Abstract
Ovines are a common animal model for preclinical evaluation of cardiovascular devices including heart valves, endovascular grafts, and ventricular assist devices. Biocompatibility is essential to the success of these devices; however, tools to assess biocompatibility in ovines are limited. To address this need, antibodies that bind to activated human and bovine platelets and annexin V protein were evaluated for potential cross-reactivity to activated ovine platelets. These candidate markers were incubated with stimulated and quiescent ovine whole blood, and binding to platelets was quantified by flow cytometry. Several antihuman CD62P antibodies including one polyclonal antibody, three monoclonal antibodies, and annexin V selectively bound to activated ovine platelets. An assay to quantify platelet microaggregates was also developed. The availability of assays to quantify ovine platelet activation can increase the quality of biocompatibility data obtainable during preclinical development of artificial organs in the ovine model, potentially aiding in the evaluation of design refinements to enhance device biocompatibility.
Keywords: Biocompatibility, Flow cytometry, Ovine platelets, Cardiovascular devices, Platelet activation
Ovines are a common animal model for preclinical testing of blood-contacting cardiovascular devices including mechanical heart valves, endovascular grafts, and ventricular assist devices (VADs) (1–4). A critical aspect in the design of these devices is the evaluation of their blood biocompatibility. Yet, the biocompatibility data that can be obtained in ovine studies is limited due to a paucity of available assays for evaluating circulating blood elements during the implant period. In particular, there has only been one report on the use of flow cytometry to evaluate ovine platelet activation in platelet-rich plasma, and there have been no reports in the literature using such techniques to assess temporal platelet activation in ovines implanted with cardiovascular devices (5).
Flow cytometry permits surface expression of platelet activation-dependent epitopes to be quantified, providing insight on circulating platelets not obtainable with platelet aggregometry and plasma assays for β-thromboglobulin and platelet factor 4 (6). Circulating activated platelets have been measured in patients with stents, mechanical heart valves, and VADs as well as in patients suffering from acute myocardial infarction and ischemic stroke (7–13). The presence of circulating activated platelets has been suggested as a marker for increased risk of thrombotic complications (10). Previously, we have described several flow cytometric assays to quantify circulating activated bovine platelets and platelet microaggregates (14). These assays have been applied to assess circulating activated platelets in calves implanted with rotary VADs (14,15). The results demonstrate the ongoing presence of circulating activated platelets after the effects of surgery (without extracorporeal circulation) have diminished, indicating a level of platelet activation that can be attributed to the VAD (15). The application of similar assays in ovines can yield a greater understanding of device effects on ovine platelet activation during preclinical testing when design changes may be made at a time of reduced regulatory and economic burden.
The objective of this work was to develop and characterize flow cytometric platelet assays that could ultimately be applied in the evaluation of cardiovascular devices undergoing in vivo testing in ovines. Specifically, we assessed antibodies and annexin V protein that recognize activated human and bovine platelets in an effort to identify cross-reactive markers that could selectively bind to activated ovine platelets. The identification and characterization of these markers using in vitro stimulation of ovine blood with several platelet agonists is reported.
MATERIALS AND METHODS
Blood collection
Eleven Dorset-cross and Cheviot sheep (three adults and eight juveniles) were used in this study. Whole blood was collected from healthy ovines by jugular venipuncture using an 18-gauge 1.5-in needle with syringe, discarding the first 3 mL. Blood (2.7 mL) was immediately added to monovette tubes containing 0.3 mL of 0.106-M trisodium citrate (Sarstedt, Newton, NC, USA). All experiments were performed at room temperature, and blood was added to test tubes within 2 h of collection. This study was approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and National Institutes of Health (NIH) guidelines for the care and the use of laboratory animals were observed.
Evaluation of monoclonal antibodies to detect ovine platelet activation
Blood (5 μL) was transferred from monovette tubes into 12 × 75 mm polystyrene tubes with each of the monoclonal antibodies listed in Table 1, 5 μL of 20-mM glycine-proline-arginine-proline (GPRP for inhibition of fibrin polymerization, Anaspec, San Jose, CA, USA) in phosphate-buffered saline (PBS; BD Biosciences, San Jose, CA, USA), and 5 μL of goat antimouse IgG-Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) that was twice the concentration of the primary antibody. Tyrode’s buffer (Electron Microscopy Services, Hatfield, PA, USA) with 1% bovine serum albumin (BSA) was added to each tube for a total volume of 50 μL for control samples. A range of concentrations or volumes was evaluated for each antibody. Antibodies that cross-reacted to ovine platelets were optimized to obtain a concentration that efficiently labeled platelets. Optimal concentrations are listed in Table 1. Antibodies that did not cross-react with ovine platelets are listed in Table 1 with the manufacturer’s suggested concentration or antibody volume.
