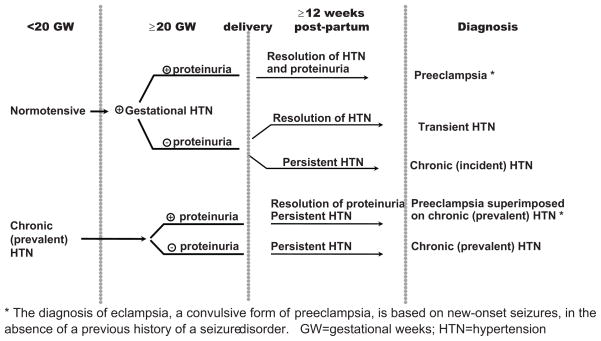
Hypertension in pregnancy covers a spectrum of conditions, including preeclampsia, gestational hypertension, chronic hypertension, and preeclampsia superimposed on chronic hypertension (Figure 1).1 Preeclampsia, unlike other hypertensive pregnancy disorders, is associated with proteinuria and affects 3–5% of all pregnancies, and remains a leading cause of both maternal and fetal mortality worldwide. It may occur both in previously healthy women (de novo preeclampsia) and in those with a history of chronic hypertension prior to their pregnancies (superimposed preeclampsia).
Figure 1.
Hypertensive Pregnancy Disorders: Classification and Diagnostic Criteria
Superimposed preeclampsia affects up to 30% of pregnancies in women with chronic hypertension, and is heralded by either the new onset of proteinuria or increase in preexisting proteinuria, worsening of blood pressure control, and/or laboratory abnormalities consistent with HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), which represents a deceptive, albeit severe, form of preeclampsia. Compared to women with preeclampsia who are normotensive at the time of conception, women with superimposed preeclampsia are at greater risk for peri-partum complications, such as placental abruption and maternal cerebrovascular incidents. The only tool currently available for the prediction of superimposed preeclampsia is a previously validated clinical prediction model, consisting of serum uric acid (>3.6 mg/dl), plasma renin activity (<4 ng/ml/hr), and systolic blood pressure (> 140 mm Hg), measured at 20 weeks of gestation.2 Few biological markers have been validated to date.3
In this issue, Perni et al.4 present results from a carefully performed longitudinal prospective study as to the role of angiogenic markers in the prediction and diagnosis of superimposed preeclampsia in 109 women with chronic hypertension that predated their pregnancies. Seminal studies of these markers have indicated that preeclampsia is associated with elevated levels of the soluble receptor for vascular endothelial growth factor (VEGF) of placental origin.5 This soluble receptor, commonly referred to as sFlt-1 (from fms-like tyrosine kinase receptor-1), may bind and neutralize VEGF and placental growth factor (PlGF), which are required for active fetal and placental angiogenesis during pregnancy. The same group also identified soluble endoglin (sEng) as another anti-angiogenic marker, which is up-regulated in preeclampsia and may amplify vascular damage by binding and neutralizing transforming factor β, thus contributing to the pathophysiology of HELLP syndrome.6 In the current study, pro-angiogenic PlGF and anti-angiogenic markers, sFlt-1 and sEng, were measured at 12, 20, 28, and 36 weeks, and postpartum. Consistent with the rates reported in the literature, superimposed preeclampsia occurred in 34% (n=37) of women, and was classified as early onset (before 34 weeks of gestation) in 9, and late onset (≥ 34 weeks) in the remaining 28 pregnancies. At the time of delivery, both women with early and late onset preeclampsia demonstrated lower PlGF levels, a higher ratio of sFlt-1/PlGF, and elevated circulating levels of sFlt-1 and sEng compared to pregnancies with uncomplicated chronic hypertension. At 20 weeks of gestation, only women who went on to develop early preeclampsia, and not those with late onset, demonstrated significantly higher sFlt-1 levels and elevated sFlt-1/PlGF ratios compared to women who did not develop preeclampsia. Finally, only the sFlt-1/PlGF ratio improved the predictive accuracy of the clinical prediction model in a clinically modest fashion (AUC increasing from 0.764 to 0.852). Perni and colleagues conclude that their findings suggest similarities in the pathogeneses of preeclampsia (de novo) and superimposed preeclampsia, and that measuring angiogenic factors to predict/diagnose superimposed preeclampsia is of potential clinical significance. Should these results affect the care of pregnant patients with a history of chronic hypertension?
