Abstract
Human herpesvirus 6 (HHV6) may be an important pathogen following allogeneic hematopoietic cell transplantation (HCT). We prospectively evaluated weekly HHV6 viremia testing after allogeneic HCT using a quantitative polymerase chain reaction (PCR)-based assay. HHV-6 viremia was detected in 46 of 82 (56%) patients at a median of 23 days post-HCT (range: day + 10 to + 168). More males (65% vs females 39%, P = .03) and recipients of umbilical cord blood (UCB 69% vs unrelated donor [URD], 46% vs sibling donor [20%] grafts, P = 0.01) reactivated HHV-6. Patients with HHV6 viremia had more cytomegalovirus (CMV) reactivation (26% vs 5.5%, P = .01) and unexplained fever and rash (23.9% vs 2.7%, P = .01) compared with patients without HHV6 viremia. High-level HHV6 (≥25,000 copies/mL) versus lower levels were associated with more culture-negative pneumonitis (72.7% vs 22.8%, P = .01). Twenty HHV6-positive patients were treated with foscarnet, ganciclovir, or cidofovir for HHV6 or other coexistent viruses. Within 2 weeks, HHV6 viremia resolved more commonly in treated (65%) than untreated patients (31%), P = .02. Survival at 3 months was similar in treated and untreated patients (90% vs 81%, P = .4). Survival at 3 and 6 months post-HCTwere not affected by HHV6 positivity (3 months HHV6 + 85% vs 78%, P = .46; 6 months HHV6+ 70% vs 72%, P = .89) or by HHV6 level (3-month high level 73% vs 89%, P = .23; 6-month high level 64% vs 71%, P = .54). Neither the occurrence of HHV6, degree of viremia, nor use of antiviral drugs influenced short-term survival after HCT.
Keywords: Human herpesvirus 6, Allogeneic transplantation, Umbilical cord blood, Foscarnet, Ganciclovir
INTRODUCTION
Human herpesvirus 6 (HHV6), a member of the Roseolovirus genus of the β-herpesvirus subfamily, establishes primary infection as exanthem subitum in the normal pediatric population [1,2]. With time, it establishes latency in CD34+ cells, monocytes, and macrophages, similar to cytomegalovirus (CMV). Over the last decade, HHV6 has been increasingly recognized as an opportunistic and potentially life-threatening pathogen after hematopoietic cell transplantation (HCT) [3-13]. Following HCT, HHV6 infections are caused by reactivation of the virus from latency [5,6]. HHV6 reactivation is detected in the blood of 40% to 60% of patients after HCT, most often by use of quantitative DNA polymerase chain reaction (qPCR) for viral-specific sequences [4,7-9,12,14].
HHV6 viremia has been reported in association with varying organ dysfunction and clinical syndromes including: delayed/impaired platelet recovery [10,12,13,15], myelosuppression [4,8], encephalitis [5,8,10-12,16-23], fever [4,14,24,25], rash [8,24-28], hepatitis [14], pneumonitis [8,25,29-31], gastroduodenitis [8], CMV reactivation, and graft-versus-host disease (GVHD) [20,28,32-34].
Treatment indications are uncertain in patients with HHV6 viremia following HCT [35]. HHV6 encephalitis is potentially fatal, and is a common indication for treatment [17,35]. Only 1 trial evaluated preemptive treatment of HHV6 based on a positive PCR test [16]. The development of reliable clinical guidelines for the management of HHV6 viremia in HCT recipients has historically been limited by the lack of specificity of viremia testing, lack of specific HHV6 clinical syndromes, and confounded by the occurrence of asymptomatic viremia, which often resolves without intervention.
In a prospective observational surveillance study, we evaluated the demographic and clinical factors associated with HHV6 infection and describe the outcome of patients with HHV6 viremia following antiviral treatment.
PATIENTS AND METHODS
Eighty-two patients who received an allogeneic HCT at the University of Minnesota between December 1, 2005, and September 30, 2006, had serial DNA PCR testing for HHV6. Patients received conditioning for transplant and prophylaxis of GVHD per active institutional protocols. All transplant protocols and written consent forms were approved by the University of Minnesota institutional review board, which also approved this specific analysis.
