Abstract
Apurinic/apyrimidinic endonuclease (Ap endo) is a key DNA repair activity that confers radiation resistance in human cells. Here we examined the association between Ap endo activity and response to radiotherapy in pediatric ependymomas, tumors for which treatment options are limited and survival rates are only about 50%. We assayed Ap endo activity in 36 ependymomas and expression of Ape1/Ref-1, the predominant Ap endo activity in humans, in 44 tumors by immunostaining. Cox proportional hazards regression models were used to analyze the association of activity or expression with progression-free survival or with overall survival. Activity varied 13-fold and was not associated with tumor or patient characteristics. In univariate models with Ap endo activity entered as a continuous variable, the hazard ratio for progression increased by a factor of 2.18 for every 0.01 unit increase in activity (P ≤ 0.003) in 24 grade II ependymomas. Risk for death increased by a factor of 1.89 (P ≤ 0.02) in the same population. The fraction of Ape1/Ref-1 immunopositive cells varied widely within individual tumors and was not associated with either progression-free or with overall survival. Suppressing Ap endo activity in pediatric ependymoma cells significantly increased radiation sensitivity, suggesting that the association of activity with radiation response reflected, at least in part, repair of radiation-induced DNA lesions. Our data indicate that Ap endo activity is predictive of outcome following radiotherapy, and suggest that Ape1/Ref-1 promotes radiation resistance in pediatric ependymomas. Our findings support the use of inhibitors of Ap endo activity to overcome resistance.
Keywords: Ape1/Ref-1, brain tumor, DNA repair, γ-ray resistance
Introduction
Ependymomas are the third most common pediatric central nervous system tumor, showing a peak incidence in children 5 to 6 years old [1]. These generally slow growing tumors arise most frequently in the posterior fossa, especially in younger patients, but also commonly occur in the cerebral hemispheres of older children, and rarely in the spine [2]. Regardless of anatomic location, the tumors are histologically similar, presumably reflecting the transformation of a common progenitor. Emerging evidence indicates that ependymomas arise from cells with functional and molecular features similar to radial glia, multipotent progenitors of normal neurons and glial cells [3]. Recent work has revealed characteristic differences in gene expression that distinguish ependymomas located in the posterior fossa from those found in the cerebrum and the spine [e.g., 2,4]. Many of the differentially expressed genes promote anatomic localization and maturation of radial glia, suggesting that ependymomas encompass distinct tumor subgroups derived from a common progenitor. The underlying heterogeneity among ependymomas is highlighted by the wide variability in clinical outcome observed among tumors that are indistinguishable histologically [1].
Despite advances in neuro-imaging, surgical techniques and radiation treatment, the overall prognosis for pediatric ependymomas remains unfavorable with 5-year survival rates less than 50% in many studies [5,6]. Gross total resection is associated with longer progression-free survival, but an appreciable fraction of completely excised tumors recur, a risk for which there are no reliable clinical or biological markers [2,7]. Moreover, in the case of posterior fossa tumors, gross total resection is frequently precluded by proximity to the brain stem or involvement of cranial nerves. While clinical trials have documented the benefit of radiation in prolonging survival, treatment of infants and very young children is restricted by a substantial risk of adverse sequelae, including cognitive impairment [1]. Effective chemotherapy regimens to be used either in lieu of radiation in newly operated tumors or for tumors that recur after radiotherapy remain to be unequivocally demonstrated [1,5]. Because of these limitations, the contemporary standard of care for pediatric ependymoma includes surgical excision to the greatest extent possible followed, when permissible, by conformal radiotherapy [1,5].
In light of the poor prognosis that accompanies a diagnosis of ependymoma, development of more effective post-operative therapies is an urgent priority. Characterization of mechanisms that promote radiation resistance will likely suggest novel approaches to improve treatment efficacy and provide new molecular markers of clinical outcome. The tumoricidal activity of radiation is mediated almost exclusively by double-strand breaks that are formed either directly [8] or, more commonly, as a consequence of oxidative free radical-induced DNA lesions that impede DNA replication fork progression [9]. Abasic (apurinic/apyrmidinic) sites and single-strand breaks are the two most abundant replication-blocking lesions produced by radiation [10,11]. Abasic sites result from free radical attack at deoxyribose or cleavage by DNA glycosylases at oxidized bases [12]. Single-strand breaks are also produced by radical attack at deoxyribose or by spontaneous hydrolysis of abasic sites. Radiation-induced single-strand breaks frequently contain deoxyribose fragments that must be removed to permit DNA repair synthesis and strand ligation [8,13]. The multifunctional DNA repair protein Ape1/Ref-1 is essential for radiation resistance in mammalian cells. Ape1/Ref-1 has a strong endonuclease activity that incises DNA 5’ to abasic sites, leaving 3’-OH and 5’-deoxyribose phosphate termini. The enzyme also excises oxidized deoxyribose fragments from single-strand breaks via a 3’-phophodiesterase activity [12]. Importantly, suppression of Ape1/Ref-1 is accompanied by hypersensitivity to radiation and oxidizing agents in human and other mammalian cells [12,14].
We have reported evidence that the activity of Ape1/Ref-1 mediates resistance to radiation in adult gliomas [15] and to radiation followed by chemotherapy in pediatric medulloblastomas [16]. In both instances, we observed a strong inverse correlation between tumor Ap endo activity and progression-free survival following adjuvant therapy, i.e., the higher the activity, the shorter the time to tumor recurrence. These findings led us to assess the contribution of Ap endo activity to radiation resistance in pediatric ependymoma. We report here an initial retrospective study of 36 childhood ependymomas. Our results indicate that Ap endo activity is predictive of outcome following radiotherapy, and suggest that Ape1/Ref-1 promotes radiation resistance in pediatric ependymomas.
Materials and Methods
Tissue
Tumors were obtained from patients operated with informed consent at Children’s Hospital and Regional Medical Center between 1994 and 2004. Diagnosis was obtained from the final neuropathology report. Demographic and treatment outcome information was obtained in accord with protocols approved by the Institutional Review Board at Children’s Hospital and Regional Medical Center. The precautions taken to preserve tissue viability and enzymatic activity during transport of tissue to the laboratory, and the procedure for determining cell number are described elsewhere [17].
