Abstract
Angiopoietin 1 (Ang-1) and angiopoietin 2 (Ang-2) have opposing effects on blood vessels, with Ang-2 being mainly induced during the endothelial barrier breakdown. It is known that spinal cord injury (SCI) induces lasting decreases in Ang-1 levels, underlying endothelial barrier disruption, but the expression of Ang-2 in spinal cord injury has not been studied. We characterized Ang-2 after SCI using a clinically relevant rat model of contusion SCI. We found that SCI induces marked and persistent upregulation of Ang-2 (up to 10 weeks after SCI), which does not reflect well-characterized temporal profile of the blood-spinal cord barrier (BSCB) breakdown after SCI, and thus suggests other role(s) for Ang-2 in injured spinal cords. Furthermore, we also found that higher Ang-2 levels were associated with more successful locomotor recovery after SCI, both in SCI rats with markedly better spontaneous motor recovery, and in SCI rats receiving a neuroprotective pharmacological intervention (amiloride), suggesting a beneficial role for Ang-2 in injured spinal cords. Immunocytochemical analyses revealed that Ang-2 was not induced in endothelial cells, but in perivascular and non-vascular cells labeled with glial fibrillary acidic protein (GFAP) or with chondroitin sulfate proteoglycan (NG2). Therefore, it is unlikely that induction of Ang-2 contributes to vascular dysfunction underlying functional impairment after SCI, but rather that it contributes to the beneficial pro-angiogenic and/or gliogenic processes underlying recovery processes after SCI.
Keywords: spinal cord injury, Ang-2, Ang-1, angiogenesis, NG2, amiloride, motor recovery
Angiopoietins are growth factors involved in blood vessel formation/maturation and in endothelial cell survival (Thomas and Augustin 2009; Hansen et al., 2008). They are also key regulators of vascular functions in the brain (Valable et al., 2005; Zacharek et al., 2007; Kim et al., 2008) and spinal cord (Hererra et al., 2010; Ritz et al., 2010; Han et al., 2010). The angiopoietin (Ang) family has four members: Ang-1, Ang-2, and Ang-4 are found in humans, while Ang-3 is a mouse ortholog of the human Ang-4 (Hansen et al., 2008; Valenzuela et al., 1999). Of these, the regulatory roles of Ang-1 and Ang-2 in vascular functions are best characterized. It has recently been shown that spinal cord injury (SCI) induces lasting decreases in Ang-1 levels (Hererra et al., 2010; Ritz et al., 2010). Given that Ang-1 suppresses vascular leakage/inflammation (Hansen et al., 2008), and facilitates maturation of blood vessels during angiogenesis (Lee et al., 2009), SCI-induced decreases in Ang-1 undoubtedly contribute to the dramatic vascular dysfunction demonstrated after SCI in several studies (Cohen et al., 2009; Popovich et al., 1996; Whetstone et al., 2003; Benton et al., 2008), which critically affects functional impairment (Sharma, 2005; Pannu et al., 2007). Therefore it is not surprising that exogenous administration of Ang-1 has beneficial effects on both vascular integrity and locomotor recovery after SCI (Han et al., 2010; Herrera et al., 2010).
However, to fully understand vascular dysfunction after SCI and the role of Ang-1 in its repair, it is also necessary to analyze Ang-2, since both angiopoietins work in concert. Ang-1 and Ang-2 exert their effects on blood vessels by competing with similar affinity for the same receptor, endothelial tyrosine kinase receptor (Tie-2 receptor; Thomas and Augustin 2009). Upon release from endothelial cells, Ang-2 exerts autocrine and paracrine effects on the Tie-2 receptor, thereby antagonizing Ang-1's effect on blood vessels. Therefore, if induced, Ang-2 could prevent the beneficial effects of endogenous or exogenous Ang-1 on vascular integrity in injured spinal cords. In contrast to Ang-1, which is constitutively expressed in normal brain vasculature (Nag et al., 2005), Ang-2 is only weakly expressed under normal conditions, and is upregulated by hypoxia and during vascular remodeling (Thomas and Augustin 2009). As SCI results in robust hypoxia and angiogenesis (Nesic et al., 2008; Casella et al., 2002; Loy et al., 2002; Mahoney et al., 2009), it would be reasonable to assume that endothelial expression of Ang-2 increases, similar to the Ang-2 changes documented in blood vessels after stroke or brain injury (Beck et al., 2000; Zhang et al., 2002; Nag et al., 2005).
However, it has recently been recognized that Ang-2, originally found primarily in blood vessels and classified only as an angiogenic factor, can also be found in neural stem cells and affect neurogenesis in vivo and in vitro (Ward and LaManna 2004; Androutsellis-Theotokis et al. 2009; Liu et al. 2009; Marteau et al., 2010); thus it can have both vascular and non-vascular functions in the central nervous system (CNS).
The effect of SCI on Ang-2 has not previously been analyzed or reported. Therefore, our study had following goals: (1) to assess the temporal and spatial changes in Ang-2 levels after SCI; (2) to compare changes in Ang-1 and Ang-2 levels with well established alterations in vascular permeability after SCI; (3) to identify the cellular localization of Ang-2 in rat spinal cords before and after SCI; (4) to shed light on the possible roles of Ang-2 in injured spinal cords.
EXPERIMENTAL PROCEDURES
Animal Surgeries
Male Sprague-Dawley rats from Harlan weighing 225-250g were anesthetized by i.p. injection of pentobarbital (50 mg/kg). A laminectomy of the tenth thoracic (T10) vertebra was performed to expose the spinal cord. Animals were contused at T10 with the Infinite Horizons (IH) Impactor using a force of 150 kdynes with 1 second dwell time, which produces moderate contusion SCI, characterized by Sheff et al., (2003). The surgical site was closed by suturing the muscle and stapling the skin, followed by the superficial application of 0.3mL of 1% lidocaine. Animals were injected subcutaneously with 2mL of 0.9% sterile saline and placed on a heating pad to maintain body temperature until they revived. Injured animals received the analgesic buprenorphin (0.1mg/kg) subcutaneously twice a day for three days. Injured (SCI) animals also received the antibiotic baytril (2.7mg/kg) subcutaneously twice a day until bladder function returned. Bladders of SCI animals were manually emptied twice daily until normal function returned, which typically occurred two weeks after injury. All procedures complied with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UTMB Animal Care and Use Committee. Control age matched animals were not subjected to any part of the surgical or post-surgical care protocols. We have decided to use only naïve rats as controls, based on our previous extensive decade-long experience with sham surgeries (laminectomy) and on published report by De Biase et al. (2005). We found that laminectomy produces an injury to the spinal cord (not published) that does not have any clinical relevance. A report by De Biase et al. (2005) describes detailed analyses of global transcriptional profiles of spinal cords of sham-treated and naïve rats. This study compellingly shows that sham surgery is not only injurious but resembles mild contusion SCI. Therefore, sham-treated rats, if used as controls may introduce false negative results in the study, or artificially reduce the effect of SCI.
Amiloride treatment (described in details in Lee et al., 2011): A 10mM amiloride (Sigma A7410) solution was made daily by dissolving in water. Daily i.p. injections of amiloride or vehicle (n=6/group) began 24hrs after injury, and continued until 35 days after injury, when animals were perfused and used for Western blot analysis. Amiloride was administered at a dose of 5mg/kg for the first 10 days after injury, after which the daily dose was increased to 10mg/kg for days 11-35 post-SCI.
Locomotor assessment
Hindlimb movement was assessed using the Basso, Beattie, and Bresnahan Scale, also known as the BBB scale (Basso et al., 1995). BBB scores were collected daily on the first 14 days after injury, and once weekly thereafter. The BBB scale assigns values to the left and right hind limbs corresponding to their locomotor ability. A score of 0/0 indicates no observable hindlimb movement, while a score of 21/21 indicates normal movement. Factors considered during the scoring process include but are not limited to: degree of joint movement, plantar paw placement, weight support, forelimb-hind limb coordination during stepping, rotation of the paw while stepping, toe dragging, trunk stability, and tail position.
Protein Extraction
Animals were anesthetized by i.p. injection of pentobarbital (150 mg/kg), and sacrificed by transcardial perfusion with 0.9% saline containing 1unit of heparin per mL. T10 (lesion epicenter) spinal cord segments were removed and stored at -80°C; samples were transferred to liquid nitrogen prior to tissue homogenization. For protein extraction from T10, 200uL of homogenization buffer (10mM HEPES, 10mM KCl, 0.1mM EDTA, 0.1mM EGTA, 1mMmM DTT, 0.5mM PMSF, 2ug/mL antipain, 2ug/mL chymostatin, 2ug/mL pepstatin, 2ug/mL leupeptin), with 1X phosphatase inhibitors (78426, Thermo Scientific), was added to each individual segment for homogenization on ice with a pestle (05-559-27, Fisher). After homogenization, samples were vortexed for 5 seconds, and centrifuged at 5,900 RCF for 8 min at 4°C. The resulting supernatant, which contained the crude cytosolic/whole cell extract, was separated from the pelleted nuclei, cellular debris, and intact cells.