Myeloablative (MA) conditioning regimens included cyclophosphamide 60 mg/kg intravenous (i.v.) daily for 2 days and 1320 cGy of total body irradiation (TBI) given in 8 fractions. For double umbilical cord blood (UCB) HCT, fludarabine 75 mg/m2 was added. In reduced-intensity conditioning (RIC) regimens, fludarabine (40 mg/m2 i.v. daily for 5 days) was used with TBI (200 cGy) with cyclophosphamide 50 mg/kg i.v. for 1 day. GVHD prophylaxis included cyclosporine (from day − 3 to at least day +100) given with either my-cophenolate mofetil (from day −3 to day +30) (n = 128) or short-course methotrexate. High dose steroids were administered for treatment of acute GVHD (aGVHD).
HHV6 viremia testing for all allogeneic HCT recipients was to be performed weekly for 4 weeks starting on day + 14. Incomplete testing was primarily because of patients’ discharge to the outpatient setting. A median of 4 testings were performed per patient in the 82 patients studied (range: 1-21). This testing involved a quantitative PCR-based evaluation of HHV6 DNA copies/mL whole blood in a certified clinical virology laboratory (Focus Diagnostics, Cypress, CA). The lower limit of detection for the assay was 500 copies/mL. Of the 82 patients screened, 14 had only 1 test performed. Of those 14 patients, only 2 individuals had a single positive value. Because the tests were performed 1 week apart, only 1 high-positive test was enough to reach the treatment threshold. Although we considered viremia >25,000 copies of HHV6 DNA as a clinically relevant cut point, we also specifically reviewed any positive HHV6 PCR to better understand the natural history of this virus in HCT patients.
HHV6 disease was defined as viremia with HHV6-compatible clinical syndromes (eg, engraftment delay, hepatitis, pneumonitis, rash, or encephalitis). HHV6 hepatitis was defined as viremia plus any aminotransferase enzyme elevation on 2 different days, or elevated bilirubin on 2 occasions, within a 1-week interval, without evidence of GVHD or alternate explanation for hepatitis. Liver biopsies were not performed on any patients in this study. If there was another reasonable explanation for liver function test (LFT) changes, then they were not attributed to HHV6. HHV6 pneumonitis was defined as viremia and evidence of unexplained cough, hypoxia, or chest radiographic changes with negative sputum or bronchial lavage cultures and microbiologic or virologic studies. Pneumonitis or pneumonia was not attributed to HHV6 viremia if a bronchoscopy or sputum culture identified an alternate microbial etiology. Fever because of HHV6 was defined as HHV6 viremia with otherwise unexplained fever, most often with a macular erythematous rash and without evidence of GVHD. HHV6 central nervous system (CNS) dysfunction and/or encephalitis were defined as mental status changes, headache, seizure/clonus, paresthesias, or compatible changes on radiologic imaging accompanying viremia or HHV6 detection in spinal fluid. Bone marrow suppression was defined as recovery of HHV6 from marrow aspirates by PCR, accompanying worsening leukopenia, thrombocytopenia, and a hypoplastic marrow.
CMV viremia testing using a quantitative CMV DNA PCR assay (Abbott Molecular, Chicago, IL) was performed weekly after day +14. Patients with a CMV-seronegative donor and CMV-seronegative recipient status were tested until day +60; CMV-seropositive recipients or those with positive donors were tested until day +100. Selected patients continued CMV testing after day +100, including patients at high risk for late CMV disease (patients treated with steroids for GVHD) or those who had received treatment for CMV before day +100.
CMV-seropositive recipients or those with seropositive donors received high-dose acyclovir prophylaxis (500 mg/m2 [10-12 mg/kg] i.v. every 8 hours or 800 mg [18 mg/kg pediatric] orally 5 times daily) until day +100 following transplantation. CMV-seronegative patients with a CMV-negative donor who were seropositive for herpes simplex virus received low (half the above) dose acyclovir prophylaxis daily until day +60. All blood products were leukoreduced by filtration, but untested for CMV status.
HHV6 viremia patients were treated according to suggested institutional guidelines, though without a formal, protocol-based therapy. Eight were treated based on the levels of HHV6 viremia and associated clinical complications. Other HHV6 positive patients received ganciclovir, foscarnet, or cidofovir for other contemporaneous viral infections. Because these drugs were active against HHV6, these patients were included in the treated HHV6 group totaling 20 patients treated for HHV6. High-level HHV6 viremia was defined as ≥25,000 copies/mL.
When antiviral therapy was required during neutropenia, foscarnet was administered at 180 mg/kg/day in divided dosing (renally adjusted as needed) for induction ×3 days, then continued as maintenance (90 mg/kg/day in divided dosing) to complete 2 weeks of therapy. HHV6 viremia was retested at 2 and 4 weeks after discontinuation of therapy. Acyclovir prophylaxis was held during foscarnet treatment.