TABLE 1. List of antibodies evaluated.
| Antibody | Antigen Target | Isotype | Concentration | Volume | Source |
|---|---|---|---|---|---|
| Monoclonal antibodies | |||||
| PAC-1 fluorescein | Activated human GPIIbIIIa | IgM | 25.0 μg/mL | 20 μL | BD Biosciences (San Jose, CA, USA) |
| Antihuman CD109-PE | Human platelet actuation factor | IgG1 | 20.0 μg/mL | 10 μL | Chemicon (Temecula, CA, USA) |
| Antihuman CD62P-PE clone # AK-4 | Human CD62P | IgG1 | * | 20 μL | BD Biosciences |
| Antihuman CD63-fluorescein | Human CD63 | IgG1 | * | 20 μL | BD Biosciences |
| BAQ 125A | Bovine activated platelet epitope | IgG1 | 15.0 μg/mL | 5 μL | Washington State University Monoclonal Antibody Center (WSUMAC; Pullman, WA, USA) |
| BAQ 56A | Bovine activated platelet epitope | IgG1 | 15.0 μg/mL | 5 μL | WSUMAC |
| GC5A | Bovine activated platelet epitope | IgG1 | 15.0 μg/mL | 5 μL | WSUMAC |
| Antibovine CD63 | Bovine CD63 | IgG1 | * | 10 μL | Serotec (Raleigh, NC, USA) |
| CAPP2A | Ovine CD41/61 | IgG1 | 7.5 μg/mL | 5 μL | Veterinary Medical Research & Development (VMRD; Pullman, WA, USA) |
| GB20A | 85 kD protein on bovine platelets | IgG1 | 7.5 μg/mL | 5 μL | VMRD |
| GB84A | Bovine CD42d | IgG1 | 7.5 μg/mL | 5 μL | WSUMAC |
| NPL44-10 | Human CD62P | IgG1 | 25.0 μg/mL | 5 μL | Takara Bio (Shiga, Japan) |
| MCA2419 | Human CD62P | IgG1 | 25.0 μg/mL | 5 μL | Serotec |
| MCA2420 | Human CD62P | IgG1 | 25.0 μg/mL | 5 μL | Serotec |
| 6F3 | Ovine CD62P | IgG1 | 25.0 μg/mL | 5 μL | Harvard University (Cambridge, MA, USA) |
| Isotype control antibodies | |||||
| IgM-fluorescein | IgM isotype control | IgM | 25.0 μg/mL | 20 μL | Southern Biotech (Birmingham, AL, USA) |
| Coli S69 | IgG1 isotype control | IgG1 | Matched for each IgG1 Ab experiment | — | WSUMAC |
| Rabbit IgG | Rabbit IgG isotype control | IgG | 30.0 μg/mL | 5 μL | Southern Biotech |
| Polyclonal antibody | |||||
| Rabbit polyclonal antihuman CD62P | Human CD62P | N/A | 30.0 μg/mL | 5 μL | BD Biosciences |
Concentrations not provided by manufacturer.
N/A, not applicable.
Activated samples were prepared as mentioned earlier, but with 5 μL less of Tyrode’s buffer and with 5 μL of agonist added for a final concentration of either 20-μM adenosine diphosphate (ADP, EMD Biosciences, San Diego, CA, USA), 68-μM thrombin receptor activating peptide-6 (TRAP, Bachem Biosciences, King of Prussia, PA, USA), or 10-μM platelet activating factor (PAF, EMD Biosciences). These agonists were prepared in PBS. Quiescent control and activated samples were incubated for 20 min in the dark with occasional gentle mixing. After incubation, the samples were washed with 1 mL of Tyrode’s buffer containing 1% BSA and 0.106-M sodium citrate and centrifuged at 132 × g for 10 min. The supernatant was then removed and the pellet was resuspended.
CAPP2A is an antibody that recognizes GpIIbIIIa (CD41/61), an antigen on the surface of resting ovine platelets (16,17). N-hydroxysulfosuccinimide-LC-LC-biotin (LC refers to a hydrocarbon chain extender to reduce steric hindrance; Pierce, Rock-ford, IL, USA) was added to CAPP2A in 20-molar excess to produce CAPP2A-biotin for use as a platelet marker. CAPP2A-biotin (5 μL at 7.5 μg/mL) and streptavidin-phycoerythrin (PE) (5 μL at 73 μg/mL, BD Biosciences) were then added and the samples were incubated and washed as before. After resuspension of the pellet, the samples were fixed with 500 μL of 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in PBS. Flow cytometric analysis occurred within approximately 2 h of fixation.
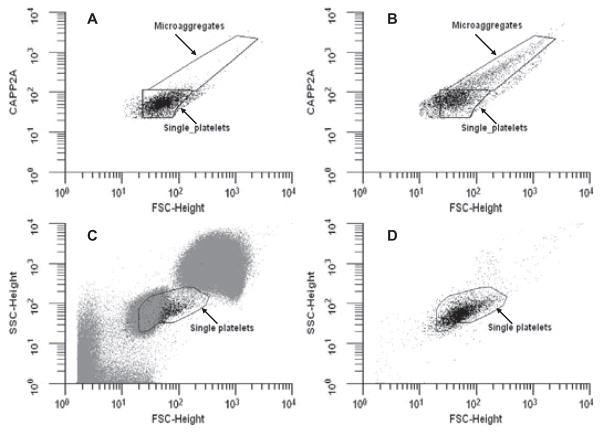
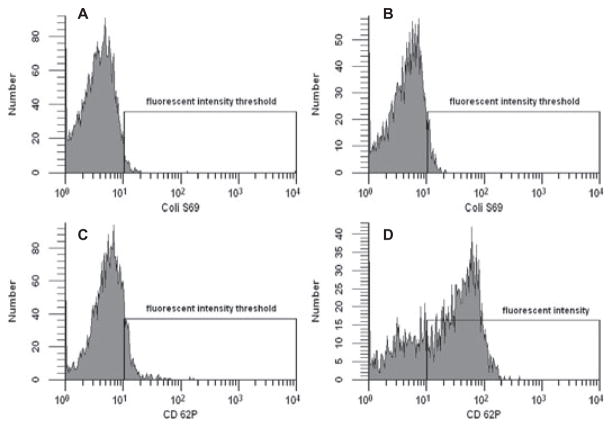
Single-platelet scattering events (3500 total) were identified using CAPP2A related PE fluorescence and forward scatter (FSC) from each sample (Fig. 1A) and were assessed for fluorescence at a second wavelength corresponding to the binding of antibodies from Table 1, using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). As shown in Fig. 2A, a standard flow cytometric technique was employed to define activated platelets—a fluorescence intensity threshold mark was set so that 2.0 ± 0.2% of the single platelet events had fluorescence intensities falling above the mark due to binding of the isotype control antibody. Fluorescence associated with each antibody for