Abstract
Epilepsy or seizure disorder is one of the most common neurological diseases in humans. Although genetic mutations in ion channels and receptors and some other risk factors such as brain injury are linked to epileptogenesis, the underlying cause for the majority of epilepsy cases remains unknown. Gene-environment interactions are thought to play a critical role in the etiology of epilepsy. Exposure to environmental chemicals is an important risk factor. Methylmercury (MeHg) is a prominent environmental neurotoxicant, which targets primarily the central nervous system (CNS). Patients or animals with acute or chronic MeHg poisoning often display epileptic seizures or show increased susceptibility to seizures, suggesting that MeHg exposure may be associated with epileptogenesis. This mini-review highlights the effects of MeHg exposure, especially developmental exposure, on the susceptibility of humans and animals to seizures, and discusses the potential role of low level MeHg exposure in epileptogenesis. This review also proposes that a preferential effect of MeHg on the inhibitory GABAergic system, leading to disinhibition of excitatory glutamatergic function, may be one of the potential mechanisms underlying MeHg-induced changes in seizure susceptibility.
Keywords: Methylmercury, environmental risk factors, seizures, epileptogenesis
Introduction
Epilepsy, a disease defined by recurrent spontaneous seizures, is one of the most common neurological disorders in humans. It affects 2.3 million Americans and approximately 50 million peoples worldwide with a prevalence rate about 0.5 – 1% (Hauser et al, 1993; Olafsson et al, 2005). Although genetic mutations in ion channels or ligand-gated receptors (for review, see Lerche et al., 2001; Hirose, 2006; Graves, 2006; Catterall et al., 2010; Mantegazza et al., 2010; Macdonald et al., 2010) and other risk factors such as head trauma, stroke, infections, brain tumor, brain developmental defects and toxins (for review, see Frey, 2003; D’Ambrosio and Perucca, 2004; Singh and Prabhakar, 2008; Lowenstein, 2009; Prince et al., 2009) have been linked to epileptogenesis, the underlying cause for most epilepsy cases remains to be identified. It is generally believed that interactions of environmental factors with genetic and other intrinsic factors play an important role in the etiology of seizures or epilepsy (Kjeldsen et al., 2002; Bener et al., 2006; Todorova et al., 1999, 2006; Nakayama, 2009; Vestergaard and Christenen, 2009; Stewardt, 2010). Exposure to some environmental chemical pollutants such as ozone (Escalantie-Membrillo and Paz, 1997), lead (Krishnamoorthy et al., 1993; Arrieta et al., 2005), nickel (Denays et al., 2005), manganese (Hernandez et al., 2003), teimethyltin (Nishimura et al, 2001), organophosphates (McDonough and Shin, 1997; Solberg and Belkin, 1997; Myhrer, 2007) or domoic acid (Tiedeken and Ramsdell, 2007; Stewardt, 2010) has been shown to initiate or promote the development of seizures or epilepsy. Clinical and epidemiological studies suggest that methylmercury (MeHg), a prominent environmental contaminant, is an important environmental risk factor contributing to epileptogenesis. This mini review will outline the effects of acute and chronic MeHg exposure, particularly developmental exposure, on the susceptibility of the brain to seizures or epilepsy based on currently available data and briefly discuss the potential mechanisms underlying MeHg-induced changes in seizure susceptibility.
Acute or chronic MeHg exposure causes epileptic seizures in human
Although the primary symptoms and signs of acute and chronic MeHg poisoning typically include visual functional disturbances (concentric constriction of the visual fields and reduced visual acuity), paresthesia, ataxia, dysarthia, and hearing impairment (Hunter and Russell, 1954; Takeuchi et al., 1959, 1962; Kurland et al., 1960; Tokuomi and Okajima, 1961; Bakir et al., 1973; Rustam and Hamdi, 1974; Chang, 1980; Nierenberg et al., 1998; Weiss et al., 2002; Clarkson et al., 2003), patients also often present with seizures or epilepsy (Harada, 1968, 1979, 1995; Marsh et al. 1987). In the Minamata Bay area of Japan, a region contaminated heavily with MeHg during 1950s, 8 – 9% of patients who suffered from Minamata disease (MD, a neurological disorder caused by chronic MeHg poisoning) experienced epileptic seizures. The types of epilepsy seen in MD patients were often atypical, many were clonic seizures with or without effects on consciousness. In Goshonoura town, a nearby area that was also contaminated by the same source of MeHg but much less heavily compared with Minamata area, 2.86% of MD patients developed epilepsy. In contrast, only 0.8% of the age- and sex-matched controls in a non-MeHg-contaminated area exhibited epilepsy (Harada, 1979). Thus, the frequency of epilepsy in MD patients of the Minamata area was >10 fold higher than that of age and sex-matched controls in the non-MeHg-contaminated area. In agreement with the increased prevalence rate of behavioral epileptic seizures, electroencephalograph (EEG) examination of MD patients also confirmed a high rate of EEG abnormality. In 106 MD patients with or without complaints of seizures, EEG examination showed abnormalities in 66 (62.2%) patients, of which 22 (33.3%) cases exhibited paroxysmal discharges (Harada, 1979).
Consistent with the notion that the developing brain is particularly sensitive to MeHg, the frequency of convulsive seizures was much higher in children with congenital MD, an infantile form of MD with high incidence of cerebral palsy-like symptoms resulting from prenatal MeHg exposure (Harada, 1979). Convulsive seizures were observed in over 50% of congenital MD patients (11 of 22 cases in the Minamata district during 1955–1957 or 5 of 6 cases in the Minamata Bay area during 1955–1962). The hair mercury content of children with congenital MD was 40.3 ± 30.3 μg/g, whereas the hair mercury content of age-matched control individuals was < 7 μg/g (Harada, 1968, 1979). Similarly, an increased incidence of convulsive seizures was observed in children with prenatal and postnatal MeHg exposure in an episode of acute MeHg poisoning in Iraq (Marsh et al., 1987). The increase in seizure incidence depended on MeHg exposure levels: when the maximum mercury content in maternal head hair was <75 ppm, none of the 53 children examined had seizures; when the maximum maternal hair mercury contents were 75–150 ppm, 1 of 6 (16.7%) children experienced seizures; when the maximum hair mercury content was >150 ppm, 6 of the 22 (27.3%) children developed seizures. Thus, the incidence of convulsive seizures among children with developmental MeHg exposure, in both Japan and Iraq MeHg poisoning episodes, was strongly associated with levels of MeHg exposure. In 1969, four children from a New Mexico family with pre- and/or postnatal exposure to MeHg following a 3-month long consumption of MeHg-contaminated pork developed severe neurological disorders (Snyder, 1971, Davis et al., 1994). Three of the four children developed seizures at one point during a 22-years follow-up study (Davis et al., 1994). Epileptoform abnormalities of EEG were observed in the two youngest children with behavioral seizures (Synder, 1971; Brenner and Synder, 1980). The total mercury (Hg) content in the cerebral cortex from one of the children who was exposed to MeHg postnatally and died 22 years later remained 50 times higher than those of the control patient (~1.595 vs 0.038 μg/g), confirming that epileptic seizures in these children were associated with developmental MeHg exposure. In contrast, their parents did not develop any overt symptoms or signs of MeHg poisoning in spite of being exposed to MeHg over same time period. Similar phenomena were also observed in parents of congenital MD patients in Japan (Harada, 1979) and children with prenatal MeHg exposure in Iraq (Marsh et al., 1987). In both Japan and Iraq cases, infants with prenatal MeHg exposure displayed overt clinical symptoms and signs of neurotoxicity while their mothers remained asymptomatic. Clearly, these data suggest that developing brains are particularly susceptible to MeHg-induced epileptic seizures.