CMV viremia was treated with ganciclovir or valganciclovir, for predefined 8-week courses (2 weeks induction, 6 weeks maintenance). In ganciclovir-resistant or neutropenic patients, foscarnet (or cidofivir) was administered for treatment of CMV [36].
Statistical Analysis
Data on transplant patient characteristics, post-transplantation complications, and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized collection procedures. Patients and transplant characteristics in each cohort were compared using chi-square or Fisher exact test for categorical data and Wilcoxon’s rank sum test for continuous data. All patients were followed up until death or last follow-up. Kaplan-Meier analysis was used to estimate overall survival (OS) [37], and OS comparisons between different groups were completed by using a log-rank test. All statistical analyses were performed with Statistical Analysis System statistical software version 9.2 (SAS Institute, Inc., Cary, NC) with P-value ≤.05 considered to be statistically significant.
RESULTS
Patients and HHV6-Associated Syndromes
The median age of patients was 26 years (range: 1-66); 66% were males (Table 1). The incidence of HHV6 viremia among the screened patients was 56% (46/82), with high-level HHV6 viremia (≥25,000 copies/mL) in 13% (11/82) (Table 1). The median time to viremia was 23 days post-HCT (range: 10-168) (Figure 1). Only 1 patient developed (low-level) HHV6 viremia before engraftment. The majority of positive values, regardless of viral load, occurred just after engraftment at the day +21 time point. No difference in marrow suppression was observed in any group surveyed. The rate of reduction in HHV6 viral load was similar among all patients treated, occurring very early in the course of therapy.
Table 1.
Human Herpesvirus 6 (HHV6) Infection following HCT
| Characteristic | HHV6 Viremia | P* = | High-Level Viremia (≥25,000 Copies/mL) | P† = |
|---|---|---|---|---|
| No. of 82 tested | 46 (56%) | 11 (13%) | ||
| Age | ||||
| Median (range) years | 24 (1-67) | .86 | 12 (1-66) | .06 |
| <18 | 17/31 (55%) | 7/31 (23%) | ||
| ≥18 | 29/51 (57%) | 4/51 (8%) | ||
| Gender | .03 | .06 | ||
| Male | 35/54 (65%) | 10/54 (19%) | ||
| Female | 11/28 (39%) | 1/28 (4%) | ||
| Conditioning regimen | .63 | .85 | ||
| Reduced intensity | 19/32 (59%) | 4/32 (13%) | ||
| Myeloablative | 27/50 (54%) | 7/50 (14%) | ||
| Donor | <.01 | .54 | ||
| Sibling | 3/15 (20%) | 0 | ||
| URD | 6/13 (46%) | 2/13 (15%) | ||
| UCB | 37/54 (69%) | 9/54 (17%) | ||
| CMV | .25 | .29 | ||
| + recipient | 25/40 (63%) | 7/40 (18%) | ||
| − recipient | 21/42 (50%) | 4/42 (10%) |
CMV indicates cytomegalovirus; URD, unrelated donor; UCB, umbilical cord blood.
P-values reflect comparisons between HHV6-positive and HHV6-negative controls.
P-values reflect comparisons between cohorts with low versus high level of HHV6 viremia.
Figure 1.
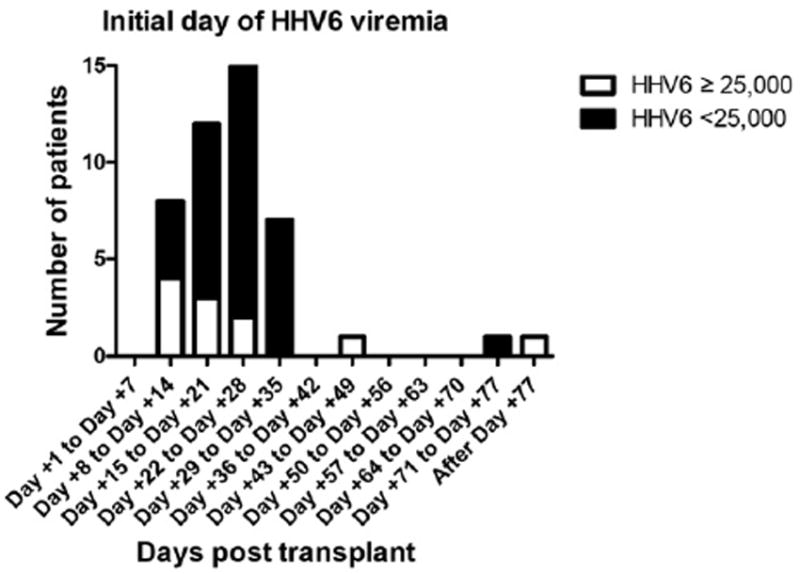
The median time to viremia was 23 days.
