Abstract
The goal of this study was to transect and immediately repair ventral roots, selected by their ability to stimulate bladder contraction, to assess the feasibility of bladder reinnervation in a canine model. Brain-derived neurotrophic factor (BDNF) was delivered via an osmotic pump (0.5 or 5 mg/mL) to a cuff surrounding the reanastomosis site to the two root bundles on one side. Electrodes were implanted bilaterally immediately proximal to the site of surgical reanastomosis. Results were compared to four root-intact, control animals that also received bilateral electrode implantation. At 6–12 months post-surgery, five of eight nerve transected and repaired animals showed increased pressure and bladder emptying during electrical stimulation of the repaired ventral roots contralateral to the BDNF delivery side. Nerve tracing studies one year postoperatively determined the repaired roots to be S1 and S2 and showed regrowth of axons from the spinal cord to nerve sites proximal to the repair site and to the bladder, and the presence of neurofilament-labeled axons growing across the ventral root repair site. In conclusion, transected ventral and dorsal roots in the sacral spine can be repaired and are capable of functionally reinnervating the urinary bladder. This feasibility study paves the way for future studies utilizing other more proximal motor nerves to bypass the transection site for bladder reinnervation.
Keywords: axon regeneration, cauda equina, motor neurons, neurogenic bladder, spinal injuries, ventral and dorsal root repair
INTRODUCTION
Urinary tract dysfunction occurs nearly universally following severe spinal cord injury (SCI), which may occur following fracture or dislocation of the spinal column as a result of trauma (e.g., motor vehicle accidents, diving accidents, acts of violence), infection, vascular injury, and other causes. Developmental abnormalities including spina bifida and meningomyelocele can also lead to similar sequelae as SCI. The prevalence of SCI is approximately 906 per million persons with motor vehicle accidents, accounting for approximately half of all cases. Falls account for another 21% with the remainder attributed to sporting-related injuries and acts of violence (Stover and Fine, 1987). Early data identified renal disease as the major cause of death in patients with SCI (Hackler, 1977) but more recent data points to pneumonia, accidents and suicides as the leading causes of death in this patient population (Stover and Fine, 1987). Although this may indicate an improvement in the urologic care of these patients, their quality of life still remains severely impaired as evidenced by the very high suicide rate. The quality of life of these patients would be significantly improved if restoration of urinary bladder emptying function could be accomplished.
Spinal cord injuries in humans above the T12-L1 vertebral level produce an upper motor neuron lesion, thus sparing the S2-4 spinal cord segments innervating the urinary bladder. This results in abnormal bladder contraction during filling (detrusor hyperreflexia) and a urinary sphincter that does not open when the bladder contracts (detrusor-sphincter dyssynergia). These types of patients can be implanted with a neuroprosthesis such as the Vocare system (NDI Medical, Cleveland, OH), which combines dorsal rhizotomy of the S2-4 sensory roots with implantable electrodes on the ventral roots that innervate the urinary bladder. The dorsal root rhizotomy at S2-4 interrupts the reflex arc that generates the detrusor dyssynergia. This allows patients with a supra-sacral spinal cord injury to empty their bladder independently using functional electrical stimulation (FES). However, many SCI patients have flaccid paralysis of their lower limbs and bladders. This is especially prevalent in children sustaining lap belt injuries and subsequent cauda equina deficits. FES in its current application cannot be used in patients with flaccid bladder paralysis since it can only restore function in SCI patients with innervated paralyzed muscles (upper motor neuron lesion).
Injuries to the conus medullaris and the cauda equina result in a lower motor neuron lesion leading to a flaccid paralysis of the urinary bladder such that the bladder does not contract when filled (detrusor areflexia). This type of injury often results in a competent but non-relaxing smooth muscle sphincter and a striated sphincter that retains some fixed tone but is not under voluntary control. Neuroprotheses such as the Vocare system cannot empty the bladder in these patients since the nerves innervating the urinary bladder, whose cell bodies are located in the injured conus medullaris, are damaged and cannot induce bladder contraction. Previous attempts to reinnervate the bladder have not led to successful functional reinnervation as defined by low-pressure bladder filling and complete bladder emptying under voluntary control (Conzen and Sollmann, 1982; Rao et al., 1971; Vorstman et al., 1986, 1987; Xiao and Godec, 1994). Since the sensory and motor innervation of the bladder including the control of spinal mechanisms by higher brain centers is extremely complex, the return of voluntary control of bladder emptying following lower motor neuron lesion by bladder reinnervation may not be possible in the foreseeable future. It is this latter group of patients with lower motor neuron injury that are the focus of this line of investigation which seeks to ultimately bypass the area of spinal cord and/or ventral root damage and reinnervate the urinary bladder using somatic nerve transfer. Bladder emptying could then be achieved by FES of the transferred somatic nerves using implanted electrodes.
The aim of this study is to repair ventral roots carrying motor innervation to the bladder immediately after transection using an end-on-end direct reanastomosis repair to determine the feasibility of normal urinary bladder reinnervation in a canine model. Due to the proximity of the dorsal roots, they were also transected and repaired with the ventral roots. BDNF was delivered via osmotic pump to a cuff surrounding the repair site on the left side only. Electrophysiological, tract tracing, histochemical and immunohistochemical techniques were used to examine the effectiveness of the root repair with and without BDNF treatment.
METHODS
All studies were approved by the Temple University Institutional Animal Care and Use Committee in accordance with the laboratory animal care guidelines of both the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care. The study subjects were fully conditioned female mongrel hounds 6–12 months of age and 18–22 kg body weight. A total of 15 dogs were used: four nerve intact controls, one nerve transection only, and 10 nerve transection with immediate reanastomosis repair.
Surgical Preparation
One day prior to surgery, animals were fasted, prophylactic antibiotics given (30 mg/kg trimethoprim and 6 mg/kg sulfadiazine p.o.) and a fentanyl patch (75–100 mg/h for a 20-kg dog) adhered to the shaved skin of the inner thigh and left in place for 3 days. On the morning of surgery, an indwelling intravenous cannula (angiocath) was inserted into the ulnar vein for intravenous administration of Ringer’s lactate containing morphine (10 mg/L) at 60–100 mL/h. For postoperative pain management starting on the second day post-surgery, 2 mg/kg ketoprofen IM was administered BID for 2 days. Propofol (6 mg/kg iv) was administered to allow insertion of an endotracheal tube for isoflurane anesthesia (0.5–4% mean alveolar concentration) using oxygen as the carrier gas.