A common theme in these MeHg poisoning episodes described above is that convulsive seizures seen in patients in Minamata Bay, Iraq, and the New Mexico family occurred after acute or chronic exposure to high levels of MeHg. Therefore, it is possible that the increased incidence rate of epilepsy or seizures in patients with MeHg poisoning could simply be secondary to MeHg-induced severe brain injury. The question then is whether chronic exposure, particularly developmental exposure, to low levels of MeHg alters the susceptibility of humans to seizures or epilepsy. This is particularly important because humans today are exposed to MeHg primarily through the consumption of MeHg-contaminated fish and seafood.
One of the main public health concerns surrounding chronic exposure to low levels of MeHg through the consumption of contaminated fish and seafood is the potential developmental neurotoxicity of MeHg in young children because the developing brains are highly vulnerable to this neurotoxicity (Harada, 1979; Marsh et al., 1987; Davis et al., 1994). To address this issue, several epidemiological studies have been carried out in Canada (McKeown-Eyssen et al., 1983), New Zealand (Crump et al., 1998), the Faroe Islands (Grandjean et al., 1997, 1998), Amazonian Basin (Grandjean et al., 1999), French Guiana (Cordier et al., 2002), the Republic of Seychelles (Davidson et al., 1998, 2000, 2006; Myers et al., 2003, 2009) and Tohoku district of Japan (Suzuki et al., 2010) in an attempt to study the effects of developmental MeHg exposure from fish consumption on child neurodevelopment. Results obtained from studies in Canada, New Zealand, the Faroe Islands, Amazonian Basin, French Guiana and Tohoku district of Japan suggest that there is a positive association between prenatal MeHg exposure and subtle neurological and/or neuropsychological deficits. In the Faroe Islands, extensive neuropsychological tests of 917 7-year-old children revealed that prenatal MeHg exposure was associated with deficits in cognitive function including primarily attention, language and memory, and to a lesser extent in visual-spatial and motor functions (Grandjean et al., 1997, 1998). However, similar extensive epidemiological studies in the Republic of Seychelles (Myers et al., 1995, 1997, 2003; Davidson et al., 1998, 2000; Axtell et al., 2000) failed to find any significant adverse effect from ocean fish consumption on child neurodevelopment. The exact reasons for the apparent disagreement between the two major cohort epidemiological studies remain unclear, but some possible confounding variables such as different types of fish consumed by the two different populations and beneficial effects of the nutrients from fish consumption may contribute to the divergent outcomes (for review, see Counter and Buchanan, 2004; Clarkson and Magos, 2006; Gradjean et al., 2010, 2011). Regardless of the disagreement between the two major cohort studies, none of these studies has reported an increased incidence of childhood seizures or epilepsy. However, no information is available to indicate that childhood seizures such as neonatal seizures and febrile seizures or epilepsy were included as a specific endpoint in these studies. In fact, children with clinically diagnosed epilepsy or neonatal seizures were pre-excluded from further analyses in both the Foroe Islands (Grandjean et al., 1997, 1998) and Seychelles studies (Marsh et al., 1995). From this point of view, the epidemiological data appear to be insufficient at present to draw any definite conclusion about the relationship between chronic exposure to low levels of MeHg and seizures (neonatal seizures or febrile seizures) or epilepsy (childhood or adult) in humans. Interestingly, an elevated rate of male cerebral palsy hospitalization has been shown in the Great Lakes communities that were associated with historic use and natural resource of mercury (Gibertson, 2004, 2009). It is known that epilepsy is highly associated with cerebral palsy; 15–60% of children with cerebral palsy also suffer epilepsy (Kwong et al., 1998; Gururaj et al. 2003). Thus, theoretically, the incidence of epilepsy in these Great Lakes areas should be higher as well. However, no such data are available yet to support this assumption.
Developmental MeHg exposure increases seizure susceptibility in animal models
Consistent with findings in epidemiological and clinical studies, developmental MeHg exposure also alters neuronal excitability and susceptibility to seizures in different animal models. Szász et al. (1999, 2002) showed that exposure of rats to low levels of MeHg (0.375 mg/kg body weight/day) during the entire mating, gestation and lactation period significantly enhanced epileptogenicity in their offspring compared with those of age-matched controls in response to chemoconvulsive agent 3- or 4-aminopyridine. Electrocorticographic examinations showed a significant increase in the frequency and summated duration of paroxysmal activity and probability of generalized seizures in MeHg-treated animals at both postnatal day 28 (PND28) and PND90; epileptic activity spread over the whole cortical surface of the brains. These data suggest that pre- and postnatal MeHg exposure significantly increased the susceptibility of both young and adult animals to seizures and facilitated propagation of epileptiform activity (Szász et al., 1999, 2002). Similar effects were also seen in the offspring of rats that were exposed to inorganic mercury (Hg2+) under similar experimental conditions, although the effect of Hg2+ on epileptogenicity appeared to be long-lasting (Szász et al., 2002). In agreement with these findings, prenatal and postnatal exposure of rats to MeHg using the same exposure paradigm as described above resulted in a decreased threshold for evoking excitatory postsynaptic potentials and spikes in neurons of neocortical slices, suggesting an increase in neuronal excitability (Világi et al., 2000). Furthermore, a single injection of 0, 6, 8 or 12 mg/kg body weight MeHg to pregnant mice on Day 10 of gestation resulted in a significantly reduced threshold to seizures induced by the convulsive agent flurothyl in the offspring (Su and Okita, 1976). Similarly, exposure of pregnant mice to a single dose of MeHg at 6 or 8 mg/kg body weight on Day 12 of gestation significantly increased the susceptibility of mouse offspring to audio-induced seizures (Menashi et al., 1982). Thus, these data suggest that prenatal MeHg exposure, regardless of whether it is single exposure at higher levels or chronic exposure at low levels, can alter the susceptibility of animals to seizure induction.
Theoretically, MeHg-induced changes in seizure susceptibility should also occur in animals following postnatal exposure, similar to children with MeHg poisoning (Harada, 1968, 1979; Snyder, 1971, Davis et al., 1994; Marsh et al., 1987). Using field potential recording techniques we have recently shown that early postnatal exposure of rats to low levels of MeHg caused a time-dependent increase in epileptiform activity of neurons in cortical slices (Dasari and Yuan, 2010). MeHg also increased sensitivity of cortical neurons to GABAergic antagonist-induced epileptiform activity and significantly reduced threshold for neuronal excitation, suggesting an increased neuronal excitability or hyperexcitibility. These data imply that postnatal MeHg exposure will also increase the susceptibility of animals to seizures. Thus, developmental MeHg exposure, either prenatal or postnatal or both, could be a potential risk factor contributing to the etiology of epileptic seizures.
Preferential effects of MeHg on GABAergic function may primarily contribute to MeHg-induced increases in seizure susceptibility
A critical gap in our understanding is how MeHg exposure alters the susceptibility of humans and animals to seizures or epilepsy. MeHg has the potential to interact with a variety of membrane proteins including enzymes, transporters, ion channels and receptors to induce a broad spectrum of neurotoxicity via specific and/or nonspecific actions at multiple target sites due partly to its high affinity and reactivity with -SH groups (For review, see Chang, 1980; Atchison and Hare, 1994; Shafer, 2000; Castoldi et al., 2001; Allen et al., 2002; Limke et al., 2004b; Atchison 2005; Aschner and Aschner, 2007; Aschner et al., 2007). Thus, we predict that multiple mechanisms contribute to MeHg-induced changes in seizure or epilepsy susceptibility.