control and activated samples was compared with respect to this threshold mark (Fig. 2B–D) and was reported as a percentage of platelets positive for binding the antibody of interest. Coli S69A (Washington State University Monoclonal Antibody Center, Pullman, WA, USA) was used as the isotype control for IgG1 antibodies, and goat antimouse IgM-fluorescein (Southern Biotech, Birmingham, AL, USA) served as the isotype control for PAC-1 fluorescein. The percentage of platelets positive for Coli S69 was 2.1% for control (Fig. 2A) and 5.7% for PAF-stimulated blood (Fig. 2B). The percentage of platelets positive for CD62P as detected by MCA2419 was 11.6% for control (Fig. 2C) and 70.7% for the PAF-stimulated sample (Fig. 2D). Because intermittent clotting of ovine blood was noted during several of the initial antibody binding experiments, 20-mM GPRP was added to the samples to prevent fibrin polymerization. Evaluated antibodies in Table 1 that were conjugated to PE were prepared as above substituting streptavidin-PE with streptavidin-fluorescein (5 μL at 100 μg/mL; [BD Biosciences]). Samples evaluating the CAPP2A, GB84A, and GB20A antibodies were prepared without the addition of a platelet marker, eliminating the second incubation and wash steps. In these experiments, 5000 total platelet scattering events were collected by FSC and side scatter (SSC) in the single platelet region shown in Fig. 1C,D.
FIG. 1.
(A) CAPP2A binding versus FSC for individual scattering events is displayed identifying ovine platelets for an unstimulated sample. (B) Scatter plot of ovine platelets gating with CAPP2A fluorescence and FSC for a 10-μM PAF-stimulated sample. Microaggregates bind more CAPP2A and have a higher FSC than single platelets. (C) FSC versus SSC plot for ovine blood cells. The region labeled “single platelets” is where platelets typically reside on such a plot. (D) FSC versus SSC plot of ovine platelets identified with CAPP2A. There are a number of cells in the single platelet region of panel C that are not in the same region in panel D, suggesting that these cells are not positive for CAPP2A and should not be considered platelets.
FIG. 2.
The y-axes represent the number of platelets having a fluorescence intensity given on the x-axis (arbitrary units) due to fluorescent antibody binding. The fluorescence intensity of platelets bound with isotype control antibody (Coli S69A) varies little between quiescent control (A) and 10-μM PAF-stimulated blood (B). The fluorescence intensity with monoclonal antihuman CD62P antibody (MCA2419) binding for unstimulated (C) and 10-μM PAF-stimulated ovine platelets (D) shows a marked increase with stimulation.
Measurement of ovine platelet microaggregates
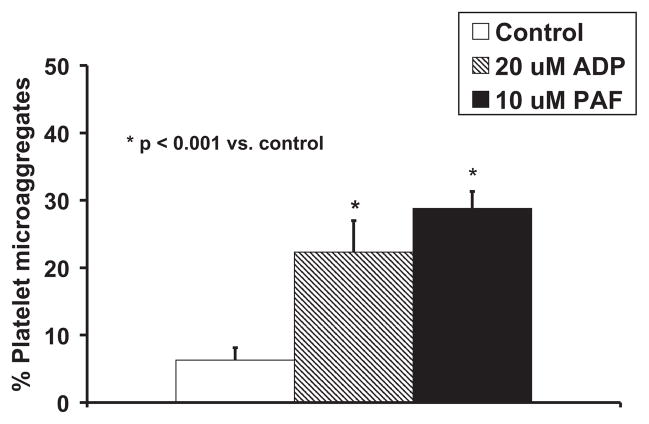
The percentage of platelet-containing microaggregates for samples evaluating NPL44-10, MCA2419, and MCA2420 antibodies was measured. Single platelets were identified with CAPP2A and FSC in control samples. Microaggregates were classified as scattering events that bound additional CAPP2A and had higher FSC than single platelets (compare Fig. 1A,B). The percentage of microaggregates was defined as the percent of microaggregate events relative to the combined number of single platelet and microaggregate events. Microaggregate percents were compared for quiescent control and agonist-stimulated samples. In Fig. 1A (quiescent control sample), the percentage of microaggregates is 2.7%, and in Fig. 1B (PAF-stimulated sample), the micro-aggregate percentage is 39%.
Evaluation of polyclonal antihuman CD62P Ab to detect ovine platelet activation
Blood (5 μL) was incubated for 20 min with 20 μL of Tyrode’s buffer with 1% BSA, 5 μL of 20-mM GPRP, 5 μL of polyclonal rabbit antihuman CD62P antibody (30 μg/mL, BD Biosciences) or 5 μL of rabbit IgG (30 μg/mL, Southern Biotech), 5 μL of goat antirabbit IgG1-A488 (30 μg/mL, Invitrogen), 5 μL of GB20A (7.5 μg/mL), and 5 μL of goat antimouse-IgG1-PE (30 μg/mL, Invitrogen) for quiescent control samples. GB20A binds to an 85-kD protein on ruminant platelets and was used as a platelet marker (17). Activated samples were prepared similarly to the quiescent control samples using 15 μL of Tyrode’s buffer with 1% BSA and 5 μL of agonist for a final concentration of 20-μM ADP or 10-μM PAF.
