Abstract
Non-psychotic individuals at increased risk for schizophrenia show alterations in fronto-striatal dopamine signaling and cortical gray matter maturation reminiscent of those seen in schizophrenia. It remains unclear however if variations in dopamine signaling influence rates of structural cortical maturation in typically developing individuals, and whether such influences are disrupted in patients with schizophrenia and their non-psychotic siblings. We sought to address these issues by relating a functional Val→Met polymorphism within the gene encoding catechol-o-methyltransferase (COMT)—a key enzymatic regulator of cortical dopamine levels—to longitudinal structural neuroimaging measures of cortical gray matter thickness. We included a total of 792 magnetic resonance imaging brain scans, acquired between ages 9 and 22 years from patients with childhood-onset schizophrenia (COS), their non-psychotic full siblings, and matched healthy controls. Whereas greater Val allele dose (which confers enhanced dopamine catabolism and is proposed to aggravate cortical deficits in schizophrenia) accelerated adolescent cortical thinning in both schizophrenia probands and their siblings, it attenuated cortical thinning in healthy controls. This similarity between COS patients and their siblings was accompanied by differences between the two groups in the timing and spatial distribution of disrupted COMT influences on cortical maturation. Consequently, whereas greater Val “dose” conferred persistent dorsolateral prefrontal cortical deficits amongst affected probands by adulthood, cortical thickness differences associated with varying Val dose in non-psychotic siblings resolved over the age-range studied. These findings suggest that cortical abnormalities in pedigrees affected by schizophrenia may be contributed to by a disruption of dopaminergic infleunces on cortical maturation.
Keywords: Neuroimaging, COMT, Schizophrenia, Siblings, Cortex, Maturation, Dopamine
Introduction
Schizophrenia is a highly heritable (Cardno et al., 1999) neurodevelopmental disorder that is strongly associated with dopaminergic dysregulation (Howes and Kapur, 2009; Simpson et al., n.d.), and maturational (Brans et al., 2008; Thompson et al., 2001; Vidal et al., 2006) and functional (Minzenberg et al., 2009) abnormalities in higher-order fronto-temporal and parietal “associative” cortices sub-serving executive function and language. Similar, but less pronounced dopaminergic (Hirvonen et al., 2006) and maturational (Gogtay et al., 2007) alterations also exist in non-psychotic individuals considered to be at elevated risk for schizophrenia based on a family history of schizophrenia, or a personal history of prodromal symptoms. Twin and family studies suggest that such alterations of dopamine (DA) signaling (Hirvonen et al., 2006) and structural cortical maturation (Brans et al., 2008; McDonald et al., 2006) directly index genetic liability for schizophrenia.
Since DA is known to influence cellular processes fundamental to structural development of the cortex (Bhide, 2009), inherited disruptions of DA signaling could contribute to the cortical dysmaturation that has been reported in people with schizophrenia during adolescence and early adulthood (Greenstein et al., 2006; Thompson et al., 2001; Vidal et al., 2006), and is also seen in non-psychotic siblings of probands in an attenuated form (Gogtay et al., 2007). We sought to test this notion by relating a genetic variant that impacts DA signaling, to cortical maturation in a large, longitudinally scanned sample of healthy controls (HCs), individuals with childhood-onset schizophrenia (COS) and the non-psychotic siblings (SIBs) of COS probands. Specifically, we hypothesized that a functional polymorphism within the gene encoding the DA catabolizing enzyme catechol-o-methyltransferase [COMT Val158Met single nucleotide polymorphism (SNP)] would modulate cortical maturation in HCs, but that these normative gene–brain relationships would be disrupted in patients with COS. We also studied the unaffected SIBs of COS probands as a way to test if any observed alterations of gene–brain relationships in COS patients reflected the consequences of active illness (e.g. treatment with DA modifying drugs) rather than familial/trait markers of risk for schizophrenia. Longitudinal assessment of the relationships between genetic and neuroanatomical variation is especially important in this context because abnormal patterns of anatomical change over time are a well-established correlate of risk for schizophrenia (Gogtay, 2008). Also, more generally, genetic influences in brain anatomy are not developmentally stable (Lenroot et al., 2009), and differ for longitudinal as opposed to static measures of brain anatomy (Brans et al., 2010).