Channelopathies or mutations in ion channels including voltage-gated Na+, Ca2+, K+ and Cl− channels and receptors for acetylcholine and γ-aminobutyric acid (GABA) are associated with a variety of seizure disorders or epilepsy syndromes (for review, see Lerche et al., 2001; Hirose, 2006; Graves, 2006; Catterall et al., 2010; Mantegazzaet al., 2010; Macdonald et al., 2010). Interestingly, MeHg affects voltage-gated Ca2+ channels (Shafer and Atchison, 1991; Sakamoto et al., 1996; Leonhardt et al., 1996; Sirois and Atchison, 2000; Shafer et al., 2002; Peng et al., 2002a,b), voltage-gated Na+ and K+ channels (Shrivastav et al., 1976; Quandt et al., 1982; Shafer and Atchison, 1992; Shafer et al., 2002; Yuan et al., 2005) and ligand-gated ion channels such as acetylcholine receptors (Von Burg et al., 1980; Quandt et al., 1982; Limke et al., 2004a; Roda et al, 2008), glutamate receptors (Yuan and Atchison, 1995, 1999; 2007) and GABAA receptors (Arakawa et al., 1991; Fonfría et al., 2001; Yuan and Atchison, 1997, 2003; Herden et al., 2008). It might be puzzling if blockade by MeHg of voltage-gated Ca2+ or Na+ channels results in neuronal hyperexcitability because the opposite effects (reduced neuronal excitability) are expected based on the roles of voltage-gated Ca2+ and Na+ channels in maintaining normal neuronal excitability and synaptic transmission. However, epilepsy resulting from loss-of-function mutations in SCN1A genes encoding voltage-gated Na1.1 channels does occur as haploinsufficiency, as shown in Scn1a+/− (Yu et al., 2006) and Scn1a mutant knock-in (Ogiwara et al., 2007) mouse models of Dravet syndrome (also called Severe Myoclonic Epilepsy of Infancy). In these mouse models, it is postulated that mutations in Nav1.1 channels selectively impair Na+ channel function in GABAergic inhibitory interneurons without detectable effects on the excitatory pyramidal neurons, leading to neuronal disinhibition and hyperexcitability. Based on this evidence, one possibility is that MeHg selectively blocks voltage-gated Ca2+ or Na+ channels in inhibitory interneurons, but not those in excitatory neurons. Although it is not impossible, no evidence to date has yet shown that MeHg specifically affects Ca2+ or Na+ channels in inhibitory interneurons and not those in excitatory neurons. The possibility for effects of MeHg on voltage-gated (Kv) and inwardly rectifying (Kir) K+ channels contributing to MeHg-induced alterations of seizure susceptibility also appears to be low since these K+ channels are generally more resistant to MeHg in vitro compared to other voltage-gated ion channels and receptors (Yuan et al., 2005). However, it remains to be determined if this is also the case in vivo. Ca2+-activated K+ channels, particularly the big and small conductance channels (BK and SK), play an important role in the regulation of neuronal excitability by coupling changes in intracellular Ca2+ concentration and membrane potentials, forming afterhyperpolarization and controlling action potential firing pattern (for review, see Vergara et al., 1998; Faber and Sah, 2003; Stocker, 2004; Berkefeld et al., 2010). Similarly, the M-channels of Kv7 (KCNQ) family of K+ channels also participate in the regulation of neuronal excitability. Mutations in KCNQ2 and KCNQ3 are associated with benign familial neonatal convulsions (BFNC) (for review, see Cooper and Jan, 2003; Burgess, 2005). However, it remains to be determined whether MeHg actually affects these ion channels in the brain to alter neuronal excitability.
Although glutamate and GABA are the predominant excitatory and inhibitory neurotransmitters, respectively, in the CNS, acetylcholine (ACh) is also an important neurotransmitter that participates in the regulation of neuronal excitability and is critical for learning and memory (for review, see Gold, 2003; Hasselmo, 2006). Activation of ACh receptors (AChRs), particularly muscarinic AChRs (mAChRs), can be excitatory or inhibitory depending on the subtypes of AChRs, and the layers and regions of the cerebral cortex (Haj-Dahmane and Andrade, 1996; Gulledge and Stuart, 2005; Eggermann and Feldmeyer, 2009; Gulledge et al., 2009). Mutations in the α4-subunit gene (CHRNA4) or the β-subunit of the neuronal nicotinic acetylcholine receptor (nAChR) are associated with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE), a focal syndrome characterized by nocturnal motor seizures (for review, see Lerche et al., 2001; Graves, 2006). MeHg has the potential to affect both nicotinic (Shamoo et al., 1976; Eldefrawi et al., 1977) and muscarinic receptors (Abd-Elfattah and Shamoo, 1981; Castodi et al., 1996; Coccini et al., 2000; Limke et al., 2004a; Roda et al., 2008). However, whether these effects of MeHg on nicotinic or muscarinic receptors lead to increased or decreased excitability remains to be determined.
Normal neuronal excitability in the brain depends on the well-regulated balance between glutamatergic excitation and GABAergic inhibition. Loss or reduction of GABAergic inhibition leading to hyperexcitability has long been considered to be an important mechanism underlying seizures or epileptogenesis. Mutations in GABAA receptors are associated with genetic epilepsies (for review, see Benarroch, 2007; Macdonald et al., 2010). MeHg has been shown to inhibit GABAA receptor-mediated currents (Arakawaet al., 1991; Yuan and Atchison, 2003; Herden et al, 2008), to modulate the benzodiazepine binding site of GABAA receptors (Komulainen et al, 1995; Fonfría et al, 2001), to cause down-regulation of mRNA levels of GABAA receptors in cerebellar granule cell cultures (Hogberg et al., 2010) and to disrupt GABAergic signaling in the brain of captive minks (Basu et al., 2010). Most importantly, MeHg appears to preferentially affect inhibitory GABAergic systems over excitatory glutamatergic systems because: 1) the GABAergic interneurons in the neocortex of rats were preferentially impaired following early postnatal MeHg exposure (O’Kusky, 1985). Consistent with this was that the activity of glutamic acid decarboxylase (GAD), a marker for GABAergic neurons, was specifically reduced in the neocortex (O’Kusky and McGeer, 1985; O’Kusky et al., 1988), suggesting a selective loss of GABAergic neurons and terminals. 2) GABAergic inhibitory synaptic transmission appears to be more sensitive to MeHg than is glutamatergic excitatory synaptic transmission in hippocampal slices (Yuan and Atchison, 1995, 1997; Fountain and Rowan, 2000). Similar phenomenon has been observed consistently in experiments in which both GABAergic and glutamatergic responses could be recorded in a given neuron simultaneously: the effects of MeHg on GABAergic responses consistently occured prior to those of MeHg on glutamatergic responses (unpublished observations). 3) Early postnatal exposure of rats to MeHg increased the sensitivity of cortical neurons to GABAA receptor antagonists and reduced the threshold for glutamatergic excitation (Dasari and Yuan, 2010). Thus, MeHg-induced preferential impairment of GABAergic inhibitory function could lead to hyperexcitability and increased seizure susceptibility.
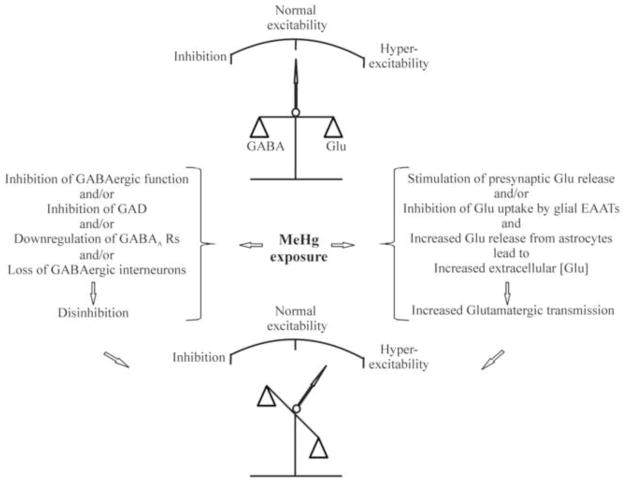
On the other hand, a direct stimulatory effect of MeHg on glutamatergic function could also contribute to increased susceptibility to seizures. In fact, inhibition by MeHg of glutamate uptake by excitatory amino acid transpoters (EAATs) in astrocytes, leading to an increase in extracellular glutamate levels, has been thought to play an important role in MeHg-induced increase in neuronal excitability (Albrecht et al., 1993; Aschner et al., 2000; Juárez et al., 2002). In addition, in vitro studies have consistently shown that MeHg always initially stimulates glutamatergic synaptic transmission including increased spontaneous release of glutamate (Yuan and Atchison, 1993, 1995, 1997, 2007; Fountain and Rowan, 2000). However, it appears that this initial stimulatory action is likely a secondary effect, at least in part, resulting from a preferential effect of MeHg on GABAergic function (Yuan and Atchison, 1995, 1997; Fountain and Rowan, 2000). Thus, a preferential effect of MeHg on GABAergic inhibitory system, leading to disinhibition of glutamatergic signaling, is predicted to be, at least partly, responsible for MeHg-induced increases in the susceptibility of mammalian brains, particularly developing brains, to seizures or epilepsy (Figure 1).