HHV6 viremia occurred more commonly in males (65% vs 39% females, P = .03). Viremia developed in 69%, 46%, and 20% patients receiving UCB, unrelated donor (URD), and sibling donor grafts, respectively (P = .01). Conditioning regimen intensity, donor-recipient gender and gender match, age, and CMV-seropositivity in recipients did not affect the incidence of HHV6 viremia (Table 1). There were trends suggested that high-level (>25,000 copies/mL) HHV6 viremia was somewhat more frequent in males and younger patients (Table 1), but these findings must be interpreted cautiously in the modestsized study cohort. UCB recipients did not have more frequent high-level viremia.
All of the patients treated for any level of HHV6 viremia had actual HHV6 disease (viremia + syndrome), that is, no patients were treated for an abnormal PCR in isolation. In fact, all patients had at least 3 symptoms compatible with HHV6 disease. The majority of patients treated for HHV6 with low-level viremia had CMV reactivation, LFT abnormalities, and marrow suppression at the time of therapy. Patients treated for HHV6 associated with high-level viremia had culture-negative pneumonitis, LFT abnormalities, and marrow suppression.
Patients who developed HHV6 viremia had more CMV reactivation (26% vs 5.5%, P = .01) and unexplained fever/rash (24% vs 3%, P = .01) compared to nonviremic patients (Table 2). Lower level HHV6 viremia was associated with slightly more frequent CMV reactivation (28% vs 18%; P = .49) (Table 2). CMV disease, rather than CMV reactivation, only occurred in 4 patients. These 4 patients had concurrent HHV6 viremia as well. For this small group of patients, the duration of foscarnet was extended because of the CMV. Of note, the reduction in HHV6 viral load was similar in both groups, occurring very early in the course of therapy. The remaining 8 patients received either ganciclovir or cidofovir at the discretion of the clinician.
Table 2.
Clinical Complications in Patients With or Without HHV6 Viremia
| No Viremia | HHV6 Viremia | P | Low-Level (<25,000) | High-Level HHV6 (≥25,000/mL) | P | |
|---|---|---|---|---|---|---|
| N | 36 | 46 | 35 | 11 | ||
| Hepatitis | 18 (50%) | 18 (39%) | .33 | 13 (37%) | 5 (45%) | .62 |
| Pneumonitis | 7 (19%) | 16 (35%) | .12 | 8 (23%) | 8 (73%) | <.01 |
| Fever/rash | 1 (3%) | 11 (24%) | .01 | 8 (23%) | 3 (27%) | .76 |
| CNS dysfunction* | 10 (28%) | 8 (17%) | .26 | 6 (17%) | 2 (18%) | .94 |
| CMV reactivation† | 2 (6%) | 12 (26%) | .01 | 10 (29%) | 2 (18%) | .49 |
| Bone marrow suppression/delayed engraftment | 4 (11%) | 7 (15%) | .59 | 6 (17%) | 1 (9%) | .52 |
| Acute GVHD grade II-IV | 7 (19%) | 16 (35%) | .12 | 13 (37%) | 3 (27%) | .55 |
GVHD indicates graft-versus-host disease; CNS, central nervous system; CMV, cytomegalovirus.
Shown is the crude incidence of the stated complications in cohorts with or without HHV6 viremia; or high- versus low-level viremia.
CNS dysfunction included: HHV6− patients, mental status changes 3, headaches 3, seizures 1, paresthesias 1, clonus 1, cerebral edema 1; HHV6 low-level patients, mental status changes 4 and headaches 2; HHV6 high-level patients, mental status changes 2.
CMV disease occurred in 4 patients, all of whom had concurrent HHV6 viremia.