Vesicostomy Procedure
For postoperative management of the neurogenic bladder, an abdominal vesicostomy was created. The bladder was located through an incision in the midline of the abdomen below the umbilicus and opened at the urachal remnant. The mucosa was everted and fixed to the skin using resorbable 4.0 chromic sutures. For bladder pressure monitoring during the surgical procedure, the vesicostomy stoma was catheterized with a 30-cc balloon Foley catheter and the urinary bladder was also catheterized per urethra for bladder filling with a syringe pump. Three successive filling cystometrograms (30 mL/min) were obtained to determine bladder capacity and the bladder was filled to approximately 60% of that capacity. Bladder pressure was continuously monitored throughout the surgical procedure. These bladder catheters were removed at the conclusion of the surgical procedure and bladder emptying occurred through this vesicostomy which remained patent throughout the post operative period of up to 1 year. In the few animals in which skin irritation ensued from this constant contact of urine with the skin of the lower abdomen and inner thighs, daily topical applications of petrolatum ointment circumvented this complication.
Surgical Procedures
Spinal surgery was performed for bladder denervation in 11 experimental animals and four sham operated, nerve intact controls. After everting the animal from the supine to the prone position and surgically prepping the skin from the base of the tail to the mid-thoracic level, an approximately 30-degree V-laminectomy of the T7 vertebral body and a partial laminectomy of the T6 and S1 vertebral bodies was performed (Fig. 1) to expose the S1 and S2 ventral roots that innervate the canine bladder. A spinal electrode (Model CES-3P-9, Ad-Tech Medical Instrument Co., Racine, WI) was positioned in the midline epidural space under the T5 vertebral body and the entire conus medullaris was stimulated electrically (30 Hz, 30 volts, 0.5 msec pulse duration) which induced a significant bladder contraction of 15–25 mm Hg peak pressure. The Ad-Tech CES-3P-9 electrode is 1.3 mm in diameter, with three cylindrical electrode contacts at the end which are 5 mm in length spaced 15 mm apart, such that optimal spinal stimulation can be attained by stimulating either the two most proximal contacts, the two most distal contacts, or the proximal and distal contacts.
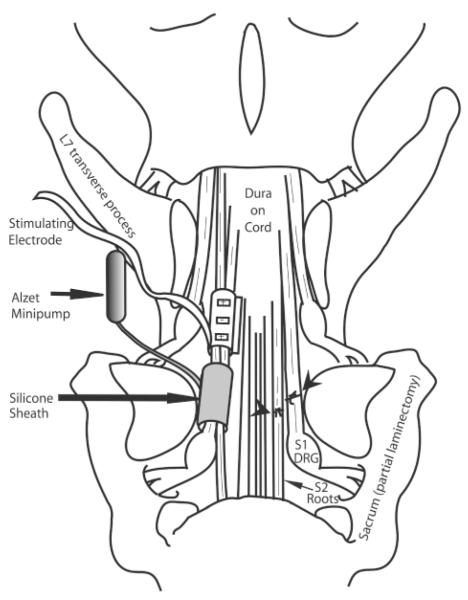
FIG. 1.
Diagram of the surgical repair methodology. A spinal laminectomy was performed, removing lumbar 6 (L6), and part of L7 and sacrum 1 (S1) lamina and spinous processes. The site of ventral and dorsal root transection and surgical repair is shown on the right side of the diagram (indicated by arrowheads). The placement of the stimulating electrode sheath and silicon sheath receiving brain-derived neurotrophic factor (BDNF) from the Alzet minipump is shown on the left side of the diagram. DRG, dorsal root ganglion.
For bladder denervation, two ventral roots that induced urinary bladder contraction upon electrical stimulation were identified extradurally, bilaterally, by intraoperative electrical stimulation (2 V, 20 Hz, 1-msec square wave trains) using a unipolar probe electrode. These ventral roots were transected along with the adjacent segmental dorsal root (two ventral/dorsal root bundles were cut per side; four per dog) at an extradural site immediately proximal to the dorsal root ganglion and the formation of the spinal nerve, a site still within the bony spinal column (Fig. 1). After transecting these roots, bladder contractions could not be induced via the epidural electrode on the conus medullaris, but could be induced by stimulating the ends of the severed roots distal to the transection site. This confirmed that all of the ventral roots inducing bladder contraction were severed. The proximal and distal segments of the transected ventral roots were approximated end-on-end, and reanastomosed microscopically by pulling the epineurium of the two cut root stumps gently together using 10-0 nylon microsutures at three to four sites around the nerve circumference.
After surgical reanastomosis, a self conforming, spiral, tripolar nerve cuff electrode (Axon Engineering, Cleveland, OH) was placed around the two root bundles on each side, proximal to the repair site, and the leads were exteriorized to the dorsal skin. Two cuff electrodes were used per animal and each cuff surrounded the two transected and repaired roots (Fig. 1). At approximately 60-day intervals postoperatively, these electrodes were stimulated (1.2 mA, 20 Hz, 0.5-msec quasi trapezoidal wave trains of 20-sec duration) under isoflurane anesthesia while monitoring bladder pressure to determine return of bladder function.
Brain-Derived Neurotrophic Factor
The repair site of the two ventral/dorsal root bundles on the left side of each spinal cord of experimental animals was surrounded by small silicone cuff connected to a silicone tube leading to an Alzet minipump (model 2002, Durect Corp., Cupertino, CA) filled with 0.5 or 5 mg/mL BDNF (a kind gift from Dr. Stanley Weigand, Regeneron Pharmaceuticals, Tarrytown, NY) in four dogs per dose of BDNF (Fig. 1). The mini-osmotic pump delivered BDNF to the root reanastomosis site at a rate of 0.5 μL (2.5 μg) per hour for 14 days. The root bundles on the right side did not receive BDNF via a cuff and thus served, at least partially, as a control for the effects of BDNF. The BDNF cuff was not used in two dogs with nerve transection and immediate repair (Table 1, dogs 6 and 7). Transection and end-on-end surgical reanastomosis of the ventral/dorsal roots was not performed on the four nerve intact, sham-operated controls. The tripolar cuff electrodes were placed around the same segmental ventral and dorsal roots in the root-intact, sham operated controls; however, the silicone cuff from the Alzet minipump was not used in one animal (Table 1, dog 1) and filled with saline in three (Table 1, dogs 2–4).