Figure 1.
Schematic diagram of a potential mechanism underlying MeHg-induced changes in neuronal excitability. Under normal conditions, neuronal excitability in the mammalian brains is maintained by well-balanced glutamatergic excitatory and GABAergic inhibitory synaptic activities (Top). Following MeHg exposure, on one hand, MeHg preferentially affects GABAergic inhibitory function result in disinhibition of glutamatergic excitatory function, leading to increased release of neurotransmitter from presynaptic terminals. On the other hand, MeHg also directly stimulates release of glutamate from presynaptic terminals, selectively inhibits glutamate uptake by astrocytes and stimulates glial release of glutamate. These actions will result in an increased extracellular glutamate concentration. Thus, the consequence of the combined effects of MeHg on GABAergic and glutamatergic systems will lead to unbalanced neuronal excitability toward to hyperexcitability direction (Bottom). Abbreviations: GABA, gamma-amino butyric acid; Glu, glutamate; GAD, glutamic acid decarboxylase; EAAT, excitatory amino acid transporters.
At high level exposure, however, MeHg is known to induce severe brain damage including a gross loss of neurons following neuronal degeneration and death, particularly the layer IV neurons in the neocortex of humans (Hunter and Russell, 1954, Takeuchi et al., 1959, 1962; Nierenberg et al., 1998) and experimental animals (Chang and Hartmann, 1972; Shaw et al., 1975; Syversen et al., 1981; Merigan et al., 1983; O’Kusky, 1985; Wakabayashi et al., 1995; Nagashima et al., 1996; Nagashima, 1997; Eto et al., 2001a, 2001b, 2002b). Therefore, it is possible that MeHg-induced neuron loss may result in reactive gliosis and/or neuronal network rewiring and forming aberrant recurrent excitatory circuits similar to those seen in chronic epilepsy resulting from brain trauma, ischemia, tumors, infection and status epilepticus (SE) in humans (for review see Parent and Murphy, 2008; Scharfman and McCloskey, 2009) and subsequently lead to hyperexcitability.
Conclusions
The importance of gene-environment interactions in epileptogenesis has been well recognized. However, what and how the environmental factors interact with genetic and other intrinsic factors to alter the susceptibility of humans and animals to epileptic seizures remain poorly understood. In terms of the roles of environmental chemicals in epileptogenesis, our knowledge is even more limited due to the lack of systematic study in this important area.
Epidemiological and animal studies have provided strong evidence that MeHg, a ubiquitous environmental contaminant, is an important risk factor contributing to epileptogenesis. Clearly, acute or chronic exposure to high levels of MeHg induces convulsive seizures. In addition, developmental exposure to relatively low levels of MeHg facilitates seizure induction in animals by other convulsive factors. Although the exact underlying mechanisms remain unclear, a preferential effect of MeHg on GABAergic functions is predicted to contribute to the etiology of epileptogenesis. However, it remains to be determined whether chronic exposure to low levels of MeHg through fish consumption also affects the susceptibility of the human brain, particular the developing brain, to seizure disorders. The latter is particularly important because: 1) the developing brain is highly sensitive to seizure, with seizures occurring more frequently in the neonatal period and early childhood than at any other stages in life (Ronen et al., 1999; Jensen 2009). 2) Coincidently, the developing brain is also highly sensitive to MeHg (Weiss et al., 2000; Myers et al., 2009).
Chronic exposure to low levels of certain environmental factors may not directly induce a disease or neurological disorder. However, chronic exposure to these factors may act as risk modifiers to alter the susceptibility and to accelerate the onset of a disease or disorder caused by other pathogens or genetic defects (for review, see Sorg and Prasad, 1997; Bell et al., 1997a, 1997b; Gilbert, 2001). In some cases, effects of early developmental environmental exposure may not be expressed until later in adult life (Vathy 2001; Landrigan et al., 2005; Doherty et al., 2009; Montandon et al., 2009; Fox et al., 2010). For instance, prenatal methamphetamine or morphine exposure affects the susceptibility of adult rats to convulsive agent-induced seizures (Vathy 2001; Šlamberová et al., 2008). Prenatal exposure to cigarette smoke affects offspring weight gain and increases risk of developing cardiovascular diseases later in life (Ng et al., 2009). Prenatal coexposure of mercury vapor and MeHg causes interactive behavioral changes in adult rats (Fredriksson et al, 1996). Developmental MeHg exposure alters behavioral and/or neurochemical sensitivity of animals to d-amphetamine and pentobarbital (Rasmussen and Newland, 2001) or amphetamine (Wagner et al., 2007) later in life. Under these circumstances, the contribution of environmental risk factors to the etiology of a neurological disorder is often ignored. This may be especially true for chemicals like MeHg that have a characteristic “latent” period before onset of its toxic effects (Rice and Gilbert, 1982; Harada, 1995; Nierenberg et al., 1998; Weiss et al., 2002). To date, however, no specific epidemiological evidence is available to support an effect of MeHg, particularly at low level MeHg exposure through the consumption of fish and seafood, on seizure susceptibility. Thus, questions remain: 1) Does exposure to low levels of MeHg from fish consumption alter the susceptibility of humans to seizures induced by other intrinsic and extrinsic factors such as gene mutations, fever, audiogenic or chemical convulsive inducers? 2) Is the prevalence rate of neonatal or childhood seizures or epilepsy in populations with long-term consumption of MeHg-contaminated fish or seafood diet higher than that in populations with low fish diet? Interestingly, the prevalence rate (8–14%) of febrile seizures in some regions of Japan and Guam (Mathai et al., 1968; Stanhope et al., 1972; Tsuboi and Okada 1984; Tsuboi, 1988; Hauser 1994) are significantly higher than the average worldwide (3–5%). Is this increased incidence rate of febrile seizures related to chronic exposure to MeHg? 3) Does early developmental exposure to MeHg affect the epileptic seizure susceptibility in adulthood? Thus, further investigation of the role of MeHg-gene interactions in alterations of seizure susceptibility is necessary. The outcomes will be helpful for better understanding of the etiology of epilepsy.