Electrophoresis and Western blotting
Samples containing 40μg of protein were mixed with an appropriate volume of 6X super denaturing sample buffer (350mM Tris-HCl, pH 6.8, 1M Urea, 6.0% 2-mercaptoethanol, 9.3% DTT, 18.0% SDS, 0.06% bromophenol blue, 30.0% glycerol), and equal amounts of protein were loaded onto a sodium dodecyl sulfate-polyacrylamide gel. Samples were not boiled to avoid aggregation of membranous proteins (Sorbo et al., 2007). Samples were separated by electrophoresis at 150V for 4.5 hours, and proteins were transferred overnight to an Immobilon-P® polyvinylidene difluoride membrane (ISEQ00010; Fisher Scientific) at 4°C and 25V.
Membranes were reversibly stained with Ponceau S (0.5% w/v Ponceau S, 1.0% acetic acid) to confirm the transfer of proteins, and destained in water. Membranes were incubated for one hour at room temperature (23°C) in blocking solution [5.0% nonfat dry milk in Tris-buffered saline with 0.2% Tween-20 (TBS-T)]. Membranes were then incubated with the appropriate primary antibody diluted in blocking solution for one hour at room temperature, or overnight at 4°C. After washing in TBS-T, membranes were subsequently incubated for one hour at room temperature with a horseradish peroxidase-conjugated secondary antibody diluted in blocking solution. Membranes were again washed in TBS-T; three twenty minute washes. Peroxidase activity was detected using the Amersham enhanced chemiluminescence lighting system (ECL, RPN2106, Amersham Biosciences) and exposure to film. Western blot films were scanned and quantified by spot densitometry using the AlphaEase program version 5.5.
Antibodies tested
We tested five different anti-rat Ang-2 antibodies (Abs), as summarized in the table below:
| Species | Company | Catalog No. | Western blot | Immunofluorescence |
|---|---|---|---|---|
| Goat | Santa Cruz | sc-7015 | Negative | Negative |
| Goat | Santa Cruz | sc-7017 | Negative | Negative |
| Goat | R&D | AF623 | Not tested | Negative |
| Rabbit | Abcam | ab65835 | Single ~50kD band | Intense neuronal staining in uninjured and injured cords (dilution 1:200; not visible in 1:1,000) |
| Rabbit | Millipore | AB3121 | ~50 and ~80 kD bands; recognizes Ang-2 protein | Glial staining (dilutions:1:200/700/1,000/5,000); Neuronal st. only with 1:200 |
Two goat anti-Ang-2 Abs (Santa Cruz; sc-7015 and sc-7017) did not give positive results in either Western blots or immunofluorescence studies (i.e. no specific fluorescence labeling or bands of the expected MW, were detected). Similarly, goat anti-Ang-2 (R&D AF623; dilution 1:200) did not produce any labeling in uninjured or injured spinal cords.
Rabbit anti-Ang-2 (Abcam ab65835; dilution 1:1000) gave a single ~50kD band in Western blots, which may represent unglycosylated Ang-2 protein (Hwang et al., 2005; predicted MW of ~57kD). The ~50 kD band had a higher intensity in some injured spinal cords, although not consistently in all injured samples. However, when tried in immunofluorescence studies, this Ab intensely labeled only neurons, predominantly motoneurons, in uninjured and injured spinal cords. However, it is difficult to reconcile the dramatic loss of motoneurons at the lesion site with increased intensity of 50kD bands in injured samples. Furthermore, the neuronal Ang-2 labeling obtained with Abcam Ang-2 Ab was strongly dependent on the dilution of the Ab, visible only with 1:200 dilution, but disappearing at 1:1,000 or 1:5,000 dilutions, which is typical for the non-specific binding, not uncommon for the polyclonal Abs. We thus assumed that the ~50kD band reflected nonspecific neuronal staining in both uninjured and injured cords, although this finding may need to be tested further.
Rabbit anti-Ang-2 Ab (Millipore AB3121; 1.7 μg/ml) recognized an expected ~80kD band, both in spinal cord samples and when recombinant Ang-2 protein was included in the Western blot (see Fig. 1A). Rat recombinant Ang-2 was not commercially available, so we used human Ang-2 protein (R&D systems 623-AN/CF), as human and rat Ang-2 proteins share 86% homology. A molecular weight of ~80kD is typically found in Ang-2 Western blots, given that Ang-2 is normally glycosylated (see hrAng-2 in Fig. 1A; Hwang et al., 2005) and contains several glycosylation sites (UniProt database). In histological analyses, Millipore Ang-2 Ab showed white matter glial staining in injured spinal cords at all antibody dilutions tested: 1:200; 1:700; 1:1,000 or 1:5,000 (n=3 uninjured /injured sections per dilution were tested). However, at high concentrations (dilution 1:200), Millipore Ang-2 Ab also recognized neuronal somata in both uninjured and injured spinal cords, similar to the Abcam Ab (described above).
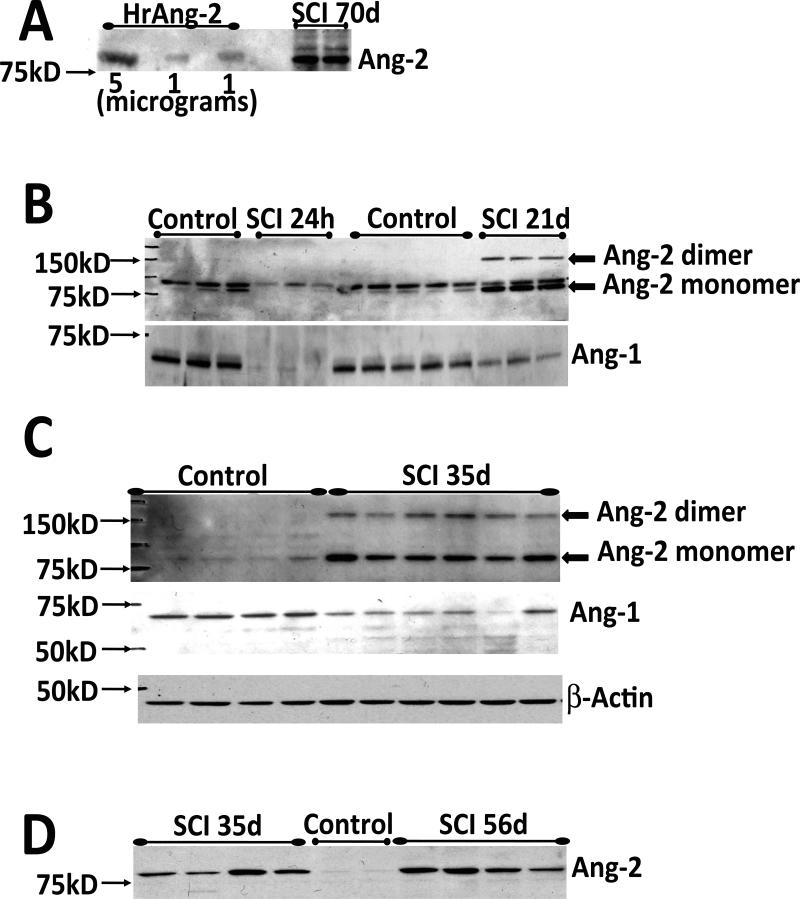
Fig. 1. SCI induces significant and persistent changes in angiopoietin levels.
A) Western blot showing that the polyclonal Ang-2 antibody (Ab, Chemicon AB3121) recognizes both human recombinant Ang-2 protein (HrAng-2; R&D systems 623-AN/CF), used here as a positive control, and Ang-2 expressed in rat spinal cord at the site of injury (T10: tenth thoracic spinal cord segment) 70 days after SCI (n=2). HrAng-2 was loaded in three lanes (two lanes with 1μg of HrAng-2 and one lane with 5 μg of HrAng-2).
B) Representative Western blots showing decreases in Ang-1 (~60kDa) protein levels at T10 and initial decreases (24h) followed by marked increases in Ang-2, 21d after SCI; 24h: control (n=3), SCI (n=3); 21 days control (n= 5), SCI (n=3). The lower band of the Ang-2 doublet was consistently seen at all time points after SCI and was therefore used for quantitation. The Ang-2 band was visible in control samples (1B) because the film exposure time for the Ang-2 Western blot was longer (60min) than in Fig. 1C or D (15 min). A 60 min exposure would yield overexposed Ang-2 bands in injured spinal cord samples at time points later than 3d post-SCI, consistent with Ang-2 being expressed at low levels in uninjured spinal cords. SCI induced marked Ang-2 upregulation at time points later than 3d post-SCI, comparable to microgram levels of loaded recombinant Ang-2 proteins (see Fig. 1A). The Ang-2 monomers and dimers are both visible and marked with arrows.