TABLE 1.
Summary of Functional Electrical Stimulation (FES) and Neurotracing Results
|
Bladder pressure (cm H2O) |
Euthanization FES/bladder pressure |
Fluorogold tracing |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dog | Surgery |
BDNF cuff (left only) |
Post op. day |
Left | Right | Both |
Electrode lead removal Post op. day |
Post op. day |
Left | Right | Both | Left | Right |
| 1 | Sham | None | 62 | 33.8 | 11.0 | 33.9 | 126 | 126 | 29.6 | 6.7 | 41.9 | + | + |
| 2 | Sham | Saline | 91 | 0.0 | 0.0 | 0.0 | 113 | 394 | 3.9 | 5.2 | 4.9 | + | + |
| 3 | Sham | Saline | 84 | 10.6 | 0.0 | 5.3 | 147 | 394 | 16.4 | 11.1 | 10.3 | + | + |
| 4 | Sham | Saline | a | 57 | 366 | 0.0 | 0.0 | 0.0 | + | + | |||
| 5 | Transect only | None | b | 233 | − | − | |||||||
| 6 | Transect-repair | None | 92 | 0 | 0 | 0 | 116 | 125 | 0 | 0 | 0 | + | + |
| 7 | Transect-repair | None | a | 12 | 49 | + | + | ||||||
| 8 | Transect-repair | 0.5 g/mL | 84 | 0.0 | 0.0 | 0.0 | 91 | 149 | 0.0 | 4.9 | 5.4 | + | + |
| 9 | Transect-repair | 0.5 g/mL | 83 | 0.0 | 0.0 | 0.0 | 174 | 365 | 0.0 | 0.0 | 0.0 | + | + |
| 10 | Transect-repair | 0.5 g/mL | 78 | 0.0 | 0.0 | 0.0 | 80 | 373 | 0.0 | 19.6 | 17.2 | + | + |
| 11 | Transect-repair | 0.5 g/mL | 118 | 0.0 | 1.8 | 1.1 | 335 | 335 | 0.0 | 1.7 | 2.0 | + | + |
| 12 | Transect-repair | 5.0 g/mL | 84 | 0.0 | 0.0 | 0.0 | 167 | 366 | 0.0 | 0.0 | 0.0 | − | + |
| 13 | Transect-repair | 5.0 g/mL | 84 | 0.0 | 0.0 | 0.0 | 290 | 366 | 0.0 | 0.0 | 0.0 | + | + |
| 14 | Transect-repair | 5.0 g/mL | 297 | 0.0 | 1.0 | 1.1 | 365 | 365 | 0.0 | 5.6 | 5.0 | + | + |
| 15 | Transect-repair | 5.0 g/mL | 78 | 0.0 | 0.0 | 0.0 | 265 | 366 | 0.0 | 13.1 | 11.6 | + | + |
Electrode leads exiting the skin became damaged prior to the first FES testing.
Electrodes were not implanted in the nerve transection only control animal.
BDNF, brain-derived neurotrophic factor; FES, functional electrical stimulation; post op., post-operative; transect-repair, nerve transection and immediate repair.
Retrograde Tract Tracing from the Bladder
To examine whether bladder motor axons regrew through the root repair site to the bladder, fluorogold retrograde neuronal tracing from the bladder to the cord was performed 12 months post-surgery on all animals. The bladder was cystoscoped through the vesicostomy, and four injections of 4% (w/v) fluorogold were made lateral to each ureteral orifice (50 μL/injection, 400 μL total per animal). Three weeks after the fluorogold injection, the dogs were re-anesthetized and the implanted electrodes stimulated as described above. The dogs were then euthanized (intravenous injection of 360 mg/kg sodium pentobarbital). The spinal column with intact cord, roots and spinal nerves was postfixed en bloc by immersion in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 3–5 days at 4°C. Lumbar and sacral spinal cord segments were cryoprotected by incubating in 30% sucrose in 0.1 M phosphate buffer (pH 7.4) for 2 days, and frozen-sectioned into 18-μm coronal sections and mounted onto coated slides. Unstained spinal cord sections were cover slipped using 80% glycerol in 0.1 M phosphate buffer, and used to measure fluorogold retrogradely labeled neurons (motor neurons). Vibratome-sectioned cord slices were examined for location of fluorogold-labeled neuronal cell bodies.
Postmortem Tract Tracing: Anterograde from Spinal Cord and Retrograde from Spinal Nerves
To determine whether repaired roots contained axons that had regrown from the cord distally through the repair site, two postmortem lipophilic dyes, Neurotrace DiI and Fast DiO, were utilized (four experimental animals and two root-intact controls). Fast DiO (Molecular Probes) was inserted either into S1 and S2 roots, 2 cm proximal to the site of reanastomosis (n = 2) or into ventro-lateral regions of unfixed lumbar spinal cords (n = 2) at the time of tissue collection (12 months post-surgery). The spinal cord, root, and nerve en bloc specimens were then postfixed 5 days in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Then, Neurotrace DiI (Molecular Probes) was applied directly onto the distal cut ends of spinal nerves (two on each side per dog), 2–2.5 cm distal to the root surgical site. Specimen was returned to fixative and incubated for 3 months at 37°C. Segments from the sacral cord were removed from the vertebral column with roots and spinal nerves still connected, cut using a vibratome into 180-μm longitudinal sections, and mounted onto uncoated slides using 80% glycerol in 0.1 M phosphate buffer. Roots and spinal nerves were examined for the presence of DiI and DiO labeled axons; spinal cord sections were examined for neuronal cell bodies double-labeled for both fluorogold and DiI.
