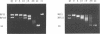Abstract
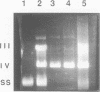
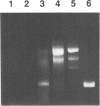
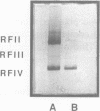
The T5 D15 exonuclease purified from an overproducing strain of E. coli was shown to possess a low level of endonucleolytic activity specific for single-stranded DNA when assayed with 1-10 mM Mg2+ as co-factor. Endonuclease activity on double-stranded circular DNA could not be detected under these conditions. Nicked circular DNA was first gapped by the enzyme's exonucleolytic activity, creating a single-stranded region. This gapped substrate was then endonucleolytically cleaved and rapidly degraded. We show that a gapped and not a nicked substrate is required for this activity as previously suggested (Moyer, R. W. and Roth, C. T. 1977, J. Virol. 24, 177-193). The single-strand endonuclease activity could be selectively suppressed by using low concentrations of Mg2+ as co-factor (less than 1 mM), thus allowing nicked double-stranded circular DNA to be gapped to a single-stranded circular species. We also report on sequence similarities between the T5 exonuclease and several prokaryotic DNA polymerases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrington J. M., Lunt M. R. Studies on the replication of bacteriophage T5. J Gen Virol. 1973 Feb;18(2):91–109. doi: 10.1099/0022-1317-18-2-91. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., McCorquodale D. J. Requirement of a phage-induced 5'-exonuclease for the expression of late genes of bacteriophage T5. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3502–3505. doi: 10.1073/pnas.70.12.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficht T. A., Moyer R. W. Isolation and characterization of a putative bacteriophage T5 transcription.replication enzyme complex from infected Escherichia coli. J Biol Chem. 1980 Jul 25;255(14):7040–7048. [PubMed] [Google Scholar]
- Frenkel G. D., Richardson C. C. The deoxyribonuclease induced after infection of Escherichia coli by bacteriophage T5. II. Role of the enzyme in replication of the pahge deoxyribonucleic acid. J Biol Chem. 1971 Aug 10;246(15):4848–4852. [PubMed] [Google Scholar]
- Kelley W. S., Joyce C. M. Genetic characterization of early amber mutations in the Escherichia coli polA gene and purification of the amber peptides. J Mol Biol. 1983 Mar 15;164(4):529–560. doi: 10.1016/0022-2836(83)90049-9. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- Leavitt M. C., Ito J. T5 DNA polymerase: structural--functional relationships to other DNA polymerases. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4465–4469. doi: 10.1073/pnas.86.12.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley M. J., Bennett S. E., Mosbaugh D. W. Characterization of the 5' to 3' exonuclease associated with Thermus aquaticus DNA polymerase. Nucleic Acids Res. 1990 Dec 25;18(24):7317–7322. doi: 10.1093/nar/18.24.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Martinez S., Diaz A., Espinosa M., Lacks S. A. Characterization of the polA gene of Streptococcus pneumoniae and comparison of the DNA polymerase I it encodes to homologous enzymes from Escherichia coli and phage T7. J Biol Chem. 1989 Mar 5;264(7):4255–4263. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Rothe C. T. Role of the T5 gene D15 nuclease in the generation of nicked bacteriophage T5 DNA. J Virol. 1977 Oct;24(1):177–193. doi: 10.1128/jvi.24.1.177-193.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. D., Timmis K. N. Highly repressible expression system for cloning genes that specify potentially toxic proteins. J Bacteriol. 1987 Oct;169(10):4457–4462. doi: 10.1128/jb.169.10.4457-4462.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Eckstein F. Properties of overexpressed phage T5 D15 exonuclease. Similarities with Escherichia coli DNA polymerase I 5'-3' exonuclease. J Biol Chem. 1990 Oct 25;265(30):18311–18317. [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]