Abstract
Objective
To define the clinical characteristics and outcome of patients with idiopathic stenosis or occlusion of the basal arteries, without moyamoya collateral formation.
Methods
We identified patients who presented to our institution from 1996 to 2005 with occlusive disease of the distal internal carotid artery or the proximal middle or anterior cerebral arteries demonstrated by digital subtraction cerebral angiography. We excluded those with evidence of atherosclerotic disease, systemic vasculitis, moyamoya phenomenon or any other condition that could otherwise explain their arterial occlusive disease. Medical records were reviewed for presenting symptoms and clinical characteristics. Outcome was determined from chart review and phone interviews.
Results
Twelve patients were identified. All presented with TIA or stroke. Eleven were women. Age at presentation ranged from 34 to 71 years. Nine had a history of hypertension. Five had unilateral intracranial disease. None of the five developed recurrent stroke on medical therapy during an average follow up of 29 months. Seven had bilateral disease. Ischemic stroke occurred between 2 and 107 months after the initial event in five of eight medically-treated hemispheres. One patient developed moyamoya collaterals on follow up angiography.
Conclusion
The clinical features and outcome of these patients are similar to those reported in large case series of North American patients with moyamoya phenomenon. These data suggest a common etiology for the basal arterial occlusive process and a variable ability to form moyamoya collaterals.
Keywords: Moyamoya, idiopathic basal arterial disease
Objective
Moyamoya phenomenon is a unique pattern of collateral formation that can attend narrowing or occlusion of the basal arteries.1,2 Patients with idiopathic basal occlusive disease and moyamoya collateral formation are often considered to have moyamoya disease, yet the underlying etiology for the occlusive vasculopathy remains undefined.3 Idiopathic basal arterial occlusive disease, without moyamoya collateral formation, is identified in some patients presenting with stroke or other ischemic symptoms. It is possible that the same underlying occlusive vasculopathy is involved in both groups of patients but that the ability to form moyamoya collaterals is variable.
The purpose of this study was to define the clinical characteristics and outcomes of patients with idiopathic basal arterial occlusive disease without moyamoya collaterals and compare these to prior reports of patients with moyamoya phenomenon. These patients may share a common underlying occlusive vasculopathy but a variable ability to form moyamoya collaterals, and therefore similar clinical features and outcome.
Methods
Patient eligibility and selection
We identified all adults (age >= 18) who underwent digital subtraction catheter angiography at our institution from 1996 through 2005 and had idiopathic basal arterial occlusive disease, defined as unilateral or bilateral smooth narrowing (>50%) or occlusion of the supraclinoid internal carotid artery, proximal anterior cerebral artery, and/or the proximal middle cerebral artery. Patients who had moyamoya collateral vessels were excluded. Patients with suspected dissections were excluded. The occlusive process was required to be isolated to the basal arteries: patients with angiographic evidence of arterial disease involving other locations, such as the cervical carotid arteries (suggesting atherosclerosis) or distal cerebral vessels (suggesting intracranial atherosclerosis or a reversible vasoconstriction syndrome) were excluded.4 Exclusion criteria also included the presence of any other medical condition that could otherwise explain the arterial steno-occlusive disease, including atherosclerosis, reversible vasoconstriction syndrome, systemic vasculitis, neurofibromatosis type-1, sickle cell anemia, Down syndrome, meningitis, and prior skull base radiation therapy. A total of twelve patients were identified.
Retrospective chart review
We reviewed the clinical records, including hospital and clinic notes, laboratory studies, and radiographic data, for each patient from presentation through 2005. As necessary, outside hospital and clinic records were also obtained and reviewed. The following terms were used to define the initial presenting symptom: transient ischemic attack (TIA) for a focal neurological deficit lasting less than 24 hours, stroke for the acute onset of a focal neurological deficit lasting greater than 24 hours, and intracranial hemorrhage if a subarachnoid, intraparenchymal, and/or intraventricular hemorrhage was noted on lumbar puncture or radiographic studies.
Baseline stroke risk factors were noted at initial presentation including a self-reported history of hypertension, hypertensive heart disease, coronary artery disease, chronic heart failure, diabetes mellitus, hyperlipidemia, significant alcohol and/or tobacco use, oral contraceptive use, and/or parental stroke death. Digital subtraction angiography was performed on all of the subjects at presentation and repeated if indicated in the course of their disease. We analyzed angiography reports for sites and extent of involvement, sources of collateral flow, and other cerebrovascular abnormalities.
Prospective follow-up
We attempted to make phone contact with all living patients. Informed verbal consent was obtained prior to phone interview via a script approved by the Human Studies Committee. We recorded any recurrent stroke events (using the Questionnaire for Verifying Stroke-Free Status),5,6 death and its cause, and any further significant change in cerebrovascular history including surgeries and medication alterations.
