture. Department of Consumer Affairs, Bureau of Home Furnishings and Thermal Insulation; North Highlands, CA: 2000. State of California. www.bhfti.ca.gov/industry/117.pdf. [Google Scholar]
- (11).Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008;18(1):2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- (12).Fraser AJ, Webster TF, McClean MD. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ. Health Perspect. 2009;117(10):1520–1525. doi: 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, et al. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006;114(10):1515–1520. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76(4):542–548. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- (15).Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ. Sci. Technol. 2010;44(14):5627–5632. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, Shakibaei M, et al. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect. 2008;116(3):308–314. doi: 10.1289/ehp.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Harley KG, Marks AR, Chevrier J, Bradman A, Sjödin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect. 2010;118(5):699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Akutsu K, Takatori S, Nozawa S, Yoshiike M, Nakazawa H, Hayakawa K, et al. Polybrominated diphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol. 2008;80(4):345–350. doi: 10.1007/s00128-008-9370-4. [DOI] [PubMed] [Google Scholar]
- (19).Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B. Polybrominated diphenylether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ. Health Perspect. 2010;118(10):1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, et al. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 2008;116(10):1376–1382. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland NT. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J. Child. Health. 2003;1(1):3–27. [Google Scholar]
- (22).Bradman A, Chevrier J, Tager I, Lipsett M, Sedgwick J, Macher J, et al. Association of housing disrepair indicators with cockroach and rodent infestations in a cohort of pregnant Latina women and their children. Environ. Health Perspect. 2005;113(12):1795–1801. doi: 10.1289/ehp.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem. 2004;76(7):1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- (24).Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr., Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- (25).Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- (27).Morland KB, Landrigan PJ, Sjödin A, Gobeille AK, Jones RS, McGahee EE, et al. Body burdens of polybrominated diphenyl ethers among urban anglers. Environ. Health Perspect. 2005;113(12):1689–1692. doi: 10.1289/ehp.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Schecter A, Päpke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005;47(3):199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- (29).She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in the San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46(5):697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- (30).Bradman A, Fenster L, Sjödin A, Jones RS, Patterson DG, Jr, Eskenazi B. Polybrominated diphenyl ether levels in the blood of pregnant women living in an agricultural community in California. Environ. Health Perspect. 2007;115(1):71–74. doi: 10.1289/ehp.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sjödin A, Päpke O, McGahee E, Focant JF, Jones RS, Pless-Mulloli T, et al. Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73(1 Suppl):S131–S136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- (32).Quirós-Alcalá L, Bradman A, Nishioka M, Harnly ME, Hubbard A, McKone TE, et al. Concentrations and loadings of polybrominated diphenyl ethers in dust from low-income households in California. Environ. Int. 2011;37(3):592–596. doi: 10.1016/j.envint.2010.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.