The samples were incubated and washed as mentioned earlier. The supernatant was removed; the pellet was resuspended, and the sample was fixed with 500 μL of 1% paraformaldehyde. Flow cytometric data acquisition was performed within approximately 2 h of fixation. Five thousand single platelets identified by FSC and GB20A binding were collected for flow cytometric analysis as above using rabbit IgG as the isotype control antibody.
Evaluation of annexin V-fluorescein
Blood (diluted 1:10 in PBS, 20 μL) was added to 265 μL of annexin V binding buffer (BD Biosciences) for quiescent control samples with 5 μL of annexin V-fluorescein (BD Biosciences), 5 μL of 7.5-μg/mL GB20A, and 5 μL of 30 μg/mL of goat antimouse IgG-PE for 20 min. Activated samples were prepared as previously mentioned using 235 μL of annexin V binding buffer along with 30 μL of one of the following agonists for a final concentration of 20-μM ADP, 10-μM PAF, 5-μM ionomycin (EMD Biosciences), or 5-μM calcium ionophore A23187 (EMD Biosciences). Ionomycin and A23187 were initially dissolved in dimethyl sulfoxide and were then diluted in PBS to the desired concentration. Flow cytometric analysis occurred within approximately 1 h of blood addition. Five thousand platelets positive for GB20A were assessed for annexin V binding. The fluorescence intensity threshold mark was set to include the upper 2% of the fluorescence from the annexin V quiescent control samples.
Statistical analysis
All data are presented as mean ± SD. Statistical analyses were performed using SPSS 12.0.1 (SPSS, Chicago, IL, USA). Comparison of means for quiescent and activated samples was calculated using one-way repeated measures analysis of variance with the F statistic and Bonferroni post hoc test. Correlations between NPL44-10, MCA2419, or MCA2420 binding and microaggregate percentage were performed using the Pearson correlation. Significance was considered to exist for P < 0.05.
RESULTS
Evaluation of antibodies to detect ovine platelet activation
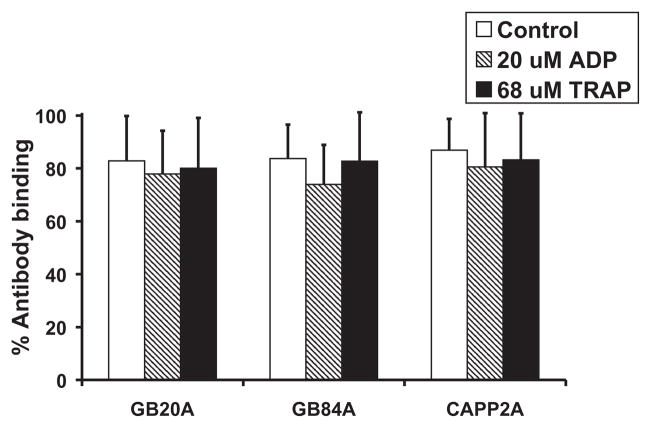
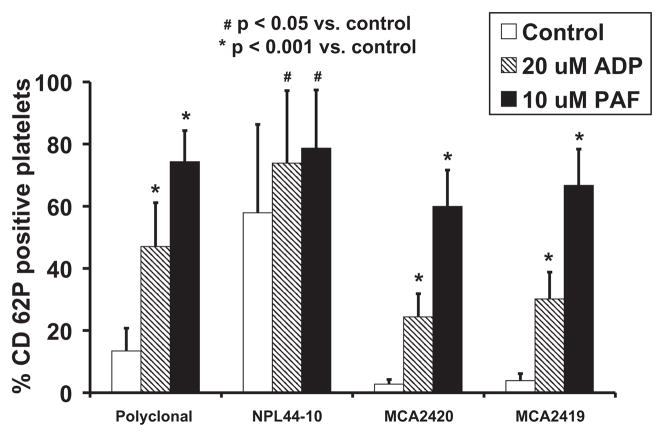
Monoclonal antibodies against human CD62P (clone# AK-4), human CD63, human CD109, human activated GPIIbIIIa (PAC-1), and antibovine CD 63 all demonstrated very low binding to quiescent control samples and did not demonstrate a significant increase in binding to ovine platelets stimulated with 20-μM ADP. Monoclonal antibodies BAQ125A, BAQ56A, GC5A, and 6F3 bound strongly to resting ovine platelets (>58%) but did not exhibit a significant increase in binding upon activation with 20-μM ADP. These results are summarized in Table 2. CAPP2A, GB84A, and GB20A all bound at least 80% of resting platelets, without a resultant increase in binding upon activation with 20-μM ADP or 68-μM TRAP as shown in Fig. 3. The polyclonal anti-human CD62P antibody and monoclonal antibodies NPL44-10, MCA2419, and MCA2420 demonstrated statistically significant increases in binding to ovine platelets stimulated by 20-μM ADP or 10-μM PAF when compared to quiescent platelets as shown in Fig. 4. The antibody concentration or volume corresponding to antibody binding results shown in Table 2 and Figs. 3 and 4 are listed in Table 1.