Results
General patient characteristics
Patient characteristics are presented in Table 1. Patients ranged in age from 34 to 71 years (mean 44, median 39). Eleven of the twelve (92%) were female. Nine (75%) were white while three (25%) were of African-American descent. All but two (83%) had hypertension, and all were free of known coronary artery disease. One had poorly controlled diabetes, one had a parental history of strokes, and another drank alcohol significantly. Two of the eleven (18%) females used oral contraceptives. Six of the twelve (50%) smoked.
Table 1.
Patient charateristics and angiography findings
| Presentation | Symptoms | Age | Gender | Historical factors | Angiographic findings | |
|---|---|---|---|---|---|---|
| 1 | Ischemic stroke | Right hemiparesis, hypesthesia, and aphasia | 39 | Female | Hypertension, smoking history, and hypercholesterolemia | Distal left ICA occlusion, distal right MCA occlusion, and proximal right MCA mild stenosis, right PCA stenosis |
| 2 | Intracerebral, intraventricular hemorrhage | Lethargy, confusion, and headaches | 37 | Male | Hypertension and smoking history | Proximal right MCA occlusion and bilateral distal ICA severe stenoses |
| 3 | TIA | Transient right hemiparesis and aphasia | 38 | Female | Hypertension | Bilateral distal ICA occlusions |
| 4 | TIA, ischemic stroke | Transient right hand numbness, then left hemiparesis and headaches | 44 | Female | Hypertension and oral contraceptive use | Proximal right MCA severe stenosis, proximal left MCA moderate stenosis and distal left MCA occlusion |
| 5 | Ischemic stroke | Right hemiparesis, hypesthesia, global aphasia, and coma | 46 | Female | Smoking history and hypercholesterolemia | Distal left ICA occlusion, left PCA occlusion, proximal right MCA severe stenosis, proximal right ACA stenosis |
| 6 | Ischemic stroke | Right hemiparesis, hypesthesia, and aphasia | 53 | Female | Hypertension and smoking history | Distal left ICA occlusion and proximal right MCA severe stenosis |
| 7 | Ischemic stroke | Right hypesthesia and left facial droop | 45 | Female | Smoking history | Proximal bilateral MCA severe stenoses |
| 8 | Ischemic stroke | Left hemiparesis | 71 | Female | Hypertension, history of pulmonary embolism, and hypercholesterolemia | Right MCA moderate stenosis, right AComA aneurysm |
| 9 | Ischemic stroke | Left hemiparesis, dysarthria, and confusion | 39 | Female | Hypertension and diabetes mellitus | Distal right ICA severe stenosis |
| 10 | TIA | Transient right hypesthesia | 37 | Female | Hypertension | Distal left ICA severe stenosis, proximal left ACA and MCA moderate stenosis |
| 11 | TIA | Left facial droop and hypesthesia | 34 | Female | Autoimmune scleritis, hypercholesterolemia, and oral contraceptive use | Distal right ICA occlusion, proximal right ACA and MCA mild stenosis |
| 12 | Ischemic stroke | Left arm paresis and hypesthesia | 46 | Female | Hypertension and smoking history | Proximal right MCA near occlusion |
Cerebrovascular events and angiographic findings at presentation
In eleven of these patients (92%) the initial cerebrovascular event was ischemic; three patients presented with TIA and eight with stroke. The twelfth patient presented with intracranial hemorrhage (Patient 2). In three patients, initial angiography revealed incidental cerebrovascular findings not related to their presenting symptoms, including one with an occlusion of the P2 segment of her posterior cerebral artery, another with a 10 × 15 mm anterior communicating artery aneurysm (Patient 8), and a third with a small petrous dural fistula in the ipsilateral hemisphere. Five of the twelve patients presented with unilateral disease while the remaining seven patients presented with bilateral stenosis of the basal arteries.
Treatment and outcomes
Patient outcomes are listed in Table 2. All five patients with unilateral stenosis were treated medically. Four of five had good outcomes. None developed recurrent strokes after an average of 29 months (range 6 – 43 months) following their initial event. Repeat angiographic imaging was obtained in two of the four. Progression to occlusion was identified in one (Patient 12). One (Patient 8) died of subarachnoid hemorrhage from rupture of her previously asymptomatic anterior communicating artery aneurysm 55 months after initially presentation to our institution.
Table 2.