HHV6 viremic patients experienced more rash/fever (24% vs 3%, P = .01) (Table 2), but the incidence of rash/fever was similar in low (23%) or high-level viremia (27%, P = .76). The rates of hepatitis, bone marrow suppression, CNS dysfunction, moderate-severe and aGVHD were similar in the HHV6 viremia positive and negative patients (Table 2). HHV6 syndromes occurred 89%, 100%, and 100% of the patients with HHV6 viremia after UCB, URD, and sibling transplantation, respectively (P = .3). Culture-negative pneumonitis was slightly more frequent in those with high- versus low-level HHV6 viremia (72% vs 22%, P < .01, vs nonviremic patients, 19%). Patients with high versus low levels of HHV6 viremia more often had 2 or more of the listed complications (45% vs 9%, P = .02). Although the incidence of CNS dysfunction was similar among the patients with or without HHV6 reactivation, mental status changes occurred more frequently in patients with any level HHV6 viremia (Table 2, no viremia N = 3, HHV6 viremia N = 6). Four patients with a viral load <25,000 copies/mL developed mental status changes. All patients in the low-level viremia group with mental status changes survived, with 1 patient receiving foscarnet (viral load of 21,000 copies/mL). Two patients with high-level viremia, 27,000 and 38,000 copies, respectively, developed mental status changes requiring foscarnet therapy. The patient with 38,000 copies/mL of HHV6 DNA died despite receiving foscarnet. HHV6 PCR was assessed in the cerebral spinal fluid for 1 patient and was undetectable.
Antiviral Treatment
Of the 46 patients with HHV6 viremia, 20 (43%) received antiviral treatment. Antiviral therapy included ganciclovir (n = 11), foscarnet (n = 8), or cidofovir (n = 1). HHV6 viremia became undetectable in 13 (65%) of the treated and in 8 (31%) of the untreated 26 patients, P = .02. The median time to clearance of the virus was 2 weeks in treated and 1.5 weeks in untreated patients. HHV6 viremia recurred in 6 patients (5 treated and 1 untreated); 5 initially had high-level viremia.
Therapy was given for viremia >25,000 copies of HHV6 DNA. For patients with viremia under 25,000 copies, the administration of antiviral therapy was at the discretion of the attending physician and infectious disease consultants who were following the patient, based on the overall clinical picture and presence of HHV6-compatible symptoms. Among the patients treated for HHV6 viremia <25,000 copies of DNA, the average PCR value was 6500 copies of HHV6 DNA, with a range of 300-21,000 copies. The average peak day of HHV6 reactivation was day +25, with a range of day +11 to day +35. Symptoms primarily included culture negative pneumonitis, transaminitis/hepatitis, and slow engraftment.
The constellation of HHV6-specific symptoms were similar for patients developing high-grade viremia (ie, PCR measuring 25,000 copies of HHV6 DNA or greater). The average peak day of viremia was within the first 2 to 3 weeks posttransplantation. The average duration of persistent viremia of any level, after reaching the threshold of 25,000 copies/mL, was 20 days. Overall, 9 of the 11 patients experienced a decrease in the HHV6 viral load, below the threshold of 25,000 copies, within 1 week of initial testing. Two of these patients did not receive any active therapy for HHV6, whereas the others received foscarnet or ganciclovir. Two patients developed persistent high-grade viremia, with viral loads remaining above 25,000 copies/mL for 9 and 45 days, respectively. The latter patient received ganciclovir and survived.
Recurrent low-level HHV6 viremia occurred more frequently in patients who had previously developed a high initial viremia. We observed that in the posttransplantation setting, 45% of patients with high primary viral loads and only 2% of patients with low primary viral loads experienced a secondary viremia. None of the episodes of recurrent HHV6 viremia reached the 25,000 copies/mL threshold. Interestingly, the majority of patients with recurrent HHV6 viremia received foscarnet during the primary infection, with the exception of the 1 patient with a low-level primary viremia. The average interval between the 2 episodes of posttransplantation HHV6 viremia was 40 days, although the patient with the initial low-level viremia recurred several months after the primary infection. One patient with recurrent viremia died of HHV6 infection, with significant CNS dysfunction and marrow suppression persisting from the time of the initial high-level viremia.
Survival
Forty (49%) of the 82 patients died at a median of 4.5 months (range: 0.7-26.0 months) following HCT. OS was similar for HHV6+ and HHV6− patients at 3 (85% vs 78%, P = .46), 6 (70% vs 72%, P = .89), 12 (63% vs 56%, P = .52), and 24 months (52% vs 53%, P = .93) post-HCT. OS was not influenced by the level of viremia at 3 months (high- vs low-level viremia, 73% vs 89%, P = .23), 6 months (64% vs 71%, P = .54), 12 months (64% vs 63%, P = .88), and 24 months (55% vs 51%, P = .98).