Immunohistochemical and Morphological Examination of Repaired Roots
To examine morphological integrity of reanastomosed ventral/dorsal roots, roots, and adjacent spinal nerves were collected and immersion fixed (four experimental animals; two root-intact controls, bilaterally) 12 months post-surgery. Roots and adjacent spinal nerves were then separated from the spinal cord to a point 1–2 cm distal to the site of root repair (a site located within the spinal nerve region), incubated in 30% sucrose in 0.1 M phosphate buffer for 2 days, frozen-sectioned into 18-μm coronal sections, and mounted onto coated slides. These sections were stained with hematoxylin and eosin (H&E) and Hema 3 stain (a modified Wright’s stain; Biochemical Science Inc). Immunohistochemistry was performed using an antibody directed against dog neurofilament 200 kDa (Chemicon). Sections were treated with 3% H2O2 for 30 min (omitted for fluorescent staining), washed, blocked with 4% goat serum 4% in phosphate-buffered saline (PBS) for 30 min at room temperature, and incubated overnight at room temperature with the antibody (1:500 dilution in 4% goat serum/PBS). After washing, sections were incubated with donkey anti-mouse anti-body conjugated to HRP or Cy3 (Jackson Immuno) diluted 1:100 in PBS for 2 h at room temperature. HRP was visualized using diaminobenzidene (DAB). HRP-DAB stained slides were counterstained with eosin and cover-slipped using DPX (BDH Laboratory Supplies); Cy3 immunofluorescent slides were cover-slipped using 80% glycerol/PBS. No primary antibody was added to negative immuno control slides. These slides were examined for integrity of repair site and presence of neurofilament immunoreactive axons.
Statistical and Quantitative Analysis
Spinal cord sections (n = 6 experimental animals; n = 3 root-intact controls) were analyzed for presence of fluorogold retrogradely labeled motor neuronal cell bodies. The smallest cross-sectional diameter of the fluorogold labeled neurons were measured bilaterally using a microscope interfaced with a bioquantitation system. Only fluorogold-labeled cells in which the nucleus was visible were measured. An average of 20 cells were measured per cord in three to four different cord non-adjacent sections per dog using 400× magnification. Means and standard deviation (SD) are presented. A repeated measures design with 11–27 cell measurements per dog (average = 20/dog) was utilized. Prior to analysis, all data were tested for normality using the Shapiro-Wilk test. The distribution of the data was statistically significantly different than the normal distribution. In order to apply ANOVA methods, a “normalized-rank” transformation was applied to the data. The rank-transformed data was analyzed using a mixed model ANOVA for repeated measures. The difference between group means was considered significantly if the probability of chance occurrence was ≤0.05 using a two-tailed test.
Fluorogold-labeled axons in roots 0.5–2.5 cm proximal or distal to the surgery site were also analyzed. The number of pixels in a defined area (at 400× magnification) expressing fluorescence above a defined threshold was measured as described in Clark et al. (2003). Three fields/root, five root bundles from three experimental animals, and three root bundles from two root-intact control animals were analyzed. Fluorogold-labeled axon area fractions were compared in the two groups using Student’s t-test, where p ≤ 0.05 was considered significant.
RESULTS
Functional Electrical Stimulation
A representative bladder pressure trace during FES at 78 and 373 days postoperatively is shown in Figure 2 in a dog that received 0.5 mg/mL BDNF to the left root reanastomosis site (dog 10 in Table 1). As can be seen, stimulation of either side at 78 days did not induce increased bladder pressure. However, at euthanization 373 days postoperatively, a significant bladder contraction was induced by FES of the right side electrodes, but not the left side where the cuff supplying BDNF was placed. This increased bladder pressure with FES was found in the right, non-BDNF treated side in three of four animals in the 0.5 mg/mL BDNF group, and in two of four animals in the 5.0 mg/mL BDNF treated group (Table 1). No bladder contractions were observed in any animals during FES of the BDNF treated left side. Average bladder pressure in the five nerve root transected and repaired dogs in which FES induced increased bladder pressure was 9.0 ± 3.3 cm H2O (mean ± SEM). Flow of saline out of the urethra was observed during FES in all 5 of these animals in which increased bladder pressure was observed during FES. Damage to the electrode leads occurred at various post operative times in all but two of the animals (Table 1, dogs 11 and 14), including complete removal of both electrodes by one animal 12 days post-operatively, prior to FES induced bladder pressure testing (Table 1, dog 7). For animals that were tested at multiple post operative time points by FES for bladder pressure responses, the bladder pressure data is shown in Table 1 for the earliest post-operative time point in which increased bladder pressure in response to FES was observed.
FIG. 2.
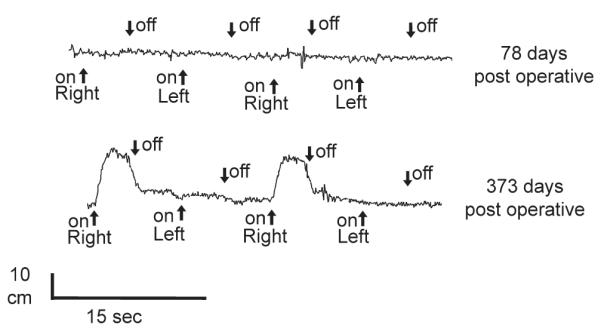
Functional electrical stimulation. Representative in vivo bladder pressure recordings (dog 10, Table 1) during functional electrical stimulation (FES) of electrodes surrounding ventral and dorsal root bundles in the sacral spine proximal to the root transection and repair site. At 373 days postoperatively, significant bladder pressure was generated with FES of the right side electrodes but not for the BDNF-treated left side (bottom trace). This was not seen in FES done at 78 days (top trace).