Acknowledgments
The author would like to specifically thank Dr. Ravindra Hajela at Michigan State University and Dr. Lori Isom and Mr. Jeffery Calhoun at University of Michigan for their critical review and comments on this manuscript. This work is supported by NIEHS grants ES013767 and Michigan State University funding 06-HBRI-II-616.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-Elfattah AS, Shamoo AE. Regeneration of a functionally active rat brain muscarinic receptor by D-penicillamine after inhibition with methylmercury and mercuric chloride. Mol Pharmacol. 1981 Nov;20(3):492–497. [PubMed] [Google Scholar]
- Albrecht J, Talbot M, Kimelberg HK, Aschner M. The role of sulfhydryl groups and calcium in the mercuric chloride-induced inhibition of glutamate uptake in rat primary astrocyte cultures. Brain Res. 1993;607:249–254. doi: 10.1016/0006-8993(93)91513-r. [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Tan KH, Aschner M. The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology. 2002;23:755–759. doi: 10.1016/S0161-813X(01)00076-6. [DOI] [PubMed] [Google Scholar]
- Arakawa O, Nakahiro M, Narahashi T. Mercury modulation of GABA-activated chloride channel and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. doi: 10.1016/0006-8993(91)90913-g. [DOI] [PubMed] [Google Scholar]
- Arrieta O, Palencia G, Garcia-Arenas G, Morales-Espinosa D, Hernandez-Pedro N, Sotelo J. Prolonged exposure to lead lowers the threshold of pentylenetetrazole-induced seizures in rats. Epilepsia. 2005;46:1599–1602. doi: 10.1111/j.1528-1167.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Methylmercury neurotoxicity: Explore potential novel targets. Open Toxicol J. 2007;1:1–10. doi: 10.2174/1874340400701010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007 Mar;40(3):285–91. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends Pharmacol Sci. 2005;26(11):549–57. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Axtell CD, Cox C, Myers GJ, Davidson PW, Choi AL, Cernichiari E, Sloane-Reeves J, Shamlaye CF, Clarkson TW. Association between methylmercury exposure from fish consumption and child development at five and a half years of age in the Seychelles Child Development Study: an evaluation of nonlinear relationships. Environ Res. 2000;84:71–80. doi: 10.1006/enrs.2000.4082. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Jhalidi A, Al-Rawi NY, et al. Methylmercury poisoning in Iraq: an interuniversity report. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Basu N, Scheuhammer AM, Rouvinen-Watt K, Evans RD, Trudeau VL, Chan LHM. In vitro and whole animal evidence that methylmercury disrupt GABAergic systems in discrete brain regions in captive mink. Comp Biochem Physiol. 2010;151 (Part C):379–385. doi: 10.1016/j.cbpc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Bell IR, Schwartz GE, Boldwin CM, Hardin EE, Klimas NG, Kline JP, et al. Individual differences in neural sensitization and the role of context in illness from low level environmental chemical exposure. Environ Health Perspect. 1997a;105 (Suppl 2):457–466. doi: 10.1289/ehp.97105s2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell IR, Rossi J, Gilbert ME, Kobal G, Morrow LA, Newlin DB, et al. Testing the neural sensitization and kindling hypothesis for illness from low levels of environmental chemicals. Environ Health Perspect. 1997b;105 (Suppl 2):539–547. doi: 10.1289/ehp.97105s2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–614. doi: 10.1212/01.wnl.0000255669.83468.dd. [DOI] [PubMed] [Google Scholar]
- Bener A, Al-Suweidi EEK, Bessisso M, Al-Gazali LI, Al-Khider A. Genetics and environmental risk factors associated with febrile seizures. J Ped Newrol. 2006;4:239–243. [Google Scholar]
- Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- Brenner RP, Synder RD. Late EEG findings and clinical status after organic mercury poisoning. Arch Neurol. 1980;37:282–284. doi: 10.1001/archneur.1980.00500540060006. [DOI] [PubMed] [Google Scholar]
- Burgess DL. Neonatal epilepsy syndromes and GEFS+: mechanistic considerations. Epilepsia. 2005;46 (Suppl 10):51–58. doi: 10.1111/j.1528-1167.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- Castoldi A, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull. 2001;55:197–203. doi: 10.1016/s0361-9230(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Candura SM, Costa P, Manzo L, Costa LG. Neurotoxicology. 1996;17:735–741. [PubMed] [Google Scholar]
- Catteral WA, Kalume F, Oakley JC. NaV1. 1 channels and epilepsy. J Physiol. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Hartmann HA. Ultrastructural studies of the nervous system after mercury intoxication. I. Pathological changes in the nerve cell bodies. Acta Neuropath. 1972;20:122–138. doi: 10.1007/BF00691129. [DOI] [PubMed] [Google Scholar]
- Chang LW. Mercury. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Neurotoxicology. New York: The Williams and Wilkins Co; 1980. pp. 508–526. [Google Scholar]
- Chouliaras L, Sierksma AS, Kenis G, Prickaerts J, Lemmens MA, Brasnjevic I, et al. Gene-environment interaction research and transgenic mouse models of Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010:859101. doi: 10.4061/2010/859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers G. The toxicology of mercury-current exposure and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Coccini T, Randine G, Candura SM, Nappi RE, Prockop LD, Manzo L. Low-level exposure to methylmercury modifies muscarinic cholinergic receptor binding characteristics in rat brain and lymphocytes: physiologic implications and new opportunities in biologic monitoring. Environ Health Perspect. 2000;108:29–33. doi: 10.1289/ehp.0010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Jan LY. M-channels: neurological diseases, neuromodulation, and drug development. Arch Neurol. 2003;60:496–500. doi: 10.1001/archneur.60.4.496. [DOI] [PubMed] [Google Scholar]
- Cooper RS. Gene–environment interactions and the etiology of common complex disease. Ann Intern Med. 2003;139:437–440. doi: 10.7326/0003-4819-139-5_part_2-200309021-00011. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, Josse D, White R, Amiel-Tison C. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Crump KS, Kjellström T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004;17:731–735. doi: 10.1097/00019052-200412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Yuan Y. Methylmercury exposure in vivo induces a long-lasting epileptiform activity in layer II/III neurons in cortical slices of rat. Toxicol Lett. 2010;193:138–143. doi: 10.1016/j.toxlet.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Palumbo D, Myers GJ, Cox C, Shamlaye CF, Sloane-Reeves J, Cernichiari E, Wilding GE, Clarkson TW. Neurodevelopmental outcomes of Seychellois children from the pilot cohort at 108 months following prenatal exposure to methylmercury from a maternal fish diet. Environ Res. 2000;84:1–11. doi: 10.1006/enrs.2000.4084. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Wilding GE, Shamlaye CF, Huang LS, Cernichiari E, Sloane-Reeves J, Palumbo D, Clarkson TW. Methylmercury and neurodevelopment: longitudinal analysis of the Seychelles child development cohort. Neurotoxicol Teratol. 2006;28:529–535. doi: 10.1016/j.ntt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, et al. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol. 1994;35:680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- Denays R, Kumba C, Lison D, De Bels D. First epileptic seizures induced by occupational nickel poisoning. Epilepsia. 2005;46:961–962. doi: 10.1111/j.1528-1167.2005.70004.x. [DOI] [PubMed] [Google Scholar]
- Doherty SP, Grabowski J, Hoffman C, Ng SP, Zelikoff JT. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 2009;1(Suppl):97–101. doi: 10.1080/13547500902965898. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc Natl Acad Sci U S A. 2009;106:11753–8. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi ME, Mansour NA, Eldefrawi AT. Interactions of acetylcholine receptors with organic mercury compounds. Adv Exp Med Biol. 1977;84:449–463. doi: 10.1007/978-1-4684-3279-4_20. [DOI] [PubMed] [Google Scholar]
- Escalantie-Membrillo C, Paz C. Development of an experimental model of epilepsy in rats exposed to ozone. Toxicol Lett. 1997;93:103–107. doi: 10.1016/s0378-4274(97)00077-5. [DOI] [PubMed] [Google Scholar]