Patient treatments and outcomes
| Angiographic findings | Initial treatment | Outcomes | Follow-up Imaging findings | Subsequent treatments | Subsequent outcomes | |
|---|---|---|---|---|---|---|
| 1 | Bilateral Disease: Distal left ICA occlusion, distal right MCA occlusion, and proximal right MCA mild stenosis, right PCA stenosis | Aspirin, warfarin, and left EDAS | Left and right hemispheric strokes 107 and 111 months after initial event | Subsequent angiogram, 4 months after initial event, worse right MCA and new L ACA stenosis. No EDAS collaterals; 2nd followup angio 4 months later – no change | Right EDAS | Right hemispheric stroke, cerebral edema, and death following right EDAS |
| 2 | Bilateral Disease: Proximal right MCA occlusion and bilateral distal ICA severe stenoses | Right STA-MCA bypass | Left hemispheric stroke 17 months after initial event New left distal vertebral occlusion | Subsequent angiogram, 17 months after initial event, new left vertebral occlusion | Aspirin | Acute myocardial infarction, left ventricular aneurysm and death 77 months after STA-MCA bypass |
| 3 | Bilateral Disease: Bilateral distal ICA occlusions | Aspirin | Left hemispheric stroke 6 months after initial event | No change on angiography 4 months after initial event | Left STA-MCA bypass Right EDAS | No further events 14 months |
| 4 | Bilateral Disease: Proximal right MCA severe stenosis, proximal left MCA moderate stenosis and distal left MCA occlusion | Clopidegrel | Right hemispheric stroke 2 months after initial event | Aspirin and warfarin | Left hemispheric stroke 66 months after initial event | |
| 5 | Bilateral Disease: Distal left ICA occlusion, left PCA occlusion, proximal right MCA severe stenosis, proximal right ACA stenosis | ASA at outside hospital; Right EDAS 9 mos later | Fatal right (ipsilateral) stroke 2 months after EDAS | |||
| 6 | Bilateral Disease: Distal left ICA occlusion and proximal right MCA severe stenosis | Aspirin and left EDAS | Right hemispheric stroke 33 months after initial event Further right proximal MCA occlusion | Angiogram 8 months after initial event, new right MCA occlusion, EDAS collaterals present | Aspirin, plavix, statin and right EDAS | No further events 10 months later |
| 7 | Bilateral Disease: Proximal bilateral MCA severe stenoses | Prednisone | No further events | Subsequent angiogram 5 months later, new right ICA occlusion with new moyamoya collaterals | Right STA- MCA, Aspirin | No further events 36 months later |
| 8 | Unilateral: Right MCA moderate stenosis, right AComA aneurysm | Aspirin | Subarachnoid hemorrhage and death 55 months later | |||
| 9 | Unilateral: Distal right ICA severe stenosis | Aspirin | No further strokes 12 months later | |||
| 10 | Unilateral: Distal left ICA severe stenosis, proximal left ACA and MCA moderate stenosis | Aspirin and plavix | No further strokes 46 months later | |||
| 11 | Unilateral: Distal right ICA occlusion, proximal right ACA and MCA mild stenosis | Aspirin | No further strokes 28 months later | Subsequent annual MRAs for next 3 years unchanged | ||
| 12 | Unilateral: Proximal right MCA near occlusion | Aspirin | No further strokes 6 months later | Subsequent MRA 1 year later showed right M1 occlusion. Unchanged on MRA 3 years later |
Six of the seven patients with bilateral disease were followed for recurrent stroke. One of the seven (Patient 5) underwent an EDAS procedure and died as a consequence of a recurrent stroke two months after surgery. Ischemic strokes occurred during follow up in five of eight medically-treated hemispheres in the six surviving patients with bilateral disease. Two of the six surviving patients with bilateral disease were not treated with surgical revascularization as their initial therapy: one was treated with clopidogrel (Patient 4) and the other with prednisone (Patient 7) for suspected vasculitis. The first patient had a recurrent stroke five years later. Medically therapy was changed to aspirin and warfarin and two months later the patient had a stroke in the contralateral, previously asymptomatic hemisphere. The second of the two patients had no further symptoms. A follow-up angiogram was obtained six months later to assess the response to therapy. New moyamoya collaterals were identified (Figure 1). A superficial temporal artery to middle cerebral artery bypass (STA-MCA) was performed on the previously symptomatic hemisphere. No new symptoms occurred during thirty-six additional months of follow up.
Figure 1. Development of moyamoya collaterals in a North American adult.