C) Western blots showing Ang-1 and Ang-2 bands at T10 in control (n=4) and SCI rats (n=6) 35 days after SCI. Equal loading of protein was confirmed by β-actin levels. Ang-1, Ang-2 and β-actin blotting were performed on the same membrane (after subsequent membrane stripping), as shown here. The same procedure was done for all other time points. Significant SCI-induced changes in Ang-1 and Ang-2 persisted into the chronic post-SCI phase, although Ang-2 changes were more robust than in the acute phase, in contrast to Ang-1 changes that subsided over time, as shown in
D) Control (n=2) and injured spinal cords at T10 35 days (n=4) and 56 days (n=4) after SCI. To confirm that increases in Ang-2 levels at 56d did not differ from Ang-2 increases at 35d after SCI, we loaded proteins from both time points on the same gel.
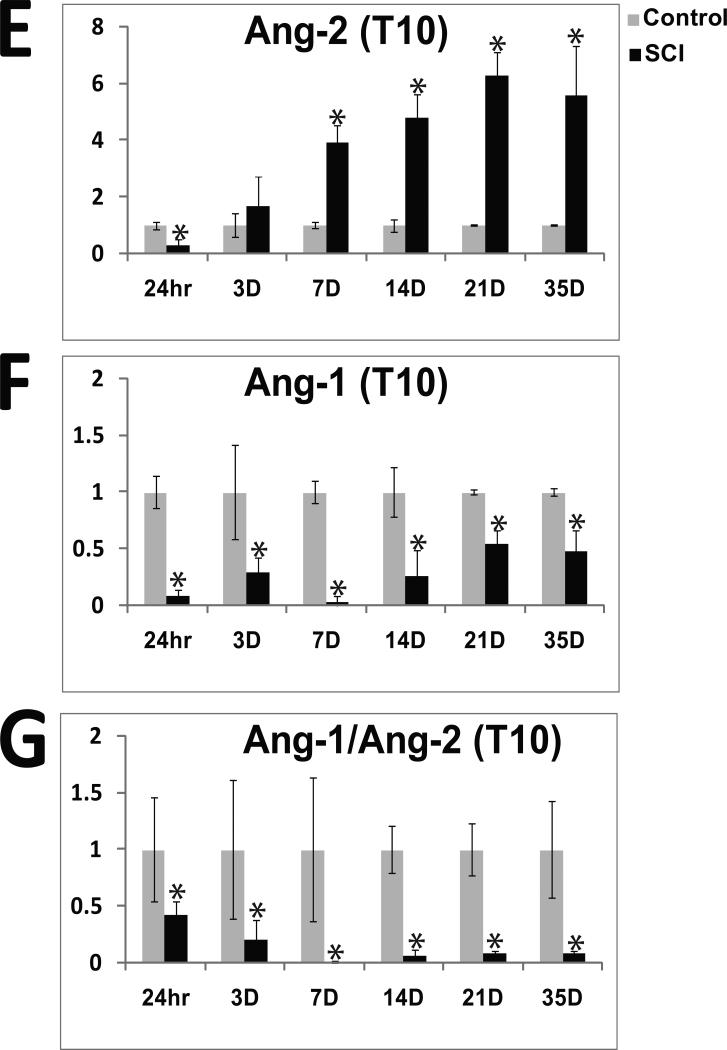
E) Quantitation of Ang-2 monomer protein expression levels at T10 in control and SCI rats 24 hr (both n=3); 3d (control n=5, SCI n=9); 7d (both n=3); 14d (both n=3); 21d (control n=5, SCI n=3); and 35d (control n=4, SCI n=6) after SCI. *=p,0.05 throughout.
F) Quantitation of Ang-1 protein expression levels at T10in control and SCI rats 24hrs (both n=3); 3d (control n=5, SCI n=9); 7d (both n=3); 14d (both n=3); 21d (control n=5, SCI n=3); and 35d (control n=4, SCI n=6) after SCI.
G) The ratio of Ang-1/Ang-2 protein levels detected in the same spinal cord samples and calculated based on data shown in E and F. The ratio represents the cumulative effects of angiopoietins on vascular stability, and reflects changes in the endothelial barrier properties. SCI induced substantial and significant decreases in Ang-1/Ang-2 levels, which should reflect almost complete and chronic blood/spinal cord barrier (BSCB) breakdown.
Here we present only data obtained with Millopore rabbit anti-Ang-2 (AB3121, Lot No. LV 1587452) in both Western blots and immunofluorescent studies. We have decided to use this Millipore Ang-2 Ab in our experiments because: (a) it recognized recombinant Ang-2 protein loaded on the gel; (b) gave a band in spinal cord Western blots with MW similar to that of the Ang-2 protein (~80kD; Fig. 1A). Furthermore, the intensity of the white matter immunolabeling with this Ab did not disappear with increased dilutions (from 1:200 to 1:5,000).
In other Western blot analyses we used goat anti-Ang-1 (Santa Cruz Biotechnology; sc-6319, dilution 1:100) and mouse anti-β−actin (Sigma A5441, dilution 1:80,000, 1hr RT). Ang-1 and Ang-2 primary Abs were incubated for 1 hr at room temperature and overnight at 4°C, with extra washing. Secondary Abs include goat anti-mouse (Southern Biotech 1034-05, dilution 1:5,000), goat anti-rabbit (Southern Biotech 4052-05, dilution 1:5,000), and donkey anti-goat (R&D Systems HAF109, dilution 1:3,000). For Western blot analyses we used 71 injured rats and 41 uninjured rats, and these numbers exclude rats used for testing different Ang-2 antibodies.
Immunofluorescence
Animals were anesthetized by i.p. injection of pentobarbital (150 mg/kg), and sacrificed by transcardial perfusion with 0.9% saline, followed by 4% paraformaldehyde (Sigma P6148-1KG) in 0.1M phosphate buffer. Spinal cords were removed, postfixed overnight at 4°C in 4% paraformaldehyde, and cryoprotected in 30% sucrose in phosphate buffer for three days. Segments were then embedded in OCT compound (Fisher 14-373-65) and cut into 30µm transverse sections on a sliding microtome. In our analyses presented here we used T9 and T10 spinal cord segments. Three 10-minute long shaking washes of the floating frozen sections were conducted in petri dishes containing cold 0.05M tris-buffered saline (TBS) and incubated in blocking solution [0.05M TBS, 0.3%Triton-X, 5% normal goat serum (NGS), 0.3% bovine serum albumin (BSA)] for 45 minutes at room temperature. Sections were then incubated with primary antibodies overnight at room temperature, with shaking, in a solution of 0.05M TBS, 0.3% Triton-X, 0.3% BSA, and 1% NGS. Sections were rinsed with 0.05M TBS three times, 10 minutes each wash, at room temperature with shaking. The appropriate secondary antibodies were diluted in 0.05M TBS, 0.3% Triton-X, 0.3% BSA, and 1% NGS for incubation with the sections for 2 hrs at room temperature with shaking; sections were covered for protection from light.
Sections were washed four times at room temperature in 0.05M TBS (ten minutes each wash, with shaking). Sections were mounted on gelatin-coated glass slides, and dried before the addition of mounting medium with the nuclear stain DAPI (Vector Laboratories H-1200), and the application of the coverslip. Slides were kept at room temperature for a minimum of 20 minutes, and subsequently kept at 4°C for a minimum of 24 hrs before storage at -20°C until viewing. Primary antibodies used include those specific for Ang-2 and Ang-1 (described above), mouse anti-RECA-1, rat endothelial cell antigen, (AbD Serotec MCA970R, 1:200), mouse anti-GFAP (1:700; Millipore MAB360) and mouse anti-NG2 (1:200; Abcam ab50009) and mouse anti-APC (Adenomatous Polyposis Coli ; a marker of mature oligodendrocytes; 1:200; Ocogene OP80). Secondary antibodies used include anti-mouse IgG AlexaFluor 488/green (Invitrogen A11001, 1:2,000), and anti-rabbit IgG AlexaFluor 568/red (Invitrogen A11011, 1:2,000). We also included a “no-primary antibody” control experiment, in which all other steps were performed, except addition of the primary antibody. However, with all antibodies used in the study, this experiment gave no staining, except for the auto-fluorescent aggregations (see Fig. 3BI). For immunocytochemical analyses we used 21 injured and 15 uninjured rats, and these numbers exclude rats used for testing different Ang-2 antibodies.