Apurinic/apyrimidinic endonuclease activity
Activity was quantified in supernatants of intact tissue homogenates using a highly sensitive assay that measures conversion of acid-treated, super-coiled plasmid DNA to relaxed form caused by incision at an abasic site. The protocol for extract preparation and activity determination, together with controls validating the assay, have been described in extensive detail elsewhere [16,18].
Immunohistochemistry
Immunoperoxidase staining was done on paraffin blocks of formalin-fixed tumors as described in Bobola et al., 2005 [16]. Briefly, de-waxed 5 µm tissue sections were treated with 0.6% hydrogen peroxide/methanol to quench endogenous peroxidase. Following rehydration, trypsin digestion, and incubation with blocking solution (10% goat serum and 3% bovine calf serum), the slides were serially incubated with a 1:5000 dilution of mouse anti-Ape1/Ref-1 monoclonal antibody (NB100-116B1; Novus) and a 1:100 dilution of a biotin-conjugated goat anti-mouse IgG antibody (BA-9200, Vector). After incubation with strepavidin-conjugated horseradish peroxidase, antibody binding was visualized by incubation with 3,3’-diaminobenzidine tetrachloride. Slides were counterstained with methyl green. Negative controls were treated identically except for omission of the primary antibody. Slides of Ape1/Ref-1-expressing medulloblastomas [16] served as positive controls. Stained slides were scanned at low power to evaluate regional variation. Five hundred cells, 100 from each of five regions chosen at random, were counted and averaged to calculate the fraction of immunopositive cells.
Suppression of Ap endo activity
Ap endo activity in the pediatric ependymoma line Res196 [18] was suppressed by two cycles of transfection with either antisense oligonucleotides (ASOs) or siRNA targeting human Ape1/Ref-1, using the same oligomer sequences and procedures similar to those we have previously described [16]. In preparation for transfection, subconfluent cultures of Res196 in 35 mm plates were incubated for 24 hr at 37 °C in humidified 95%/5% air/CO2 in 3 mL of Opti-MEM containing 5% iron supplemented bovine serum. Immediately prior to transfection cells were washed once with serum-free Opti-Mem. Optimal oligonucleotide concentrations and transfection procedures were established for Res196 as follows.
For transfection with ASOs, separate 50 µl aliquots of unsupplemented Opti-MEM (Life Technologies, Inc) were mixed with 20 µL of 14.5 µM ASO (5’-TTTCCCACGCTTCGGCATTCC-3’ complementary to the translation start site) or with 20 µL of cationic lipid (Lipofectin; Life Technologies, Inc) and held at room temperature for 30 min. The oligonucleotide and cationic lipid mixtures were gently combined and held overnight at 4 °C. The oligonucleotide mixture, diluted to 2.9 mL with unsupplemented Opti-MEM (final ASO concentration, 1 µM), was added to washed cells and incubation continued for 18 h. Three mL of Opti-MEM supplemented with 10% iron-supplemented bovine serum was then added and incubation continued for 30 hr; this cycle was repeated. Transfection with a random oligomer of the same nucleotide composition as the ASOs (i.e., a scrambled oligonucleotide) served as a control; the scrambled oligomer yielded no reduction in Ap endo activity relative to untransfected cells (0.16 ± 0.09 vs 0.18 ± 0.03 fmol abasic sites incised/cell/min).
For transfection with siRNA, 8 µL of 20 µM siRNA (5’-CUUCAGGAGCUGCCUGGACUC-3’, 3’-UUGAAGUCUCGACGGACCUG-5’, corresponding to nucleotides 532–552 of the human Ape1/Ref-1 cDNA sequence), was mixed with 42 µL of unsupplemented Opti-MEM; in a separate tube 10 µL of cationic lipid was mixed with 40 µL Opti-MEM. After 20 min at room temperature, the two mixtures were combined and incubated an additional 30 minutes. Washed cultures in 1.9 mL of Opti-MEM were incubated with the siRNA mixture for 4 to 18 hr; final siRNA concentration was 80 nM. Two mL of Opti-MEM containing 10% iron supplemented bovine serum was then added and incubation continued. This cycle was repeated 48 hours after initiating siRNA treatment. Transfection with an inactive siRNA duplex (5’-AAUGUGGAUGGGCUUCGAGCC-3’, 3’-CCUUACACCUACCCGAAGCUC-5’, corresponding to nucleotides 412–432 of the human Ape1/Ref-1 cDNA sequence) served as a control; this sequence yielded no reduction in Ap endo activity relative to untransfected cells (0.17 ± 0.07 vs 0.18 ± 0.03 fmol abasic sites incised/cell/min).
Radiation sensitivity
The effect of suppressing of Ap endo activity on the radiation sensitivity of Res196 was determined by clonogenic assay essentially as previously described in detail [20]. Briefly, 24 hr after initiating the second cycle of transfection with ASOs or siRNA, 500 to 20,000 cells were sub-cultured into 35 mm plates. Twenty-four hr later cells were exposed to 137Cs-γ-ray irradiation at 1 Gy/min under ambient conditions. Incubation was continued for approximately 14 days to allow formation of colonies ≥ 50 cells. Survival (mean ± SD) is the ratio of colony-forming ability of treated cells to that of untreated cells.
Survival curves were determined using the linear-quadratic model [21] from which the following parameters were derived that quantitatively describe sensitivity: D1, the initial rate of cell killing, D0, the final rate of killing, Dq, the quasi-threshold dose below which cells are insensitive to radiation killing, and D10, the dose required to reduce surviving fraction to 10%.
Statistical analysis
Statistical procedures were performed using Stata (Stata Corporation). Comparison of means was by Student’s t-test assuming unequal variances. Relationships between continuous variables were assessed by regression analysis. In these analyses, the outcome variables progression-free and overall survival are measures of time from initiation of radiotherapy to tumor progression assessed by radiologic imaging and date of death, respectively. Tumor progression was defined as: appearance of tumor growth in the case of gross total resection; increase in the largest dimension of residual tumor by at least 25% and/or tumor growth at a different site in the case of sub-total resection. Observations were censored at the last documented follow-up time if progression or death was not yet observed. Median survival was determined by the method of Kaplan and Meier. The hazard ratio (HR) for tumor progression as a function of Ap endo activity was calculated using Cox regression analysis. Ap endo was entered into the regression models as a continuous variable and scaled (i.e., multiplied by 100) so that the tabulated HR represents the relative change in hazard for a 0.01 unit change in measured Ap endo activity. Possible confounding by patient and tumor characteristics was examined by using multivariate Cox regression analysis. Statistically significant relationships were determined at the 95% confidence level.