Evidence of Regrowth of Axons from Cord to Bladder
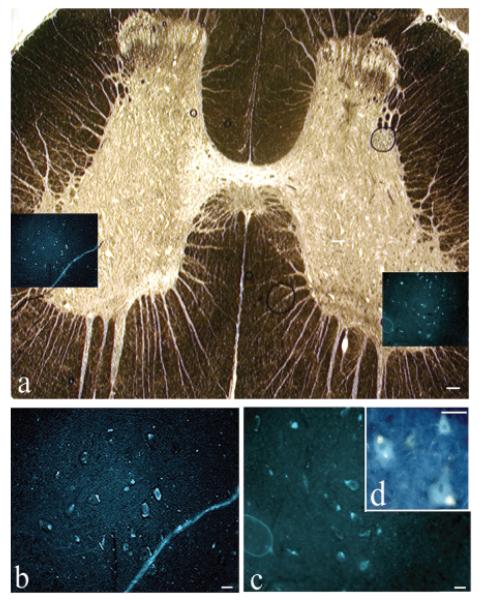
In all 10 experimental dogs that received surgical transection/repair of ventral and dorsal roots, fluorogold-labeled neuronal cell bodies were observed in the lateral ventral horns and in the zona intermedia of upper sacral spinal segments, indicating regrowth of motor axons to the bladder (Fig. 3; Table 2). For the composite micrograph shown in Figure 3A, a bright-field image of spinal cord cross-section is overlaid with the fluorescence image of fluorogold-labeled cell bodies in the same section. Higher power examples of the fluorogold-labeled neurons are shown in Figure 3B–D. Only a subset of cord neurons were retrogradely labeled with fluorogold in the experimental and root-intact control animals (all having received fluorogold injections into the bladder wall). This subset was located in lateral ventral horns of sacral segments, and in the lateral zona intermedia. The mean diameter of the fluorogold-labeled neurons in the experimental group was not statistically significantly different from that found in the root-intact control group (Table 2; p = 0.12).
FIG. 3.
Reinnervation of the bladder from the cord. Fluorogold retrogradely labeled neuronal cell bodies in spinal cord following injection into the bladder 3 weeks before euthanasia, which was 12 months postoperative. (A) Cross-section of a sacral cord segment. A bright-field low-magnification photomicrograph of an unstained (other than the fluorogold, which is not visible in bright field) wet-mounted cord that had been cover slipped with 80% glycerol in phosphate-buffered saline (PBS). Insets in A show fluorogold (FG)–labeled neuronal cell bodies located bilaterally in lateral ventral horns. (B,C) Enlargement of insets in panel A showing fluorogold-labeled neuronal cell bodies. (D) Higher power of neuronal cell bodies showing cytosolic localization of fluorogold dye in neuronal cell bodies. Scale bar 100 μm (A), 50 μm (B,C).
TABLE 2.
Results of Histological Analyses Examining Nerve Regrowth
| Histological analyses | Experimental (ventral root repair) group | Root-intact controls | |
|---|---|---|---|
| No. of dogs with fluorogold- labeled spinal neuronal cell bodies |
n = 9, bilaterally n = 1, right side only |
n = 4, bilaterally | |
| Diameter fluorogold- labeled spinal neuronal cell bodies |
22.31 ± 8.96 (mean ± SD; n = 6 examined) |
17.82 ± 6.10 (mean ± SD; n = 3 examined) |
|
| No. of dogs with DiI-labeled spinal neuronal cell bodiesb |
4/4 dogs, bilaterally | 2/2 dogs, bilaterally | |
| Number of fluorogold- labeled axons in ventral roots |
Distal to repair site, 20.43 ± 0.98 (mean ± SD) |
Proximal to repair, 6.83 ± 2.49a (mean ± SD) |
Intact ventral root, 16.11 ± 4.73 (mean ± SD) |
p ≤ 0.05.
DiI was inserted into spinal nerves distal to ventral root repair site.
In all but one dog, fluorogold-labeled cell bodies were observed bilaterally in sacral cord segments (Table 1, dog 12; Table 2). In that one dog, the silicone cuff surrounding the roots and supplying the BDNF to the site of root reanastomosis for 14 days post-operatively had become misaligned and had constricted the repaired roots. This constriction explains the absence of any fluorogold labeled neurons in the left cord of this animal. The FES data for this dog indicates an absence of bladder reinnervation on the left side (Table 1).
Evidence of Regrowth from Cord through Root Repair Site
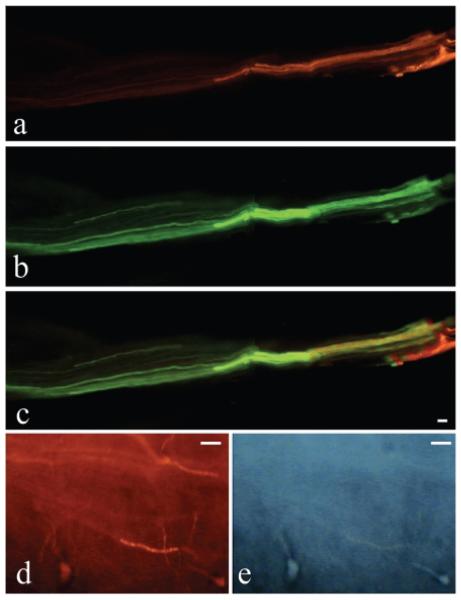
Post euthanasia dissection showed that the repaired roots were S1 and S2 spinal segmental levels (Fig. 1). Postmortem tracing studies were performed 12 months postoperatively to further examine axonal regrowth through the repair site. As described, DiI was inserted into spinal nerves 2 cm distal to the root transection/repair site in four experimental animals (two root bundles/side/dog), while DiO was inserted into either the spinal cord (n = 2 experimental animals) or into the roots (n = 2 experimental animals) at a site 2 cm proximal to the ventral/dorsal root surgery site. Similar labeling was performed in four ventral and dorsal roots at the same root segmental level (S1 and S2) in two root-intact control dogs, which showed passage of these postmortem lipophilic dyes through the intact roots (Table 2). In each of the four experimental dogs, DiI-labeled processes were visualized in the roots proximal to the transection/repair site (Fig. 4A,C; Table 2), indicating intact axons across the repair site. The dog from which Figure 4 was photographed, also received DiO labeling in the lumbar spinal cord. Figure 4B shows DiO-labeled processes in the same ventral root, indicating lumbar spinal cord axons are growing distally from the cord to this region located proximal to the root repair site. There was overlap of the dyes within this ventral root (Fig. 4C). Neuronal cell bodies were observed in the spinal cord that were retrogradely double-labeled with fluorogold and DiI (Fig. 4D,E, arrows; n = 3 experimental animals), indicating regrowth of the repaired ventral roots.
