Abstract
Genome-wide association studies have identified associations between type 1 diabetes and single nucleotide polymorphisms (SNPs) at chromosome 12q13, surrounding the gene ERBB3. Our objective was to fine map this region to further localize causative variants. Re-sequencing identified more than 100 putative SNPs in an 80 kb region at 12q13. By genotyping 42 SNPs, spanning approximately 214 kb, in 382 affected sibling pair type 1 diabetes families, we were able to genotype or tag 67 common SNPs (MAF ≥ 0.05) identified from HapMap CEU data and CEU data from the 1000 Genomes Project, plus additional rare coding variants identified from our re-sequencing efforts. Fifteen SNPs provided nominal evidence for association (P≤ 0.05) with type 1 diabetes. The most significant associations were observed with rs2271189 (P = 4.22×10−5), located in exon 27 of the ERBB3 gene, and an intergenic SNP rs11171747 (P= 1.70×10−4). Follow-up genotyping of these SNPs in 2 740 multiplex type 1 diabetes families validated these findings.
After analyzing variants spanning more than 200 kb, we have replicated associations from previous GWAS and provide evidence for novel associations with type 1 diabetes. The associations across this region could be entirely accounted for by two common SNPs, rs2271189 and rs11171747.
Keywords: ERBB3, Association, Gene, Type 1 Diabetes, 12q13
Introduction
Type 1 diabetes is an autoimmune disease resulting from the actions of both genetic and environmental risk factors (1). Genome wide association studies (GWAS), have identified a substantial number of loci contributing to type 1 diabetes disease susceptibility (2–7), including a location on chromosome 12q13, near the gene ERBB3.
The first report of association between SNPs at chromosome 12q13 and type 1 diabetes identified a highly significant association with SNP rs11171739, located between the RPS26 and ERBB3 genes (2). Subsequent studies, including a meta analysis, have replicated the reported association at rs11171739 and identified a second, more significantly associated SNP, rs2292239, located in intron 7 of ERBB3 (6–8). Based upon CEU data from the International HapMap project (9), alleles at rs11171739 and rs2292239 are correlated (r2 = 0.71) and are located within a block of significant linkage disequilibrium (LD) that includes a number of additional SNPs. Although these two SNPs in or near ERBB3 provide the strongest evidence for association with type 1 diabetes, the strong LD across flanking genes leaves unresolved which genes in the 12q13 region may be involved in type 1 diabetes risk. The candidate genes include ERBB3, IKZF4, RPS26, and PA2G4.
ERBB3 encodes a member of the epidermal growth factor receptor family of receptor tyrosine kinases that is capable of ligand binding but lacks an active kinase domain. Heterodimerization of the ERBB3 protein with other members of the epidermal growth factor family results in the activation of pathways resulting in cell proliferation and differentiation. ERBB3 is also known to interact with several proteins in lymphocytes that are involved in cell signaling such as the Syk kinases, ZAP70, and SH2B3 (10). SH2B3 is, itself, also a candidate type 1 diabetes susceptibility gene based on GWAS data (8). Previous studies suggest that insulin deficiencies increase ERBB3 mRNA and protein levels (11). The protein encoded by IKZF4 is a member of the Ikaros family of DNA-binding proteins that are required for correct development of B and T lymphocytes (12). RPS26 gene expression has been suggested to influence risk for developing type 1 diabetes (13–14). The product of the fourth gene in this region, PA2G4, is ERBB3-binding protein 1 (EBP1) which interacts with the cytoplasmic domain of the ERBB3 receptor and may contribute to the transduction of growth regulatory signals (15).
The distribution of confirmed SNPs in the ERBB3 region is atypically sparse. Therefore, to identify potentially disease-associated SNPs from the 12q13 region, we carried out re-sequencing of the region in type 1 diabetes patients, and extracted and evaluated sequence and SNP data from both HapMap and 1000 Genomes Project (16) testing the SNPs identified for association with type 1 diabetes.
Results
Re-sequencing of an 80 kb region at 12q13 identified 113 SNPs, of which 68 SNPs were present in dbSNP (Build 129). Among those SNPs not present in dbSNP, ten were also detected in the 1000 Genomes project data, while 35 were unique to this study (Supplementary Table 1). Of the 113 variants identified by sequencing, only five were coding variants (rs2271189, rs773123, Novel ERBB3 Exon 6 (A/G), rs2229045, and rs2229046).