Results
Patient and tumor characteristics
Thirty-six ependymomas were obtained from patients ranging in age from 10 months to 16 years (mean ± SD = 7.3 ± 4.6 years; median = 6.6 years). A majority of tumors (57%) were from males in accord with previous observations [22]. Twenty-nine (81%) were grade II by WHO criteria while 4 (11%) displayed anaplastic features characteristic of grade 3 tumors [23]. Three tumors (8%) met the histological criteria for WHO grade I (2 myxopapillary ependymomas and one subependymoma). Twenty-two tumors (54%) were located in the posterior fossa, the most prevalent site of occurrence [2], 14 (38%) in the cerebral hemispheres, and 3 (8%) in the spine. Twenty-five tumors were newly diagnosed and 11 had received prior therapy. Of the previously treated tumors, 5 had recurred after prior surgery alone, 2 after surgery and radiotherapy, 3 after surgery and chemotherapy and one after surgery, radiotherapy and chemotherapy.
Association of Ap endo activity with patient and tumor characteristics
Ap endo activity in the 36 tumors ranged 13-fold from 0.005 to 0.066 fmol/cell/min (Table 1). The mean was 0.022 ± 0.015 fmol/cell/min, and, as shown in Table 1, did not significantly differ between the 25 newly operated and the 11 previously treated tumors (0.023 ± 0.016 vs. 0.020 ± 0.013 fmol/cell/min; P ≤ 0.60). Activity also showed no dependence on gender, age, tumor grade or anatomic location (Table 1); essentially identical results were obtained if these analyses were limited to newly operated tumors (data not shown).
Table 1.
Ap endo activity by patient and tumor characteristics
| Activitya | Immunopositive (%) | |||||
|---|---|---|---|---|---|---|
| N | Mean ± SD | Range | N | Mean ± SD | Range | |
| All tumors | 36 | 0.022 ± 0.015 | 0.005 – 0.066 | 44 | 53 ± 9 | 25 – 69 |
| Newly operated | 25 | 0.023 ± 0.016 | 0.005 – 0.066 | 27 | 52 ± 8 | 25 – 64 |
| Previously treated | 11 | 0.020 ± 0.013 | 0.005 – 0.048 | 17 | 52 ± 11 | 35 – 69 |
| Gender | ||||||
| Female | 15 | 0.021 ± 0.018 | 0.005 – 0.066 | 17 | 55 ± 7 | 45 – 69 |
| Male | 21 | 0.023 ± 0.013 | 0.005 – 0.048 | 27 | 50 ± 10 | 25 – 64 |
| Age | ||||||
| Infants (< 3 y) | 8 | 0.016 ± 0.014 | 0.005 – 0.038 | 10 | 49 ± 7 | 35 –58 |
| Children (≥ 3, < 12 y) | 22 | 0.025 ± 0.015 | 0.006 – 0.066 | 28 | 53 ± 10 | 25 – 69 |
| Adolescents (≥ 12 y) | 6 | 0.019 ± 0.014 | 0.010 – 0.043 | 6 | 55 ± 7 | 48 – 63 |
| Histology | ||||||
| Grades 1 | 3 | 0.023 ± 0.023 | 0.010 – 0.050 | 2 | 56 | 48 – 63 |
| Grade 2 | 29 | 0.021 ± 0.014 | 0.005 – 0.066 | 36 | 52 ± 10 | 25 – 69 |
| Grade 3 | 4 | 0.029 ± 0.15 | 0.016 – 0.048 | 6 | 54 ± 6 | 46 – 60 |
| Anatomic location | ||||||
| Supratentorial | 14 | 0.025 ± 0.018 | 0.005 – 0.065 | 13 | 54 ± 8 | 44 – 69 |
| Infratentorial | 22 | 0.020 ± 0.013 | 0.004 – 0.050 | 21 | 53 ± 9 | 35 – 64 |
fmol abasic sites incised/cell/min
Ap endo activity is inversely associated with progression-free and overall survival following radiotherapy
We have previously reported that Ap endo activity is inversely associated with progression-free survival in grade III adult gliomas treated with radiotherapy alone [15] and in pediatric medulloblastomas treated with radiotherapy and chemotherapy [16]. To examine the relationship between Ap endo and response to radiation, we analyzed the association of activity with progression-free survival (PFS) following radiotherapy in 24 of the 29 grade II ependymomas for which outcome data were available. The tumors were from 16 male and 8 female patients ranging in age from 10 months to 16 years (mean ± SD = 6.6 ± 4.4 years; median = 6.0 years). Mean Ap endo activity was 0.018 ± 0.012 fmol/cell/min (range, 0.0049 – 0.048 fmol/cell/min) and did not differ between genders or anatomic locations (data not shown). Twenty-two tumors had received no prior adjuvant therapy. Two tumors had recurred after chemotherapy and were re-operated prior to radiation treatment; Ap endo activity was determined in the re-operated tumor. All patients received 54–55.8 Gy of fractionated radiotherapy. Fourteen tumors progressed and 10 were censored; median time at risk for progression determined by the method of Kaplan-Meier was 31 months (range, 4.5 – 144 months); median time at risk for death was 34 months (range, 6 – 144 months).