FIG. 4.
Postmortem labeling of ventral and dorsal root axons. Photomicrographs show a root immediately proximal to the repair site. (A) Postmortem retrograde labeling of axons (red DiI) is visible in the ventral root, as determined anatomically, after dye injection into the spinal nerve 2 cm distal to the ventral root transection/repair site. (B) Postmortem anterograde labeling of axons from the spinal cord (green DiO) is visible in the same ventral root as shown in A. (C) A merger of both A and B, and shows overlap of these 2 dyes. (D,E) High-power pictures from sacral spinal cord segments showing two fluorogold-labeled motor neuronal cell bodies that were retrogradely labeled from the bladder (arrows in D). These same neurons are also labeled with DiI which had been inserted into a ventral root distal to the site of reanastomosis (E). Scale bar = 50 μm.
Morphological Examination of Root Repair Site
Histochemical examination of the transection/repair site at 12 months postoperatively showed healthy roots and adjacent spinal nerves (Fig. 5). In most roots, successful regrowth across the repair site was apparent (Fig. 5A; arrows indicate sutures at repair site). In these same roots, neurofilament-stained axons were visible distal to the site of repair (Fig. 5B, arrow indicates suture).
FIG. 5.
Integrity of repaired roots. Photomicrographs of longitudinally sectioned ventral and dorsal roots and adjacent spinal nerves showing integrity of repair site. (A) Examination of hematoxylin and eosin (H&E)–stained root bundle of one animal reveals excellent healing across the site of reanastomosis. Small black arrows indicate sutures. (B) Neurofilament immunofluorescence staining (red) of a nearby section of same animal as shown in A shows continuity of axons across the site of repair. White arrow indicates a suture. (C) H&E staining of a root from another animal that had received brain-derived neurotrophic factor (BDNF) from a cuff at the site of root repair shows a separation between proximal (upper half of picture) and distal (lower half of picture) stumps. The root is surrounded by clumps of inflammatory cells (indicated by asterisk). (D) Neurofilament immunostaining of an adjacent section of root as shown in panel C, reveals axons growing around the scar site (black arrowheads trace the path of the neurofilament stained axons). (E,F) Higher-power examination of inflammatory site indicated by the asterisk in panel C reveals leukocytes (indicated as L in panel E, H&E staining), as well as macrophages (mac) and neutrophils (n; F, Hema 3 stain). Scale bar = 50 μm.
In approximately one third of the repaired ventral and dorsal root bundles, a small gap still existed between the proximal and distal segments of the transected root (Fig. 5C). However, neurofilament immunohistochemistry usually revealed neurofilament-stained axons passing around the repair site through the surrounding connective tissue (Fig. 5D). This pattern of axonal growth was typically seen at the BDNF cuff site (left side), but not in the contralateral repaired roots not surrounded by the BDNF cuff. The BDNF appeared to create circuitous axonal outgrowth within the cuff in the connective tissue surrounding the root repair site (Fig. 6) and occasionally outside of the cuff.
FIG. 6.

Circuitous growth pattern at brain-derived neurotrophic factor (BDNF) delivery site. (A) Low-power photo of BNDF cuffed nerve in cross section showing nerve bundle (arrows) and surrounding connective tissues. Unstained bright-field photo. (B) Higher power of circled area shown in panel A. (C) Same section as shown in panel B photographed using epifluorescence light. Circled area contains FG labeled axons. (D) Higher-power fluorescence photo of circled area showing fluorogold (FG)–labeled axons in connective tissues outside of main nerve bundle. Scale bar = 50 μm.
H&E and Hema staining revealed an immune cell response around the repaired roots that had received the BDNF cuff (left sides; Fig. 5E,F). These cells were lymphocytes (Fig. 5E), neutrophils (Fig. 5F), and monocytes/macrophages (Fig. 5F). This immune response was absent around repaired roots not enclosed by the BNDF cuff (right sides; Fig. 5A,B). The second repaired root on the same side as the one shown in Figure 5C–F had better regrowth, providing a mechanism for transport of fluorogold from the bladder to the cord in that side (Table 1, dog 14).
Figure 7A–C shows a cross-section of a ventral root of one animal as it nears a caudal sacral cord segment. Figure 7A shows the fluorogold labeled axons in the ventral root cross-section, Figure 7B shows the bright field image, while Figure 7C shows the merged images. Quantification of the mean number of fluorogold-labeled axons in the roots revealed that the number in root-intact controls was not significantly different in experimental animals at 0.5–2.5 cm distal to the root repair site (Table 2). However, the number of fluorogold labeled axons at 0.5–2.5 cm proximal to the root repair site contained significantly fewer labeled axons than controls (Table 2; p < 0.05). These results show that only a subset of axons in the roots are fluorogold labeled (Fig. 7A), and that some of these are able to traverse the root repair site (Table 2).
FIG. 7.
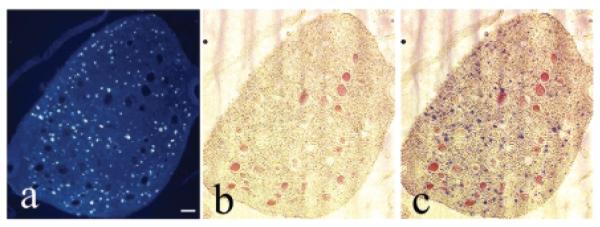
Number of new axons in repaired root. Fluorogold (FG)–labeled axons in ventral and dorsal roots. (A–C) Cross-section of a root as it nears the cord, a root region that is proximal to the site of reanastomosis. (A) Small fluorescence blue profiles in root are fluorogold (FG) retrogradely labeled axons (labeled after injection into the bladder). (B) Bright-field image of same root; red filled circles are blood vessels. (C) Images in panels A and B are shown superimposed. Scale bar = 50 μm.