Collectively, 78 SNPs were present in data from either HapMap or the 1000 Genomes project. Twenty-six of these SNPs were located in repetitive sequence or ambiguously mapped to the reference genome. Three of the known SNPs (rs66911160, rs61003310, and rs72247105) were insertions/deletions (indels). These indels are present but not validated in dbSNP, also mapped to repetitive sequence, and were detected only as discrepancies observed in assembling the reference sequence. These indels were not amenable to SNP based genotyping, therefore they were excluded from further analyses.
A total of 42 SNPs were genotyped in 1,744 individuals from 382 type 1 diabetes multiplex families. Excluding SNPs that were not designable on Illumina or Nanogen genotyping platforms, we were able to genotype or tag 67 common variants (MAF ≥ 0.05) identified from HapMap CEU data and CEU data from the 1000 Genomes Project, plus additional rare coding variants identified from our re-sequencing efforts. After data cleaning, four of these SNPs (rs10876864, rs1131017, rs3759094, rs773121) were eliminated from further analyses due to excessive numbers of Mendelian errors, indicative of a failed assay. The remaining 38 SNPs were tested for association with type 1 diabetes. Evidence of association with type 1 diabetes was observed for fifteen SNPs, with P-values ranging from 0.0219 – 4.22×10−5 (Table 1). The most significant associations were observed with rs2271189 (P = 4.22×10−5), a synonymous coding SNP located in exon 27 of ERBB3, and an intergenic SNP rs11171747 (P = 1.70×10−4) located approximately 21 kb 3′ of ERBB3.
Table 1.
PLINK TDT association results for the 15 significantly associated SNPs in the screening panel of affected sibling pair families.
| Marker | Position | Gene | Minor Allele Frequency | Transmitted Minor Allele Count | Untransmitted Allele Count | TDT P-Value | Odds Ratio (95% CI) | rs2271189 Adjusted P-Value | rs11171747 Adjusted P-Value |
|---|---|---|---|---|---|---|---|---|---|
| rs773107 | 54655773 | RAB5B | 0.32 | 348 | 282 | 0.0086 | 1.23 (1.05 – 1.44) | 0.8712 | 0.5505 |
| rs1701704 | 54698754 | Intergenic | 0.34 | 358 | 286 | 0.0046 | 1.25 (1.07 – 1.46) | 0.7606 | 0.4306 |
| rs2456973 | 54703195 | IKZ4 | 0.34 | 357 | 283 | 0.0034 | 1.26 (1.08 – 1.47) | 0.7048 | 0.3831 |
| ERBB3rsx29 | 54714511 | IKZ4 | 0.09 | 134 | 99 | 0.0219 | 1.35 (1.04 – 1.76) | 0.9678 | 0.5237 |
| rs705704 | 54721679 | Intergenic | 0.34 | 264 | 196 | 0.0015 | 1.35 (1.12 – 1.62) | 0.4748 | 0.2061 |
| rs11171739 | 54756892 | Intergenic | 0.42 | 386 | 321 | 0.0145 | 1.20 (1.04 – 1.39) | 0.2563 | 0.4860 |
| rs2271194 | 54763961 | ERBB3 | 0.42 | 378 | 314 | 0.0150 | 1.20 (1.04 – 1.40) | 0.3413 | 0.6014 |
| rs10876870 | 54764269 | ERBB3 | 0.42 | 390 | 322 | 0.0108 | 1.21 (1.05 – 1.40) | 0.2511 | 0.5115 |
| ERBB3rsx44 | 54766741 | ERBB3 | 0.09 | 127 | 93 | 0.0219 | 1.37 (1.05 – 1.78) | 0.9782 | 0.4762 |
| rs2292239 | 54768447 | ERBB3 | 0.35 | 350 | 288 | 0.0141 | 1.22 (1.04 – 1.42) | 0.8289 | 0.5070 |
| rs705708 | 54775180 | ERBB3 | 0.49 | 411 | 315 | 3.67×10−4 | 1.31 (1.13 – 1.51) | 0.8417 | 0.2842 |
| rs2292238 | 54780089 | ERBB3 | 0.45 | 351 | 274 | 0.0021 | 1.28 (1.10 – 1.50) | 0.0824 | 0.2518 |
| rs2271189 | 54781258 | ERBB3 | 0.43 | 388 | 282 | 4.22×10−5 | 1.38 (1.18 – 1.60) | 1.0000 | 0.0721 |
| rs11171747 | 54804675 | Intergenic | 0.36 | 278 | 374 | 1.70×10−4 | 0.74 (0.64 – 0.87) | 0.1132 | 1.0000 |
| rs7960225 | 54851077 | SMARCC2 | 0.34 | 264 | 333 | 0.0047 | 0.79 (0.67 – 0.93) | 0.1690 | 0.6075 |
Adjusted P-Values are calculated using the Main Effects option implemented in the program UNPHASED. SNPs denoted with ERBB3rsx represent novel SNPs identified in 1000 Genomes data, not present in dbSNP.