- Eto K, Yasutake A, Kuwana T, Korogi Y, Akima M, Shimozeki T, Tokunaga H, Kaneko Y. Methylmercury poisoning in common marmosets-a study of selective vulnerability within the cerebral cortex. Toxicol Pathol. 2001a;29:565–573. doi: 10.1080/019262301317226375. [DOI] [PubMed] [Google Scholar]
- Eto K, Yasutake A, Nakano A, Akagi H, Tokunaga H, Kojima T. Reappraisal of the historic 1959 cat experiment in Minamata by the Chisso factory. Tohoku J Exp Med. 2001b;194:197–203. doi: 10.1620/tjem.194.197. [DOI] [PubMed] [Google Scholar]
- Eto K, Yasutake A, Korogi Y, Akima M, Shimozeki T, Tokunaga H, Juwana T, Kaneko Y. Methylmercuty poisoning in common marmosets-MRI findings and peripheral nerve leisions. Toxicol pathol. 2002;30:723–734. doi: 10.1080/01926230290166814. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- Fonfría E, Rodriguez-Farré E, Suñol C. Mercury interaction with the GABAA receptor modulates the benzodiazepine binding site inprimary cultures of mouse cerebellar granule cells. Neuropharmacology. 2001;41:819–833. doi: 10.1016/s0028-3908(01)00130-7. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Effects of sequential exposure to multiple concentrations of methylmerrcury in the rat hippocampal slice. Ecotoxicol Environ Safety. 2000;47:130–136. doi: 10.1006/eesa.2000.1941. [DOI] [PubMed] [Google Scholar]
- Fox DA, Opannashuk L, Zharkovsky A, Weiss B. Gene-chemical interactions in the developing mammalian nervous system: effects on proliferation, neurogenesis and differentiation. Neurotoxicology. 2010;31:589–597. doi: 10.1016/j.neuro.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Dencker L, Archer T, Danielsson BRG. Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changers in adult rats. Neurotoxicol Teratol. 1996;18:129–134. doi: 10.1016/0892-0362(95)02059-4. [DOI] [PubMed] [Google Scholar]
- Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44 (Suppl 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gibertson M. Male cerebral palsy hospitalization as a potential indicator of neurological effects of methylmercury exposure in Great Lakes communities. Environ Res. 2004;75:375–384. doi: 10.1016/j.envres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Gibertson M. Index of congenital Minamata disease in Canadian areas of concern in the Great Lakes: an eco-social epidemiological approach. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:246–275. doi: 10.1080/10590500903310120. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. Does the kindling model of epilepsy contribute to our understanding of multiple chemical sensitivity? Ann N Y Acad Sci. 2001;933:68–91. doi: 10.1111/j.1749-6632.2001.tb05815.x. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Herz KT. Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. Mt Sinai J Med. 2011;78:107–118. doi: 10.1002/msj.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves TD. Ion channel and epilepsy. Q J Med. 2006;99:201–217. doi: 10.1093/qjmed/hcl021. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci. 2005;25:10308–10320. doi: 10.1523/JNEUROSCI.2697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururaj AK, Sztriha L, Bener A, Dawodu A, Eapen V. Epilepsy in children with cerebral palsy. Seizure. 2003;12:110–114. doi: 10.1016/s1059131102002558. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harada Y. Study Group of Minamata Diseas. Minamata disease. Kumamoto University; Japan: 1968. Congenital (or fetal) Minamata Bay disease; pp. 73–91. [Google Scholar]
- Harada Y. Congenital Minamata Disease. In: Tsubaki T, Irukayama K, editors. Minamata disease: methylmercury poisoning in Minamata and Niigata, Japan. New York: Elsevier; 1979. pp. 209–269. [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35:S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Herden CJ, Pardo NE, Hajela R, Yuan Y, Atchison WD. Differential effects of methylmercury on GABAA receptor currents in rat cerebellar granule cells and cerebral cortical neurons in culture. J Pharmacol Exp Ther. 2008;324:517–528. doi: 10.1124/jpet.107.123976. [DOI] [PubMed] [Google Scholar]
- Hernandez EH, Discalzi G, Dassi P, Jarre L, Pira E. Manganese intoxication: the cause of an inexplicable epileptic syndrome in a 3 year old child. Neurotoxicology. 2003;24:633–639. doi: 10.1016/S0161-813X(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Hirose S. A new paradigm of channelopathy in epilepsy syndromes: Intracellular trafficking abnormality of channel molecules. Epilepsy Res. 2006;70S:S206–S217. doi: 10.1016/j.eplepsyres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Hogberg HT, Kinsner-Ovaskainen A, Coecke S, Hartung T, Bal-Price AK. mRNA expression is a relevant tool to identify developmental neurotoxicants using an in vitro approach. Toxicol Sci. 2010;113:95–115. doi: 10.1093/toxsci/kfp175. [DOI] [PubMed] [Google Scholar]
- Hunter D, Russell DS. Focal cerebral and cerebellar atrophy in a human subject due to organic mercury compounds. Neurol Neurosurg Psychiat. 1954;17:235–241. doi: 10.1136/jnnp.17.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900. vii. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–765. doi: 10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez BI, Martínez ML, Montante M, Dufour L, García E, Jiménez-Capdeville ME. Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol. 2002;24:767–771. doi: 10.1016/s0892-0362(02)00270-2. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Davis R, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevent of common diseases with environmental causes? Am J Epidemiol. 2005;161:779–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- Kjeldsen MJ, Kyvik KO, Friis ML, Christensen K. Genetic and environmental factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. 2002;51:167–177. doi: 10.1016/s0920-1211(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Komulainen H, Bondy SC. Increased free intrasynaptosomal Ca2+ by neurotoxic organometals: distinctive mechanism. Toxicol Appl Pharmacol. 1987;88:77–86. doi: 10.1016/0041-008x(87)90271-7. [DOI] [PubMed] [Google Scholar]
- Komulainen H, Keränen A, Saano V. Methylmercury modulates GABAA receptor complex differentially in rat cortical and cerebellar membranes in vitro. Neurochem Res. 1995;20:659–662. doi: 10.1007/BF01705532. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy MS, Parthiban N, Muthu P, Paul V, Balagopal G, Kumaravel TS. Effect of acute pretreatment of lead on picrotoxin-induced convulsions in rats. J Appl Toxicol. 1993;13:155–159. doi: 10.1002/jat.2550130303. [DOI] [PubMed] [Google Scholar]
- Kuneš J, Zicha J. The Interaction of Genetic and Environmental Factors in the Etiology of Hypertension. Physiol Res. 2009;58(Suppl 2):S33–S41. doi: 10.33549/physiolres.931913. [DOI] [PubMed] [Google Scholar]
- Kurland LT, Faro SN, Siedler H. Minamata Disease. The outbreak of a neurologic disorder in Minamata, Japan, and its relationship to the ingestion of seafood contaminated by mercuric compounds. World Neurol. 1960;1:370–395. [PubMed] [Google Scholar]
- Kwong KL, Wong SN, So KT. Epilepsy in children with cerebral palsy. Pediatr Neurol. 1998;19:31–36. doi: 10.1016/s0887-8994(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande LM, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspec. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt R, Haas H, Büsselberg D. Methyl mercury reduces voltage-activated currents of rat dorsal root ganglion neurons. Naunyn-Schmiedeberg’s Arch Pharmacol. 1996;354:532–538. doi: 10.1007/BF00168447. [DOI] [PubMed] [Google Scholar]
- Lerche H, Jurkat-Rott K, Lehmann-Horn F. Ion channel and epilepsy. Amer J Med Jenet. 2001;106:146–159. doi: 10.1002/ajmg.1582. [DOI] [PubMed] [Google Scholar]
- Limke TL, Bearss JJ, Atchison WD. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol Sci. 2004a;80:60–68. doi: 10.1093/toxsci/kfh131. [DOI] [PubMed] [Google Scholar]
- Limke TL, Heidemann SR, Atchison WD. Disruption of intraneuronal divalent cation regulation by methylmercury: are specific targets involved in altered neuronal development and cytotoxicity in methylmercury poisoning? Neurotoxicology. 2004b;25:741–760. doi: 10.1016/j.neuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50 (Suppl 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol (Lond) 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Rusconi R, Scalmani P, Avanzini G, Franceschetti S. Epileptogenic ion channel mutations: From bedside to bench and, hopefully, back again. Epilepsy Res. 2010;92:1–29. doi: 10.1016/j.eplepsyres.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Marsh DO, Clarkson TW, Cox C, Myers GJ, Amin-Zaki L, Al-Tikriti S. Fetal methylmercury poisoning. Relationship between concentration in single strands of maternal hair and child effects. Arch Neurol. 1987;44:1017–1022. doi: 10.1001/archneur.1987.00520220023010. [DOI] [PubMed] [Google Scholar]