DISCUSSION
These results indicate regrowth of axons following acute sacral root transection with immediate end-on-end reanastomosis. Spinal motor processes regrew through the transection/repair site from the cord to the bladder in all animals. Evidence of regrowth include (1) FES-induced bladder contractions through the repaired ventral roots, (2) fluorogold-labeled neuronal cell bodies in sacral spinal cord and fluorogold-labeled axons in ventral roots proximal to the transection/repair site following retrograde transport of this dye from the bladder, and (3) postmortem lipophilic dye tracing through the site of repair from distal nerve regions. Cell bodies in the sacral cord double-labeled with both fluorogold and DiI further confirms regrowth of motor axons from the spinal cord to the bladder. Since neurofilament-200 is a structural protein typically down regulated in degenerating nerves (Sotelo-Silveira et al., 2000), its presence in the ventral root distal to the site of repair is also a sign of root regrowth. The location of the fluorogold-labeled neuronal cell bodies in the cord in this study closely match results seen by other investigators examining the location of spinal neurons innervating the bladder (de Groat et al., 1988; Petras and Cummings, 1978).
End-on-end root and nerve repair has been shown to be a feasible method of nerve reanastomosis for recovery of limb motor function (de Medinaceli et al., 1993; Huang et al., 2003). However, optimal results are obtained with immediate reanastomosis, as was performed in our study, before nerve stiffness develops (Hentz et al., 1993; Toby et al., 1996). Our results also confirm that motor neuron and axon survival is enhanced when a minimum of at least 4 mm of axon remains in the ventral root being repaired (Gu et al., 1997; Huang et al., 2003). The presence of a peripheral nerve (via direct reanastomosis, autograft, or nerve transfer) sutured to the distal end of the proximal root or nerve stump aids regrowth compared to a short gap or bridging via conventional sutures (Huang et al., 2003; Liu et al., 2001; Scherman et al., 2001; Zhang et al., 2000). Since our aim is to develop methods of functional reinnervation of the bladder after conus medullaris and cauda equina injury, our results demonstrate that the present repair strategy makes possible long-term regeneration and functional reinnervation of structures innervated by the caudal spinal cord.
The generation of bladder contractions requires the presence of intact motor roots that transmit excitatory information from either the sacral spinal cord or from the site of root stimulation to the bladder (Kontani and Hayashi, 1997). Since the dye tracing and morphological results of our study clearly show that most of the transected-reanastomosed roots have reinnervated the bladder by 1 year postsurgery, we can only conclude that the inability to successfully stimulate bladder contractions in all of the experimental animals was hindered by electrode failure. One possible cause may have been the presence of an inflammatory response and scar tissue at the repair site, since scar tissue around a nerve hinders conduction. This response was observed in most ventral roots cuffed for delivery of BDNF. Since an inflammatory response induced by root/nerve transection resolves within 7–14 days (Griffin et al., 1992; Tetzlaff et al., 1991; Zochodne, 2000), our 1-year post-surgery model rules out transection-induced inflammation. Since BDNF promotes rescue and regeneration of injured motor neurons and axons (Blits et al., 2004; Funakoshi et al., 1993), BDNF delivery is not likely to be the cause of the leukocyte infiltration. It is more likely that the silicone cuff created a non-specific inflammatory reaction. Different cuff materials should be explored in future experiments using a similar neurotrophic factor delivery paradigm. On the other hand, others have postulated that an inflammatory reaction around a peripheral nerve may be considered as a conditioning lesion, stimulating nerve regeneration (Dahlin, 1992; Kato et al., 2001).
Our results suggest that BDNF contributed to misdirected axon growth. Other studies have also shown that infusion of BDNF promotes sprouting and axonal growth toward the site of BDNF delivery (Blits et al., 2004). We still achieved axonal regrowth in all but one of the repaired ventral roots, despite this misdirected type growth. Apparently, enough of the axons were still able to grow across the ventral root repair site to allow retrograde labeling from distal sites to occur following the end-on-end repair. Results by Amara et al. (2000) suggest that misdirected axonal growth is reduced by correct stump-end alignment without rotation during nerve repair.
In our study, the number of fluorogold labeled axons in regions proximal to the site of transection/repair are lower than in distal regions (Table 2). The fluorogold axon count most likely underestimated the actual total number of axons that had retrogradely transported the fluorogold from the bladder since the dye does not remain permanently in axons following transport. Underestimated or not, though, the presence of fluorogold-labeled axons proximal to the repair site supports a conclusion of regrowth of at least some of the axons across the repair site.
This study shows that transected ventral roots from the sacral spinal cord can be repaired surgically via end-on-end surgical re-approximation, and are capable of reinnervating the urinary bladder. These reinnervated ventral roots can induce bladder emptying via functional electrical stimulation. This feasibility study paves the way for future studies utilizing other more proximal motor nerves to bypass the transection site for bladder reinnervation.
ACKNOWLEDGMENTS
The expert veterinary care provided by Bernadette Simpkiss, who was responsible for all aspects of the immediate post operative intensive veterinary care as well as the ongoing veterinary care of these animals, is greatly appreciated. Mamta Amin is also acknowledged for her expert technical assistance in the histological studies. Dr. John Gaughan, in the Department of Biostatistics, is acknowledged for his performance of the biostatistical analyses. We would like to thank Dr. Stanley Wiegand and Regeneron Pharmaceuticals for the BDNF used in this investigation. This work was supported by research grants from the Shriners Hospitals and National Institutes of Health (RO1-DK43333; to M.R.R.).