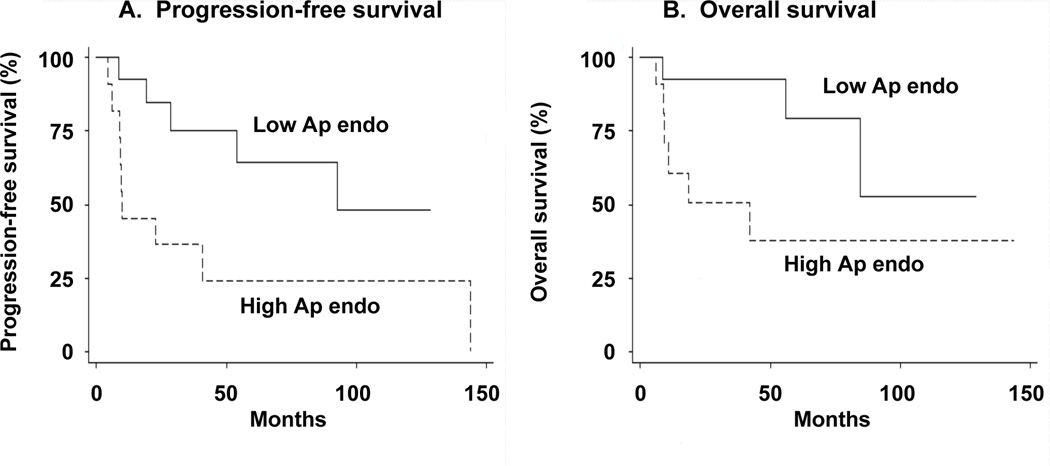
With Ap endo entered as a continuous variable in a univariate Cox proportional hazards regression model, the hazard ratio (HR) for progression increased by a factor of 2.18 for every 0.01 unit increase in activity (95 % CI, 1.32–3.61; P ≤ 0.003; Table 2). Thus, greater Ap endo activity was associated, on average, with an increasing risk for tumor progression. For example, the tumor with the highest activity was ~29 times [i.e., 2.18(0.048–0.0049)/0.01] more likely to progress than the tumor with the lowest activity. The HR was unchanged by eliminating the two samples with the [1] lowest activity (HR = 2.24; 95% CI, 1.36–3.71; P ≤ 0.002); [2] highest activity (HR = 2.21; 95% CI, 1.32–3.69; P ≤ 0.003); [3] shortest progression-free survival (PFS) (HR = 2.19; 95% CI, 1.27–3.77; P ≤ 0.005); [4] longest PFS (HR = 2.24; 95% CI, 1.36–3.71; P ≤ 0.002), indicating that the association is not determined by a limited number of outliers. The inverse association of Ap endo activity with PFS is illustrated by comparing survival in the 12 tumors with highest (i.e., > 0.013 fmol/cell/min) and lowest Ap endo activity (Fig. 1A, Table 2) that revealed a significantly greater risk of progression (HR = 3.91; 95% CI, 1.18–13.0; P ≤ 0.03) in the high activity group. Including age, extent of resection or infratentorial localization had no effect on the association in bivariate Cox regression models (data not shown).
Table 2.
Association of Ap endo activity with survival following radiotherapy in grade II ependymomasa
| Survival | Ap endo | HRb | 95% CIc | P |
|---|---|---|---|---|
| Progression-free | Continuous variable | 2.18 | [1.32, 3.61] | ≤ 0.003 |
| Overall | Continuous variable | 1.89 | [1.12, 3.16] | ≤ 0.02 |
| Progression-free | High vs low activity | 3.91 | [1.18, 13.0] | ≤ 0.03 |
| Overall | High vs low activity | 4.17 | [1.0, 17.6] | ≤ 0.05 |
Ap endo activity was measured in 24 tumors. Activity was also dichotomized into groups of 12, using 0.013 fmol/cell/min as the cut point.
Hazard ratio.
Confidence interval
Figure 1. Progression-free survival (A) and overall survival (B) according to Ap endo activity for grade II ependymomas treated with radiotherapy.
Tumors were divided into two equal groups (N = 12), with low (< 0.013 fmol/cell/min; solid line) and high (> 0.013 fmol/cell/min; dashed line) activity. Activity was measured in tumors obtained prior to radiation treatment. Curves were calculated by the method of Kaplan-Meier.
Ap endo activity was also predictive of overall survival in the 24 radiation-treated ependymomas (Table 2). In a univariate Cox regression model with Ap endo entered as a continuous variable, the HR for death increased by 1.89 for every 0.01 unit increase in activity (95% CI, 1.12–3.16; P ≤ 0.02). As shown in Fig. 1B, the 12 high activity tumors (i.e., activity > 0.013 fmol/cell/min) had a greater risk for death than the 12 low activity tumors (HR = 4.17; 95% CI, 1.0–17.6; P ≤ 0.05), illustrating the inverse association between activity and overall survival.
Ape1/Ref-1 is localized in tumor cell nuclei
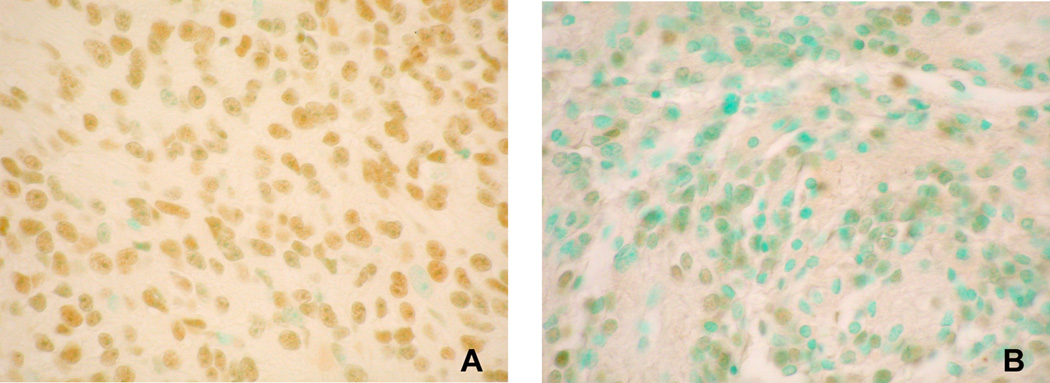
Intracellular localization of Ape1/Ref-1, the predominant Ap endo activity in human cells [12, 24], was examined in 44 ependymomas (2 grade I, 36 grade II and 6 grade III), including 30 (2 grade I, 24 grade II and 4 grade III) tumors described above for which Ap endo activity was determined. Immunopositivity for Ape1/Ref-1 was observed in all tumors examined, and as illustrated in Fig. 2, occurred almost exclusively in the nucleus. The mean fraction of immunopositive cells was 53% ± 8% and varied from 25% to 69% between specimens. As with Ap endo activity, immunopositivity for Ape1/Ref-1 displayed no association with tumor or patient characteristics (Table 1). The fraction of immunopositive cells in different areas of the tumors displayed little heterogeneity, varying on average only 11%. However, for most specimens, the intensity of immunostaining varied markedly among cells (Fig. 2), suggesting that expression of Ape1/Ref-1 and Ap endo activity is not uniform within a tumor, a conclusion consistent with the absence of an association between activity and fraction of immunopositive cells (r = 0.002; P = 0.99).