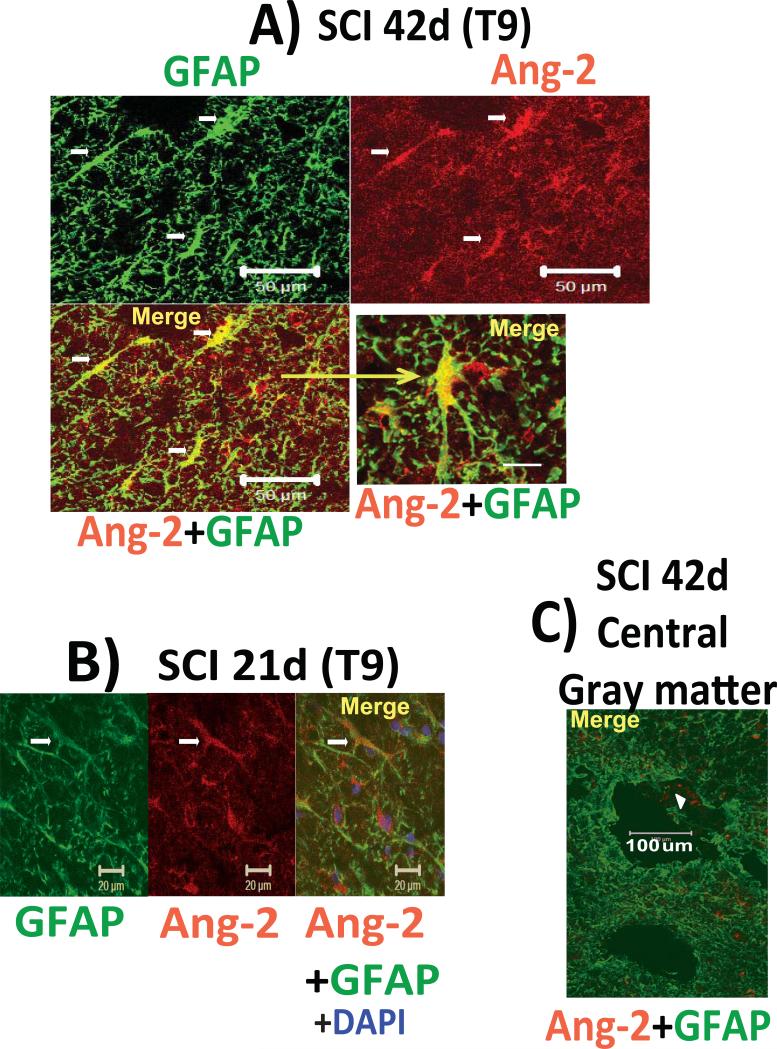
Fig. 3. Glial Ang-2.
A) Co-localization of Ang-2 (red) and GFAP (green) in a T9 segment of the injured spinal cord white matter, 42d after SCI. Calibration line: 50 μm. High-magnification image (lower right, calibration line: 10 μm), shows a stellate, Ang-2/GFAP-labeled cell.
B) GFAP/Ang-2 co-labeled cells were found irrespective of the post-SCI time point analyzed. Here we show lateral white matter of a spinal cord cross section with cells that morphologically resembled GFAP-positive radial glia activated after SCI (Wu et al., 2005). Calibration line: 20 μm. Nuclei stained with DAPI counter stain (blue).
C) Low-magnification image of the GFAP labeling in the central gray matter of injured spinal cord (42d after SCI) with a large cavity (calibration line: 100 μm). Astrocytes in the central gray matter region and around cavities were devoid of Ang-2. However, sparse Ang-2 labeling was observed (marked with white arrowhead), but the identity of these Ang-2-expressing structures was not established.
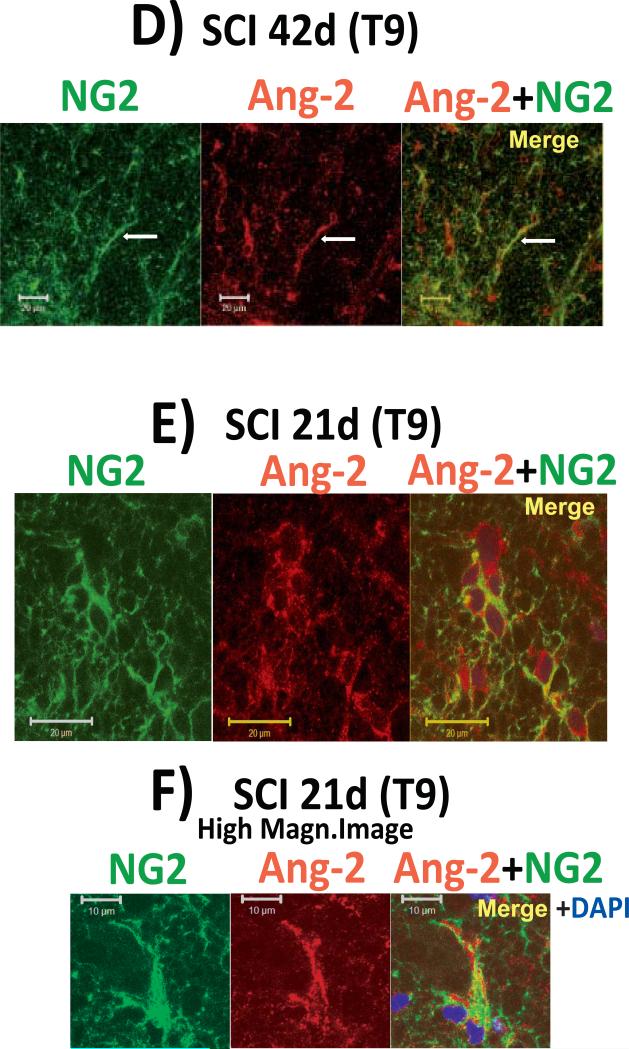
D) Ang-2-positive white matter cells (red) were also co-labeled with NG2 (green; a marker of oligodendrocyte progenitor cells, pericytes or macrophages) in T9, 42d after SCI. Calibration line: 20 μm.
E) Ang-2 labeled white matter cells at T9, 21d after SCI, which, based on their shape and large hypertrophic nuclei, resemble microglial/macrophages that may be both pro-inflammatory cells, but also non-phagocytic progenitor cells found in neonatal and injured brains (Yokoyama et al. 2006; Matsumoto et al., 2008). Calibration line: 20 μm. F) High-magnification image (calibration line: 10 μm) showing Ang-2 labeling in both the peri-nuclear cytoplasm and in processes labeled with NG2.
Confocal Imaging
Images were collected using a Zeiss LSM-510 META confocal microscope with a 20X, 0.75 numerical aperture objective, 40X, 0.95 numerical aperture objective, 63X, 1.4 numerical aperture objective, or with a 100X, 1.40 numerical aperture oil immersion objective (Optical Microscopy Core at UTMB). The images were obtained using excitation lines at 364, 488, 543nm and three different channels of emission with sequential acquisition. After excitation with the 364 nm laser line, emission was measured with a 385-470 nm filter. After excitation with the 488 nm laser line, emission was measured with a 505-530 nm filter, and after excitation with the 543 laser line, emission was measured with a 560-615 nm filter. Z-stack acquisition was done at Z-steps of 0.5μm. 3D rendering was done using Imaris 7.0 software.
Statistical Analysis
All data were graphed with standard deviations displayed. For multiple-group comparisons, data were analyzed using one-way analysis of variance (one-way ANOVA; SPSS statistical package, ver. 11.0). The LSD multiple comparisons post-hoc test was used to determine p values; p values less than 0.05 were considered significant.