Catechol-o-methyltransferase has been intensely investigated in schizophrenia genetics as a functional candidate gene because of its active role in DA metabolism (Weinshilboum et al., 1999), and as a structural candidate gene because it sits within a chromosome 22q11 deletion that results in velocardiofacial syndrome (VCFS); a neurogenetic disorder that shows 30-fold increased risk for schizophrenia-like psychosis (Murphy, 2002). The catabolic action of COMT is particularly important in regulating DA levels in the pre-frontal cortex (PFC) (Lewis et al., 2001). The Val158Met SNP within COMT (rs4680) encodes a Valine→Methionine substitution at protein residue 158 of the membrane-bound COMT isoform that predominates in brain tissue. This substitution impacts COMT protein thermostability, such that the Val allele encodes a protein with approximately 40% greater enzymatic activity than the Met allele, as demonstrated in lymphocytes as well as postmortem human dorsolateral prefrontal cortex (dlPFC) tissue (Chen et al., 2004). Increased DA catabolism imparted by the Val allele is thought to result in reduced synaptic DA availability, with consequences for PFC function that vary according to background dopaminergic tone (Tunbridge et al., 2006). Thus, comparing the relationship between Val158Met genotype and developmental changes in cortical anatomy between people with COS and HCs would provide information about the integrity of dopaminergic influences upon cortical maturation in schizophrenia. Preliminary evidence that these influences are disrupted in people with schizophrenia comes from cross-sectional neuroimaging reports that the structural (Ohnishi et al., 2006), and functional (Prata et al., 2009) cortical correlates of Val158Met genotype differ between people with schizophrenia and HCs in cingulate, dlPFC and lateral temporal regions. Our current study represents the first to (i) relate to Val158Met genotype to longitudinal measures of anatomical change in people with schizophrenia, and (ii) also do so in the healthy siblings of probands as a way of assessing gene–brain relationships in the absence of disease-related confounds.
Cortical thickness (CT) was studied as a developmentally sensitive (Raznahan et al., 2010) sMRI index of cortical structure that can be mapped at high-spatial resolution and is well established to vary in a regionally specific manner according to genetic (Lenroot et al., 2009; Rimol et al., 2010; Shaw et al., 2009), cognitive (Shaw et al., 2006) and pathological influences (Gogtay et al., 2007; Greenstein et al., 2006).
Materials and methods
Subjects
Subject characteristics are summarized in Table 1, and detailed in supplementary on-line material [Supplementary Table 1]. Subject recruitment, and the gathering of behavioral and neuroimaging data have been described in detail elsewhere (Kumra, 2000;McKenna et al., 1994), and will only be briefly addressed here.
Table 1.
Summary of participant characteristics.
| Characteristic | Group | ||
|---|---|---|---|
| HC | COS | SIB | |
| Total number of participants | 208 | 83 | 62 |
| Males | 118 | 48 | 32 |
| Right handeda | 181 | 62 | 56 |
| IQ, mean (SD) | 114 (12.8) | 74.5 (18.7) | - |
| Vocabulary score, mean (SD)a | 12.3 (2.8) | 6.4 (3.55) | 11.2 (2.9) |
| Number of participants by Val158Met genotype | |||
| MM | 60 | 12 | 13 |
| VM | 91 | 42 | 30 |
| VV | 57 | 29 | 19 |
| Number of participants by number of scans | |||
| 1 scan | 67 | 22 | 29 |
| 2 scans | 61 | 23 | 13 |
| ≥ 3 scans | 80 | 38 | 29 |
| Total number of scans | 475 | 193 | 124 |
| Age distribution of scans, years | |||
| Mean (SD) | 14.7 (3.6) | 16.7 (3.0) | 15.8 (3.9) |
| Range | 9.0–22.8 | 9.2–22.9 | 9.1–22.7 |
| Age at each scan in years, mean (SD) | |||
| 1st scanb | 12.6 (2.9) | 14.6 (2.3) | 14.3 (3.9) |
| 2nd scanb | 15.1 (2.8) | 17.1 (2.5) | 15.9 (3.4) |
| 3rd scanb | 17.2 (2.6) | 18.5 (2.4) | 18.2 (2.7) |
| 4th scan | 18.7 (2.4) | 20.5 (1.4) | 20.5 (2.2) |
Handedness was established using Physical and Neurological Examination of Soft Signs). Estimates of Full-Scale IQ were not available for SIBs, although vocabulary score was a common cognitive measure across all three groups. HC (Healthy Controls), COS (Childhood Onset Schizophrenia), SIBs (Unaffected siblings of COS probands).
p = 0.05.
p < 0.005.
Controls
We included 208 healthy controls (HCs), with a total of 475 structural magnetic resonance imaging (sMRI) brain scans between the ages of 9 and 22 years. Any neurological or psychiatric illnesses were exclusionary (Giedd et al., 1999). Seventy-seven of the 208HCs included in this study had been part of an earlier, smaller study by our group that related Val dose to CT within HCs (Shaw et al., 2009). Our present study includes more HCs than our earlier study, with more sMRI scan for each year of the age-range under examination.
COS subjects and their non-psychotic siblings
Patients with COS were recruited through nationwide referral and extensive prescreening. Diagnoses were made using unmodified DSM-IIIR/IV criteria for schizophrenia with the onset of psychosis before age 13. Any history of significant medical/neurological problems, substance abuse, or premorbid IQ below 70 was exclusionary. Full, non-psychotic siblings (SIBs) of COS subjects also participated in the study and were scanned prospectively along with probands, at approximately two-year intervals. The SIB group showed no evidence of schizophrenia spectrum disorders for either Axis I (mental illnesses) and Axis II (personality disorder and “mental retardation”) diagnoses (Gogtay et al., 2007). We included all COS probands and SIBs with DNA and at least one MRI scan resulting in 83 COS probands and 62 SIBs with a total of 193 and 124 sMRI brain scans respectively. The institutional review board of the National Institutes of Health approved the study and written informed consent and assent were obtained from parents and children respectively.
