Abstract
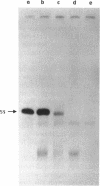
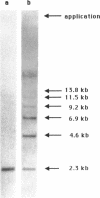
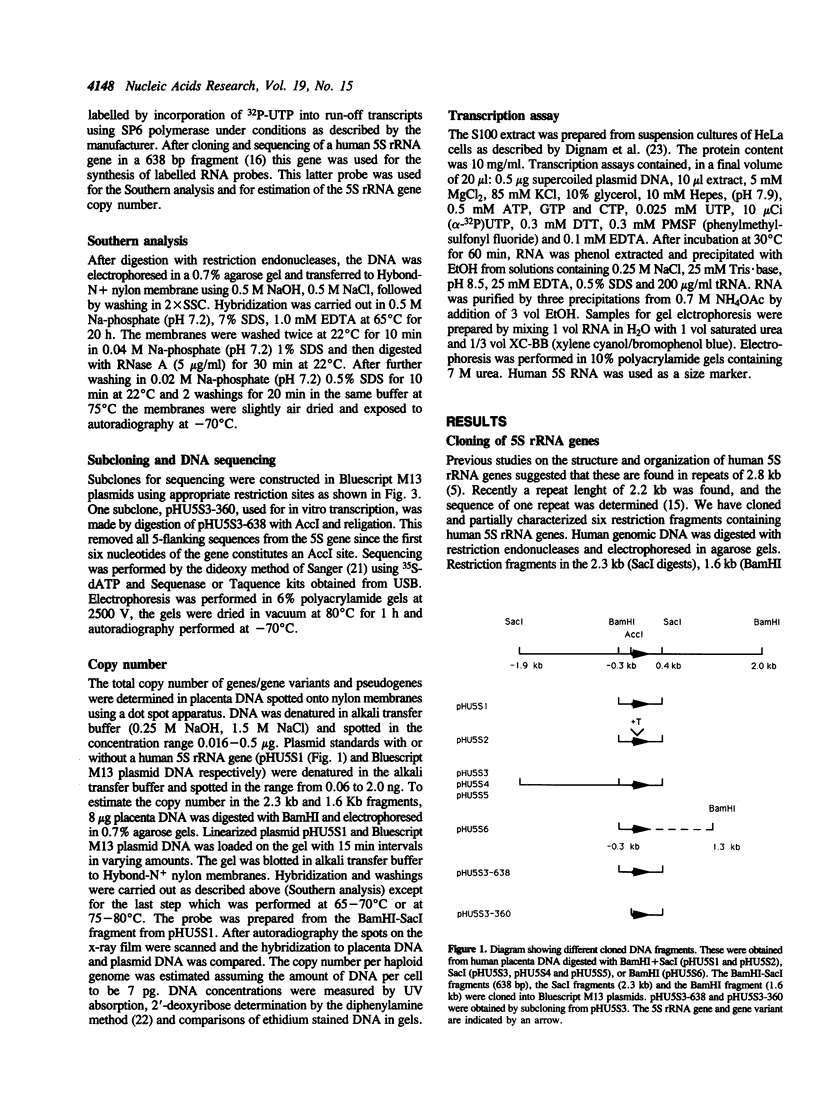
The human 5S rRNA genes are found in clusters of tandem repeated units. We have cloned and partially characterized six restriction fragments from two clusters of 2.3 kb and 1.6 kb repeats, respectively. Four fragments from the cluster of 2.3 kb repeats contain a 5S rRNA gene and one fragment contains a gene variant with an additional nucleotide in the internal control region. A fragment from the 1.6 kb cluster contains a gene and is highly homologous to the 2.3 kb repeats, except for a large deletion in the 3'-flanking region starting 12 bp downstream of the gene. The number of genes and closely related gene variants is found to be 300-400 per haploid human genome. 100-150 of these are found in 2.3 kb repeats and 5-10 are found in 1.6 kb repeats. The total number of 5S rRNA sequences, including pseudogenes, is 1700-2000 per haploid genome. The genes and the gene variant are transcribed equally efficient in a HeLa cell extract. If 5'-flanking sequences, including a GC-motif in the -40 region, are removed from the genes, transcription is reduced with a factor 10 or more, suggesting that sequences upstream of the coding region are important for the level of transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold G. J., Kahnt B., Herrenknecht K., Gross H. J. A variant gene and a pseudogene for human 5S RNA are transcriptionally active in vitro. Gene. 1987;60(1):137–144. doi: 10.1016/0378-1119(87)90221-6. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Schedl P., Tschudi C., Pirrotta V., Steward R., Gehring W. J. The 5S genes of Drosophila melanogaster. Cell. 1977 Dec;12(4):1057–1067. doi: 10.1016/0092-8674(77)90169-6. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran J. L., Bingle W. H., Roy K. L. The nucleotide sequences of two human 5S rRNA pseudogenes. Nucleic Acids Res. 1987 Aug 11;15(15):6297–6297. doi: 10.1093/nar/15.15.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Roeder R. G. Isolation and genomic arrangement of active and inactive forms of mammalian 5 S RNA genes. J Biol Chem. 1984 Jun 25;259(12):7916–7925. [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Hart R. P., Folk W. R. Structure and organization of a mammalian 5 S gene cluster. J Biol Chem. 1982 Oct 10;257(19):11706–11711. [PubMed] [Google Scholar]
- Hatlen L., Attardi G. Proportion of HeLa cell genome complementary to transfer RNA and 5 s RNA. J Mol Biol. 1971 Mar 28;56(3):535–553. doi: 10.1016/0022-2836(71)90400-1. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Shu M. D. Epstein-Barr virus small RNA (EBER) genes: unique transcription units that combine RNA polymerase II and III promoter elements. Cell. 1989 Jun 2;57(5):825–834. doi: 10.1016/0092-8674(89)90797-6. [DOI] [PubMed] [Google Scholar]
- Korn L. J. Transcription of Xenopus 5S ribosomal RNA genes. Nature. 1982 Jan 14;295(5845):101–105. doi: 10.1038/295101a0. [DOI] [PubMed] [Google Scholar]
- Lazar E., Haendler B., Jacob M. Two 5S genes are expressed in chicken somatic cells. Nucleic Acids Res. 1983 Nov 25;11(22):7735–7741. doi: 10.1093/nar/11.22.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah R., Frederiksen S., Engberg J., Sørensen P. D. Nucleotide sequence of a mouse 5S rRNA variant gene. Nucleic Acids Res. 1990 Dec 25;18(24):7441–7441. doi: 10.1093/nar/18.24.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. D., Braaten D. C. Genomic organization of human 5 S rDNA and sequence of one tandem repeat. Genomics. 1989 Apr;4(3):376–383. doi: 10.1016/0888-7543(89)90345-5. [DOI] [PubMed] [Google Scholar]