RESULTS
I) SCI induces significant and persistent changes in angiopoietin protein levels
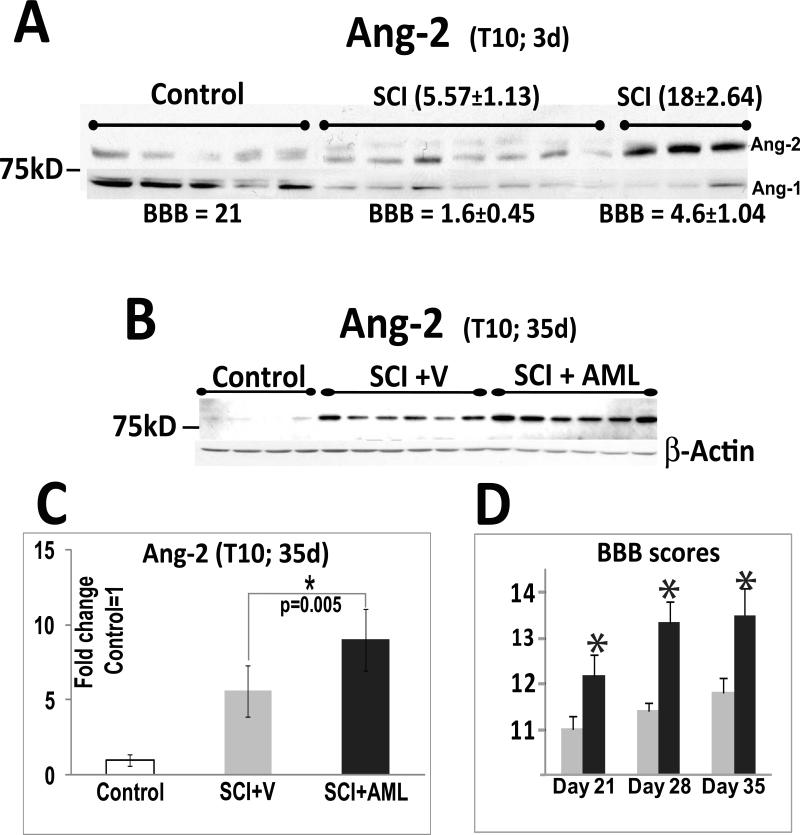
We used Ang-2 Western blots to assess possible changes in Ang-2 protein levels at the lesion site (T10) at different times after SCI, from 24h to 70d (Fig. 1). In all Western blot experiments we used only SCI rats whose motor recovery was similar at specific time point post-injury, as shown by BBB scores (Basso et al., 1995) within the group differing by only ±1 BBB unit. To test the specificity of the polyclonal Ang-2 Ab employed in our experiments, we used human recombinant Ang-2 protein (HrAng-2; Fig. 1A) and analyzed it together with proteins extracted from injured rat spinal cords (T10; 70d after SCI; Fig. 1A). Structural studies have shown that the amino-terminus of Ang-2 is important for Ang-2 oligomerization, and that active Ang-2 is released as a dimer or an oligomer (Maisonpierre et al., 1997; Barton et al., 2005). We have also detected the Ang-2 dimer in our rat spinal cord Western blots (~150kD; Fig. 1B; C). In some Western blots the ~80kD monomer band appeared as a doublet (Fig. 1B), which may represent two Ang-2 isoforms (Kim et al. 2000), different Ang-2 glycosylation levels, or the appearance of non-specific bands. However, only the “lower” 80kD Ang-2 bands and the dimer bands were consistently detected in all our Ang-2 Western blots (Fig. 1C). The dimer band intensities mirrored the intensities of the lower monomer band, so only the lower ~80kD monomer band was used for quantitative analysis of SCI-induced changes in Ang-2 levels at different times after SCI.
We found that SCI induced significant decreases in Ang-2 protein levels 24hrs after SCI at the lesion site (T10; Fig 1B; 1E). However, starting 3d after SCI, Ang-2 levels gradually began to increase over time, (Fig. 1E), with maximum levels obtained 21d after SCI (Fig. 1B, E). The Ang-2 protein level increases persisted, being unchanged at 56d (Fig. 1D) or 70d after SCI (Fig. 1A). Quantitative analysis of Ang-2 levels at different times after SCI (Fig. 1E) showed that SCI-induced increases in Ang-2 protein levels were statistically significant at all time points (*p<0.05; except at 3d post-injury).
In contrast to Ang-2, Ang-1 levels were significantly decreased at all time points analyzed, in agreement with Herrera et al., (2010). At 24 hrs after injury the Ang-1 signal was almost completely gone (Fig. 1B). However, in contrast to data reported by Hererra et al. (2010), in our experiments the Ang-1 signal intensity gradually returned (Fig. 1F), although it never reached pre-injury levels.
Since the two angiopoietins influence vascular integrity in an opposite manner by affecting the activity of the Tie-2 receptor, the ratio of Ang-1/Ang-2 is often calculated as an indicator of vascular (in)stability and edema formation (Tait and Jones 2004). We calculated the Ang-1/Ang-2 ratio (Figs. 1E, F, G) at different time points after SCI. Given that Ang-1 is constitutively expressed in the CNS, while Ang-2 expression is induced during vascular remodeling (Nag et al., 2005; Thomas and Augustin 2009), Ang-1 levels are normally higher than Ang-2 levels. This results in an Ang-1/Ang-2 ratio that is markedly shifted towards Ang-1 in normal physiological conditions, consistent with our data for uninjured spinal cords (Figs. 1B, C, G). However, due to decreases in Ang-1 and concomitant increases in Ang-2 after SCI, the Ang-1/Ang-2 ratio was significantly decreased (by ~60%) 3d after SCI. At 7d after SCI the ratio decreased even further (by ~95%), and remained similarly reduced (~90%) for weeks after SCI (Fig. 1G).
II) Vascular Ang-1 and glial Ang-2
In agreement with decreased Ang-1 levels after SCI (Fig. 1) we found only slight Ang-1 labeling at the lesion site (T10) 21 or 42d after SCI (n=3 per time point). Although rare, distinctly punctate Ang-1 labeling (red; Fig. 2A) was usually closely associated with blood vessels (green; Fig. 2A), and found on the abluminal surface of endothelial cells.
Fig. 2. Cellular localization of angiopoietins.
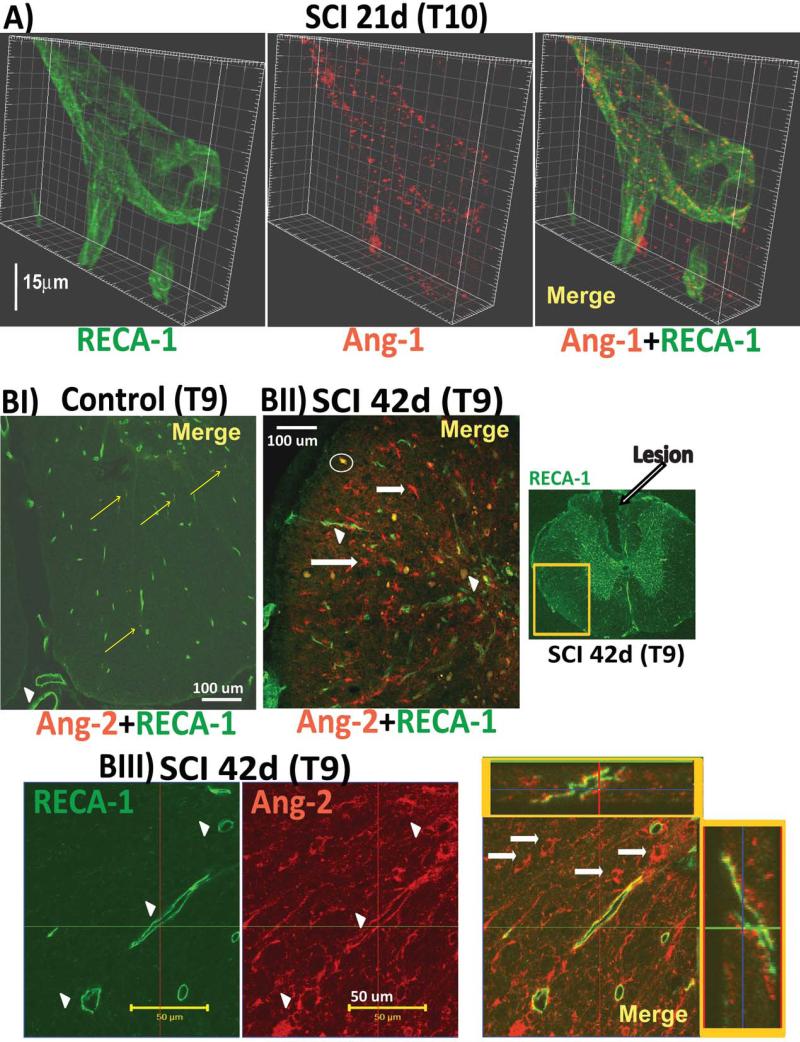
A) Close apposition of Ang-1 punctate staining (red) on the outer surface of blood vessels, whose endothelial cells were labeled with RECA-1 (green) in injured spinal cord at the lesion site (T10) 21d after SCI. Similar punctate Ang-1 staining has already been reported in brain vessels (Nourhaghighi e al., 2003). Calibration line: 15 μm. Z-stack image acquisitions were done with a Zeiss LSM-510 META confocal microscope with a 63X oil, 1.4 numerical aperture, objective at optical slices of X and at Z-steps of 0.6uM. 3D rendering was done with Imaris software version 7.0.
BI) An uninjured spinal cord (ventral white matter area, T9, age-matched control rat) labeled with RECA-1 was almost completely devoid of Ang-2 staining, regardless of animal age, spinal segments analyzed, or the size of parenchymal or pial blood vessels (the large posterior artery and vein are marked with a white arrowhead).
BII) Ang-2 labeling (red) was clearly visible in injured spinal cords (T9, 42d after SCI) in the lateral white matter of a spinal cord cross section (the ventrolateral white matter area shown in BI is marked as a yellow square in a low-magnification image of the RECA-1-labeled T9 injured spinal cord section with visible lesion is shown to the right). Co-labeling injured spinal cord sections with RECA-1 (green) showed blood vessels associated with Ang-2 (marked with arrowhead). However, Ang-2 was mostly not associated with RECA-1-labeled blood vessels (see red labeling in Figs. BI and BIII), indicating that SCI mainly stimulates non-vascular Ang-2. White arrows mark white matter cells intensely labeled with Ang-2.