Clinical studies of urine and serum measurements of circulating angiogenic markers using current techniques have not provided a reliable screening tool for preeclampsia, 7, 8 leading to studies that have combined biomarkers and other clinical tests in an attempt to improve upon the predictive values of individual angiogenic markers. For example, combining angiogenic markers in to a single angiogenic index, such as the sFlt-1/PlGF ratio, 9 which captures their reciprocal changes in preeclampsia, has demonstrated a better predictive value for preeclampsia than any single marker. Most published studies suggest that measurements of these markers, while not predictive of late-onset preeclampsia in full-term pregnancies, may be useful in predicting early-onset and severe disease. These findings raise a possibility to use these tests in special patient subgroups, such as those with chronic hypertension, who may be at a higher than average risk for preeclampsia and preeclampsia-related complications. Of note, a study of the role of angiogenic markers in predicting superimposed preeclampsia in 369 women with chronic hypertension concluded that corresponding sensitivities and/or positive predictive values were low, rendering these markers not clinically useful.3 It is important to emphasize that Perni et al., rightly so, acknowledge that further studies regarding the potential roles of angiogenic markers in predicting/diagnosing superimposed preeclampsia are required due to the limitations of their data, including lack of normotensive pregnant controls and small sample size. In conclusion, due to insufficient and conflicting evidence regarding the use of angiogenic markers in prediction of preeclampsia superimposed on chronic hypertension, further studies are needed to determine their clinical usefulness.
The utility of angiogenic markers in the prediction and diagnosis of preeclampsia must be interpreted in the context of their potential roles in causing preeclampsia. Despite the increasing research efforts in the field, the etiology and pathogenesis of preeclampsia remain elusive, resulting in a failure to develop specific screening, preventive, and treatment strategies. The relationship between endothelial dysfunction and preeclampsia, first introduced by Roberts in the 1980’s, 10 seems to play a central role in the pathophysiology of this disease, leading to a systemic disease with multi-organ involvement. Placental hypoxia, caused by abnormal placentation, may act as an early event, which may cause placental production of soluble factors that enter the maternal circulation leading to endothelial dysfunction. Consequently, anti-angiogenic markers are commonly viewed as the missing link between placental ischemia on one side, and endothelial dysfunction, mediated by neutralization of angiogenic factors, on the other.
Central to the potential role of angiogenic markers in predicting preeclampsia is the question as to whether or not the dysregulation of these markers is the cause or a consequence of placental ischemia in preeclampsia. A fall in sFlt-1 levels is known to occur after delivery and removal of the placenta, reaching pre-pregnancy levels by 72 hours postpartum. Therefore, the sFlt-1 theory does not explain the occurrence of preeclampsia in the postpartum period, which is a recognized clinical entity of unknown exact incidence, but reported to occur in up to 6% of all preeclampsia cases.11 While it has been postulated that retained placental tissue may serve as the source of sFlt-1 in these patients, it is widely recognized that sFlt-1 levels may correlate with placental mass, thus making it difficult to conclude that a small amount of retained tissue would be able to produce sFlt-1 levels above and beyond what is normal for pregnancy. However, data on angiogenic factor levels in postpartum preeclampsia are not available to further support this theory. Placental ischemia, which is central for the sFlt-1 theory, is not present in all cases of preeclampsia, nor is it specific for this disease, occurring in other disease entities, such as intrauterine growth retardation, but in the absence of preeclampsia.
In summary, the available data support the relationship between angiogenic markers and the pathophysiology of the disease, rather than direct causality. On the clinical side, compared to the non-pregnant state, normal pregnancy, near the time of delivery, is characterized by elevated levels of anti-angiogenic markers. These fulfill the important physiological role of neutralization of pro-angiogenic effectors which are no longer required. The use of angiogenic markers for preeclampsia screening in the general pregnant population is limited by the significant overlap in PlGF and sFlt-1 values between the mild forms of preeclampsia and normotensive pregnancies, leading to both false positive and false negative screening test results. The study by Perni et al. in this issue suggests that angiogenic markers may provide important prognostic and diagnostic tools for specific subgroups of patients, such as those with chronic hypertension at risk for superimposed preeclampsia. The strength of this study is the authors’ diligence in establishing clinically relevant phenotypes in a longitudinal, prospective manner, which provides solid preliminary data for an adequately powered study to investigate the role of angiogenic markers in the prediction/diagnosis of preeclampsia superimposed on chronic hypertension.
Acknowledgments
Sources of funding
The work was supported by Award Number K08HD051714 (Vesna D. Garovic) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Disclosures None
Conflicts of interest: None
References
- 1.Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy.[see comment] Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 2.August P, Helseth G, Cook EF, Sison C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666–1672. doi: 10.1016/j.ajog.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MVC, Martins-Costa, Bartz J, de Barros Santos C, Cecatti JG, Costa R, Ramos JG, Spinnato JA., II Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008;199:268.e261–268.e269. doi: 10.1016/j.ajog.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 4.Perni US, Sharma K, Helseth V, Hawfield G, Suthanthiran A, August M, Angiogenic P. Factors in Superimposed Preeclampsia: A Longitudinal Study of Women with Chronic Hypertension during Pregnancy. Hypertension. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia.[see comment] J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 7.Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the Theories of Preeclampsia and the Role of Angiogenic Factors: A Systematic Review. Obstet Gynecol. 2007;109:168–180. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 8.Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112:359–372. doi: 10.1097/AOG.0b013e3181801d56. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, Group CS. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 11.Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190:1464–1466. doi: 10.1016/j.ajog.2004.02.037. [DOI] [PubMed] [Google Scholar]