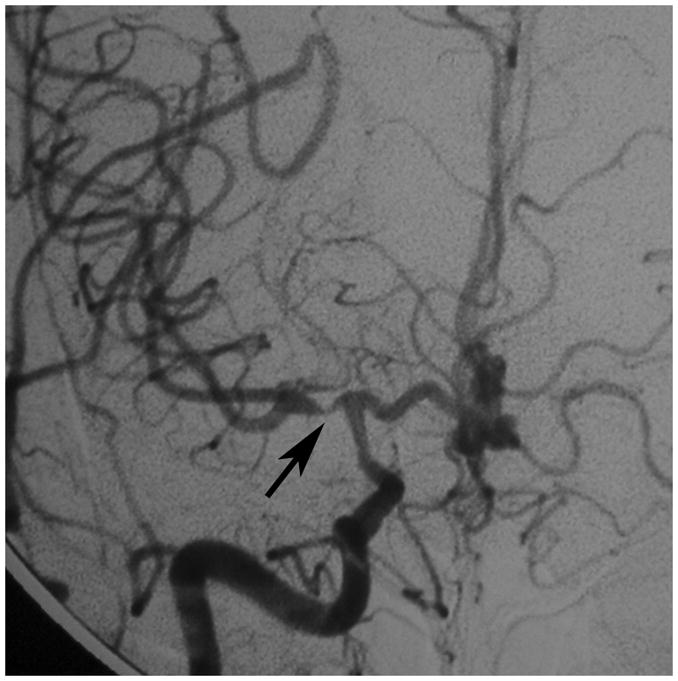
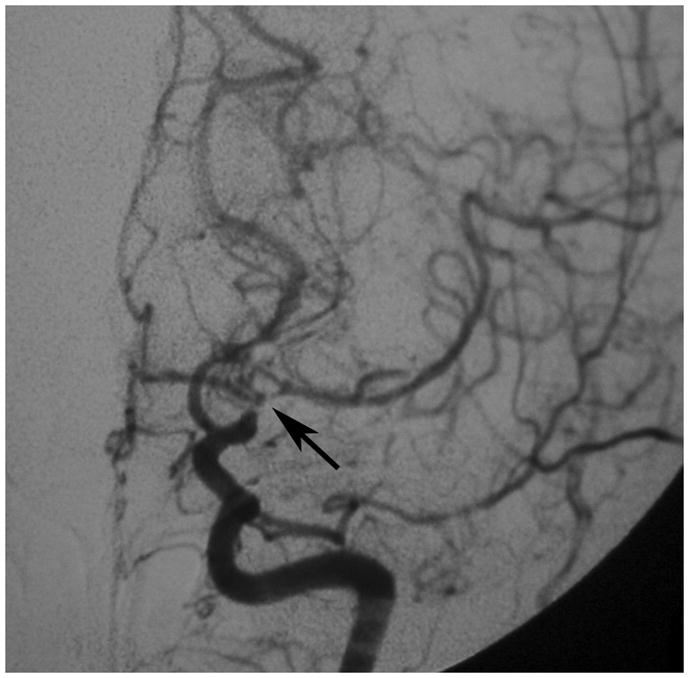
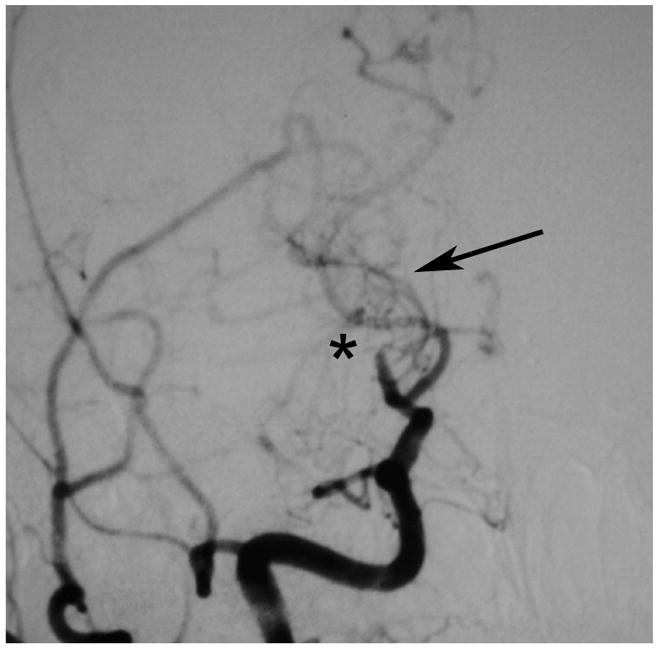
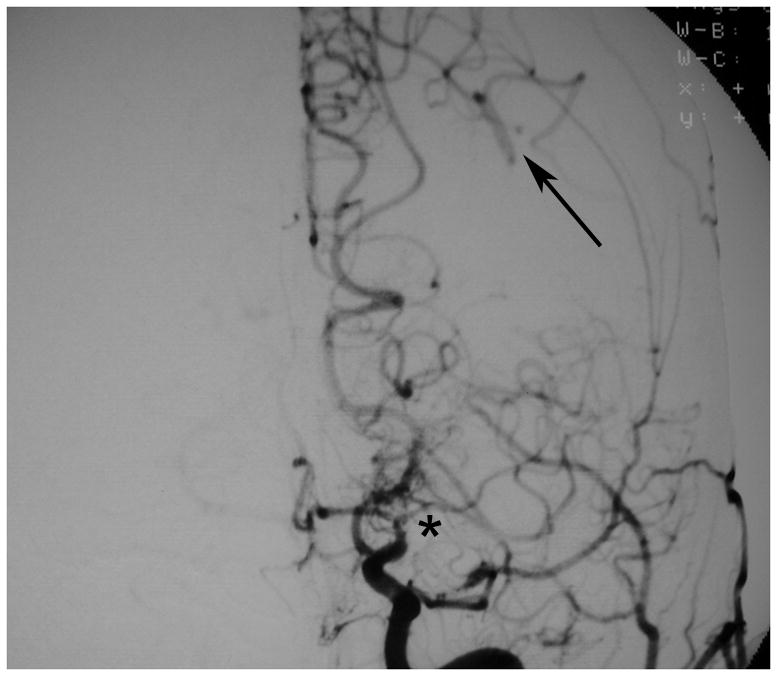
Legend: A 45 year old female patient initially presented with ischemic stroke. Cerebral angiography demonstrated proximal stenosis of the middle cerebral arteries bilaterally (A and V). Figure 1A, AP projection after right common carotid artery injection, demonstrates severe narrowing of the proximal middle cerebral artery (black arrow). Figure 1B, AP projection after left common carotid artery injection, shows similar changes of the distal internal and proximal middle and anterior cerebral arteries (black arrow). She was treated with steroids for presumed vasculitis. On repeat angiography approximately 6 months later, the stenotic lesions markedly progressed with the interval development of moyamoya collaterals (C and D). Figure 1C, AP projection after right common carotid artery injection, shows occlusion of the distal internal carotid artery (asterisk) with moyamoya collaterals (black arrow). Figure 1D, AP projection after left common carotid artery injection, demonstrates similar changes (middle cerebral artery occlusion with moyamoya collateral formation, asterisk) with pial collaterals from the posterior artery branches to the middle cerebral artery territory (black arrow).