Occasional round spots (e.g. one marked with a white circle) showing complete co-localization and intense yellow color after merging of Ang-2 (red) and RECA-1 (green) images were non-specific autofluorescing aggregates that were found as fluorescently labeled spots regardless of the filters used, including in control experiments in which the primary antibody was omitted. We analyzed Ang-2 labeling at the site of injury (T10) and segments around the lesion (T9) at all time points. Here we are presenting primarily results of Ang-2 immunolabeling from the T9 segments, as T10 sections (the lesion itself) contained a large number of autofluorescent agglomerations (white circle in Fig. 2BI) that obscured Ang-2-specific labeling,
BIII) High-magnification (60x) image of white matter area of an injured spinal cord (T9; 42d after SCI) showing RECA-1-labeled blood vessels associated with Ang-2 (marked with white arrowheads). Both orthogonal projections (x-horizontal; y-vertical; framed in yellow boxes) of the long blood vessel in the merged image showed punctate Ang-2 staining on the outer surface of the blood vessels, This image also shows several round Ang-2-labeled cells (marked with white arrows) not associated with blood vessels, consistent with Ang-2 being induced in non-vascular cells after SCI (Fig. 2BII).
Ang-2 (red) in uninjured cords was almost undetectable (Fig. 2 BI), except for scarce and isolated appearance of red puncta in small blood vessels (labeled with RECA-1; green; co-localization was marked with yellow arrows), consistent with previous reports on brain Ang-2 (Nag et al.2005). Ang-2 immuno-labeling (red) was markedly increased in injured spinal cords (Fig. 2 BII), consistent with the results shown in Fig. 1. SCI induced Ang-2 expression associated with some blood vessels (arrow heads in Fig. 2BII and 2BIII) primarily in injured white matter, at all time points that were analyzed immunocytochemically: 14, 21 and 42d after SCI (n=3 per time point and for age-matched uninjured control spinal cord sections). Although both orthogonal projections (x and y, shown in yellow squares in Fig. 2BIII) showed Ang-2 labeling on the outer surface of endothelial cells labeled with RECA-1 (green), suggesting Ang-2 induction in perivascular cells, the endothelial expression of Ang-2 could not be ruled out. However, given that the morphological characteristics of the perivascular Ang-2 labeling in injured spinal cords (Fig. 2BIII) were similar to those of perivascular astrocytes, we tested the hypothesis that Ang-2 is localized to astrocytes.
Indeed, Ang-2-positive cells in injured spinal cords were partially co-labeled with the astrocytic marker glial fibrillary acidic protein (GFAP/green; Fig. 3; n=9). Here we show GFAP-labeled white matter astrocytes 42d (Fig. 3A) or 21d (Fig. 3B; n=3 for both control and SCI) after SCI. Some Ang-2 labeling was found around the lesion epicenter/ fluid-filled cavity in central gray matter and dorsal columns (Fig. 3C), despite marked accumulation of reactive astrocytes. Interestingly, some Ang-2 labeling was found within a fluid-filled cavity (marked with a white arrowhead in Fig. 3C).
A large population of Ang-2 positive cells was not localized around blood vessels (white arrows in Figs. 2BII and 2BIII), but in white matter parenchymal cells co-labeled with GFAP (Fig. 3A/B and with NG2 (Fig. 3D/E; 21 and 42d after SCI; n=3 per time point and for age-matched uninjured control spinal cord sections). NG2 is an integral membrane chondroitin sulfate proteoglycan expressed by oligodendrocyte progenitor cells (OPCs; Nishiyama et al., 1999), pericytes (LaManna et al., 2004) and microglia/macrophages (Polverini et al., 1977; Sunderkotter et al., 1994; Matsumoto et al., 2008). Induction of Ang-2 in GFAP- and NG2-labeled cells in injured spinal cords can thus explain marked increases in Ang-2 levels (Fig. 1), given that those cells in uninjured spinal cords do not express Ang-2 (note the complete lack of Ang-2 labeling in uninjured spinal cords; Fig. 2BII), and that there is robust proliferation of GFAP- or NG2- expressing cells after SCI (Zai and Wratahall, 2005).
III) Higher Ang-2 levels were associated with better functional recovery after SCI
We compared Ang-2 levels in two groups of SCI rats: SCI rats whose combined hind limb locomotor BBB scores (Basso et al., 1995) 3d after SCI averaged 1.6±0.45 (n=7), and SCI rats whose average BBB scores were 3 times higher, 4.6±1.04, at the same time after SCI (3d, n=3; Fig. 4A), although the initial impact of contusion injury was the same in all SCI rats, with BBB scores of zero 24h after SCI. After obtaining BBB scores, T10 segments were isolated and used for Ang-2 Western blots. We found significant 3.2-fold higher Ang-2 levels (18±2.64 vs. 5.57±1.13; *p=0.005) in SCI rats with markedly better motor recovery, suggesting that early and robust induction of Ang-2 was associated with better functional recovery after SCI. Interestingly, Ang-1 levels were not affected.
Fig. 4. Ang-2 and better functional recovery.
A) Western blot showing ~80kDa Ang-2 bands 3d after SCI at T10 (the site of injury) in uninjured rats (n=5), and in two groups of SCI rats: those with poor locomotor ability (n=7) and those with markedly better locomotion (n=3). The combined hind limb locomotor BBB scores (average±SD) for each group of rats is indicated below the Ang-2 bands. Numbers above the Ang-2 bands represent quantitative analysis of Ang-2 band intensities in each experimental group; average±SD. Ang-2 levels 3d after SCI were significantly higher in SCI rats with preserved locomotion than in SCI rats with poor locomotion or uninjured rats (p<0.001). However, 3d after SCI the Ang-2 levels in severely impaired rats did not differ significantly from those in uninjured rats, suggesting a delayed induction of Ang-2. B) Western blot showing Ang-2 protein levels at T10 35 days after injury in three groups of rats: uninjured rats (control, n=4), SCI rats treated with vehicle (V, n=6), and SCI rats treated with amiloride (AML, n=6). Amiloride was administered i.p. daily from 24h until 35d after SCI. The dose administered was 5 mg/kg for the first 10d, followed by an increased dose of 10mg/kg (see Lee et al., 2011).
C) Quantified signal intensity of the ~ 80kDa Ang-2 bands in Fig. 5B. The intensity of the ~80kDa band was normalized first to the β-actin levels, and then to uninjured values (uninjured signal intensity =1; mean ± SD). Ang-2 levels in amiloride-treated SCI rats were significantly higher than in vehicle-treated SCI rats (*, p=0.005).
D) BBB scores (mean±SD) at 21, 28 and 35d after SCI in vehicle- (gray, n=6) and amiloride-treated SCI rats (black, n=6) whose Ang-2 levels were analyzed and shown in Fig. 4B/C. Amiloride treatment induced a significant improvement in locomotor ability.
We have recently reported that daily i.p. administration of amiloride results in significantly better motor recovery of moderately contused SCI rats (Lee et al., 2011), so we asked if amiloride intervention also affected Ang-2 levels, not previously investigated. As shown in Fig. 4B, we analyzed Ang-2 Western blots in four control rats (that did not receive any treatment; white bars in Fig. 4C), six SCI rats that received vehicle (gray bars in Figs. 4C/D) and six SCI rats that received amiloride (black bars in Figs. 4C/D). As shown in Fig. 4D, amiloride treatment improved motor recovery after SCI, as it significantly increased BBB scores 21, 28 and 35d after SCI (but not sooner than 21d after SCI; Lee et al., 2011). As shown in Figs. 4B and C, amiloride treatment significantly increased Ang-2 levels by 1.6- fold compared to vehicle–treated SCI rats 35d after SCI at the lesion site (T10) associated with improved functional recovery, further supporting a beneficial role of Ang-2 increases after SCI.
DISCUSSION
Ang-2 induction has largely been associated with vascular barrier breakdown underlying different pathological processes, especially if coupled with decreases in Ang-1 levels. A significantly reduced Ang-1/Ang-2 ratio (shifted towards Ang-2) is often used as a predictor not only of vascular dysfunction, but also of disease severity in various pathological conditions (Ricciuto et al., 2011; Ong et al., 2010; David et al., 2009; Szarvas et al., 2008; Lim et al., 2004), including in the CNS. For example, Nourhaghighi et al. (2003) found that marked decreases in Ang-1 and increases in Ang-2 (i.e. a decreased Ang-1/Ang-2 ratio) coincide with marked blood-brain barrier breakdown after brain injury. It has been well established that the effect of Ang-2 on the barrier properties depends on its induction in endothelial cells in various tissues, including the CNS. For example, endothelial upregulation of Ang-2 and consequent blood-brain barrier deregulation has been documented in stroke (Beck et al., 2000; Zhang et al., 2002), brain injury (Nag et al., 2005), and brain tumors (Sie et al., 2009).