Antiviral Treatment and Survival
Among the 46 patients with HHV6 viremia, survival was similar at 3 months in the treated and the untreated patients (90% vs 81%, P = .4); a trend toward improved OS at 6 months (85% vs 58%, P = 0.06), but no difference at 1 year (54% vs 75%, P = .14) and 2 years (60% vs 46%, P = .29).
DISCUSSION
This prospective observational study revealed that HHV6 reactivation was common (56%) after allogeneic HCT, similar to earlier series reporting a 40% to 60% HHV6 incidence [4,7-9,12]. Viremia occurred early, most often within the first month [5,7,9-11,29,34,38]. Some reports suggest that HHV6 reactivation occurs close to neutrophil engraftment [10], but even with our larger number of UCB graft recipients who often have slower neutrophil recovery and presumably no adoptive anti-HHV6 immunity, we cannot distinguish differences in the time of onset or evolution of any HHV6 syndrome. The rate of reduction in HHV6 viral load was similar among all patients treated, occurring very early in the course of therapy. By giving a short course of therapy early after identification of new high-grade viremia, with a rapid reduction in the viral load, we were able to avoid giving excess treatment to some patients. All of the cases of recurrent HHV6 viremia after foscarnet therapy occurred with low viral loads, suggesting that induction followed by a brief maintenance period is adequate in this setting. The 1 death occurring with low-level recurrent viremia was likely because of persistent end-organ damage from the primary infection.
Reported risk factors associated with HHV6 reactivation are uncertain, but include: younger age [5], underlying disease [5], sex mismatch [5], HLA mismatch [10,11], steroid treatment [5,10], URD [10,11] or UCB grafts [11,15,25,28], and low pre-HCT anti-HHV6 IgG levels [11]. We observed more frequent HHV6 viremia in those receiving UCB grafts and in males. HHV6 infection after UCB transplantation is most likely from endogenous viral reactivation rather than transmission through the graft because HHV6 is rarely detected in UCB [39-41]. Reactivation of CMV, another β-Herpesvirinae family member, was common in patients with HHV6 viremia [42,43]. However, we observed a similar incidence of pretransplantation CMV seropositivity in those with or without HHV6 viremia. The coincidentally recognized CMV reactivation in the HHV6 viremic patients may reflect impaired T cell immunity, a recognized risk factor for reactivation of either virus [42,44-46]. However, in 2 previous independent analyses we noted no increased risk of CMV reactivation in recipients of UCB grafts [47,48]. Moreover, 1 recent report suggested that HHV6, but not CMV infections, may be increased after UCB compared with URD HCT [15]. Anti-HHV6 antibodies are uncommon in UCB compared with adult donor grafts, but the specific mechanisms associating HHV6 reactivation with UCB HCT remain uncertain.
HHV6 infection has been associated with several clinical syndromes, but these associations are only sometimes definitive with viral DNA isolated from the affected tissues, most often marrow or CNS. In the present analysis, mental status changes were the manifestation of CNS dysfunction for 3 of 10 patients with no viremia and for 6 of 8 patients with viremia. (Headaches were the manifestation of CNS dysfunction for 2 of 6 patients with low viremia and for 0 of 2 patients with high-grade viremia.) Some of these syndromes may be associated with a higher viral load as well. We observed that HHV6 viremia occurred in common with CMV reactivation and rash/fever, and that high-level HHV6 patients had more culture-negative pneumonitis.
Only a few studies evaluated treatment of HHV6 following allogeneic HCT [16,34,49]. Zerr et al. [49] treated 11 patients with CNS dysfunction using ganciclovir or foscarnet leading to decreased HHV6 levels in cerebrospinal fluid (CSF), but only 5 patients survived. Ogata et al. [16] treated 6 patients with a high level of HHV6 (>104 copies/mL) with ganciclovir, yet resolution of viremia was neither accelerated nor more common in the treated patients. However, a few patients had developed encephalopathy before detectable plasma HHV6. We observed clearance of HHV6 more commonly in treated patients, but neither viral recurrence nor survival was influenced by antiviral therapy. Recurrence of viremia developed in only 1 of 35 patients with low HHV6 levels, but in 5 of 11 patients with initially high levels.
There remains uncertainty about whether HHV6 viremia increases mortality [1,8,10,11,13]. Most studies, as did ours, found no association between mortality and HHV6 infection [8,10,11,13], likely because of the frequent occurrence of spontaneously resolving viremia and the complex, often multifactorial interaction of immune deficiency, GVHD, and coexisting complications in HCT recipients who develop HHV6 viremia. The pathogenetic association of HHV6 viremia and related complications remains uncertain. Additional prospective studies are required to determine whether anti-HHV6 therapy (empirical or targeting HHV6 disease) clears HHV6 and influences survival for these patients. We suggest, however, that PCR surveillance in higher-risk patients demonstrating otherwise unexplained syndromes compatible with HHV6 is a rationale for therapy. Preemptive therapy in otherwise asymptomatic viremic patients, regardless of degree of viremia, may not be needed.