Genotyping
DNA was extracted from previously prepared lymphoblastoid cell lines using standard methods (Qiagen, MD, USA). It has been established that conversion of cells into lymphoblastoid lines does not cause errors into SNP genotyping (Herbeck et al., 2009). Genotyping was performed by Prevention Genetics (Marshfield, WI, USA). Allele frequencies were: Val 0.53, Met 0.47. Genotype frequencies were: ValVal (VV) homozygotes 0.3, ValMet (VM) heterozygotes 0.46, and MetMet (MM) homozygotes 0.24, and did not deviate from Hardy-Weinberg equilibrium (p = 0.2) (i.e. genotype frequencies were as would be predicted from allele frequencies in the absence of factors such as non-random mating or selection).
Neuroimaging
Of all 353 participants, 67% had 2 or more scans and 42% had 3 or more brain MRI scans acquired at approximately 2-year intervals All sMRI scans were T-1 weighted images with contiguous 1.5 mm axial slices and 2.0 mm coronal slices, obtained on the same 1.5-T General Electric (Milwaukee, WI) Signa scanner using a 3D spoiled gradient recalled echo sequences. Native MRI scans were submitted to the CIVET pipeline to generate separate cortical models for each hemisphere as described previously (Giedd et al., 2007). Briefly, this fully automated and previously validated (Lerch and Evans, 2005; Shaw et al., 2008) set of algorithms consists of the following stages; Masking-out non-brain tissues from native-space sMRI scans using a Brain Extraction Tool method (Smith, 2002); registration of native scans into MNI-ICBM152 stereotaxic space by 9-parameter linear transformation (Collins et al., 1994); correction for non-uniformity artifacts (Sled et al., 1998); and image classification into white matter, grey matter and CSF using a neural-net classifier (Zijdenbos et al., 2002). Next, surface meshes representing the gray/white and gray/pial surfaces were generated with a Constrained Laplacian Anatomic Segmentation Using Proximities surface-extraction procedure (MacDonald et al., 2000). This approach begins with an ellipsoid polyhedral mesh located outside the brain, which is then shrunk to approximate the gray/white surface by minimization of an objective function that includes weighted terms for stretching, bending, self-proximity and prevents self-intersection. This process starts with a coarse mesh that consists of 320 triangles, which through an iterative process of deformation and sub-sampling results in a representation of the surface in question, with a mesh of 81,920 triangles defined by 40,962 vertices. This mesh is then expanded with constraints to define the gray/pial surface. Cortical thickness was calculated in native space as the root mean square of the distance between homologous vertices on each surface. In the absence of prior evidence regarding the spatial extent of genotype effects on cortical maturation, a 30mm-bandwidth blurring kernel was applied over the 2D manifold, based on the findings of an earlier statistical power analysis that identified the optimal kernel size for maximize statistical power and spatial resolution, while minimizing false positives (Lerch and Evans, 2005). Surface representations for individual scans were then aligned using a 2D surface-based registration algorithm to maximize vertex-correspondence across individuals with respect to cortical folding patterns (Lyttelton et al., 2007).
Statistical analysis
In line with molecular data (Chen et al., 2004), and previous imaging-genetic studies (Egan et al., 2001), we modeled COMT Val158Met genotype in an ordinal manner (i.e. 0/1/2 Val allele “dosage”). Mixed model regression was chosen over traditional methods to examine cortical thickness development (i.e. repeated measures ANOVA, fixed effects regression models) because it permits the inclusion of multiple measurements per person, missing data, and irregular intervals between measurements (Pinheiro and Bates, 2000). Linear (rather than non-linear) age-terms were used in all analyses because (i) preliminary analyses within this dataset established that higher order age terms were not better able to predict variance in CT than linear age, and (ii) our previous work has demonstrated that over the age range included in this study—the predominant effect of age is linear (Shaw et al., 2008).
In all analyses, mixed models were run separately at each vertex. We first established the relationship between Val dose and cortical maturation in HCs, by modeling CT for ith family's jth individual's kth time-point as:
Regions where the t-statistic associated with the β4 coefficient (differences in the rate of CT change with increasing Val dose) was significant after False Discovery Rate correction for multiple comparisons (q = 0.05) (Genovese et al., 2002), were visualized by projection onto a standard cortical surface.
Then, to contrast the relationship between Val158Met genotype and cortical development between different participant groups, CT for ith family's jth individual's kth time-point was modeled as:
This model was run once for each possible contrast between the three clinical groups: HC, COS probands and SIBs. Regions where the t-statistic associated with the β8 coefficient (differences between two clinical groups in the rate of CT change with increasing Val dose) was significant after False Discovery Rate correction for multiple comparisons (q = 0.05) were visualized by projection onto a standard cortical surface.
Results
Sample demographics are summarized in Table 1 (and detailed in Supplementary Table 1). There were significant difference across the three clinical groups in; the common cognitive measure of vocabulary (p = 0.0005) [driven by both the COS (p = 0.005), and the SIB group (p = 0.02) having reduced scores compared to HCs], and race distribution (p = 0.0005) [due to proportionally less people of European descent in COS and SIB groups relative to HCs]. Vocabulary score did vary significantly by Val158Met genotype. More extensive cognitive measure available within the HC group showed a statistically non-significant trend for step-wise reduction in performance IQ with increasing Val load (114/113/111 for 0/1/2 Val alleles, p = 0.3), which was not seen for full-scale (p = 0.96) or verbal (p = 0.48) IQ. The Val allele was significantly over-represented in the COS (p = 0.04), but not in the SIB (p = 0.5) group relative to the HC group.