TABLE 2. Antibody binding to ovine platelets.
| Antihuman platelet monoclonal antibodies (n = 3)
| |||||
|---|---|---|---|---|---|
| Human CD63 | Human CD62P (clone # AK-4) | Human CD109 | PAC-1 FITC | ||
| Quiescent | 0.2 ± 0.2 | 3.3 ± 1.5 | 0.4 ± 0.5 | 1.0 ± 0.2 | |
| 20-μM ADP | 0.3 ± 0.4 | 3.5 ± 1.6 | 1.0 ± 1 | 1.5 ± 1.0 | |
|
| |||||
| Antibovine and ovine platelet monoclonal antibodies (n = 3)
| |||||
| Bovine CD63 | BAQ125A | BAQ56A | GC5A | 6F3 | |
|
| |||||
| Quiescent | 2.0 ± 0 | 97 ± 2 | 58.0 ± 7.0 | 99 ± 1 | 97 ± 2 |
| 20-μM ADP | 2.1 ± 0.8 | 95 ± 3 | 65.0 ± 0.3 | 95 ± 7 | 94 ± 4 |
FIG. 3.
GB20A, GB84A, and CAPP2A binding to unstimulated, 20-μM ADP, and 68-μM TRAP-stimulated ovine platelets (n = 7).
FIG. 4.
The percentage of unstimulated and stimulated ovine platelets positive for p-selectin as indicated by the binding of polyclonal and monoclonal antihuman CD62P antibodies. The percentage of CD62P-positive platelets was significantly increased (P < 0.001) in 10-μM PAF and 20-μM ADP samples compared to unstimulated controls for the polyclonal CD62P antibody (n = 8), MCA2419 (n = 7), and MCA2420 (n = 7). The percentage of CD62P-positive platelets as indicated by NPL44-10 binding was also significantly increased (P < 0.05) for samples stimulated with 10-μM PAF (n = 8) and 20-μM ADP (n = 8).
Measurement of ovine platelet microaggregates
The percentage of platelet microaggregates increased significantly upon stimulation with 20-μM ADP or 10-μM PAF when compared to quiescent control samples as shown in Fig. 5. There also were significant correlations found between the percentage of platelet microaggregates detected and the binding of the respective monoclonal antihuman CD62P antibodies: MCA2419 (r = 0.919, P < 0.001) and MCA2420 (r = 0.908, P < 0.001). The correlation between binding of the NPL44-10 antibody on platelets with percentage of platelet microaggregates was not statistically significant: r = 0.304, P = 0.149.
FIG. 5.
The percentage of ovine platelet microaggregates increased significantly with stimulation using 20-μM ADP and 10-μM PAF (P < 0.001 vs. unstimulated). n = 8 for control and stimulated samples.
Annexin V-fluorescein binding
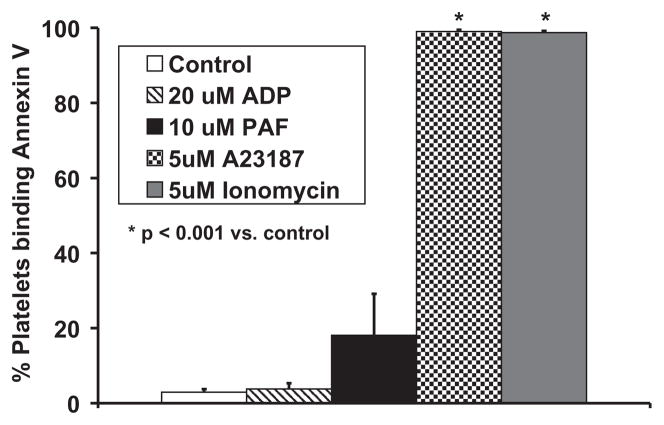
Annexin V exhibited statistically significant binding to ovine platelets stimulated by 5-μM calcium ionophore A23187 and 5-μM ionomycin compared to unstimulated platelets as seen in Fig. 6. Annexin V binding to 20-μM ADP and 10-μM PAF-stimulated samples was not statistically different from control samples.
FIG. 6.
Annexin V binding to ovine platelets was significantly increased for samples stimulated with 5-μM ionomycin (n = 9, P < 0.001) and 5-μM calcium ionophore A23187 (n = 9, P < 0. 001). Annexin V binding to 20-μM ADP (n = 7) or 10-μM PAF-stimulated platelets (n = 8) was not statistically different from binding to control samples.
DISCUSSION
The use of animals to evaluate cardiovascular devices is an essential part of device development as success in animals can provide some assurance that a device will be safe when used in humans. A drawback to the use of animals for biocompatibility testing is that animals and human platelets respond differently to external stimuli. Goodman reported that ovine and porcine platelets adhere and spread differently than human platelets on several common biomaterials (1). Pelagalli et al. reported differences in the adhesion of animal and human platelets to immobilized fibrinogen (3). These differences must be considered in interpreting the animal platelet response to artificial organs and in extrapolating this information to predict human platelet behavior. It seems likely, however, that general trends in platelet activation would hold true: design modifications that reduce platelet activation in animals would most likely cause a reduction in human platelet activation.
A major advantage in using animals for in vivo studies is that they are typically healthy before device implantation. This is rarely the case in humans receiving a device who are suffering from a variety of cardiac disorders and typically have ongoing inflammation and coagulopathies. The relative health of the animal makes it possible to elucidate device effects on platelet activation once the effects of the implantation surgery have dissipated. This has previously been demonstrated in comparing platelet activation between calves receiving a rotary VAD and those receiving a sham surgery (15). Platelet activation associated with the sham surgery could be discounted to show that there was ongoing platelet activation in the VAD-implanted calves. Because the calves were healthy before VAD implantation, ongoing platelet activation could be directly attributed to the device.