Conditional analysis, using the “main effects” test of Cordell and Clayton (22), as implemented in the program UNPHASED (23), was used to evaluate the independence of the observed associations with type 1 diabetes. Upon conditioning on either rs2271189 or rs11171747, no additional SNPs provided residual evidence of association with type 1 diabetes (Table 1). To replicate our initial observations we further tested rs2271189 and rs11171747 for association in our complete set of 2,740 type 1 diabetes families. The evidence for association with type 1 diabetes for both SNPs was, again, significant (rs2271189 P= 1.06×10−9, OR= 1.23 (1.15–1.32); rs11171747 P=4.24×10−11, OR= 0.79 (0.74–0.85)) (Table 2). Conditional analyses in this larger dataset indicate that the two SNPs are independently associated with type 1 diabetes.
Table 2.
PLINK TDT association results for rs2271189 and rs11171747 in the complete data set (2,740 type 1 diabetes families).
| Marker | Minor Allele Frequency | Transmitted Minor Allele Count | Untransmitted Allele Count | TDT P- Value | Odds Ratio (95% CI) | rs2271189 Adjusted P- Value | rs11171747 Adjusted P- Value |
|---|---|---|---|---|---|---|---|
| rs2271189 | 0.43 | 1871 | 1516 | 1.06×10−9 | 1.23 (1.15–1.32) | 1.0000 | 0.0110 |
| rs11171747 | 0.36 | 1437 | 1813 | 4.24×10−11 | 0.79 (0.74–0.85) | 0.0301 | 1.0000 |
Adjusted P-Values are calculated using the Main Effects option implemented in the program UNPHASED.
Discussion
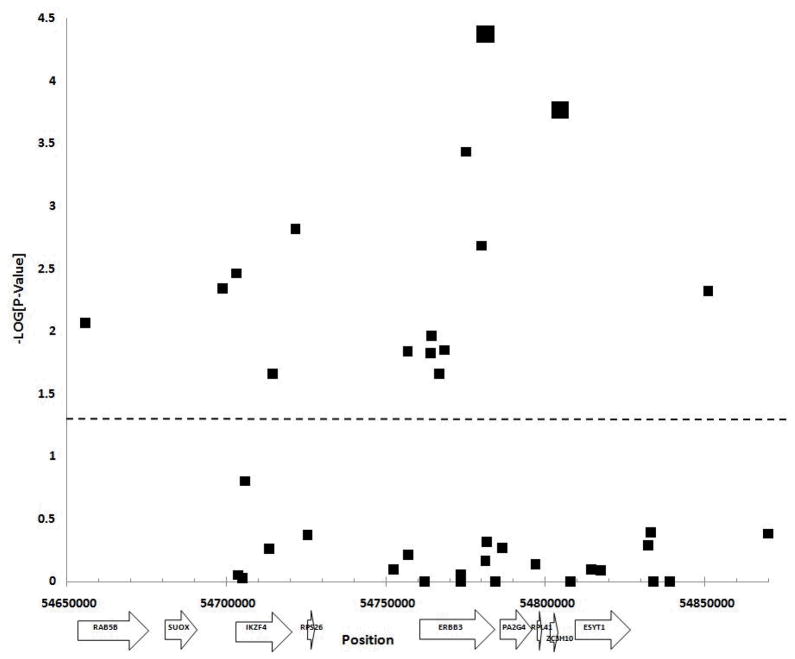
We have investigated genetic variation across approximately 214 kb surrounding the ERBB3 gene at 12q13, in 382 affected sibling pair type 1 diabetes families from the Type 1 Diabetes Genetics Consortium in order to fine map potential risk variants. The association of this region with type 1 diabetes risk is already well established at genome-wide significance levels, and there is similar evidence for association in this region with rheumatoid arthritis and thyroiditis (2, 24–25). Evidence of association with type 1 diabetes was observed for fifteen of the 42 SNPs genotyped (Figure 1). The most statistically significant associations were observed with a synonymous coding SNP in exon 27 of ERBB3 and an intergenic SNP. Alleles at the two SNPs are common, with MAF >0.35 and are modestly correlated (r2= 0.42). Although eight additional SNPs located outside of the coding region of ERBB3 were nominally associated with type 1 diabetes, upon conditioning on either rs2271189 or rs11171747, there was no significant residual evidence of association at these SNPs. This included rs2292239 and rs11171739 that were reported to be associated with type 1 diabetes in prior GWAS.