The remaining four patients with bilateral disease had unilateral encephalodural synangiosis (EDAS) (Patients 1 and 6) or STA-MCA bypass (Patients 2 and 3) on the symptomatic hemisphere as their initial therapy. Three of these four patients suffered subsequent strokes in their medically-treated, contralateral hemisphere. One of the two patients treated with a STA-MCA bypass procedure (Patient 2) remained stroke-free for 86 months. Unfortunately, he died of a myocardial infarction and resultant left ventricular aneurysm. The remaining patient (Patient 3) had a contralateral stroke 6 months after successful STA-MCA bypass. She underwent an EDAS procedure on that contralateral hemisphere and has remained symptom-free for 14 months.
The two remaining patients underwent unilateral EDAS procedures after initial presentation and received aspirin post operatively. One was administered warfarin as well. Both had subsequent ischemic strokes, one ipsilateral and contralateral (Patient 1) and one contralateral to the treated hemisphere (Patient 6). One stroke occurred ipsilateral to the EDAS at 107 months (Patient 1). This patient went on to have a contralateral stroke in the right cerebral middle cerebral artery territory 4 months later. The patient underwent an EDAS procedure of the right hemisphere and died as a complication of the surgery. Blood pressure was closely monitored and maintained during and after the procedure. Despite this, the patient suffered a massive infarction of the entire vascular territory of the right internal carotid artery (right anterior and middle cerebral arteries, as well as the left anterior cerebral artery that was supplied across the anterior communicating artery). Patient 6 had an ischemic stroke in the untreated contralateral hemisphere 33 months after presentation. She underwent an EDAS procedure at that point and has remained symptom free for 10 months.
Overall EDAS was performed in six hemispheres of four patients with bilateral steno-occlusive disease of the basal arteries. One death occurred as a direct result of the surgery (Patient 1, discussed above). Two recurrent ischemic strokes occurred in the five remaining EDAS-treated hemispheres (2 months, Patient 5, and 107 months after the procedure, Patient 1).
Discussion
These data demonstrate important similarities in both the clinical phenotype and outcome between recent descriptions of North American and European adults with moyamoya phenomenon and patients who have idiopathic occlusion of the basal arteries but fail to develop moyamoya collateral vessels. This finding supports the hypothesis that the ability to form moyamoya collaterals may be variable, despite a similar occlusive vasculopathy or vasculopathies.
The baseline clinical characteristics of the patients in the present study are very similar to recent series of North American and European patients with moyamoya.7–10 A large majority of the patients in the present series were female and between the ages of 30 and 50. The racial demographics corresponded to those of our region. Similar to North American and European moyamoya case series, almost all of our patients presented with ischemic symptoms. In the series from St. Louis, Missouri, reported by Hallemeier et al., 24 of 34 adult moyamoya presented with ischemic symptoms, and 25 were women with a mean age of 42 at presentation.7 In the series from Houston, Texas, reported by Chiu et al., 23 of 26 adult patients presented with ischemic symptoms, 25 of the 35 total patients were women (this study included all idiopathic moyamoya patients, including 7 children), and the mean age was 32.8 In the series from New York, reported by Starke et al., 42 of 43 adult patients presented with ischemic symptoms, and 28 were women with a mean age of 40 years.9 In the Essen, Germany, series of 21 European patients reported by Kraemer et al., all were of northern European ancestry, all presented with ischemic symptoms, and 17 were women with a mean age of 34 years.10 These ethnic and demographic features differ substantially from Asian series in several regards: children are affected much more frequently than adults; adults are more likely to present with hemorrhage than ischemia (50 – 60%); and there is only a slight female preponderance.2,11
Baseline stroke risk factors primarily included hypertension in most and tobacco use in some of the patients in the present series. Diabetes, family history of stroke, and coronary artery disease were not prominent features in their history. Less than 20% of our females used oral contraceptives, providing no evidence that this may represent a major risk factor for steno-occlusive disease of intracranial arteries. These frequencies of stroke risk factors are similar to the published series of North American and European moyamoya patients.7–10
Unilateral versus bilateral disease
The extent of stenosis, i.e. unilateral versus bilateral, had a striking effect on stroke recurrence and overall outcome. Like patients with moyamoya disease, bilateral disease conferred greater risk of stroke recurrence and grave prognosis than unilateral involvement.7 Three of the six surviving patients with bilateral involvement suffered a recurrent ischemic stroke (ipsilateral or contralateral) within two years of their initial event. Two of the remaining three had a recurrence after two years and the final patient later developed moyamoya collaterals.