Flow cytometry provides potentially more sensitive information about device effects on platelets because it can provide some degree of temporal resolution of platelet activation trends in vivo, whereas assessment of platelet deposition on an implanted device and detection of end organ infarcts can only be adequately assessed during necropsy (14). In fact, flow cytometric assays to detect circulating activated platelets were able to discern trends in platelet activation between calves having uneventful VAD implantation periods and calves experiencing thrombotic obstruction of the VAD (15). Flow cytometric assays were also sensitive enough to detect significant differences in levels of circulating activated platelets for different blood-contacting surface coatings in VAD-implanted calves (18). Similar experiments in ovines using the assays described in this report could suggest materials or surface coatings that would reduce device-related platelet activation. Experiments evaluating the effects of fluid path, shear stress, and type of blood-contacting materials on platelet activation may help to elucidate underlying contributors to platelet activation observed with artificial organs, and may provide insight into potential design modifications that could enhance device biocompatibility before the device is tested in humans.
In bovines and humans, identifying platelet events during flow cytometric analysis can be carried out by simply gating the scattering events using FSC and SSC. However, ovine red blood cells are smaller than human red blood cells (4.5- vs. 8.0-μm diameter), while platelets are similar in size, making the discrimination of ovine platelets in whole blood by FSC and SSC alone a challenge (Fig. 1) (19,20). To reliably detect ovine platelets, a platelet specific marker is required. CAPP2A binds to CD41/61 on ovine platelets, and GB84A and GB20A bind to an 85-kD protein on the surface of bovine and ovine platelets (16,17). The antigen target for GB84A is CD42d, and this is also the presumed antigen target for GB20A, although no confirmation has been reported (personal communication, William C. Davis; 21). Each of these antibodies bound more than 80% of cells in the FSC versus SSC region that contain platelets in quiescent control samples. The percentage of cells that these antibodies bound decreased in ADP-stimulated samples. This is probably indicative of single platelets forming platelet microaggregates that, because of their increased size, no longer reside in the FSC versus SSC region typically occupied by single platelets. This further illustrates the need for a platelet marker to discriminate ovine single platelets and ovine platelet microaggregates from other cells. CAPP2A and GB20A were subsequently utilized to label platelets in experiments evaluating the binding of various activation-specific antibodies and annexin V protein.
The goal of our in vitro characterization experiments was to investigate the ability of candidate antibodies and annexin V to increase in binding to activated ovine platelets. The stimulation of platelets with agonists that would likely result in secondary generation of thrombin and other agonist release was an inherent feature of the study. To specifically avert fibrin polymerization while allowing thrombin generation, GPRP peptide was utilized. The use of GPRP to prevent fibrin polymerization caused by thrombin has become an accepted practice in the assessment of platelet function using flow cytometry in whole blood (22–24). TRAP, a peptide fragment of the tethered ligand receptor for thrombin, can directly activate platelets without the generation of a fibrin clot and is an alternative to using GPRP to inhibit the secondary effects of thrombin (6). However, in our hands, TRAP did not induce expression of activation-dependent epitopes on ovine platelets.
While it is of interest to allow thrombin generation following stimulation with agonists in these studies, it would not be desirable to have unanticipated thrombin generation occurring prior to agonist stimulation for assay characterization. Because fibrin formation was sporadically observed in some blood draws (these samples were not utilized), the question arises as to whether there might be artifactual activation due to the presence of thrombin in some samples that did not rise to the level necessary for fibrin formation. Our results suggest that such artifact does not appear to be relevant for the assays that are showing strong sensitivity to platelet activation and that thus may find further use in device evaluation. Specifically, if thrombin were variably present, then the unstimulated control samples for the MCA2419 and MCA2420 assays (Fig. 4) would not show low levels of activation and would have low variance. Of note, the values for these assays were not normalized to the control; rather, all values including control were normalized to isotype-control antibody binding. Activation due to consistently uncontrolled or variably uncontrolled thrombin would show up in the magnitude and variance of the unactivated control data, respectively. Furthermore, if the platelets were activated by thrombin, it is unlikely that they would be able to respond to the extent observed with agonist addition. In future use of these assays, it may be reasonable to draw the blood into a potent anti-platelet and antithrombin mixture and to have such inhibitors present in any incubation buffers if there is concern that background platelet activation is occurring. The presence of such inhibitors may preclude agonist stimulation studies, however.
P-selectin (CD62P) is located within the platelet alpha granule and mediates platelet-neutrophil adhesion. Upon activation, the alpha granule fuses with the cell membrane, expressing p-selectin on the cell surface (6,25). 6F3 was the only antibody in this study that was specifically raised against an ovine antigen (p-selectin); however, its very high binding on the resting platelet precluded its use as an effective marker for ovine platelet activation (26). The monoclonal antibody against human CD62P (clone # AK-4) showed no cross-reactivity to ovine platelets. The polyclonal antihuman CD62P antibody, NPL44-10, MCA2419, and MCA2420 were also raised against human p-selectin; however, these antibodies cross-reacted and selectively bound to activated ovine platelets. MCA2419 and MCA2420 exhibited the lowest quiescent control binding and produced the greatest fold increase in relative binding between control and activated samples. The higher control binding with the polyclonal CD62P antibody was not unexpected given that a number of antigens would be targeted. The higher background binding seen with NPL44-10 was less expected. In addition, NPL44-10 demonstrated much higher variation than the other cross-reactive CD62P antibodies. Because of this variation, it seems that NPL44-10 is not the ideal antibody for quantifying p-selectin expression on ovine platelets temporally. For the purposes of one-time in-vitro experiments, however, NPL44-10 may still be useful. The differences in cross-reactivity and affinity for the resting platelet between the monoclonal antihuman CD62P antibodies may be due to the different epitopes to which the different antibody clones bound.