Figure 1. Plot for Association with T1D.
Association plot for single SNP associations with type 1 diabetes. The SNP position and -LOG (P-Value) are plotted on the X and Y axis respectively. All symbols above the dashed line represent a significant association (P ≤ 0.05). SNPs rs2271189 and rs11171747 are represented by larger black squares.
The distinct locations of the two implicated SNPs (23 kb apart), one within the coding region of ERBB3 and one located in the intergenic region between ZC3H10 and ESYT1, as well as the residual evidence of association at each SNP after conditioning on the other, suggests that the two SNPs have at least some independent effects on risk. Although rs2271189 is located within the coding region of ERBB3, it does not alter the amino acid sequence. Thus, if it is acting to affect ERBB3 function, the e most plausible explanation is via an effect on mRNA expression, splicing, or stability. The second SNP is not located within a known coding region.
Previous studies searching for loci affecting the quantitative expression of genes on a genome-wide basis without particular reference to type 1 diabetes (http://eqtl.uchicago.edu) have identified 21 variants within the 12q13 region of interest, as cis-acting expression quantitative trait loci (eQTLs). Of these 21 SNPs, 16 were genotyped or tagged by SNPs present in our study. Although neither rs2271189 nor rs11171747 were among the previously identified eQTLs, there is detectable linkage disequilibrium between alleles at these SNPs and four eQTL variants (r2 = 0.22–0.39). Notably, a number of these reported eQTL, while physically located within the ERBB3 gene, affect expression of nearby flanking genes, in particular the RPS26 gene. These reports suggest that caution is appropriate in interpreting the locations of the disease associated SNPs identified here with regard to their possible effects on the expression of specific genes.
In summary, in the current study we evaluated variants spanning approximately 214 kb of the IKZF4-RPS26-ERBB3-PA2G4 gene region to further refine the previously reported regional association with type 1 diabetes susceptibility. Two SNPs were identified that display significantly stronger associations with type 1 diabetes than those reported in prior GWAS studies. Alleles at these two SNPs are only modestly correlated and conditional analyses suggest that they may confer independent effects on risk for type 1 diabetes. The nature of the two SNPs, one coding but synonymous and the other intergenic, suggest that their most likely mechanism for affecting disease risk would be through effects on gene expression.
Materials and Methods
Subjects
This study was conducted under Institutional Review Board approval from the University of Virginia. DNA from 192 samples, recruited from both the Virginia Mason Medical Center and Puget Sound Blood Center (Seattle, WA, USA) was utilized for next generation DNA sequencing. Affected sibling pair (ASP) families obtained from the Type 1 Diabetes Consortium (T1DGC) and Human Biological Data Interchange (HBDI) repository (17) were used for subsequent association studies. Information regarding identification, clinical characteristics, and recruitment of the type 1 diabetes ASP families, is accessible from the T1DGC web site (www.t1dgc.org).
Re-sequencing and SNP selection
The International HapMap project (HapMap B35) (9) genotyped 25 common SNPs (MAF>0.05), spanning approximately 96 kb of the 12q13 region, in the CEU population. Using a pairwise tagging approach (r2 threshold = 0.8) in Haploview 3.2 (18), nine tagging SNPs capture the 25 SNPs, with an average r2 = 0.958.
Due to the limited number of common SNPs genotyped in the HapMap CEU population, next generation sequencing was utilized to identify additional SNPs throughout the region. DNA pooling and long range PCR (Roche Diagnostics, Indianapolis IN, USA) approaches were used to isolate an 80 kb region encompassing ERBB3, IKZF4, RPS26 and PA2G4. Eight PCR primer pairs were designed to amplify overlapping fragments ~10kb in size, spanning the 80 kb region, in forty-eight DNA pools, each containing DNA from 4 individuals (192 individuals total). The PCR products were then mixed in equimolar amounts, sheared by sonication and adaptors added.