- Marsh DO, Clarkson TW, Myers GJ, Davidson PW, Cox C, Cernichiari E, Tanner MA, Lednar W, Shamlaye C, Choisy O, Hoareau C, Berlin M. The Seychelles study of fetal methylmercury exposure and child development: introduction. Neurotoxicology. 1995;16:583–96. [PubMed] [Google Scholar]
- Mathai KV, Dunn DP, Kurland LT, Reeder FA. Covulsive disorders in the Mariana Islands. Epilepsia. 1968;9:77–85. doi: 10.1111/j.1528-1157.1968.tb05130.x. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Shin T. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McKeown-Eyssen GE, Ruedy J, Neims A. Methyl mercury exposure in northern Quebec. II. Neurologic findings in children. Am J Epidemiol. 1983;118:470–479. doi: 10.1093/oxfordjournals.aje.a113652. [DOI] [PubMed] [Google Scholar]
- Menashi M, Ornoy A, Yanai J. Transplacental effects of methylmercury chloride in mice with specific emphasis on the audiogenic seizure response. Dev Neurosci. 1982;5:216–221. doi: 10.1159/000112679. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maurissen JPJ, Weiss B, Eskin T, Lapham LW. Neurotoxic actions of methylmercury on the primate visual system. Neurobehav Toxicol Teratol. 1983;5:649–658. [PubMed] [Google Scholar]
- Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in Schizophrenia: The role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Horner RL, Kinkead R, Bairam A. Caffeine in the neonatal period induces long-lasting changes in sleep and breathing in adult rats. J Physiol (Lond) 2009;587:5493–5507. doi: 10.1113/jphysiol.2009.171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Marsh DO, Cernichiari E, Lapham LW, Berlin M, Clarkson TW. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995;16:711–716. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, Choi A, Cox C, Clarkson TW. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18:819–829. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, Clarkson TW. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30:338–349. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrer T. Neuronal structures involved in the induction and propagation of seizures caused by nerve agents: Implications for medical treatment. Toxicology. 2007;239:1–14. doi: 10.1016/j.tox.2007.06.099. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Fujii Y, Tsukamoto T, Nukuzuma S, Satoh M, Fujita M, Fujioka Y, Akagi H. Apoptotic process of cerebellar degeneration in experimental methylmercury intoxication of rats. Acta Neuropathol. 1996;91:72–77. doi: 10.1007/s004010050394. [DOI] [PubMed] [Google Scholar]
- Nagashima K. A review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicologic Pathol. 1997;25:624–631. doi: 10.1177/019262339702500613. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Cellular and molecular mechanisms of Prkinson’s disease:Neurotoxin, causative gene and inflammatory cytokines. Cell Mol Neurobiol. 2006;26:781–802. doi: 10.1007/s10571-006-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J. Progress in searching for the febrile seizure susceptibility genes. Brain Dev Brain Dev. 2009;31:359–365. doi: 10.1016/j.braindev.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Ng SP, Conklin DJ, Bhatnagar A, Bolanowski DD, Lyon J, Zelikoff T. Prenatal exposure to cigarette smoke induces diet- and sex-dependent dyslipidemia and weight gain in adult murine offspring. Environ Health Perspect. 2009;117:1042–1048. doi: 10.1289/ehp.0800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, et al. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N Engl J Med. 1998;338:1672–1676. doi: 10.1056/NEJM199806043382305. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Furtinger A, Lmai H, Kato N, Sperk G. Changes in the GABA-ergic system induced by trimethyltin application in the rat. Mol Brain Research. 2001;97:1–6. doi: 10.1016/s0169-328x(01)00278-9. [DOI] [PubMed] [Google Scholar]
- O’Ksky JR, McGeer EG. Methylmercury poisoning of the developing nervous system in the rat; decreased activity of glutamic acid decarboxylase in cerebral cortex and neostriatum. Develop Brain Res. 1985;21:299–306. doi: 10.1016/0165-3806(85)90219-6. [DOI] [PubMed] [Google Scholar]
- O’Kusky JR. Synapse elimination in the developing visual cortex: a morphometric analysis in normal and dark-reared cats. Brain Res. 1985;354:81–91. doi: 10.1016/0165-3806(85)90071-9. [DOI] [PubMed] [Google Scholar]
- O’Kusky, Radke JM, Vincent SR. Methylmercury-induced movement and postural disorders in developing rat: loss of somatostatin-immunoreactive interneurons in the striatum. Develop Brain Res. 1988;40:11–23. doi: 10.1016/0165-3806(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorffer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4:627–634. doi: 10.1016/S1474-4422(05)70172-1. [DOI] [PubMed] [Google Scholar]
- Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia. 2008;49(Suppl 5):19–25. doi: 10.1111/j.1528-1167.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- Peng S, Hajela RK, Atchison WD. Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells. Mol Pharmacol. 2002;62:1418–1430. doi: 10.1124/mol.62.6.1418. [DOI] [PubMed] [Google Scholar]
- Peng S, Hajela RK, Atchison WD. Effects of methylmercury on human neuronal L-type calcium channels transiently expressed in human embryonic kidney cells (HEK-293) J Pharmacol Exp Ther. 2002;302:424–432. doi: 10.1124/jpet.102.032748. [DOI] [PubMed] [Google Scholar]
- Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50 (Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt FN, Kato E, Narahashi T. Effects of methylmercury on electrical responses of neuroblastoma cells. NeuroToxicology. 1982;3:205–220. [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to d-amphetamine and pentobarbital in adult rats. Neurtoxicl Teratol. 2001;23:45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Early chronic low-level methylmercury poisoning in monkeys impairs spatial vision. Science. 1982;216:759–761. doi: 10.1126/science.7079739. [DOI] [PubMed] [Google Scholar]
- Roda E, Coccini T, Acerbi D, Castoldi A, Bernocchi G, Manzo L. Cerebellum cholinergic muscarinic receptor (subtype-2 and -3) and cytoarchitecture after developmental exposure to methylmercury: an immunohistochemical study in rat. J Chem Neuroanat. 2008;35:285–294. doi: 10.1016/j.jchemneu.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–75. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- Rustam H, Hamdi T. Methyl mercury poisoning in Iraq: a neurological study. Brain. 1974;97:499–510. [PubMed] [Google Scholar]
- Sakamoto M, Ikegami N, Nakano A. Protective effects of Ca2+ channel blockers against methyl mercury toxicity. Pharmacol Toxicol. 1996;78:193–199. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]; Epilepsy Res. 2009;85:150–61. doi: 10.1016/j.eplepsyres.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, McCloskey DP. Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Methylmercury blocks N- and L- type Ca++ channels in nerve-growth factor-differentiated pheochromocytoma (PC12) cells. J Pharmacol Exp Ther. 1991;258:149–157. [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Effects of methylmercury on perineurial Na+ and Ca2+-dependent potentials at neuromuscular junctions of the mouse. Brain Res. 1992;595:215–219. doi: 10.1016/0006-8993(92)91052-g. [DOI] [PubMed] [Google Scholar]
- Shafer TJ. Methylmercury effects on ion channels and electrical activity in neurons: future directions. Cell Mol Biol (Noisy-le-grand) 2000;46:855–864. [PubMed] [Google Scholar]
- Shafer TJ, Meacham CA, Barone SJ. Effects of prolonged exposure to nanomolar concentrations of methylmercury on voltage-sensitive sodium and calcium currents in PC12 cells. Brain Res Dev Brain Res. 2002;136:151–164. doi: 10.1016/s0165-3806(02)00360-7. [DOI] [PubMed] [Google Scholar]
- Shamoo AE, Maclennan DH, Elderfrawi ME. Differential effects of mercurial compounds on excitable tissues. Chem Biol Interact. 1976;12:41–52. doi: 10.1016/0009-2797(76)90065-x. [DOI] [PubMed] [Google Scholar]
