Abstract
Aberrant Wnt/β-catenin signaling is widely implicated in numerous malignancies, including cancers of the gastrointestinal (GI) tract. Dysregulation of signaling is traditionally attributed to mutations in Axin, APC (adenomatous polyposis coli), and β-catenin that lead to constitutive hyperactivation of the pathway. However, Wnt/β-catenin signaling is also modulated through various other mechanisms in cancer, including crosstalk with other altered signaling pathways. A more complex view of Wnt/β-catenin signaling and its role in GI cancers is now emerging as divergent phenotypic outcomes are found to be dictated by temporospatial context and relative levels of pathway activation. This review summarizes the dysregulation of Wnt/β-catenin signaling in colorectal carcinoma, hepatocellular carcinoma, and pancreatic ductal adenocarcinoma, with particular emphasis on the latter two. We conclude by addressing some of the major challenges faced in attempting to target the pathway in the clinic.
The Wnt/β-catenin signaling pathway plays a pivotal role in regulating cellular processes involved in development, differentiation, and adult tissue homeostasis.1 Aberrant Wnt/β-catenin signaling is also widely implicated in cancer and other disease states.2, 3 Since the molecular aspects of Wnt/β-catenin signaling have been the subject of numerous comprehensive reviews,4–7 here we focus on the similarities and differences of this pathway in the context of three specific GI cancers: colorectal carcinoma (CRC), hepatocellular carcinoma (HCC), and pancreatic ductal adenocarcinoma (PDAC). These tumor types illustrate how the timing, pattern, and levels of Wnt/β-catenin signaling impact normal and cancerous cells in different tissues, providing a framework for understanding the complexities faced in attempting leverage this pathway in the clinic.
An overview of the Wnt/β-catenin signaling pathway
The portmanteau “Wnt”, derived from the Drosophila gene for wingless (wg) and the murine oncogene int-1, was coined after the seminal discovery that these two genes were in fact conserved orthologs.8 This finding facilitated our present understanding that dysregulation of pathways directing the specification of normal adult structures is involved in critical aspects of oncogenesis and cancer progression.
Wnt/β-catenin signaling is highly conserved from nematodes to humans, and has been reviewed in detail in numerous publications.1 At the core of this pathway is the versatile and tightly regulated protein β-catenin, encoded by CTNNB1. β-catenin is variably detected in three distinct pools: 1) at cellular adherens junctions, where it directly interacts with E-cadherin; 2) in the cytosolic space; and 3) in the nucleus. In the absence of activated Wnt/β-catenin signaling, cytosolic β-catenin is rapidly phosphorylated by a complex of proteins collectively termed the “destruction complex”, comprised of the core proteins AXIN, APC (adenomatous polyposis coli), GSK3 (glycogen synthase kinase), and CK1 (casein kinase 1).7 The destruction complex phosphorylates the N-terminus of β-catenin,9 targeting the protein for proteasomal degradation and thereby maintaining low baseline cytosolic levels. The binding of certain “canonical” Wnt ligand isoforms1 to cognate receptors of the Fzd (frizzled) and LRP (low-density lipoprotein receptor-related protein) families inhibits β-catenin phosphorylation, thereby allowing β-catenin to escape degradation, accumulate in the cytosol, and translocate to the nucleus (Figure 1). In the nucleus, β-catenin interacts primarily with members of the TCF/LEF family of transcription factors to trans-activate target genes. By influencing diverse cellular processes including differentiation, proliferation, migration, and adhesion, these target genes mediate the effects of Wnt/β-catenin signaling in normal and diseased cells. The binding of “non-canonical” Wnt ligand isoforms to Fzd or alternative receptors including receptor tyrosine kinase-like orphan receptor 2 (Ror2) separately regulates cell polarity, asymmetric cell division, and developmental morphogenesis in a β-catenin-independent manner.1 While acknowledging that β-catenin-independent signaling plays important roles in tumor progression,10, 11 this review focuses primarily on “canonical” Wnt signaling, perhaps more accurately denoted as “Wnt/β-catenin-dependent signaling.”
Figure 1.
Schematic illustrating the Wnt/β-catenin pathway. (A) In the absence of a Wnt signal, β-catenin is bound to E-Cadherin (E-CAD) at adherens junctions or is phosphorylated by a destruction complex comprised of the core proteins AXIN, APC (adenomatous polyposis coli), GSK3 (glycogen synthase kinase), and CK1 (casein kinase 1). N-terminal phosphorylated β-catenin is targeted for ubiquitination and subsequent proteasomal degradation, maintaining low levels of cystosolic and nuclear β-catenin. Expression of Wnt/β-catenin target genes via activation of TCF/LEF (T-cell factor/lymphoid enhancer factor) transcription factors is inhibited by the transcriptional repressor Groucho. (B) Wnt ligand initiates signaling through FZD (Frizzled) receptor and LRP (low-density lipoprotein receptor-related protein) co-receptor, activating and recruiting DVL (Dishevelled) and Axin to the membrane, thereby disrupting the destruction complex. Higher cytosolic levels of β-catenin result in its translocation into the nucleus, where it binds TCF/LEF transcription factors and displaces Groucho to trans-activate Wnt/β-catenin target gene expression.