The resulting sequencing library had an average fragment size of 550 bp. Paired-end, 35 bp sequencing was performed using the Genome AnalyzerIIx (Illumina, Inc. San Diego, CA, USA). The resulting paired-end sequences (readpairs) were aligned to the human reference genome (Build 36) using the program Mosaik (unpublished; http://code.google.com/p/mosaik-aligner/). To minimize false positive SNPs, subsequent analyses were restricted to readpairs that aligned uniquely to the targeted region. SNPs were identified among the aligned readpairs using a custom script based upon the SAMTOOLS “pileup” program (19). For a given SNP to be included in our analyses, we required a minimum posterior SNP probability of 0.99 (i.e., pileup SNP quality of at least 20) and at least ten aligned reads at the polymorphic locus.
Additional novel SNPs were identified from 1000 Genomes Project data (denoted with the prefix ERBB3rsx). Sequencing data, derived from 36 unrelated CEU individuals, were downloaded from ftp://ftp-trace.ncbi.nih.gov/1000genomes/ftp/release/2009_02/Pilot1/ (February 2009 data release). Custom scripts were developed which extracted 65 common SNPs across 12q13 (i.e. chromosome 12, from 54.6Mb to 54.9Mb).
Genotyping
Forty-two SNPs were genotyped in 382 affected sibling pair type 1 diabetes families using Eclipse (Nanogen) and Illumina GoldenGate BeadXpress (Illumina Inc,) genotyping platforms. SNPs with poor clustering or significant deviations from Hardy-Weinberg equilibrium were excluded from further analysis. The two most significant SNPs were further genotyped for replication purposes in the complete set of 2,740 type 1 diabetes families.
Statistical analyses
Families with Mendelian errors, as determined using Pedcheck (20), were zeroed out prior to performing tests for association, using the TDT option in PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) (21). The “main effects” test of Cordell and Clayton (22), as implemented in the program UNPHASED (23), was used to perform association tests conditioning on markers rs2271189 and rs11171747.
Supplementary Material
Acknowledgments
This research utilizes resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD), and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. This work was supported by grant DK46635 and a Juvenile Diabetes Research Foundation Postdoctoral Fellowship (KLK).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at Genes and Immunity’s website.
References
- 1.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 2.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, et al. A genome-wide association of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JD, Smyth DJ, Smiles AM, Plagnol V, Walker NM, Allen JE, et al. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet. 2008;40:1399–1401. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 6.Hakonarson H, Qu HQ, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. A Novel Susceptibility Locus for Type 1 Diabetes on Chr12q13 identified by a Genome-Wide Association Study. Diabetes. 2008;57:1143–1146. doi: 10.2337/db07-1305. [DOI] [PubMed] [Google Scholar]
- 7.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15:413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carver RS, Mathew PM, Russell WE. Hepatic expression of ErbB3 is repressed by insulin in a pathway sensitive to PI-3 kinase inhibitors. Endocrinology. 1997;138:5195–5201. doi: 10.1210/endo.138.12.5601. [DOI] [PubMed] [Google Scholar]
- 12.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 13.Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plagnol V, Smyth DJ, Todd JA, Clayton DG. Statistical independence of the colocalized association signals for type 1 diabetes and RPS26 gene expression on chromosome 12q13. Biostatistics. 2009;10:327–334. doi: 10.1093/biostatistics/kxn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, et al. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lernmark A, Ducat L, Eisenbarth G, Ott J, Permutt MA, Rubenstein P, et al. Family cell lines available for research. Am J Hum Genet. 1990;47:1028–1030. [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordell HJ, Clayton DG. A unified stepwise regression procedure for evaluating the relative effects of polymorphisms with a gene using case/control or family data: application to HLA in type 1 diabetes. Am J Hum Genet. 2002;70:124–141. doi: 10.1086/338007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–122. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- 24.Barton A, Thomson W, Ke X, Eyre S, Hinks A, Bowes J, et al. Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat Genet. 2008;40:1156–1159. doi: 10.1038/ng.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awata T, Kawasaki E, Tanaka S, Ikegami H, Maruyama T, Shimada A, et al. Association of type 1 diabetes with two loci on 12q13 and 16p13 and the influence coexisting thyroid autoimmunity in Japanese. J Clin Endocrinol Metab. 2009;94:231–235. doi: 10.1210/jc.2008-0718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.