In contrast, the patients with unilateral disease and without moyamoya vessels did not suffer any recurrent ipsilateral ischemic strokes. In the similar cohort of moyamoya patients with unilateral involvement, two of twelve (17%) experienced subsequent ipsilateral ischemic strokes.7 Becker and colleagues report the outcome of 8 symptomatic patients, age 32 – 56 years with isolated middle cerebral artery stenosis and no evidence of atherosclerotic disease. No further strokes occurred during a follow up of 39 – 82 months.12 Overall, both in moyamoya patients and in the present cohort, risk of recurrent stroke was highly related to the presence of bilateral disease while unilateral involvement, at least in these patients, generally conferred a good prognosis. We suggest that bilateral non-atherosclerotic occlusive disease of the basal arteries imparts a considerably worse prognosis, while unilateral disease is less likely to result in recurrent stroke.
The effect of surgical revascularization
The conclusions that may be drawn regarding the effects and role for surgical revascularization are extremely limited, owing to the small sample size, and the retrospective nature of the study. In addition, the patients were not investigated for hemodynamic impairment. Finally, owing to a lack of consensus regarding the utility of indirect or direct revascularization procedures in patients with non-atherosclerotic idiopathic occlusive disease without moyamoya collaterals, there was considerable variability in treatment (medical versus surgical).
EDAS was performed in six hemispheres among four patients who presented with bilateral stenosis. Of these, two patients died, one owing to complications of the procedure (Patient 1). This patient may not be typical. She was the only one with recurrent ipsilateral ischemic stroke after EDAS. In addition, these symptoms occurred 8 years after the procedure. Contralateral symptoms developed 4 months later. The other patient suffered a fatal stroke in the surgically-treated hemisphere 2 months after surgery. Poor outcomes after indirect revascularization procedures were reported by Komotar et al. in 12 patients with atherosclerotic intracranial arterial disease.13 Ten suffered new strokes or TIA after the procedure. All had evidence of hemodynamic impairment on pre-operative testing and five demonstrated worsening after the procedure. As discussed below, patients with either idiopathic or atherosclerotic basal arterial occlusive disease and no moyamoya collaterals may lack the necessary vessel proliferative signals that allow the indirect bypasses to form. Alternatively, as suggested by Komotar et al., this may also be related to the time-lag in the development of indirect anastamoses.13 Further investigation in this area is warranted.
The three patients treated with STA-MCA bypass had good neurological outcomes. However, it is not possible to draw any firm conclusions from this experience owing to the limited number of procedures performed. These data suggest that STA-MCA bypass may represent a feasible method of treatment for patients with bilateral steno-occlusion of the basal arteries without moyamoya collateral vessels. Success rates for extracranial-intracranial bypass procedures may be further improved by stratifying higher risk patients with physiologic testing for hemodynamic impairment (i.e. via positron emission tomography or transcranial Doppler imaging).14 Finally, the high rate of contralateral events suggests a potential benefit for performing bilateral revascularization procedures, either at the time of initial treatment or in a staged fashion.
Though not attempted in any of these patients, endovascular treatments including angioplasty and possibly stenting may be useful adjuncts to antiplatelet and surgical therapy, particularly in the setting of bilateral idiopathic basal arterial occlusive disease. A few case reports suggest that such treatment may be possible in select patients.14,15 The restenosis rates may be high, however. In a large prospective registry of patients treated with angioplasty and stenting for symptomatic intracranial stenosis, severe restenosis, often more severe than the initial lesion, was strongly associated with younger age (<55 years) and the supraclinoid carotid location.16,17 The role of gender was not reported. Many of these patients may have had a non-atherosclerotic vasculopathy. Further investigations are warranted here as well.
Relationship to moyamoya disease
The common phenotype at presentation and good correspondence of outcomes on medical therapy between the patients presented here and those of similar cohorts of North American and European patients with moyamoya phenomenon suggests that the underlying occlusive vasculopathy may be similar between these two groups, while the ability to form moyamoya collaterals may be variable.
There has been considerable confusion regarding the relationship of patients with isolated idiopathic occlusion of the basal arteries to patients who meet full criteria for moyamoya disease. Suzuki and Takaku had initially proposed six stages of moyamoya disease.18 Neither the first nor the last stage required the presence of moyamoya vessels, both being characterized by stenosis and occlusion, respectively, of the carotid fork and the proximal middle cerebral arteries. Later, however, the Research Committee on Spontaneous Occlusion of the Circle of Willis included moyamoya collateral vessels in their criteria for moyamoya disease.19 The relevance of unilateral versus bilateral involvement for definitive diagnosis of moyamoya disease has been discussed extensively elsewhere and further complicates the matter.