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
References
- 1.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 2.Campadelli-Fiume G, Mirondaol P, Menoti L. Human Herpesvirus 6: an emerging pathogen. Emerg Infect Dis. 1999;5:353–366. doi: 10.3201/eid0503.990306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadakia MP, Rybka WB, Stewart JA, et al. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation. Blood. 1996;87:5341–5354. [PubMed] [Google Scholar]
- 4.Imbert-Marcille BM, Tang XW, Lepelletier D, et al. Human herpesvirus 6 infection after autologous or allogeneic stem cell transplantation: a single-center prospective longitudinal study of 92 patients. Clin Infect Dis. 2000;31:881–886. doi: 10.1086/318142. [DOI] [PubMed] [Google Scholar]
- 5.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S, Manna P. Quantitative detection and differentiation of human herpesvirus 6 subtypes in bone marrow transplant patients by using a single real-time polymerase chain reaction assay. Biol Blood Marrow Transplant. 2005;11:530–541. doi: 10.1016/j.bbmt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Ihira M, Yoshikawa T, Suzuki K, et al. Monitoring of active HHV-6 infection in bone marrow transplant recipients by real time PCR; comparison to detection of viral DNA in plasma by qualitative PCR. Microbiol Immunol. 2002;46:701–705. doi: 10.1111/j.1348-0421.2002.tb02754.x. [DOI] [PubMed] [Google Scholar]
- 8.Hentrich M, Oruzio D, Jager G, et al. Impact of human herpesvirus-6 after haematopoietic stem cell transplantation. Br J Haematol. 2005;128:66–72. doi: 10.1111/j.1365-2141.2004.05254.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa T, Asano Y, Ihira M, Suzuki K, Ohashi M, et al. Human herpesvirus 6 viremia in bone marrow transplant recipients: clinical features and risk factors. J Infect Dis. 2002;185:847–853. doi: 10.1086/339411. [DOI] [PubMed] [Google Scholar]
- 10.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193:68–79. doi: 10.1086/498531. [DOI] [PubMed] [Google Scholar]
- 11.Yamane A, Mori T, Suzuki S, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant. 2007;13:100–106. doi: 10.1016/j.bbmt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774–781. [PubMed] [Google Scholar]
- 13.Radonic A, Oswald O, Thulke S, et al. Infections with human herpesvirus 6 variant B delay platelet engraftment after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2005;131:480–482. doi: 10.1111/j.1365-2141.2005.05788.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomonari A, Takahashi S, Ooi J, et al. Human herpesvirus 6 variant A infection with fever, skin rash, and liver dysfunction in a patient after unrelated cord blood transplantation. Bone Marrow Transplant. 2005;36:1109–1110. doi: 10.1038/sj.bmt.1705184. [DOI] [PubMed] [Google Scholar]
- 15.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2010;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 16.Ogata M, Satou T, Kawano R, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant. 2008;41:279–285. doi: 10.1038/sj.bmt.1705907. [DOI] [PubMed] [Google Scholar]
- 17.Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:309–317. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]
- 18.Fujimaki K, Mori T, Kida A, et al. Human herpesvirus 6 meningoencephalitis in allogeneic hematopoietic stem cell transplant recipients. Int J Hematol. 2006;84:432–437. doi: 10.1532/IJH97.06072. [DOI] [PubMed] [Google Scholar]
- 19.Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 20.Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, et al. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:705–709. doi: 10.1038/sj.bmt.1705666. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain MC, Chowdhary S. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2008;70:491–492. doi: 10.1212/01.wnl.0000304028.19061.46. [DOI] [PubMed] [Google Scholar]
- 22.Wang FZ, Linde A, Hagglund H, Testa M, Locasciulli A, Ljungman P. Human herpesvirus 6 DNA in cerebrospinal fluid specimens from allogeneic bone marrow transplant patients: does it have clinical significance? Clin Infect Dis. 1999;28:562–568. doi: 10.1086/515142. [DOI] [PubMed] [Google Scholar]
- 23.Drobyski WR, Knox KK, Majewski BS, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus 6 in a bone marrow transplant recipient. N Engl J Med. 1994;330:1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- 24.Cone RW, Huang ML, Corey L, Zeh J, Ashley R, Bowden R. Human herpesvirus 6 infections after bone marrow transplantation: clinical and virologic manifestations. J Infect Dis. 1999;179:311–318. doi: 10.1086/314581. [DOI] [PubMed] [Google Scholar]