Subsidiary analyses were conducted to investigate the possibility of confounds arising from HCs having a different racial composition to COS and SIB groups. First, because in this sample, and in prior studies of racially mixed samples (Palmatier et al., 1999), the Val allele is over represented in non-white groups (Val dose in HCs of European vs. non-European descent, Chi-squared = 13, p = 0.002), race rather than genotype could potentially explain differences in cortical anatomy between genotype groups. To examine this possibility, we ran the HC-only analysis after excluding all HCs of non-European descent and found that our result were unchanged (Supplementary Figure 1). Second, because clinical groups in this study varied in their racial composition, observed differences between HCs and COS probands, or between HCs and SIBs could reflect race rather than disease status. We tested for and found no evidence of such a confound, as there were no statistically significant group-by-race-by-genotype interactions for the rate of CT change.
Val dose and cortical maturation in HCs
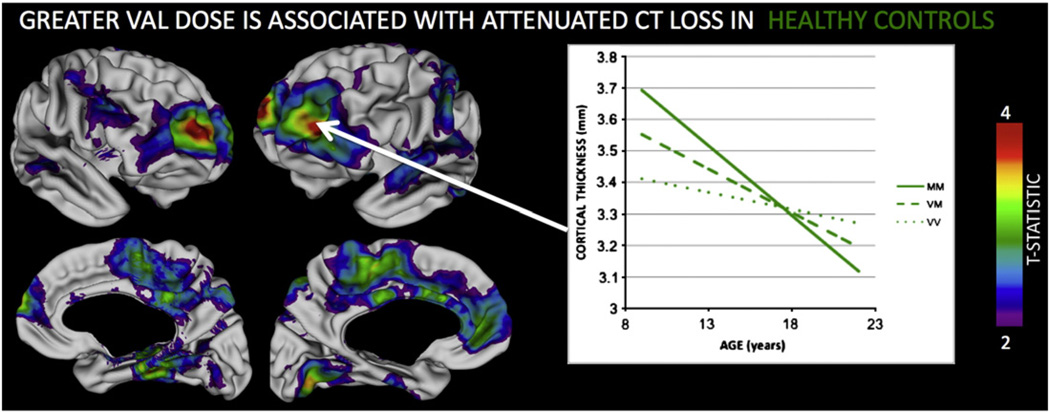
As seen in Fig. 1, HCs displayed a step-wise attenuation (slowing) in the rate of CT loss with increasing Val dose within widespread bilateral PFC (medial and lateral aspects), temporal, and superior parietal regions (co-ordinates for peak vertices in each region are provided in Supplementary Table 2). As a result, although increasing Val dose was significantly associated with less CT in these cortical areas at age 10 years, this relationship was lost (bilateral temporal, left lateral PFC and anterior cingulate) or inverted (bilateral middle cingulate, left superior parietal and right lateral PFC) by age 20 years.
Fig. 1.
Lateral and medial views of the cortical surface summarizing regions where Val158Met genotype was associated with significant (FDR corrected q < 0.05) difference in the rate of CT loss in Healthy Controls (HCs). Colored regions indicate where HCs show a step-wise reduction in the rate of CT loss for each extra Val allele. The inset plot illustrates this for the left dorsolateral prefrontal cortex.
Val dose and Cortical Maturation in COS probands and non-psychotic SIBs
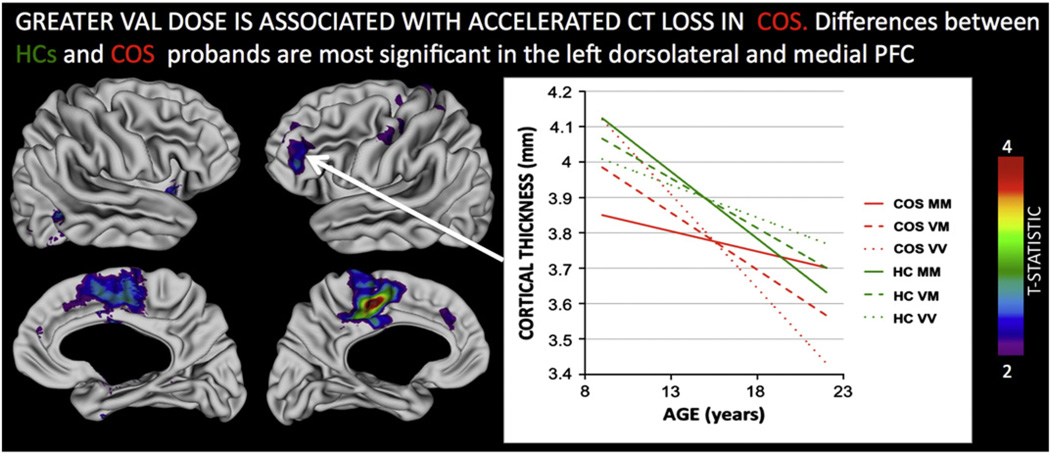
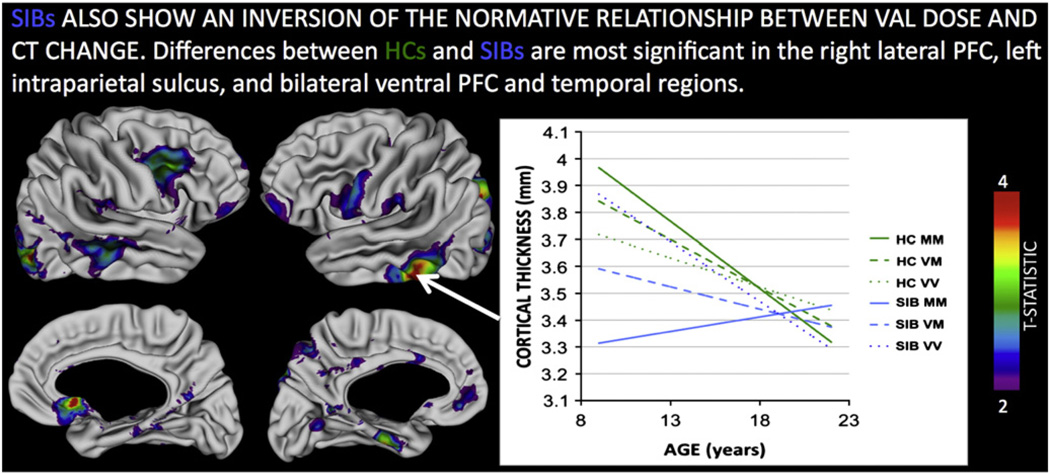
Both COS probands and SIBs showed significant differences from HCs in the relationship between Val dose and CT change. In contrast to HCs (where CT loss was slower with increasing Val dose) within both COS probands and SIBs, greater Val dose was associated with accelerated CT loss. These significant group-by-gene-by-age interactions were most marked between HCs and COS probands within left dlPFC and bilateral cingulate (Fig. 2, co-ordinates for peak vertices in each region are provided in Supplementary Table 2), and between HCs and SIBs within left intraparietal sulcus (IPS) bilateral inferior frontal gyrus (IFG), anterior cingulate and lateral temporal regions (Fig 3, coordinates for peak vertices in each region are provided in Supplementary Table 2). A direct comparison between COS probands and their SIBs, highlighted that significant disruptions of the normative relationship between Val dose and CT in anterior cingulate, lateral temporal and IPS regions where were only seen in unaffected SIBs, and not in COS probands (Supplementary Figure 2).