REFERENCES
- AMARA B, DE MEDINACELI L, LANE GB, MERLE M. Functional assessment of misdirected axon growth after nerve repair in the rat. J. Reconstr. Microsurg. 2000;16:563–567. doi: 10.1055/s-2000-8396. [DOI] [PubMed] [Google Scholar]
- BLITS B, CARLSTEDT TP, RUITENBERG MJ, et al. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol. 2004;189:303–316. doi: 10.1016/j.expneurol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- CLARK BD, BARR AE, SAFADI FF, et al. Median nerve trauma in a rat model of work-related musculoskeletal disorder. J Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONZEN MA, SOLLMANN H. Reinnervation of the urinary bladder after microsurgical reconstruction of transsected caudal fibres. An experimental study in pigs. Urol. Res. 1982;10:141–144. doi: 10.1007/BF00255957. [DOI] [PubMed] [Google Scholar]
- DAHLIN LB. Stimulation of regeneration of the sciatic nerve by experimentally induced inflammation in rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1992;26:121–125. doi: 10.3109/02844319209016001. [DOI] [PubMed] [Google Scholar]
- DE GROAT WC, ARAKI I, VISSARD MA, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 1988;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- DE MEDINACELI L, PRAYON M, MERLE M. Percentage of nerve injuries in which primary repair can be achieved by end-to-end approximation: review of 2,181 nerve lesions. Microsurgery. 1993;14:244–246. doi: 10.1002/micr.1920140406. [DOI] [PubMed] [Google Scholar]
- FUNAKOSHI H, FRISEN J, BARBANY G, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN JW, GEORGE R, LOBATO C, TYOR WR, YAN LC, GLASS JD. Macrophage responses and myelin clearance during Wallerian degeneration: relevance to immune-mediated demyelination. J. Neuroimmunol. 1992;40:153–165. doi: 10.1016/0165-5728(92)90129-9. [DOI] [PubMed] [Google Scholar]
- GU Y, SPASIC Z, WU W. The effects of remaining axons on motoneuron survival and NOS expression following axotomy in the adult rat. Dev. Neurosci. 1997;19:255–259. doi: 10.1159/000111214. [DOI] [PubMed] [Google Scholar]
- HACKLER RH. A 25-year prospective mortality study in the spinal cord injured patient: comparison with the long-term living paraplegic. J. Urol. 1977;117:486–488. doi: 10.1016/s0022-5347(17)58506-7. [DOI] [PubMed] [Google Scholar]
- HENTZ VR, ROSEN JM, XIAO SJ, McGILL KC, ABRAHAM G. The nerve gap dilemma: a comparison of nerves repaired end to end under tension with nerve grafts in a primate model. J. Hand Surg. Am. 1993;18:417–425. doi: 10.1016/0363-5023(93)90084-G. [DOI] [PubMed] [Google Scholar]
- HUANG MC, CHEN KC, CHUANG TY, et al. Cervical root repair in adult rats after transection: recovery of forelimb motor function. Exp. Neurol. 2003;180:101–109. doi: 10.1016/s0014-4886(02)00026-2. [DOI] [PubMed] [Google Scholar]
- KATO N, NEMOTO K, KAWAGUCHI M, AMAKO M, ARINO H, FUJIKAWA K. Influence of chronic inflammation in peripheral target tissue on recovery of crushed nerve injury. J. Orthop. Sci. 2001;6:419–423. doi: 10.1007/s007760170008. [DOI] [PubMed] [Google Scholar]
- KONTANI H, HAYASHI K. Urinary bladder response to hypogastric nerve stimulation after bilateral resection of the pelvic nerve or spinal cord injury in rats. Int. J. Urol. 1997;4:394–400. doi: 10.1111/j.1442-2042.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- LIU S, AGHAKHANI N, BOISSET N, SAID G, TADIE M. Innervation of the caudal denervated ventral roots and their target muscles by the rostral spinal motoneurons after implanting a nerve autograft in spinal cordinjured adult marmosets. J. Neurosurg. 2001;94:82–90. doi: 10.3171/spi.2001.94.1.0082. [DOI] [PubMed] [Google Scholar]
- PETRAS JM, CUMMINGS JF. Sympathetic and parasympathetic innervation of the urinary bladder and urethra. Brain Res. 1978;153:363–369. doi: 10.1016/0006-8993(78)90416-x. [DOI] [PubMed] [Google Scholar]
- RAO CR, BRUCE AW, LYWOOD DW, ROBERTSON DM. Reinnervation of the neurogenic bladder with somatic motor nerves. Invest. Urol. 1971;9:59–63. [PubMed] [Google Scholar]
- SCHERMAN P, LUNDBORG G, KANJE M, DAHLIN LB. Neural regeneration along longitudinal polyglactin sutures across short and extended defects in the rat sciatic nerve. J. Neurosurg. 2001;95:316–323. doi: 10.3171/jns.2001.95.2.0316. [DOI] [PubMed] [Google Scholar]
- SOTELO-SILVEIRA JR, CALLIARI A, KUN A, et al. Neurofilament mRNAs are present and translated in the normal and severed sciatic nerve. J. Neurosci. Research. 2000;62:65–74. doi: 10.1002/1097-4547(20001001)62:1<65::AID-JNR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- STOVER SL, FINE PR. The epidemiology and economics of spinal cord injury. Paraplegia. 1987;25:225–228. doi: 10.1038/sc.1987.40. [DOI] [PubMed] [Google Scholar]
- TETZLAFF W, ALEXANDER SW, MILLER FD, BISBY MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBY EB, MEYER BM, SCHWAPPACH J, ALVINE G. Changes in the structural properties of peripheral nerves after transection. J. Hand Surg. Am. 1996;21:1086–1090. doi: 10.1016/S0363-5023(96)80320-0. [DOI] [PubMed] [Google Scholar]
- VORSTMAN B, SCHLOSSBERG SM, KASS L, DEVINE CJ., JR. Urinary bladder reinnervation. J. Urol. 1986;136:964–969. doi: 10.1016/s0022-5347(17)45141-x. [DOI] [PubMed] [Google Scholar]
- VORSTMAN B, SCHLOSSBERG S, KASS L. Investigations on urinary bladder reinnervation. Historical perspective and review. Urology. 1987;30:89–96. doi: 10.1016/0090-4295(87)90168-3. [DOI] [PubMed] [Google Scholar]
- XIAO CG, GODEC CJ. A possible new reflex pathway for micturition after spinal cord injury. Paraplegia. 1994;32:300–307. doi: 10.1038/sc.1994.52. [DOI] [PubMed] [Google Scholar]
- ZHANG Z, SOUCACOS PN, BERIS AE, BO J, IOACHIM E, JOHNSON EO. Long-term evaluation of rat peripheral nerve repair with end-to-side neurorrhaphy. J. Reconstr. Microsurg. 2000;16:303–311. doi: 10.1055/s-2000-7338. [DOI] [PubMed] [Google Scholar]
- ZOCHODNE DW. The microenvironment of injured and regenerating peripheral nerves. Muscle Nerve Suppl. 2000;9:S33–S38. doi: 10.1002/1097-4598(2000)999:9<::aid-mus7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]