- Shaw CM, Mottet NK, Body RL, Luschei ES. Variability of neuropathologic lesions in experimental methylmercurial encephalopathy in primates. Am J Pathol. 1975;80:451–470. [PMC free article] [PubMed] [Google Scholar]
- Shrivastav BB, Brodwick BS, Narahashi T. Methylmercury: effects on electrical properties of squid axon membranes. Life Sci. 1976;18:1077–1081. doi: 10.1016/0024-3205(76)90141-7. [DOI] [PubMed] [Google Scholar]
- Singh G, Prabhakar S. The association between central nervous system (CNS) infections and epilepsy: epidemiological approaches and microbiological and epileptological perspectives. Epilepsia. 2008;49 (Suppl 6):2–7. doi: 10.1111/j.1528-1167.2008.01749.x. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Atchison WD. Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxical Appl Pharmcol. 2000;167:1–11. doi: 10.1006/taap.2000.8967. [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Bernášková K, Matĕjovská I, Schutová B. Does prenatal methamphetamine exposure affect seizure susceptibility in adult rats with acute administration of the same drugs? Epi Res. 2008;78:33–39. doi: 10.1016/j.eplepsyres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Solberg Y, Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. Trends Pharmacol Sci. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Prasad BM. Potential role of stree and sensitization in the development and expression of multiple chemical sensitivity. Environ Health Perpect. 1997;105 (Suppl 2):467–471. doi: 10.1289/ehp.97105s2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Hannan AJ. Nature, nurture and neurology: gene environment interactions in neurodegenerative disease FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2347–2361. doi: 10.1111/j.1742-4658.2005.04677.x. [DOI] [PubMed] [Google Scholar]
- Stanhope JM, Brody JA, Brink E. Convulsions among the Chamorro people of Guam, Mariana islands. I. Seizure disorders. Am J Epidemiol. 1972;95:292–298. doi: 10.1093/oxfordjournals.aje.a121396. [DOI] [PubMed] [Google Scholar]
- Stewardt I. Environmental risk factors for temporal lobe epilepsy - Is prenatal exposure to the marine algal neurotoxin domoic acid a potentially preventable cause? Med Hypoth. 2010;74:466–481. doi: 10.1016/j.mehy.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Su M-Q, Okita GT. Behavioral effects on the progeny of mice treated with methylmercury. Toxico Appl Pharmacol. 1976;38:195–205. doi: 10.1016/0041-008x(76)90173-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakai K, Sugawara T, Nakamura T, Ohba T, Shimada M, Hosokawa T, Okamura K, Sakai T, Kurokawa N, Murata K, Satoh C, Satoh H. Neurobehavioral effects of prenatal exposure to methylmercury and PCBs, and seafood intake: neonatal behavioral assessment scale results of Tohoku study of child development. Environ Res. 2010;110:699–704. doi: 10.1016/j.envres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Synder RD. Congenital mercury poisoning. N Engl J Med. 1971;284:1014–1016. doi: 10.1056/NEJM197105062841806. [DOI] [PubMed] [Google Scholar]
- Syversen TLM, Totland G, Flood PR. Early morphological changes in rat cerebellum caused by a single dose of methylmercury. Arch Toxicol. 1981;47:101–111. doi: 10.1007/BF00332352. [DOI] [PubMed] [Google Scholar]
- Szász A, Barna B, Szupera Z, De Visscher G, Galbács Z, Kirsch-Volders M, Szente M. Chronic low-dose maternal exposure to methylmercury enhances epileptogenicity in developing rats. Int J Dev Neurosci. 1999;17:733–742. doi: 10.1016/s0736-5748(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Szász A, Barna B, Szupera Z, De Visscher G, Galbács Z, Kirsch-Volders M, Szente M. Effects of continuous low-dose exposure to organic and inorganic mercury during development on epileptogenicity in rats. NeuroToxicology. 2002;23:197–206. doi: 10.1016/s0161-813x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Kambara T, Morikawa N, Matsumoto H, Shiraishi Y, Ito H. Pathologic observations of the Minamata disease. Acta Pathol J. 1959;9:769–783. doi: 10.1111/j.1440-1827.1959.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Morikawa N, Matsumoto H, Shiraishi Y. A patholgical study of Minamata Disearse in Japan. Acta Neuropathol. 1962;2:40–57. [Google Scholar]
- Tiedeken JA, Ramsdell JS. Embryonic Exposure to Domoic Acid Increases the Susceptibility of Zebrafîsh Larvae to the Chemical Convulsant Pentylenetetraz. Environ Health Perspect. 2007;115:1547–1552. doi: 10.1289/ehp.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova MT, Mantis JG, Le M, Kim CY, Seyfried TN. Genetic and environmental interactions determine seizure susceptibility in epileptic EL mice. Gens Brain Behav. 2006;5:519–527. doi: 10.1111/j.1601-183X.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- Todorva MT, Burwell TJ, Seyfried TN. Environmental risk factors for multifactorial epilepsy in EL mice. Epilepsia. 1999;40:1697–1707. doi: 10.1111/j.1528-1157.1999.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Tokuomi H, Okajima T. Minamata Disease. World Neurol. 1961;2:536–545. [PubMed] [Google Scholar]
- Tsuboi T, Okada S. Seasonal variation of febrile convulsion in Japan. Acta Neurol Scand. 1984;69:285–292. doi: 10.1111/j.1600-0404.1984.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi T. Prevalence and incidence of epilepsy in Tokyo. Epilepsia. 1988;29:103–110. doi: 10.1111/j.1528-1157.1988.tb04404.x. [DOI] [PubMed] [Google Scholar]
- Vathy I. Prenatal morphine exposure induces age- and sex-dependent changes in seizure susceptibility. Prog Neuro-psychopharmacol Bio Psychiat. 2001;25:1203–1226. doi: 10.1016/s0278-5846(01)00187-7. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Christensen J. Register-based studies on febrile seizures in Denmark. Brain Dev. 2009;51:372–377. doi: 10.1016/j.braindev.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Világi I, Dóczi J, Banczerowski-Pelyhe I. Altered electrophysiological characteristics of developing rat cortical neurones after chronic methylmercury chloride treatment. Int J Dev Neurosci. 2000;18:493–499. doi: 10.1016/s0736-5748(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Von Burg R, Northington FK, Shamoo A. Methylmercury inhibition of muscarinic receptor. Toxicol Appl Pharmacol. 1980;53:285–292. doi: 10.1016/0041-008x(80)90428-7. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Ming X, Halladay AK. Behavioral and neurochemical sensitization to amphetamine following early postnatal administration of methylmercury (MeHg) Neurotoxicology. 2007;28:59–66. doi: 10.1016/j.neuro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Wakabayshi K, Kakita A, Sakamoto M, Su M, Iwanaga K, Ikuta F. Variability of brain lesions in rats administered methylmercury at various postnatal development phases. Brain Res. 1995;705:267–272. doi: 10.1016/0006-8993(95)01208-7. [DOI] [PubMed] [Google Scholar]
- Weiss B. Vulnerability of children and the developing brain to neurotoxic hazards. Environ Health Perspect. 2000;108(Suppl 3):375–381. doi: 10.1289/ehp.00108s3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110:851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Disruption by methylmercury of membrane excitability and synaptic transmission in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993;120:203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury act at multiple sites to block hippocampal synaptic transmission. J Pharmacol Exp Ther. 1995;275:1308–1316. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Action of methylmercury on GABA(A) receptor-mediated inhibitory synaptic transmission is primarily responsible for its early stimulatory effects on hippocampal CA1 excitatory synaptic transmission. J Pharmacol Exp Ther. 1997;282:64–73. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury differentially affects GABAA receptor-mediated spontaneous IPSCs in Purkinje and granule cells of rat cerebellar slices. J Physiol (Lond) 2003;550:191–204. doi: 10.1113/jphysiol.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Otero-Montañez JKL, Yao A, Herden CJ, Sirois JE, Atchison WD. Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury. Neurotoxicology. 2005;26:439–454. doi: 10.1016/j.neuro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury-induced increase of intracellular Ca2+ increases spontaneous synaptic current frequency in rat cerebellar slices. Mol Pharmacol. 2007;71:1109–1121. doi: 10.1124/mol.106.031286. [DOI] [PubMed] [Google Scholar]