- 25.Sashihara J, Tanaka-Taya K, Tanaka S, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. 2002;100:2005–2011. [PubMed] [Google Scholar]
- 26.Yoshikawa T, Suga S, Asano Y, et al. Human herpesvirus-6 infection in bone marrow transplantation. Blood. 1991;78:1381–1384. [PubMed] [Google Scholar]
- 27.Volin L, Lautenschlager I, Juvonen E, Nihtinen A, Anttila VJ, Ruutu T. Human herpesvirus 6 antigenaemia in allogeneic stem cell transplant recipients: impact on clinical course and association with other betaherpesviruses. Br J Haematol. 2004;126:690–696. doi: 10.1111/j.1365-2141.2004.05101.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomonari A, Takahashi S, Ooi J, et al. Human herpesvirus 6 variant B infection in adult patients after unrelated cord blood transplantation. Int J Hematol. 2005;81:352–355. doi: 10.1532/IJH97.04183. [DOI] [PubMed] [Google Scholar]
- 29.Cone RW, Hackman RC, Huang ML, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med. 1993;329:156–161. doi: 10.1056/NEJM199307153290302. [DOI] [PubMed] [Google Scholar]
- 30.Carrigan DR, Drobyski WR, Russler SK, Tapper MA, Knox KK, Ash RC. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991;338:147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- 31.Buchbinder S, Elmaagacli AH, Schaefer UW, Roggendorf M. Human herpesvirus 6 is an important pathogen in infectious lung disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;26:639–644. doi: 10.1038/sj.bmt.1702569. [DOI] [PubMed] [Google Scholar]
- 32.Pereira CM, de Almeida OP, Correa ME, Costa FF, de Souza CA, Barjas-Castro ML. Detection of human herpesvirus 6 in patients with oral chronic graft-vs-host disease following allogeneic progenitor cell transplantation. Oral Dis. 2007;13:329–334. doi: 10.1111/j.1601-0825.2006.01294.x. [DOI] [PubMed] [Google Scholar]
- 33.de Pagter PJ, Schuurman R, Meijer E, van Baarle D, Sanders EA, Boelens JJ. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2008;43:361–366. doi: 10.1016/j.jcv.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 34.de Pagter PJ, Schuurman R, Visscher H, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14:831–839. doi: 10.1016/j.bbmt.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Ljungman P, de la Camara R, Cordonnier C, et al. European Conference on Infections in Leukemia. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42:227–240. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 36.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 38.Boutolleau D, Fernandez C, Andre E, et al. Human herpesvirus-6 and HHV-7: two closely related viruses with different infection profiles in stem cell transplantation recipients. J Infect Dis. 2003;187:179–186. doi: 10.1086/367677. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg A, Enomoto L, Li S, Shen D, Coll J, Shpall EJ. Risk of transmission of herpesviruses through cord blood transplantation. Biol Blood Marrow Transplant. 2005;11:35–38. doi: 10.1016/j.bbmt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Adams O, Krempe C, Kogler G, Wernet P, Scheid A. Congenital infections with human herpesvirus 6. J Infect Dis. 1998;178:544–546. doi: 10.1086/517470. [DOI] [PubMed] [Google Scholar]
- 41.Daibata M, Miyoshi I. Presence of human herpesvirus 6 DNA in cord blood cells. J Infect Dis. 1999;179:1046–1047. doi: 10.1086/314687. [DOI] [PubMed] [Google Scholar]
- 42.Tormo N, Solano C, de la Cámara R, et al. An assessment of the effect of human herpesvirus-6 replication on active cytomegalovirus infection after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:653–661. doi: 10.1016/j.bbmt.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Humar A, Malkan G, Moussa G, Greig P, Levy G, Mazzulli T. Human herpesvirus-6 is associated with cytomegalovirus reactivation in liver transplant recipients. J Infect Dis. 2000;181:1450–1453. doi: 10.1086/315391. [DOI] [PubMed] [Google Scholar]
- 44.Brown J, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstituion. Clin Immunol. 2008;127:286–297. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabolcs P, Niedzwiecki D. Immune reconstitution in children after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trivedi HN, Hay Glass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 47.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:309–317. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]