Figure 2. Ape1/Ref-1 immunoreactivity in grade II ependymoma cells.
Tumor nuclei expressing detectable Ape1/Ref-1 are brown (stained with 3,3’-diaminobenzidine tetrachloride); negative nuclei are green (stained with methyl green). Fraction of immmunopositive cells was 82% for A and 40% for B. All images 40X power.
Ape1/Ref-1 immunopositivity is not associated with radiation response
Some, but not all, studies have suggested an association between the fraction of Ape1/Ref-1 immunopositive cells and treatment outcome in diverse pediatric and adult tumors [12,14,24]. PFS following radiotherapy was available for 30 grade II ependymomas examined for Ape1/Ref-1 by immunostaining. This population included the 24 grade II tumors that displayed an inverse association between Ap endo and PFS. Univariate analysis revealed no association between the fraction of immunopositive cells and PFS (HR = 0.90; 95 % CI, 0.95–1.02; P = 0.55). In addition, no association between immunopositive fraction and overall survival was observed for 19 of the tumors (HR = 1.02; 95 % CI, 0.95–1.09; P = 0.63).
Suppression of Ap endo activity increases the radiosensitivity of ependymoma cells
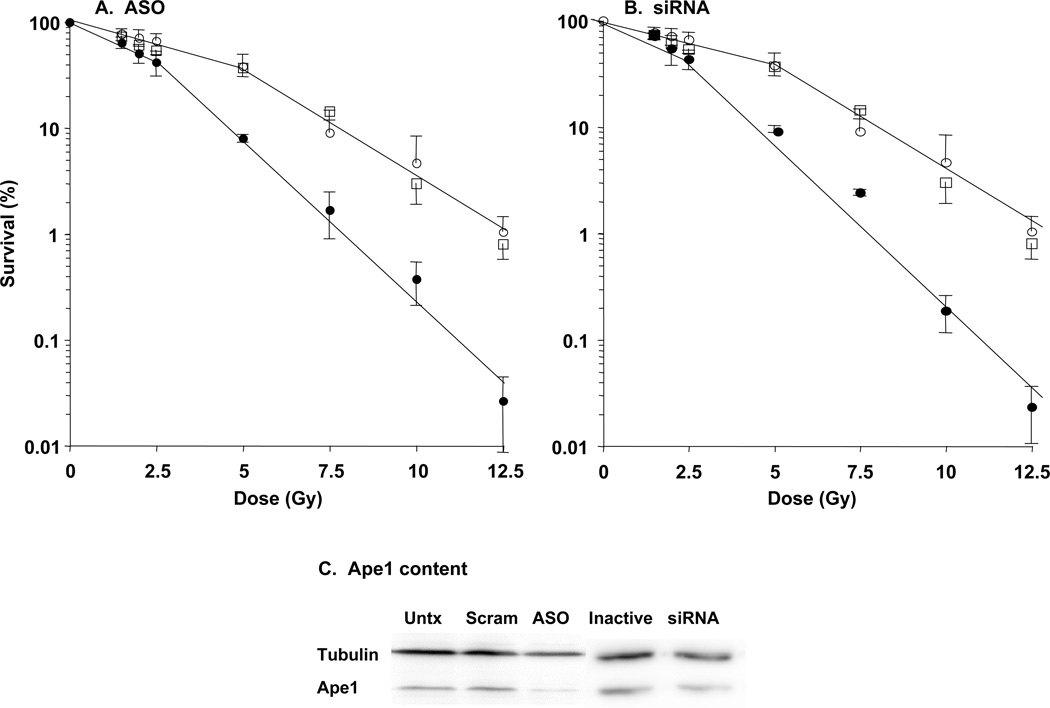
The inverse association between Ap endo activity and survival following radiotherapy suggests that repair of oxidative lesions that are substrates of Ape1/Ref-1 (e.g., abasic sites, single-strand breaks; [12–14]) promote clinical radioresistance in ependymoma. To address this hypothesis, we determined the effect of suppressing Ap endo activity on sensitivity to 137Cs-γ-rays by clonogenic assay in the pediatric ependymoma-derived line Res196 [16]. The effect of transfection with either ASOs or siRNAs targeting Ape1/Ref-1 is illustrated in Fig. 3 and summarized in Table 3. Compared to control cells, Ap endo activity was reduced 2.7-fold by ASOs and 3,2-fold by siRNAs, changes that are statistically significant; it is important in this regard that Ape1/Ref-1 constitutes the major Ap endo activity (~95%) in human cells (12–14). Reduction in biochemical activity was accompanied by significantly greater γ-ray sensitivity, as evidenced by a 1.5-fold reduction in D10 for both ASO- and siRNA-treated cells. Greater sensitivity reflected a 2.4- to 2.8-fold decrease in D1 and a 1.8-fold reduction in D0, measures of the rate of cell killing on the initial and final linear portions, respectively, of the survival curves in Fig. 3. Suppression of Ap endo activity had no detectable effect on the quasi-threshold dose Dq, indicating that activity is not a prominent contributor to resistance at very low radiation doses. Importantly, suppression of activity produced significant decreases in survival at 2 Gy, the standard fractionated radiotherapy dose (Table 3). The radiosensitivity of untransfected cells (open squares, Fig. 3A,B) was indistinguishable from that of control cells treated with scrambled oligonucleotide or inactive siRNA, demonstrating that transfection of control oligomers did not affect intrinsic radiosensitivity. Like radiosensitivity, Ape1/Ref-1 content (Fig. 3C and legend) did not differ between untransfected cells and cells treated with control sequences. Comparable reductions in Ap endo activity and radioresistance were observed in a preliminary experiment with Res254, another pediatric ependymoma line (data not shown).