Fig. 2.
Regions where the relationship between COMT Val158Met Val allele dose and cortical thickness change is significantly different in healthy controls (HCs) as compared to probands with Childhood Onset Schizophrenia (COS). The inset plot illustrates this interaction for the left dorsolateral prefrontal region, where increased Val dose attenuates cortical thinning on HCs, but accelerates it in probands with COS. Note that by adulthood, COS Val homozygotes have persistent cortical thickness deficits compared to HCs, whereas Met homozygotes do not. All colored regions shown survive False Discovery Rate correction for multiple comparisons at q < 0.05.
Fig. 3.
Regions where the relationship between COMT Val158Met Val allele dose and cortical thickness change is significantly different in healthy controls (HCs) as compared to the unaffected siblings (SIBs) of probands with schizophrenia. The inset plot illustrates this interaction for the left inferior temporal region, where increased Val dose attenuates cortical thinning on HCs, but accelerates it in SIBs. Note that by adulthood, cortical thickness trajectories have converged between HC and SIB groups—regardless of Val dose. All colored regions shown survive False Discovery Rate correction for multiple comparisons at q < 0.05.
Thus, while both COS probands and their unaffected SIBs showed an inversion of the relationship between Val dose and rate of CT loss in adolescence, compared to HCs, the regions where this inversion was most marked were different in COS and SIBs. Furthermore, visual comparison (inset plots on Figs. 2 and 3) shows that the timing of Val influences on CT change was different in COS and SIB groups because the three genotype groups “crossed-over” earlier in COS probands than in SIBs. Resultantly, greater Val dose resulted in persistence of CT deficits in early adulthood amongst COS probands (e.g. t = −3.0, p = 0.003 for CT difference at age 20 years in dlPFC), but not amongst healthy SIBs where they disappeared by age 20 (t = 0.4, p = 0.7 for CT differences in inferior temporal cortex).
Discussion
In this, the largest schizophrenia imaging-genetic study conducted to date, we provide the first longitudinal evidence to link a genetic variant impacting DA signaling to structural cortical deficits in children with schizophrenia as well as in their non-psychotic siblings. This suggests that disruption of the causal pathways linking variation in DA signaling to cortical maturation may partly index primary risk factors for schizophrenia rather than solely resulting from the presence of active disease.