There are many potential factors that may play a role in the ability of a given patient to develop moyamoya collaterals in response to an occlusive vasculopathy.20 Age at onset of the vasculopathy may be important: children appear to commonly form moyamoya collaterals (given the Asian phenotype), young adults less frequently, and older adults even less. The pathologic nature of the occlusive vasculopathy is also likely to be important. For example, a non-atherosclerotic process may be more likely to lead to a proliferative response than atherosclerosis. The progression seen in the patient reported in this series while on corticosteroids may have been incidental, or related in some way to inflammation or the inhibition of inflammation. Genetic factors are almost certainly involved. An epidemiological survey of moyamoya disease in Japan in 2003 identified a family history in 12% of patients.21 An autosomal dominant pattern of inheritance was reported in 15 Japanese families, with a linkage analysis identifying chromosome 17q25.22 Finally, the degree of hemodynamic impairment may also play a role.
As mentioned above, the experience of Komotar, et al., regarding the poor performance of indirect anastamosis in patients with atherosclerotic intracranial occlusive disease and hemodynamic impairment raises interesting issues.13 Angiographic follow up was obtained in two of the patients in the present series after EDAS. One had no collateral formation (Patient 1) while the other did (Patient 6). It may be that indirect anastamoses require vascular proliferative signals in order to be effective, and that these signals are related to moyamoya collateral formation as well. In other words, patients with basal arterial occlusive disease can develop moyamoya collaterals if there are appropriate proliferative signals, and these may be the same signals required for the formation of indirect anastamoses. The data regarding the role of proliferative factors in moyamoya are incomplete, however.23 Several growth factors have been associated with moyamoya formation, including hepatocyte growth factor, platelet-derived growth factor, and hypoxia inducible factor 1a.24–27
Moyamoya vessels provide a source of collateral flow, bypassing stenotic or occluded segments of the internal carotid and middle cerebral arteries. In nearly all cases, the bulk of the cortical flow is provided by pial collaterals from the posterior circulation. The clinical importance of moyamoya vessels is unclear. Given the similar outcomes in the present series, albeit very small, compared to the prior series of North American moyamoya, these collaterals may not provide adequate collateral flow. In addition, it appears that long-standing flow through these small vessels may predispose to hemorrhage. Hemodynamic studies of Asian adults presenting with hemorrhage tend to show an absence of severe cortical hemodynamic impairment, as compared with children presenting with ischemic symptoms.28,29
We recognize several limitations to the present study. This study was retrospective with variable patient characteristics and treatments, precluding any definitive statement on the benefits of particular treatments. The etiology of intracranial stenosis in the patients of the present series is unknown. A reversible vasoconstriction syndrome, such as Call-Fleming, is unlikely but could have been present in some of these patients. This disorder tends to involve multiple cortical vessels in addition to the basal arteries.4 Though we excluded patients with obvious signs of atherosclerotic disease or dissection, only histopathological confirmation could exclude such etiologies definitively. Despite the results above, the relationship between these patients and the moyamoya phenomenon remains speculative in all but the one patient who later developed the characteristic moyamoya collaterals. Further longitudinal study of the other patients, including follow-up angiography, would permit more exploration of this hypothesis. The mean follow up in the present series was slightly more than two years. We performed no systematic analysis of hemodynamic status in this cohort. As mentioned above, the presence of severe hemodynamic impairment might be a factor in the development of moyamoya collaterals. The poor outcome on medical therapy in patients with bilateral occlusive disease suggests that many likely had impaired hemodynamics, however.
Summary
The clinical characteristics, anatomic features, and outcome of North American adult patients with idiopathic occlusive disease of the basal arteries are similar to those reported in large recent case series of North American and European patients with moyamoya phenomenon. This, as well as the progression to moyamoya in one patient, suggests a common etiology for the arterial occlusive process with a variable ability to form moyamoya collateral vessels. Patients with unilateral involvement appear to have a good outcome with medical therapy. Patients with bilateral disease at presentation have a high likelihood of recurrent ischemic stroke.