Figure 3. Suppression of Ap endo activity increases radiosensitivity in the ependymoma cell line Res196.
Subconfluent cultures were transfected with ASOs (A), siRNA (B) or control sequences. Ap endo activity and radiation sensitivity were determined 48 hr after initiating the second transfection cycle as described in Materials and Methods. Data are the mean ± SD of triplicate determinations in three separate experiments, i.e., nine determinations. Open squares, untransfected cells; open circles, control oligonucleotides; closed circles, ASO or siRNA. Ape1/Ref-1 protein content (C) was assayed by Western blotting with chemilumuniscent detection, carried out essentially as we have previously described (19). Gels were loaded with whole cell extracts equivalent to 50,000 cells, harvested 48 hr after initiating the second transfection cycle. Densitometry (19) revealed that Ape1/Ref-1 signal intensity, normalized to that of tubulin, was reduced ca. 50% in ASO- and siRNA-treated cells relative to their control counterparts, and did not differ among untransfected cells and cells transfected with control sequences. Untx, untransfected; Scram, scrambled oligonucleotide control; Inactive, inactive siRNA control.
Table 3.
Suppression of Ape1/Ref-1-catalysed Ap endo activity increases radiosensitivity in pediatric ependymoma cellsa
| Activityb | P ≤ | D10c | P ≤ | D1c | P ≤ | D0c | P ≤ | Dqc | P ≤ | S2Gyd | P ≤ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controle | 0.16 ± 0.09 | – | 7.3 ± 0.8 | 5.3 ± 2.3 | 2.3 ± 0.8 | 2.0 ± 0.8 | 71 ± 12 | |||||
| ASO | 0.06 ± 0.05 | 0.01 | 4.8 ± 0.3 | 0.001 | 2.2 ± 1.2 | 0.01 | 1.3 ± 0.3 | 0.01 | 1.7 ± 0.3 | 0.20 | 51 ± 10 | 0.01 |
| siRNA | 0.05 ± 0.04 | 0.02 | 4.9 ± 0.2 | 0.001 | 1.9 ± 1.1 | 0.01 | 1.3 ± 0.3 | 0.01 | 1.9 ± 0.2 | 0.60 | 55 ± 17 | 0.05 |
See legend to Fig 3 and Materials and Methods for details.
fmol abasic sites incised/cell/min;
Gy;
percent survival following 2 Gy.
Activity did not differ significantly among cells that were transfected with control oligonucleotides and cells that were not transfected (0.16 ± 0.09 vs 0.18 ± 0.03 fmol abasic sites incised/cell/min).
Discussion
The prognosis for pediatric ependymoma remains relatively poor with 5-year survival rates ranging from 35–65% [5]. The unfavorable prognosis reflects the high likelihood (~50%) of recurrence even after gross total resection and post-operative radiotherapy [1,5]. Progress in treating ependymoma has been hampered by the lack of consensus in studies seeking associations between treatment response and a number of clinical, demographic and histological characteristics (reviewed in [2,5]). The heterogeneous course of ependymoma has heightened interest in identifying genetic (e.g., [2]) and biochemical (e.g., [7]) markers that better characterize the underlying biological determinants of clinical outcome.
Radiotherapy is the only clinically proven, effective adjuvant treatment for childhood ependymoma [1,5]. The tumoricidal activity of radiation is mediated by double-strand breaks that arise in large part as a consequence of impeded DNA replication at radiation-induced abasic sites and single-strand breaks [12,13]. This fundamental consideration, together with our previous observations in adult gliomas [15] and pediatric medulloblastomas [16], led us to hypothesize that tumor Ap endo activity is a mechanistically relevant marker of response to radiotherapy in ependymomas. The work here supports this hypothesis and suggests that Ap endo activity may be a useful predictor of radiation response and a target for anti-resistance therapies.
A role for Ap endo activity in clinical resistance is supported by the strong inverse association between ependymoma Ap endo activity and progression-free and overall survival following standard conformal radiotherapy (Fig. 1). Based on extensive genetic and biochemical studies [reviewed in 12–14], particularly in human primary brain tumor cells [15,16,19], the relationship between activity and radiation response likely reflects, at least in part, repair of abasic sites, a common lesion produced by ionizing radiation and other oxidizing agents, e.g., bleomycin [13]. The cytotoxicity of unrepaired abasic sites has been extensively documented by the hypersensitivity to radiation and chemical oxidizers displayed by bacteria, yeast and mammalian cells deficient in repair of this lesion [12,14,19]. Additional evidence that Ap endo activity promotes radiation resistance is provided by our demonstration that suppression of activity by transfection with antisense oligonucleotides or siRNA targeting Ape1/Ref-1, the DNA repair protein that constitutes ~95% of Ap endo activity in human cells [12], significantly increases γ-ray sensitivity in ependymoma cells (Fig. 3). This observation is in accord with previous reports that suppression of Ape1/Ref-1 increases sensitivity to radiation and oxidants in both rodent and human cells (reviewed in [12,14]), including human glioma cells [19]. The association between Ap endo activity and radiation response may also reflect the repair of lesions in addition to abasic sites. Ape1/Ref-1 possesses 3’-phosphodiesterase, 3’-phosphatase and 3’-exonuclease activities catalyzed by an active site in common with Ap endo activity. Importantly, the 3’-phosphodiesterase has been shown to excise fragmented deoxyribose phosphate moieties at single-strand breaks [13], suggesting that Ape1/ref-1 plays an important role in the repair of radiation-induced single strand breaks.
Ape1/Ref-1 may also modulate radiation response by mechanisms other than repair of oxidative DNA lesions. The reduction-oxidation activity Ref-1, located at the amino terminus of the protein, promotes resistance to oxidative stress by facilitating signal transduction that maintains important stress-related transcription factors (e.g., c-Jun, NFκB, HIF-1α) in a reduced, active state (reviewed in [12,24]). Ref-1 has also been implicated in regulating the transactivation and proapoptotic functions of p53. Emerging evidence suggests that Ape1/Ref-1 also participates in Ca+2-dependent regulation of gene expression, ribosome function and regulation of c-myc mRNA expression. Thus, it is possible that the association between Ap endo activity and radiation response we observed in ependymomas reflects, to some degree, resistance mediated by one or more of these Ref-1-mediated functions. Of particular interest is a report implying that Ref-1 promotes neovascularization in response to hypoxia, a hallmark of solid tumors, including malignant human brain tumors (25). By restoring oxygenation, Ref-1 function in this instance may paradoxically promote radiosensitivity. What association, if any, these distinct functions may have with response to radiotherapy remains to be fully delineated with the advent of inhibitors that specifically target either the nuclease or re-dox activities.