In support of our first hypothesis we show that variation in Val158Met genotype modulates the tempo of cortical maturation in health brain development. These relationships are particularly prominent in fronto-temporal cortical regions that are relevant to the neurobiology of schizophrenia [e.g. dlPFC, medPFC, cingulate and STS (Ellison-Wright and Bullmore, 2010)], and sub-serve cognitive tasks which tap DA-sensitive aspects of prefrontal information processing such as working memory (Mattay et al., 2003). Other structural neuroimaging studies in HCs have also found anatomical differences in similar fronto-temporal regions as a function of Val158Met genotype (Cerasa et al., 2010; Honea et al., 2009; Zinkstok et al., 2006). These cross-sectional studies all examine older samples than ours, and almost all (Cerasa et al., 2010) focus on cortical volume rather than CT. While methodological differences between prior studies and our current report preclude more detailed comparison of findings, there is clear convergence of results across studies in support of the notion that Val158Met genotype plays a particularly important role in the structural development of lateral prefrontal, cingulate and superior temporal cortices. These findings in HCs also overlap those of our prior preliminary report (Shaw et al., 2009). However, our present study uses an expanded and largely independent sample of HCs, with a greater density of imaging data (33 vs 23 scans per year of age-range) over a tighter age range (9 to 22 years vs 3 to 22 years), to identify more symmetric and dynamic relationships between Val dose and cortical maturation in typical development.
It is notable that the bilateral PFC sub-regions where these dynamic relationships are strongest, are also (i) where variation in the dynamics of gray matter change in childhood and adolescence has been inked to intellectual ability (Shaw et al., 2006): (ii) important for working memory and executive function tasks which are negatively impacted by increasing Val in HCs (Barnett et al., 2007) and (iii) where other studies of HCs have found the Val158 allele to be associated with decreased activational ‘efficiency’ in meta-analysis of functional imaging data (Mier et al., 2009), decreased functional interconnectivity during rest (Liu et al., 2010), altered oscillatory activity during electroencephalography accompanied by worse executive functioning(Bodenmann et al., 2009), and decreased signal to noise ratio during executive functioning(Winterer et al., 2006). We speculate therefore that step-wise slowing of adolescent CT loss with increasing Val load in HCs, may be a maturational mechanism underlying observed associations between the Val allele and markers of reduced PFC functioning in typically developing groups.
In support of our second hypothesis, we find that both COS probands and their non-psychotic SIBs show a complete reversal of the relationships that exists between Val158Met genotype and CT maturation in HCs. This inversion is most pronounced in fronto-temporal sub-regions where existing cross-sectional studies in schizophrenia have found Val158Met genotype to modulate cortical structure (McIntosh et al., 2007; Ohnishi et al., 2006) and function (Prata et al., 2009) in a manner that differs from that seen in HC groups (Ohnishi et al., 2006; Prata et al., 2009). Moreover, abnormalities of structural maturation (Brans et al., 2008) and function (Fusar-Poli et al., 2007) within these same cortical regions appear to index genetic risk for schizophrenia, whether active disease is present or not. Our findings imply that shared fronto-temporal abnormalities in persons with schizophrenia and their unaffected relatives could, in part, arise due to shared disruptions of the mechanisms through which Val158Met genotype normally influences cortical development during childhood and adolescence. Because these disruptions are evident in both probands and unaffected relatives, they likely reflect shared genetic and/or environmental risk factors for schizophrenia, rather than secondary consequences of either active illness or its treatment. However, it is also possible that shared disruptions in COS probands and SIBs could reflect shared exposures that do not directly reflect risk factors for psychosis (e.g. changes in rearing environment due to parental responses to caring for a child with severe neurodevelopmental difficulties).
We also find notable differences between COS and SIB groups in the regional distribution and developmental timing of disrupted relationships between Val dose and CT maturation. Differences between COS and SIB groups in the age at which CT trajectories for Val158Met genotype groups “cross-over”, would suggest that Val carriage confers persistence of dlPFC CT deficits in COS probands relative to HCs, whereas in SIBs, CT deficits resolve over the same developmental period, regardless of Val dose. This observation echoes our earlier findings that whereas both COS probands and SIBs show fronto-temporal gray matter deficits relative to HCs in early adolescence, these persist into adulthood in COS probands, but resolve over time in non-psychotic SIBs (Gogtay et al., 2003; Greenstein et al., 2006). Thus, cortical maturation in COS appears to be especially sensitive to Val dose within the PFC, in a manner that is not seen in non-psychotic SIBs. This finding is consistent with the prominent contribution of COMT to DA regulation in the PFC, and notion that PFC hypodopaminergia is more marked in people with schizophrenia and their healthy relatives (Hirvonen et al., 2006).
Different cortical regions are highlighted by our statistical comparison of COS probands and HCs, than for SIBs and HCs. This is most notable in anterior cingulate, lateral temporal and parietal region. These differences could be due to several mechanisms. They could reflect factors operating in COS probands but not SIBs, such as; a greater burden of genetic or environmental risk for psychosis; the direct consequences of active psychosis on brain development; or medication treatment. Alternatively, differences between COS probands and their non-psychotic SIBs could reflect active compensatory processes in SIBs, or the “unmasking” in SIBs of primary psychosis risk correlates that are obscured by active disease in probands. A longitudinal discordant monozygotic twin study design could test these various accounts more clearly, although the necessarily limited sample size of such a study would complicate the modeling of anatomical change.