Monoclonal antibodies BAQ56A, BAQ125A, and GC5A selectively bound to unknown epitopes on the surface of activated bovine platelets and were successfully applied to assess circulating activated platelets in calves implanted with VADs (14,15). In ovines, these antibodies demonstrated much higher binding to quiescent platelets than was observed with calves, and selective binding to activated ovine platelets was not observed. NPL44-10 and the anti-bovine CD63 antibody also selectively bound to activated bovine platelets (18). In ovines, NPL44-10 selectively bound to activated ovine platelets, but had high background binding, whereas the anti-CD63 antibody did not cross-react to a meaningful degree.
The percentage of platelet microaggregates increased upon stimulation with PAF and ADP. Formation of platelet-platelet and possibly platelet-leukocyte microaggregates can follow platelet activation. This phenomenon was noted in our study; a significant correlation was observed between ovine platelets expressing p-selectin (assessed with MCA2419 and MCA2420) and the formation of platelet microaggregates after stimulation with ADP and PAF. Quantification of platelet microaggregates provides an additional index with which to assess platelet activation and, at least in bovines, appears to be a marker of very high levels of in vivo platelet activation (15).
Annexin V binds to negatively charged phospholipids including phosphatidyl serine, which serve as a catalytic surface for coagulation reactions on platelets (27,28). Annexin V selectively bound to ovine platelets stimulated by the calcium ionophores A23187 and ionomycin. Calcium ionophores increase intracellular calcium, inducing the translocation of phosphatidyl serine to the platelet surface among other effects. Somewhat surprisingly though, annexin V did not selectively bind to ADP or PAF-stimulated platelets, suggesting that these agonists did not stimulate the translocation of phosphatidyl serine onto the ovine platelet surface, despite inducing p-selectin expression and microaggregate formation. Annexin V’s response to ADP-stimulated ovine platelets was more consistent with the response to ADP-stimulated human platelets than with ADP-stimulated bovine platelets where annexin V has been shown to bind preferentially (15,28).
CONCLUSIONS
This work summarizes our effort to develop flow cytometric assays for assessment of platelet activation in ovines. Platelets could not be adequately identified by FSC and SSC alone; therefore, a platelet marker was necessary. CAPP2A, GB20A, and GB84A bound to platelets regardless of activation state and could be used for platelet labeling. Several proteins that bound to activated human and bovine platelets cross-reacted and selectively bound to activated ovine platelets with statistical significance. These proteins included NPL44-10, MCA2419, MCA2420, annexin V, and a polyclonal antihuman CD62P antibody, and their binding was indicative of surface expression of p-selectin and a procoagulant platelet surface. An assay to detect microaggregates was also developed, and the percentage of aggregates was shown to increase upon stimulation. The developed flow cytometric assays to quantify activated platelets and platelet microaggregates provide additional tools for improving the performance and safety of blood-contacting devices in the ovine model of preclinical testing.
Acknowledgments
The authors would like to thank the entire PediaFlow consortium for their technical advice and support. The authors would also like to thank Kevin Affum, Sang Ho Ye, PhD, and Mitch Barnett for their technical assistance. This work was supported by NIH Contract No. HHSN268200448192C (N01-HV-48192), NIH Small Business Innovation Research (SBIR) Phase I Award R41 HL077028-01, NIH SBIR Phase II Award R44 Hl071376-02, and a NIH Diversity Graduate Research Assistant Supplement Award (Johnson).
References
- 1.Goodman SL. Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J Biomed Mater Res. 1999;45:240–50. doi: 10.1002/(sici)1097-4636(19990605)45:3<240::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Narayanaswamy M, Wright KC, Kandarpa K. Animal models for atherosclerosis, restenosis, and endovascular graft research. J Vasc Interv Radiol. 2000;11:5–17. doi: 10.1016/s1051-0443(07)61271-8. [DOI] [PubMed] [Google Scholar]
- 3.Pelagalli A, Belisario MA, Tafuri S, et al. Adhesive properties of platelets from different animal species. J Comp Pathol. 2003;128:127–31. doi: 10.1053/jcpa.2002.0615. [DOI] [PubMed] [Google Scholar]
- 4.James NL, van der Meer AL, Edwards GA, et al. Implantation of the VentrAssist implantable rotary blood pump in sheep. ASAIO J. 2003;49:454–8. [PubMed] [Google Scholar]
- 5.Mateo A, Perez de la Lastra JM, Garrido JJ, Llanes D. Platelet activation studies with anti-CD41/61 monoclonal antibodies. Vet Immunol Immunopathol. 1996;52:357–62. doi: 10.1016/0165-2427(96)05587-0. [DOI] [PubMed] [Google Scholar]
- 6.Michelson AD, Barnard MR, Krueger LA, Frelinger AL, III, Furman MI. Evaluation of platelet function by flow cytometry. Methods. 2000;21:259–70. doi: 10.1006/meth.2000.1006. [DOI] [PubMed] [Google Scholar]