Our understanding of Wnt/β-catenin signaling continues to evolve with technological advances and the further identification of novel regulators of this pathway. Traditionally, the pathway has been shown to be dysregulated in multiple ways, including genetic alterations of core signaling components or misexpression of Wnt ligands and secreted inhibitors of the pathway (Figure 2). While this traditional view of Wnt/β-catenin pathway regulation is often depicted as a linear set of defined events, the advent of systems biology and high throughput genetic and proteomic techniques have revealed that Wnt/β-catenin signaling is further modulated by countless protein interactions at various levels, including the extracellular environment, membrane, cytoplasm, and nucleus.12–14 Crosstalk with other signaling pathways further influence Wnt/β-catenin pathway activation at various levels (Figure 3).
Figure 2.
Common mechanisms by which the Wnt/β-catenin pathway is dysregulated in cancer. (A) Loss-of-function mutations in APC (adenomatous polyposis coli) lead to a breakdown of the destruction complex, accumulation of β-catenin in the cytoplasm, translocation of β-catenin into the nucleus, and constitutive expression of Wnt/β-catenin-dependent genes. (B) Gain-of-function mutations in β-catenin, often occurring in exon 3, prevent its N-terminal phosphorylation thus averting its ubiquitination and degradation. (C) Overexpression of FZD (frizzled) receptors or WNT ligands can lead to increased activation of the pathway. (D) Underexpression of secreted inhibitors of the pathway (i.e., secreted frizzled-related proteins, sFRPs) can also lead to increased sensitivity to Wnt ligands and increased pathway activation.
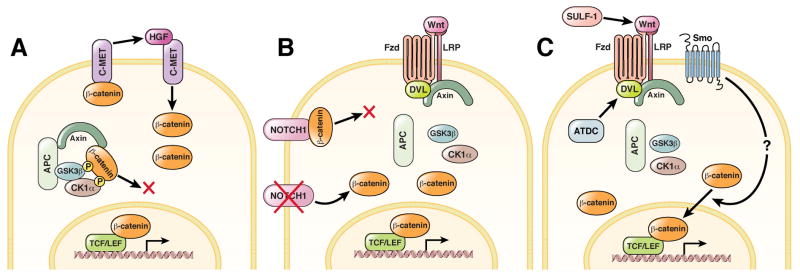
Figure 3.
Additional mechanisms by which Wnt/β-catenin signaling can be modulated in cancer. (A) In hepatocytes, β-catenin can be released from an additional membrane bound pool associated with the receptor C-MET. When engaged by HGF (hepatocyte growth factor), C-MET releases β-catenin into the cytoplasm with its eventual translocation into the nucleus. (B) Membrane-bound NOTCH1 can bind activated β-catenin and cause its lysosomal destruction, thereby inhibiting Wnt/β-catenin signaling. A decrease in NOTCH1 expression may therefore potentiate Wnt/β-catenin signaling in certain types of cancer. (C) Interactions with numerous proteins can modulate Wnt/β-catenin signaling at various levels in the pathway. SULF-1 (Sulfatase-1) can increase the efficiency of Wnt ligands by modulating their interactions with heparin sulfate proteoglycans in the extracellular environment. ATDC (Ataxia telangiectasia group D-complementing) protein can potentiate pathway activation through its interaction with DVL (Dishevelled). SMO (Smoothened) can increase Wnt/β-catenin signaling by an unknown mechanism. Undoubtedly, novel mechanisms will be uncovered to explain the complex context dependency of Wnt/β-catenin signaling.
The three gastrointestinal cancers discussed in this review arise in organs where Wnt/β-catenin signaling is critical for normal embryonic development and adult tissue homeostasis. By examining these GI cancers, we will illustrate how the phenotypic consequences of Wnt/β-catenin activation or inhibition are highly context dependent, which has critical implications for therapies attempting to target the pathway.