Our results showed a markedly reduced Ang-1/Ang-2 ratio that began soon after SCI, but persisted unchanged for weeks and months after injury, primarily due to permanently elevated Ang-2 levels. These SCI-induced changes in the Ang-1/Ang-2 ratio would suggest that the blood-spinal cord barrier (BSCB) remains equally compromised from the acute into the chronic post-SCI phase. However, this is not consistent with other reports that have analyzed vascular integrity in injured spinal cords. (Popovich et al., 1996; Whetstone et al., 2003; Casella et al., 2002; Loy et al., 2002; Benton et al., 2008; Mahoney et al., 2009; Cohen et al., 2009), or with our analysis of the time course of vasogenic edema formation after SCI (Nesic et al., 2006). Early and massive disruption of the BSCB occurs as early as 35 min post-injury (Whetstone et al., 2003), but the barrier is gradually and partially restored by one month post-SCI (Noble and Wrathall 1989; Popovich et al., 1996; Mautes et al. 2000; Whetstone et al., 2003; for a review see Sharma, 2005), thus closely paralleling changes in Ang-1. Recent MRI imaging of injured rat spinal cords (Cohen et al., 2009) suggests the existence of aberrant BSCB in chronically injured cords months after SCI. Evidence has accumulated that chronic disturbances in the BSCB result from an inadequate maturation of the newly formed blood vessels after SCI (Benton et al., 2008), which is consistent with our finding that Ang-1 levels do not return to pre-SCI, basal levels.
Therefore, our data showing a lack of temporal correspondence between BSCB changes and Ang-2 levels, and association of higher Ang-2 levels with better functional recovery after SCI cumulatively suggest a beneficial role of Ang-2 upregulation, which, although rarely, has been reported in injured CNS.
Ang-2 and angiogenesis
It has been shown that Ang-2 has a pro-angiogenic role in the injured CNS (Nourhaghighi et al. 2003), and that angiogenesis contributes to the recovery after SCI. Zhang et al. (1997) have demonstrated that angiogenesis promotes neural regeneration in injured spinal cords, and more recently, Han et al. (2010) have elegantly shown that pro-angiogenic interventions result in significantly improved functional recovery after SCI. In the injured brain proangiogenic Ang-2 has been identified in cells other than endothelial, such as astrocytes (Mandriota et al, 2000; Beck et al, 2000), pericytes (LaManna et al., 2004) and macrophages (Matsumoto et al., 2008; Polverini et al, 1977; Sunderkotter et al, 1994), consistent with our data, as Ang-2 was found in GFAP-labeled astrocytes, and in NG2-positive cells, which can be pro-angiogenic pericytes (Ozerdam et al., 2001) and/or activated microglia/macrophages (Matsumoto et al., 2008).
SCI-induced angiogenesis is a response to hypoxia and ischemia resulting from direct damage to the blood vessels and from the posttraumatic hypoperfusion and hypotension. It has been shown that SCI results in a massive loss of endothelial cells 1day after SCI (Whetstone et al. 2003), including loss of blood vessels normally expressing Ang-2 (see Fig. 2BI), which can explain the decrease in Ang-2 24h after SCI. However, lost blood vessels are replaced within one week after SCI, as robust angiogenesis takes place between 3 and 7 d after SCI (Whetstone et al., 2003; Loy et al., 2002), paralleling the time profile of Ang-2 induction at the lesion epicenter. However, newly formed blood vessels are not fully mature (Benton et al., 2008), and thus they maintain chronic hypoxic conditions in injured spinal cords. Given that hypoxia stimulates Ang-2 synthesis (Pichiule et al., 2004), it is possible that hypoxia induces persistent Ang-2 upregulation after SCI, which may underlie both early angiogenic response following the loss of blood vessels that occurs within 3-7 days (Loy et al., 2002), and a secondary angiogenic wave resulting from the formation of immature vessels that occurs at 14-60 days after SCI (Popovich et al., 1996; Loy et al., 2002). Interestingly, the second angiogenic wave is found only within the injured white matter (Popovich et al., 1996; Loy et al., 2002), in agreement with our data showing Ang-2 induction in chronically injured cords -predominantly in the white matter.
Our results also suggest that better recovery of SCI rats is associated not only with higher Ang-2 levels, but also with the early onset of Ang-2 induction. SCI rats with modest functional recovery (BBB scores ~1.5 at 3d) had Ang-2 levels increased by ~1.7-fold at 3d (Fig. 4A), and ~3-fold at 7d after SCI (Fig. 1E). However, SCI rats whose Ang-2 levels had already increased ~3-fold at 3d after SCI recovered locomotion extraordinarily better (Fig. 4A). Taken together, our results suggest that earlier and more robust induction of Ang-2 predicts markedly better functional recovery after SCI, consistent with the early wave of angiogenesis being relevant to the functional recovery after SCI (Loy et al., 2002; Han et al., 2010).
Ang-2 and progenitor cells
Liu et al. (2009) have shown that stroke induces Ang-2 expression in neural stem cells in the subventricular zone, while a number of reports indicate that Ang-2 in progenitor cells can stimulate neurogenesis in vivo and in vitro (Ward and LaManna 2004; Androutsellis-Theotokis et al. 2009; Liu et al. 2009; Marteau et al., 2010). Although SCI did not induce Ang-2 in ependymal or subependymal spinal cord cells, which are analogous to the subventricular neural stem cells (Kulbatski et al., 2005), we found Ang-2 in NG2 cells that morphologically closely resemble white matter-specific NG2-positive oligodendrocyte progenitor cells (OPCs), characterized and described in the study by Wu et al., (2005). It has been well established that NG2-labeled OPCs generate myelinating oligodendrocytes (Horner et al. 2000; Nishiyama et al. 2002; Reynolds et al. 2002), whose contribution to the functional recovery after SCI is well established (Cao et al., 2010; Jiang et al., 2008). Although additional markers should be tested to confirm that Ang-2+/NG2+ cells are OPCs, we can hypothesize that an amiloride-induced increase in myelin levels 35d after SCI (Lee et al., 2011) resulted from an increased abundance of Ang-2-expressing OPCs in amiloride-treated SCI, as this medication also significantly increased Ang-2 levels. This may also explain the delayed onset of the beneficial amiloride effect on the motor recovery (seen only 21+ days after SCI; Lee et al., 2011), since remyelination processes relying on prior OPC proliferation and differentiation take place not in the acute but in the subchronic phase post-SCI (Totoiu and Keirstead 2005). Therefore it is possible that Ang-2 has different roles in diverse and time-dependent recovery processes after SCI, such as in stimulating early angiogenesis and/or late gliogenesis, an issue that remains to be examined in our future studies.
Given that treatments targeting angiogenesis or OPCs have shown significant therapeutic potential in SCI (Han et al., 2010; Cao et al., 2010; Sharp et al., 2010), further characterization of the Ang-2 role in SCI is timely and warranted.
SCI induces persistent upregulation of Ang-2.
Higher Ang-2 levels are associated with better functional recovery after SCI.
Ang-2 is induced in parenchymal cells labeled with GFAP or NG2.
Ang-2 induction likely contributes to pro-angiogenic and/or gliogenic processes.
Acknowledgements
We would like to thank Dr. Claire E. Hulsebosch (Department of Neuroscience and Cell Biology, UTMB) for the generous use of her laboratory space for animal housing. This work was funded by the Mission Connect, a project of TIRR Foundation, Moody Center for Brain and Spinal Cord Injury and Craig Nielsen Foundation. We also thank Dr. David Konkel for critically editing the manuscript.