Regardless of study design, use of in-vivo neuroimaging means that the proximal cellular and molecular mechanisms underlying observed relationships between Val158Met genotype and cortical development remain to be determined. However, the factors driving any observed genetic association exist on a theoretical spectrum that extends between two extremes—total environmental mediation and total independence from environmental influences. Existing studies of the COMT Val158Met variant argue for a mixed model. For example, the possibility of environmental mediation is raised by a recent report that Val dose in HCs is associated with an increased tendency to respond to uncertainty in an exploratory (“trying a new tack to see what happens”) rather than exploitative (“sticking to what you know”) fashion (Frank et al., 2009). These genotype-related differences in behavioral style could result in the accrual of greater experiential diversity across the lifespan, with consequences for cortical development (Bennett et al., 1969). On the other hand, variation in DA signaling arising from allelic differences at Val158Met is also likely to have direct consequences for basic DA-sensitive cellular processes that are fundamental to neurogenesis and neuronal and inter-neuron differentiation (Bhide, 2009).
The idea that Val dose might influence cortical maturation in HCs by directly modifying dopaminergic influences on neurogenesis and neuronal differentiation provides a parsimonious account for how the disrupted gene–brain relationships we observe in COS probands and SIB groups could arise. For example, many dopaminergic influences on cortical development are D1-mediated (Bhide, 2009), and both patients with schizophrenia and their healthy monozygotic co-twins show increased cortical D1 density compared to HCs (Hirvonen et al., 2006). These D1 abnormalities are hypothesized to be a response to cortical hypodopaminergia, and are more severe in affected probands compared to unaffected relatives (Hirvonen et al., 2006). Thus, heritable abnormalities of DA signaling shared by COS probands and SIBs could disrupt the relationship between Val dose and cortical development in both groups compared to HCs. Under this model, more atypical cortical DA signaling in affected probands relative to their healthy relatives could account for our finding that the higher-functioning Val allele is associated with more persistent CT deficits in COS probands than in unaffected SIBs. Although highly speculative, this proposal has implications for the two most prominent theories of schizophrenia—the “dopamine hypothesis” and the “developmental hypothesis” (Murray et al., 2008). Dopamine dysregulation in those at manifest and latent risk for schizophrenia is most commonly studied with respect to its potential to cause dysfunction within meso-cortical, meso-striatal and meso-limbic circuits that are thought to underlie many of the cardinal clinical features of the syndrome (Howes and Kapur, 2009; Simpson et al., n.d.). Our data do not argue against this notion, but raise the additional possibility that DA dysregulation may also contribute to schizophrenia pathogenesis by directly derailing structural development of “higher” associative cortices during childhood and adolescence. This possibility provides a mechanistic bridge between the dopamine hypothesis of schizophrenia and the neurodevelopmental hypothesis of schizophrenia, which sees aberrant early brain development before the onset of illness as being central to the neurobiology of schizophrenia (Rapoport et al., 2005).
Our study has some limitations. Firstly, we lacked behavioral measures that selectively target performance in executive function and working memory domains that others have found to vary by Val158Met genotype(Barnett et al., 2007). Secondly, we did not have a sufficient sample size to examine differences between HCs, COS probands and SIBs in how allelic variation at Val158Met interacts with other genetic variants of potential relevance for dopaminergic systems both within (Meyer-Lindenberg et al., 2006), and beyond [e.g. DRD2, (Fazio et al., 2011)] the COMT gene—although we hope to address these issues in the future using larger samples. Thirdly, although (based on earlier developmental studies) we modeled CT change with age in a linear manner, it is conceivable that genetic influences in CT change may be non-linear in nature. Larger longitudinal datasets than ours will be required to more formally test for the presence of these more complex, and as yet hypothetic genotypic effects on structural maturation. Finally, our approach to image analysis focused in CT and different methods will be required to determine how Val158Met genotype modulates other aspects of cortical morphometry such as surface area and gyrification, which are known to be shaped by genetic influences that are partially distinct from those that influence CT (Panizzon et al., 2009).
Despite these limitations, our study is one of the largest schizoph0renia neuroimaging studies conducted to date, and provides the first longitudinal evidence that the normative neurodevelopmental correlates of genetic variants impacting dopamine signaling are disrupted in people with schizophrenia as well as their unaffected relatives. Our findings further suggest that while some of these disruptions reflect primary genetic or environmental risks shared between probands and siblings, others only arise in the context of active disease.
Supplementary Material
Acknowledgments
This study was funded through the National Institutes of Health, National Institute of Health Intramural Research, and a UK Medical Research Council Clinical Research Training Fellowship (Author A.R—G0701370). The authors wish to thank the participants who took part in this study.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.neuroimage.2011.05.032.