- 7.Dewald O, Schmitz C, Diem H, et al. Platelet activation markers in patients with heart assist device. Artif Organs. 2005;29:292–9. doi: 10.1111/j.1525-1594.2005.29050.x. [DOI] [PubMed] [Google Scholar]
- 8.Houel R, Mazoyer E, Boval B, et al. Platelet activation and aggregation profile in prolonged external ventricular support. J Thorac Cardiovasc Surg. 2004;128:197–202. doi: 10.1016/j.jtcvs.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm CR, Ristich J, Kormos RL, Wagner WR. Monocyte tissue factor expression and ongoing complement generation in ventricular assist device patients. Ann Thorac Surg. 1998;65:1071–6. doi: 10.1016/s0003-4975(98)00111-8. [DOI] [PubMed] [Google Scholar]
- 10.Gawaz M, Neumann FJ, Ott I, May A, Rudiger S, Schomig A. Role of activation-dependent platelet membrane glycoproteins in development of subacute occlusive coronary stent thrombosis. Coron Artery Dis. 1997;8:121–8. doi: 10.1097/00019501-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Maugeri N, Santarelli MT, Lazzari MA. Circulating platelet/polymorphonuclear leukocyte mixed-cell aggregates in patients with mechanical heart valve replacement. Am J Hematol. 2000;65:93–8. doi: 10.1002/1096-8652(200010)65:2<93::aid-ajh1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Htun P, Fateh-Moghadam S, Tomandl B, et al. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke. 2006;37:2283–7. doi: 10.1161/01.STR.0000236638.75591.61. [DOI] [PubMed] [Google Scholar]
- 13.Gawaz M, Neumann FJ, Ott I, Schiessler A, Schomig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996;93:229–37. doi: 10.1161/01.cir.93.2.229. [DOI] [PubMed] [Google Scholar]
- 14.Baker LC, Davis WC, Autieri J, et al. Flow cytometric assays to detect platelet activation and aggregation in device-implanted calves. J Biomed Mater Res. 1998;41:312–21. doi: 10.1002/(sici)1097-4636(199808)41:2<312::aid-jbm17>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Snyder TA, Watach MJ, Litwak KN, Wagner WR. Platelet activation, aggregation, and life span in calves implanted with axial flow ventricular assist devices. Ann Thorac Surg. 2002;73:1933–8. doi: 10.1016/s0003-4975(02)03549-x. [DOI] [PubMed] [Google Scholar]
- 16.Mateo A, Pintado CO, Perez de la Lastra J, et al. Ruminant cluster CD41/CD61. Vet Immunol Immunopathol. 1996;52:251–3. doi: 10.1016/0165-2427(96)05570-5. [DOI] [PubMed] [Google Scholar]
- 17.Mateo A, Perez de la Lastra J, Moreno A, et al. Biochemical characterization of antigens detected with anti-platelet monoclonal antibodies. Vet Immunol Immunopathol. 1996;52:363–70. doi: 10.1016/0165-2427(96)05588-2. [DOI] [PubMed] [Google Scholar]
- 18.Snyder TA, Tsukui H, Kihara S, et al. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res A. 2007;81:85–92. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 19.Marascalco PJ, Ritchie SP, Snyder TA, Kameneva MV. Development of standard tests to examine viscoelastic properties of blood of experimental animals for pediatric mechanical support device evaluation. ASAIO J. 2006;52:567–74. doi: 10.1097/01.mat.0000242248.66083.48. [DOI] [PubMed] [Google Scholar]
- 20.Smith JE, Mohandas N, Shohet SB. Variability in erythrocyte deformability among various mammals. Am J Physiol. 1979;236:H725–30. doi: 10.1152/ajpheart.1979.236.5.H725. [DOI] [PubMed] [Google Scholar]
- 21.Washington State University Monoclonal Antibody Center. [Accessed September 15, 2006];Taxonomic Key Program (GB84A [CD42d] + GB20A) Available at: http://www.vetmed.wsu.edu/tkp/Search.aspx.
- 22.Lalko CC, Deppe E, Ulatowski D, et al. Equine platelet CD62P (P-selectin) expression: a phenotypic and morphologic study. Vet Immunol Immunopathol. 2003;91:119–34. doi: 10.1016/s0165-2427(02)00287-8. [DOI] [PubMed] [Google Scholar]
- 23.Michelson AD. Platelet activation by thrombin can be directly measured in whole blood through the use of the peptide GPRP and flow cytometry: methods and clinical applications. Blood Coagul Fibrinolysis. 1994;5:121–31. doi: 10.1097/00001721-199402000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kestin AS, Ellis PA, Barnard MR, Errichetti A, Rosner BA, Michelson AD. Effect of strenuous exercise on platelet activation state and reactivity. Circulation. 1993;88:1502–11. doi: 10.1161/01.cir.88.4.1502. [DOI] [PubMed] [Google Scholar]
- 25.Michelson AD, Furman MI. Laboratory markers of platelet activation and their clinical significance. Curr Opin Hematol. 1999;6:342–8. doi: 10.1097/00062752-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Nagashima M, Shin’oka T, Nollert G, et al. Effects of a monoclonal antibody to P-selectin on recovery of neonatal lamb hearts after cold cardioplegic ischemia. Circulation. 1998;98(19 Suppl):II391–7. [PubMed] [Google Scholar]
- 27.Tait JF, Smith C, Wood BL. Measurement of phosphatidylserine exposure in leukocytes and platelets by whole-blood flow cytometry with annexin V. Blood Cells Mol Dis. 1999;25:271–8. doi: 10.1006/bcmd.1999.0254. [DOI] [PubMed] [Google Scholar]
- 28.Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990;265:17420–3. [PubMed] [Google Scholar]