- Mao J., Appel B., Schaack J., Sharp S., Yamada H., Söll D. The 5S RNA genes of Schizosaccharomyces pombe. Nucleic Acids Res. 1982 Jan 22;10(2):487–500. doi: 10.1093/nar/10.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. G., Sprague K. U. In vitro transcription of a silkworm 5S RNA gene requires an upstream signal. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5519–5522. doi: 10.1073/pnas.81.17.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietfeld W., Digweed M., Mentzel H., Meyerhof W., Köster M., Knöchel W., Erdmann V. A., Pieler T. Oocyte and somatic 5S ribosomal RNA and 5S RNA encoding genes in Xenopus tropicalis. Nucleic Acids Res. 1988 Sep 26;16(18):8803–8815. doi: 10.1093/nar/16.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei S. L., Pieler T. A transcription stimulatory factor binds to the upstream region of Xenopus 5 S RNA and tRNA genes. J Biol Chem. 1990 May 5;265(13):7485–7491. [PubMed] [Google Scholar]
- Reddy R., Henning D., Rothblum L., Busch H. Some gene variants for 5 S RNA are dispersed in the rat genome. J Biol Chem. 1986 Aug 15;261(23):10618–10623. [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifart K. H., Wang L., Waldschmidt R., Jahn D., Wingender E. Purification of human transcription factor IIIA and its interaction with a chemically synthesized gene encoding human 5 S rRNA. J Biol Chem. 1989 Jan 25;264(3):1702–1709. [PubMed] [Google Scholar]
- Selker E. U., Yanofsky C., Driftmier K., Metzenberg R. L., Alzner-DeWeerd B., RajBhandary U. L. Dispersed 5S RNA genes in N. crassa: structure, expression and evolution. Cell. 1981 Jun;24(3):819–828. doi: 10.1016/0092-8674(81)90107-0. [DOI] [PubMed] [Google Scholar]
- Sharp S. J., Garcia A. D. Transcription of the Drosophila melanogaster 5S RNA gene requires an upstream promoter and four intragenic sequence elements. Mol Cell Biol. 1988 Mar;8(3):1266–1274. doi: 10.1128/mcb.8.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., Garcia A., Cooley L., Söll D. Transcriptionally active and inactive gene repeats within the D. melanogaster 5S RNA gene cluster. Nucleic Acids Res. 1984 Oct 25;12(20):7617–7632. doi: 10.1093/nar/12.20.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen P. D., Simonsen H., Frederiksen S. Nucleotide sequence of a human 5S rRNA gene. Nucleic Acids Res. 1990 May 25;18(10):3060–3060. doi: 10.1093/nar/18.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. M. Transcription of Neurospora crassa 5 S rRNA genes requires a TATA box and three internal elements. J Mol Biol. 1987 Aug 20;196(4):801–811. doi: 10.1016/0022-2836(87)90406-2. [DOI] [PubMed] [Google Scholar]
- Wingender E., Frank R., Blöcker H., Wang L. R., Jahn D., Seifart K. H. Complete synthesis and transcription in vitro of a gene coding for human ribosomal 5S RNA. Gene. 1988 Apr 15;64(1):77–85. doi: 10.1016/0378-1119(88)90482-9. [DOI] [PubMed] [Google Scholar]
- Wormington W. M., Bogenhagen D. F., Jordan E., Brown D. D. A quantitative assay for Xenopus 5S RNA gene transcription in vitro. Cell. 1981 Jun;24(3):809–817. doi: 10.1016/0092-8674(81)90106-9. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]