Acknowledgments
Acknowledgments and Funding
Sources of funding for the research: NINDS NS051631
Footnotes
Conflicts of Interest/Financial Disclosure: None
References
- 1.Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000;20:S61–4. doi: 10.1046/j.1440-1789.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki J, Kodama N. Moyamoya disease - a review. Stroke. 1983;14:104–109. doi: 10.1161/01.str.14.1.104. [DOI] [PubMed] [Google Scholar]
- 3.Peerless SJ. Risk factors of Moyamoya disease in Canada and the USA. Clin Neurol Neurosurg. 1997;99:S45–8. doi: 10.1016/s0303-8467(97)00039-5. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–40. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 6.Meschia JF, Brott TG, Chukwudelunzu FE, Hardy J, Brown RD, Jr, Meissner I, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 7.Hallemeier CL, Rich KM, Grubb RL, Jr, Chicoine MR, Moran CJ, Cross DT, 3rd, et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke. 2006;37:1490–6. doi: 10.1161/01.STR.0000221787.70503.ca. [DOI] [PubMed] [Google Scholar]
- 8.Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347–51. doi: 10.1161/01.str.29.7.1347. [DOI] [PubMed] [Google Scholar]
- 9.Starke RM, Komotar RJ, Hickman ZL, Paz YE, Pugliese AG, Otten ML, et al. Clinical features, surgical treatment, and long-term outcome in adult patients with moyamoya disease. J Neurosurg. 2009;111(5):936–42. doi: 10.3171/2009.3.JNS08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer M, Heienbrok W, Berlit P. Moyomoya disease in Europeans. Stroke. 2008;39:3193–3200. doi: 10.1161/STROKEAHA.107.513408. [DOI] [PubMed] [Google Scholar]
- 11.Ikezaki K, Han DH, Kawano T, Kinukawa N, Fukui M. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke. 1997;28:2513–2517. doi: 10.1161/01.str.28.12.2513. [DOI] [PubMed] [Google Scholar]
- 12.Becker VU, Eckert B, Thie A. Isolated symptomatic stenosis of the middle cerebral artery in younger adults. A clinical and ultrasonic follow-up study of eight patients. Eur Neurol. 1996;36:65–70. doi: 10.1159/000117209. [DOI] [PubMed] [Google Scholar]
- 13.Komotar RJ, Starke RM, Otten ML, Merkow MB, Garrett MC, Marshall RS, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. J Neurosurg. 2009;110:896–904. doi: 10.1016/j.clineuro.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Kornblihtt LI, Cocorullo S, Miranda C, Lylyk P, Heller PG, Molinas FC. Moyamoya syndrome in an adolescent with essential thrombocythemia: successful intracranial carotid stent placement. Stroke. 2005;36:E71–3. doi: 10.1161/01.STR.0000174193.89864.55. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez GJ, Kirmani JF, Ezzeddine MA, Qureshi AI. Primary percutaneous transluminal angioplasty for early moyamoya disease. J Neuroimaging. 2007;17:48–53. doi: 10.1111/j.1552-6569.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 16.Albuquerque FC, Levy EI, Turk AS, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23–7. doi: 10.1227/01.NEU.0000335067.53190.A2. [DOI] [PubMed] [Google Scholar]
- 17.Turk AS, Levy EI, Albuquerque FC, Pride GL, Jr, Woo H, Welch BG, et al. Influence of patient age and stenosis location on wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008;29(1):23–7. doi: 10.3174/ajnr.A0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–99. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 19.Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99:S238–40. [PubMed] [Google Scholar]
- 20.Choi HY, Lee JE, Jung YH, Cho HJ, Kim DJ, Heo JH. Progression of isolated middle cerebral artery stenosis into moyamoya disease. Neurology. 2007;68:954. doi: 10.1212/01.wnl.0000244412.18039.03. [DOI] [PubMed] [Google Scholar]
- 21.Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42–47. doi: 10.1161/STROKEAHA.107.490714. [DOI] [PubMed] [Google Scholar]
- 22.Mineharu Y, Liu W, Inoue K, Matsuura N, Inoue S, Takenaka K, et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 2008;70:2357–2363. doi: 10.1212/01.wnl.0000291012.49986.f9. [DOI] [PubMed] [Google Scholar]
- 23.Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: a summary. Neurosurg Focus. 2009;26(4):E11. doi: 10.3171/2009.1.FOCUS08310. [DOI] [PubMed] [Google Scholar]
- 24.Hojo M, Hoshimaru M, Miyamoto S, Taki W, Nagata I, Asahi M, et al. Role of transforming growth factor-beta1 in the pathogenesis of moyamoya disease. J Neurosurg. 1998;89:623–629. doi: 10.3171/jns.1998.89.4.0623. [DOI] [PubMed] [Google Scholar]
- 25.Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo) 2007;47:1–4. doi: 10.2176/nmc.47.1. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Aoyagi M, Tajima S, Wachi H, Fukai N, Matsushima Y, et al. Increase in elastin gene expression and protein synthesis in arterial smooth muscle cells derived from patients with Moyamoya disease. Stroke. 1997;28:1733–1738. doi: 10.1161/01.str.28.9.1733. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Houkin K, Takahashi A, Abe H. Angiogenic factors in moyamoya disease. Stroke. 1996;27:2160–2165. doi: 10.1161/01.str.27.12.2160. [DOI] [PubMed] [Google Scholar]
- 28.Taki W, Yonekawa Y, Kobayashi A, Ishikawa M, Kikuchi H, Nishizawa S, et al. Cerebral circulation and metabolism in adults’ moyamoya disease--PET study. Acta Neurochir (Wien) 1989;100(3–4):150–4. doi: 10.1007/BF01403603. [DOI] [PubMed] [Google Scholar]
- 29.Ikezaki K, Matsushima T, Kuwabara Y, Suzuki SO, Nomura T, Fukui M. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg. 1994;81(6):843–50. doi: 10.3171/jns.1994.81.6.0843. [DOI] [PubMed] [Google Scholar]