We have previously described a strong, statistically significant inverse association between Ap endo activity and progression-free survival in pediatric medulloblastomas/primitive neuroectodermal tumors (PNETs) [16] and adult anaplastic (grade III) gliomas [15]. In the case of medulloblastomas/PNETs the hazard for progression varied 25-fold among the 50 tumors treated with radiation and alkylating agent-based chemotherapy (HR = 1.07; P ≤ 0.001). For the adult gliomas, hazard for progression varied by 14-fold among 44 tumors treated with radiotherapy alone (HR = 1.07; P ≤ 0.008) and by 13-fold in 30 tumors treated with alkylating agents following recurrence after prior surgery and radiotherapy (HR = 1.09; P ≤ 0.003). These results together with our findings presented here suggest that Ap endo activity may promote resistance to adjuvant radiotherapy and to chemotherapy that produces abasic sites (e.g., temozolomide), and provide a predictor of treatment outcome in a variety of primary brain tumors. In accord with this conclusion, our preliminary data indicate a statistically significant inverse association between Ap endo activity and progression-free survival following radiotherapy in 44 adult glioblastomas (HR = 1.24; P = 0.005; Bobola and Silber, unpublished observations).
We observed an approximately 3-fold range among ependymomas in the fraction of cells that were immunopositive for Ape/Ref-1 (Table 1). While the fraction of immunopositive cells within a tumor did not vary widely, the intensity of staining within a tumor was not uniform, and often ranged from intense to faint within a single section. Endogenous oxidative stress has been implicated as a modulator of Ap endo activity and Ape1/Ref-1 content in cell lines and tissue [12,14,24], and is a likely mechanism contributing to the variability in stained cell fraction and intensity of staining in ependymoma. Inter- and intra-tumoral heterogeneity in Ape1/Ref-1 expression may reflect differences in oxidative stress associated with cell proliferation or transient episodes of hypoxia due to inadequate or defective vasculature. In this regard it is notable that the Ap endo activity of ependymomas, typically slow growing tumors, is 5-fold less than that of rapidly proliferating medulloblastomas/PNETs (0.022 ± 0.015 vs 0.12 ± 0.12 fmol/cell/min; [16]).
Ape1/Ref-1 expression determined by immmunohistochemistry has been associated with treatment outcome in a variety of human tumors, including non-small cell lung cancer, osteosarcoma, cervical carcinoma and hepatocellular carcinoma (reviewed in 12,14,24). However, a Cox univariate model did not reveal a significant association between the fraction of immunopositive cells and either progression-free survival or overall survival in 30 grade II ependymomas following radiotherapy (See Results). This negative finding contrasts with the strong inverse association between Ap endo activity and both progression-free and overall survival (Table 2, Fig. 1). The lack of association with immunopositivity may reflect the relatively small number of tumors examined, the narrow range in immunopositivity among tumors and/or the heterogeneity of Ape1/Ref-1 staining intensity within tumors. In particular, the heterogeneity of staining intensity between cells within a tumor is likely to obscure any association between fraction of immunopositive cells and treatment outcome. These findings are similar to those for medulloblastomas/PNETs that also revealed no association between treatment response and fraction of Ape1/Ref-1 immunopositive cells, even though in this instance the intensity of immunostaining was uniform within tumors [16].
Our results suggest that Ap endo activity may be useful in directing adjuvant therapy in ependymoma. High activity tumors may be candidates for novel, potentially more effective therapies that would spare toxicity caused by radiation. Alternatively, high activity tumors may be targets for anti-resistance therapies targeting Ape1/Ref-1. The potentiation of radiation sensitivity effected by ASO-and siRNA-mediated suppression of Ap endo activity that we observed here in the ependymoma line Res196 provides proof of principle of the potential clinical benefit afforded by circumventing radiation resistance. Importantly, significant increases in sensitivity to a single, 2 Gy dose of γ-rays required only partial suppression of Ap endo activity (Table 3). If this is also the case in vivo, it could facilitate clinically effective suppression of activity during radiotherapy.
The multiple demonstrations of increased sensitivity to radiation and alkylating agents produced by suppression of Ape1-mediated Ap endo activity in human tumor cells (reviewed in 12, 14, 24), including human primary brain tumors cells (e.g., 16, 19), has stimulated interest in developing small molecule inhibitors of Ape1 function. Some efforts to this end employ the structural data available for Ape1 bound to an abasic site in DNA to guide the rational design of active site inhibitors (e.g., 26) while others employ high through-put assays that screen large chemical libraries for active compounds (reviewed in 24). These techniques have revealed a large number of potential candidates that inhibit the Ap endo activity of Ape1 in vitro and increase drug or radiation sensitivity in cultured cells. For example, Kelley and co-workers have recently described a compound, designated AR03, that increases the cytotoxicity of the methylating agents methylmethane sulfonate and temozolomide in the human malignant glioma cell line SF767 (27). Importantly, greater cell killing was accompanied by a greater abundance of abasic sites, indicating that toxicity was due, at least in part, to inhibition of Ape1 Ap endo activity. Inhibitors of Ref-1 have also been described such as E3330 which when used in combination with proapoptotic TNF family members promote cell killing in human cancer cells (28). While the identification of potential Ape1 and Ref-1 inhibitors is an important step in designing strategies to increase the therapeutic efficacy of radiation and chemotherapeutic drugs, clinical utilization will be dependent upon a circumventing a wide variety of pharmacological limitations (e.g., stability, solubility, excretion, toxicity, inability to cross physiological barriers). The recent development of pharmacologically compatible nanoparticles as delivery vehicles that easily cross the blood-brain barrier and specifically target brain tumor cells has been described [29] and may afford a novel approach to deliver treatment-sensitizing concentrations of inhibitors.