Abbreviations
- Ang-1
angiopoietin 1
- Ang-2
angiopoietin 2
- BBB scale/scores
Basso, Beattie, and Bresnahan locomotive rating scale (Basso et al., 1995)
- BSCB
blood spinal cord barrier
- GFAP
glial fibrylary acidic protein
- NG2
a transmembrane chondrotin sulfate proteoglycan expressed on several types of immature progenitor cells
- OPC
oligodendrocyte progenitor cells
- SCI
spinal cord injury
- T10
tenth thoracic segment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott NH, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Amiloride protects against pentylenetetrazole-induced kindling in mice. Br J Pharmacol. 2005;145:880–884. doi: 10.1038/sj.bjp.0706291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Pillai KK, Ahmad FJ, Dua Y, Khan ZI, Vohora D. Comparative efficacy of liposome-entrapped amiloride and free amiloride in animal models of seizures and serum potassium in mice. Eur Neuropsychopharmacol. 2007;17:227–229. doi: 10.1016/j.euroneuro.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb D, Poser SW, Walbridge S, Munasinghe J, Koretsky AP, Lonser RR, McKay RD. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci USA. 2009;106:13570–75. doi: 10.1073/pnas.0905125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rueger MA, Park DM, Boyd JD, Padmanabhan R, Campanati L, Stewart CV, LeFranc Y, Plenz D, Walbridge S, Lonser RR, McKay RD. Angiogenic factors stimulate growth of adult neural stem cells. PLoS One. 2010;5:e9414. doi: 10.1371/journal.pone.0009414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, Bowlby MR, Dunlop J, Monaghan M. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis. 2008;31:334–341. doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Barton WA, Tzvetkova D, Nikolov DB. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure. 2005;13:825–32. doi: 10.1016/j.str.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Worth CA, Mahoney ET, Hagg T, Whittemore SR. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008;28:1771–85. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Chen MT, Huang HC, Hsiao MC, Chen H. A Method for unit recording in the lumbar spinal cord during locomotion of the conscious adult rat. J Neurosci Methods. 2009;182:49–54. doi: 10.1016/j.jneumeth.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, Kim DH, Whittemore SR. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neuronal parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–41. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kümpers P, Hellpap J, Horn R, Leitolf H, Haller H, Kielstein JT. Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am J Kidney Dis. 2009;53:770–8. doi: 10.1053/j.ajkd.2008.11.030. [DOI] [PubMed] [Google Scholar]
- De Biase A, Knoblach SM, Di Giovanni S, Fan C, Molon A, Hoffman EP, Faden AI. Gene expression profiling of experimental traumatic spinal cord injury as a function of distance from impact site and injury severity. Physiol Genomics. 2005 Aug 11;22(3):368–81. doi: 10.1152/physiolgenomics.00081.2005. Epub 2005 Jun 7. [DOI] [PubMed] [Google Scholar]
- Falgairolle M, Seze M, Juvin L, Morin D, Cazalets JR. Coordinated network functioning in the spinal cord: An evolutionary perspective. J Physiol Paris. 2006;100:304–316. doi: 10.1016/j.jphysparis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Han S, Arnold SA, Sithu SD, Mahoney ET, Geralds JT, Tran P, Benton RL, Maddie MA, D'Souza SE, Whittemore SR, Hagg T. Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain. 2010;133:1026–42. doi: 10.1093/brain/awq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TM, Moss AJ, Brindle NP. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res. 2008;5:236–45. doi: 10.2174/156720208786413433. [DOI] [PubMed] [Google Scholar]
- Herrera J, Sundberg LM, Zentilin L, Giacca M, Narayana P. Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J Neurotrauma. 2010;27:2067–2076. doi: 10.1089/neu.2010.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. Comp Neurol. 2006;498(4):525–38. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Choi HH, Kim KT, Hong HJ, Koh GY, Lee GM. Expression and purification of recombinant human angiopoietin-2 produced in Chinese hamster ovary cells. Protein Expr Purif. 2005;39:175–83. doi: 10.1016/j.pep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Jiang S, Ballerini P, Buccella S, Giuliani P, Jiang C, Huang X, Rathbone MP. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell Transplants Remyelinate and Restore Locomotion after Spinal Cord Injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Kim JH, Ryu YS, Jung SH, Nah JJ, Koh GY. Characterization and expression of a novel alternatively spliced human angiopoietin-2. J Biol Chem. 2000;275:18550–18556. doi: 10.1074/jbc.M910084199. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee JM, Park JS, Jo SA, Kim YO, Kim CW, Jo I. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem Biophys Res Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Kulbatski I, Mothe AJ, Nomura H, Tator CH. Endogenous and exogenous CNS derived stem/progenitor cell approaches for neurotrauma. Curr Drug Targets. 2005;6:111–26. doi: 10.2174/1389450053345037. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004 Aug;207(Pt 18):3163–9. doi: 10.1242/jeb.00976. Review. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Myung JS, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009;276:4622–35. doi: 10.1111/j.1742-4658.2009.07174.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Mokkapati VU, Johnson KM, Nesic-Taylor DO. Amiloride improves locomotor recovery after spinal cord injury. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1921. e-pub ahead of print 2 May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–24. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, Lu M, Zhang ZG. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009;284:22680–22689. doi: 10.1074/jbc.M109.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnell JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–24. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Mahoney ET, Benton RL, Maddie MA, Whittemore SR, Hagg T. ADAM8 is selectively up-regulated in endothelial cells and is associated with angiogenesis after spinal cord injury in adult mice. J Comp Neurol. 2009;512:243–255. doi: 10.1002/cne.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Marteau L, Pacary E, Valable S, Bernaudin M, Guillemot F, Petit E. Angiopoietin-2 regulates cortical neurogenesis in the developing telencephalon. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq243. e-pub ahead of print 2 December 2010. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Chuai M, Imai Y, Takahashi H, Tanaka J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2008;28:149–163. doi: 10.1038/sj.jcbfm.9600519. [DOI] [PubMed] [Google Scholar]
- N'Gouemo P. Amiloride delays the onset of pilocarpine-induced seizures in rats. Brain Res. 2008;1222:230–232. doi: 10.1016/j.brainres.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Papneja T, Venugopalan R, Stewart DJ. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab Invest. 2005;85:1189–98. doi: 10.1038/labinvest.3700325. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Ye Z, Unabia GC, Rafati D, Hulsebosch CE, Perez-Polo JR. Acute and chronic changes in aquaporin 4 expression after spinal cord injury. Neuroscience. 2006;143:779–92. doi: 10.1016/j.neuroscience.2006.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, Hulsebosch CE, Perez-Polo JR. Aquaporin 1 - a novel player in spinal cord injury. J Neurochem. 2008;105:628–640. doi: 10.1111/j.1471-4159.2007.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–24. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med. 2010;38:1845–51. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu R, Christie DK, Barbosa E, Singh I, Singh AK. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J Neurochem. 2007;101:182–200. doi: 10.1111/j.1471-4159.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Bradford TS. A quantitative spatial analysis of the blood-spinal cord barrier. 1. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Ricciuto DR, Dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, Lafferty EI, Cook DJ, Fox- Robichaud A, Kahnamoui K, Kain KC, Liaw PC, Liles WC. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011 doi: 10.1097/CCM.0b013e318206d285. e-pub ahead of print 14 January 2011. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Graumann U, Gutierrez B, Hausmann O. Traumatic spinal cord injury alters angiogenic factors and TGF-beta1 that may affect vascular recovery. Curr Neurovasc Res. 2010;7:301–310. doi: 10.2174/156720210793180756. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord injury. Brain Res: Brain Res Rev. 2002;40:257–266. doi: 10.1016/s0165-0173(02)00208-4. [DOI] [PubMed] [Google Scholar]
- Scheff Stephen W, Rabchevsky Alexander G., Fugaccia Isabella, Main John A., Lumpp James E., Jr Experimental Modeling of Spinal Cord Injury: Characterization of a Force-Defined Injury Device. Journal of Neurotrauma. 2003 February;20(2):179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29:6722–33. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des. 2005;11:1353–1389. doi: 10.2174/1381612053507837. [DOI] [PubMed] [Google Scholar]
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–63. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie M, Wagemakers M, Molema G, Mooij JJ, de Bont ES, den Dunnen WF. The angiopoietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiforme. J Neurosurg. 2009;110:147–55. doi: 10.3171/2008.6.17612. [DOI] [PubMed] [Google Scholar]
- Song HS, Son MJ, Lee YM, Kim WJ, Lee SW, Kim CW, Kim KW. Oxygen tension regulates the maturation of the blood-brain barrier. Biochem Biophys Res Commun. 2002;290:325–331. doi: 10.1006/bbrc.2001.6205. [DOI] [PubMed] [Google Scholar]
- Sorbo JG, Moe SE, Holen T. Early upregulation in nasal epithelium and strong expression in olfactory bulb glomeruli suggest a role for aquaporin-4 in olfaction. FEBS Lett. 2007;581:4884–4890. doi: 10.1016/j.febslet.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Szarvas T, Jäger T, Tötsch M, vom Dorp F, Kempkensteffen C, Kovalszky I, Romics I, Ergün S, Rübben H. Angiogenic switch of angiopietins-Tie2 system and its prognostic value in bladder cancer. Clin Cancer Res. 2008;14:8253–62. doi: 10.1158/1078-0432.CCR-08-0677. [DOI] [PubMed] [Google Scholar]
- Tait CR, Jones PF. Angiopoietins in tumors: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1419–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Huang T, Papadopoulos N, Maisonpierre PC, Davis S, Yancopoulos GD. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–9. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–84. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–8. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–83. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and would healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Shibuya S, Miyamoto O, Itano T, Yamamoto T. Increase of NG2-positive cells associated with radial glia following traumatic spinal cord injury in adult rats. J Neurocytol. 2005;34:459–69. doi: 10.1007/s11068-006-8998-4. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Zhu X, Chu X, Minami M, Hey J, Wei W, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–68. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;50:247–57. doi: 10.1002/glia.20176. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–3. doi: 10.1038/35097593. Review. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and Angiopoietin Expression With Disruption of Blood–Brain Barrier and Angiogenesis After Focal Cerebral Ischemia. J Cereb Blood Flow Metab. 2002;22:379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]