References
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol. Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–826. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin. Cell Dev. Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Bodenmann S, Rusterholz T, Durr R, Stoll C, Bachmann V, Geissler E, Jaggi-Schwarz K, Landolt HP. The functional Val158Met polymorphism of COMT predicts interindividual differences in brain alpha oscillations in young men. J. Neurosci. 2009;29:10855–10862. doi: 10.1523/JNEUROSCI.1427-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, Kahn RS, Schnack HG, van Baal GC, Posthuma D, van Haren NE, Lepage C, Lerch JP, Collins DL, Evans AC, Boomsma DI, Hulshoff Pol HE. Brain plasticity and intellectual ability are influenced by shared genes. J. Neurosci. 2010;30:5519–5524. doi: 10.1523/JNEUROSCI.5841-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch. Gen. Psychiatry. 2008;65:1259–1268. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch. Gen. Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Cherubini A, Quattrone A, Gioia MC, Tarantino P, Annesi G, Assogna F, Caltagirone C, Spalletta G. Met(158) variant of the catechol-O-methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.02.040. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75(5):807–821. doi: 10.1086/425589. [erratum appears in Am J Hum Genet. 2005 Jun;76(6):1089] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Egan MF, Goldbert TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val 108/158 Met genotype on frontal lobe function and risk for schizophrenia. PNAS. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Fazio L, Blasi G, Taurisano P, Papazacharias A, Romano R, Gelao B, Ursini G, Quarto T, Lo Bianco L, Di Giorgio A, Mancini M, Popolizio T, Rubini G, Bertolino A. D2 receptor genotype and striatal dopamine signaling predict motor cortical activity and behavior in humans. NeuroImage. 2011;54:2915–2921. doi: 10.1016/j.neuroimage.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat. Neurosci. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW, Stayer C, Evans AC, Samango-Sprouse CA. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr. Bull. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch. Gen. Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Greenstein D, Giedd JN, Lenane M, Gochman PA, Zijdenbos A, Rapoport JL. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am. J. Psychiatry. 2003;160:569–571. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gogtay N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J. Child Psychol. Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Herbeck JT, Gottlieb GS, Wong K, Detels R, Phair JP, Rinaldo CR, Jacobson LP, Margolick JB, Mullins JI. Fidelity of SNP array genotyping using Epstein Barr virus-transformed B-lymphocyte cell lines: implications for genome-wide association studies. PLoS One. 2009;4:e6915. doi: 10.1371/journal.pone.0006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M, Lonnqvist J, Kaprio J, Cannon TD, Hietala J. Brain dopamine d1 receptors in twins discordant for schizophrenia. Am. J. Psychiatry. 2006;163:1747–1753. doi: 10.1176/ajp.2006.163.10.1747. [DOI] [PubMed] [Google Scholar]
- Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. NeuroImage. 2009;45:44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S. The diagnosis and treatment of children and adolescents with schizophrenia. “My mind is playing tricks on me”. Child Adolesc Psychiatr Clin N Am. 2000;9:183, 199. x. [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J. Comp. Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T. Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J. Neurosci. 2010;30:64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. U.S. A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chappele B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional Brain Morphometry in Patients With Schizophrenia or Bipolar Disorder and Their Unaffected Relatives. [References] Am. J. Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, Moorhead TW, Owens DG, Miller P, Porteous D, Lawrie SM, Johnstone EC. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol. Psychiatry. 2007;61:1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, Mattay VS, Egan M, Weinberger DR. Impact of complex genetic variation in COMT on human brain function. Molecular Psychiatry. 2006;11(9):867–877. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry. 2009 doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC. Schizophrenia and velo-cardio-facial syndrome. Lancet. 2002;359:426–430. doi: 10.1016/S0140-6736(02)07604-3. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lappin J, Di Forti M. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur. Neuropsychopharmacol. 2008;18 Suppl 3:S129–S134. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Iida H, Noguchi H, Nakabayashi T, Hori H, Ohmori M, Tsukue R, Anami K, Hirabayashi N, Harada S, Arima K, Saitoh O, Kunugi H. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129:399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol. Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Howes O, Kravariti E, Demjaha A, Toulopoulou T, Diforti M, Murray RM, Collier DA, McGuire PK. Opposite effects of catechol-O-methyltransferase Val158Met on cortical function in healthy subjects and patients with schizophrenia. Biol. Psychiatry. 2009;65:473–480. doi: 10.1016/j.biopsych.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr. Psychiatry Rep. 2005;7:81–82. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U.S.A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, Hagler DJ, Lyons MJ, Neale MC, Pacheco J, Perry ME, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Kremen WS, Dale AM. Cortical thickness is influenced by regionally specific genetic factors. Biol. Psychiatry. 2010;67:493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Wallace GL, Addington A, Evans A, Rapoport J, Giedd JN. Effects of the Val158Met catechol-O-methyltransferase polymorphism on cortical structure in children and adolescents. Mol. Psychiatry. 2009;14:348–349. doi: 10.1038/mp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Rapoport JL, Hayashi KM, Geaga JA, Sui Y, McLemore LE, Alaghband Y, Giedd JN, Gochman P, Blumenthal J, Gogtay N, Nicolson R, Toga AW, Thompson PM. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch. Gen. Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu. Rev. Pharmacol. Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, Gallinat J, Dahmen N, Weinberger DR. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. NeuroImage. 2006;32:1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, Schmitz N, van AT, de WM, van den BW, Baas F, Linszen D. The COMT val158met polymorphism and brain morphometry in healthy young adults. Neurosci. Lett. 2006;405(1–2):34–39. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.