Acknowledgements
Grant support: This work was supported by funding from the American Cancer Society (RSG 01 9101 CCE, MSB) and the from NIH (CA82622 and CA104593, JRS)
Abbreviations
- Ap endo
apurinic/apyrimidinic endonuclease
- ASO
antisense oligonucleotide
- CI
confidence interval
- HR
hazard ratio
- PFS
progression-free survival
Footnotes
Conflicts of interest: None
References
- 1.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75:287–299. doi: 10.1007/s11060-005-6753-9. [DOI] [PubMed] [Google Scholar]
- 2.Kilday JP, Rahman R, Dyer S, Ridley L, Lowe J, Coyle B, Grundy R. Pediatric ependymoma: biological perspectives. Mol Cancer Res. 2009;7:765–786. doi: 10.1158/1541-7786.MCR-08-0584. [DOI] [PubMed] [Google Scholar]
- 3.Poppleton H, Gilbertson RJ. Stem cells of ependymoma. Br J Cancer. 2007;96:6–10. doi: 10.1038/sj.bjc.6603519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack SC, Taylor MD. The genetic and epigenetic basis of ependymoma. Childs Nerv Syst. 2009;25:1195–1201. doi: 10.1007/s00381-009-0928-1. [DOI] [PubMed] [Google Scholar]
- 5.Tamburrini G, D'Ercole M, Pettorini BL, Caldarelli M, Massimi L, Di Rocco C. Survival following treatment for intracranial ependymoma: a review. Childs Nerv Syst. 2009;25:1303–1312. doi: 10.1007/s00381-009-0874-y. [DOI] [PubMed] [Google Scholar]
- 6.Zacharoulis S, Moreno L. Ependymoma: an update. J Child Neurol. 2009;24:1431–1438. doi: 10.1177/0883073809339212. [DOI] [PubMed] [Google Scholar]
- 7.Ridley L, Rahman R, Brundler MA, Ellison D, Lowe J, Robson K, Prebble E, Luckett I, Gilbertson RJ, Parkes S, Rand V, Coyle B, et al. Children's Cancer and Leukaemia Group Biological Studies Committee. Multifactorial analysis of predictors of outcome in pediatric intracranial ependymoma. Neuro Oncol. 2008;10:675–689. doi: 10.1215/15228517-2008-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward JF. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 9.O'Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 10.Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Radic Biol Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 11.Drabløs F, Feyzi E, Aas PA, Vaagbø CB, Kavli B, Bratlie MS, Peña-Diaz J, Otterlei M, Slupphaug G, Krokan HE. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair. 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Abbotts R, Madhusudan S. Human AP endonuclease 1 (APE1): From mechanistic insights to druggable target in cancer. Cancer Treat Rev. 2010;36:425–435. doi: 10.1016/j.ctrv.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 14.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 15.Bobola MS, Emond MJ, Blank A, Meade EH, Kolstoe DD, Berger MS, Rostomily RC, Silbergeld DL, Spence AM, Silber JR. Apurinic endonuclease activity in adult gliomas and time to tumor progression after alkylating agent-based chemotherapy and after radiotherapy. Clin Cancer Res. 2004;10:7875–7883. doi: 10.1158/1078-0432.CCR-04-1161. [DOI] [PubMed] [Google Scholar]
- 16.Bobola MS, Finn LS, Ellenbogen RG, Geyer JR, Berger MS, Braga JM, Meade EH, Gross ME, Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;11:7405–7414. doi: 10.1158/1078-0432.CCR-05-1068. PMID: 16243814. [DOI] [PubMed] [Google Scholar]
- 17.Silber JR, Bobola MS, Ghatan S, Blank A, Kolstoe DD, Berger MS. O6-methylguanine-DNA methyltransferase activity in adult gliomas: relation to patient and tumor characteristics. Cancer Res. 1998;58:1068–1073. [PubMed] [Google Scholar]
- 18.Bobola MS, Silber JR, Ellenbogen RG, Geyer JR, Blank A, Goff RD. O6-methylguanine-DNA methyltransferase, O6-benzylguanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res. 2005;11:2747–2755. doi: 10.1158/1078-0432.CCR-04-2045. [DOI] [PubMed] [Google Scholar]
- 19.Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 2002;8:3008–3018. [PubMed] [Google Scholar]
- 20.Bobola MS, Blank A, Berger MS, Silber JR. Contribution of O6-methylguanine-DNA methyltransferase to monofunctional alkylating-agent resistance in human brain tumor-derived cell lines. Mol Carcinog. 1995;13:70–80. doi: 10.1002/mc.2940130203. [DOI] [PubMed] [Google Scholar]
- 21.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th ed. Philidelphia: Lippincott, Wiiliams and Wilkins; 2006. [Google Scholar]
- 22.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg. 2009;110:725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 23.Louis N, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumors of the Central Nervous System. 3rd ed. Geneva: WHO Press; 2007. [Google Scholar]
- 24.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JL, Wang X, Van Adamson, Zhao Y, Gibbons GH. Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-kappab-independent and -dependent pathways. Circ Res. 2001;88:1247–1253. doi: 10.1161/hh1201.091796. [DOI] [PubMed] [Google Scholar]
- 26.Zawahir Z, Dayam R, Deng J, Pereira C, Neamati N. Pharmacophore guided discovery of small-molecule human apurinic/apyrimidinic endonuclease 1 inhibitors. J Med Chem. 2009;52:20–32. doi: 10.1021/jm800739m. [DOI] [PubMed] [Google Scholar]
- 27.Bapat A, Glass LS, Luo M, Fishel ML, Long EC, Georgiadis MM, Kelley MR. Novel small molecule inhibitor of Ape1 endonuclease blocks proliferation and reduces viability of glioblastoma cells. J Pharmacol Exp Ther. 2010;334:988–998. doi: 10.1124/jpet.110.169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu N, Sugimoto K, Tang J, Nishi T, Sato I, Hiramoto M, Aizawa S, Hatakeyama M, Ohba R, Hatori H, Yoshikawa T, Suzuki F, et al. High-performance affinity beads for identifying drug receptors. Nat Biotechnol. 2000;18:877–881. doi: 10.1038/78496. [DOI] [PubMed] [Google Scholar]
- 29.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]