Colorectal Carcinoma
The role of Wnt/β-catenin signaling in intestinal development, intestinal adult homeostasis, and CRC has been extensively reviewed elsewhere.15–19 In CRC, ninety percent of all tumors have a mutation in a key regulatory factor of the Wnt/β-catenin pathway, most often in APC or CTNNB1, resulting in activation of the pathway. Up to 80% of tumors have nuclear accumulation of β-catenin.20–22 Interestingly, APC and CTNNB1 mutations are mutually exclusive events23 and associate with different types of colorectal tumors. Mutations CTNNB1 are more often found in small colorectal adenomas than in invasive carcinomas,24 whereas others have found that CTNNB1 mutations associate with CRC in hereditary non-polyposis colorectal cancer syndrome.25 In mouse models, tumors secondary to mutations in Apc, but not Ctnnb1, can be inhibited by Ctnnb1 silencing alone using inducible shRNAs.26, 27 These findings highlight an important theme that although mutations in APC and CTNNB1 both result in pathway activation, these mutations are not functionally equivalent. APC has many different functions in addition to regulating the Wnt/β-catenin pathway, such as roles in cell migration, adhesion, chromosome segregation and spindle assembly.28 In summary, APC and CTNNB1 mutations confer different levels of pathway activation, require a different subset of cooperating mutations to drive tumor progression, and may promote tumor progression by different mechanisms such as, for example, the increase in chromosomal instability of a cell seen upon loss of APC.29, 30
The level of β-catenin signaling activity has important consequences on tumor development. Analysis of the second hit in familial adenomatous polyposis polyps reveals that the APC geneotypes that are selected during tumor formation retain some ability to down regulate β-catenin signaling over genotypes that completely lose the ability to regulate β-catenin signaling.31 This “just right” signaling model has been validated in various Apc mutant mice that confer different amounts of β-catenin signaling and result in different tumor phenotypes.32 Interestingly, hypomorphic mutant Apc mice with intermediate levels of Wnt/β-catenin signaling (Apc+/1572T and Apcfl/fl) do not develop intestinal cancer but instead develop liver tumors,33, 34 supporting the idea that specific levels of β-catenin signaling initiate tumorigenesis in a tissue specific manner. It should be noted that Apc mutant mice tend to form benign adenomas in the small intestine,35 so they are very helpful to study intestinal cancer and the role of β-catenin signaling in tumor development, but they are not a perfect model of human CRC. Mice with multiple mutations (i.e. Apc and Kras)36 or treatment of Apc mutant mice with carcinogens37 will increase the incidence of tumor progression to carcinoma.
Even within a tumor, the amount of β-catenin signaling exhibits heterogeneity. CRC tumors harboring activating mutations in the Wnt/β-catenin pathway show variability in levels of signaling, implying that additional regulatory cues (i.e., epigenetic silencing, extrinsic signals from the microenvironment, or pathway crosstalk) modulate pathway activation.38, 39 One example of a modulator is members of the R-spondin protein family. Recent findings have determined that Lgr4 and Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 4 and 5) function as R-spondin receptors, associate with the Frizzled/Lrp receptor complex, and potentiate Wnt/β-catenin signaling by enhancing Wnt-induced LRP6 phosphorylation.40–42 The four secreted proteins of the R-spondin protein family are strong synergizers of Wnt/β-catenin signaling.43, 44 Given that Lgr5 marks the small intestinal stem cells at the crypt base, is a Wnt target gene, and potentiates R-spondin mediated enhancement of Wnt/β-catenin signaling, a feed forward mechanism can be established. Overexpression of Lgr5 has been reported in several types of cancer, including CRC45, 46 and HCC,47 and highlight the importance of future studies looking at the interplay between Wnt, Lgr5, and R-spondins in malignancy.
Crosstalk between the Wnt/β-catenin pathway and other developmental signaling pathways may also modulate β-catenin signaling in CRC. Kwon et al. have shown that membrane bound Notch1 can bind to active β-catenin and negatively regulate it in stem and progenitor populations, as well as in human colorectal cancer cell lines.48 Evidence suggests that the Hedgehog pathway also may also regulate the Wnt/β-catenin pathway in CRC, although there are conflicting reports regarding the polarity of this interaction.49, 50 In one provocative study, the increase in Wnt/β-catenin signaling in Apc+/δ716 mice was dependent on Smoothened (Smo), a mediator of Hedgehog (Hh) signaling.49
In summary, while CRC serves as the prototypic example of the oncogenic nature of Wnt/β-catenin signaling, it is evident that the activity of the pathway is not solely dictated by mutations in canonical members of the pathway. Importantly, specific levels of Wnt/β-catenin signaling are necessary and confer tissue-specific tumorigenesis. This brief background on CRC provides a good starting point and yardstick for comparing the role of Wnt/β-catenin signaling in hepatocellular carcinoma and pancreatic adenocarcinoma.
Hepatocellular Carcinoma
Dysregulation of the Wnt/β-catenin pathway has been implicated in the pathogenesis of HCC for over a decade, although its precise role in HCC progression remains unresolved.51 In particular, the different pathologic states that underpin the development of cirrhosis and HCC (i.e., viral hepatitis, steatohepatitis, genetic disorders, etc.) further complicate attempts to generalize the functional activity of Wnt/β-catenin signaling in hepatocellular carcinogenesis.
Genetics of the Wnt/β-catenin pathway in HCC
Anywhere from 3–44% of tumors in human HCC contain mutations of β-catenin in exon 3,52–54 resulting in constitutively-active N-terminal deletions that lack the sites normally phosphorylated to target the protein for proteasomal degradation.55 Mutations in AXIN1 are observed in 5–25% of HCC cases and most often occur in tumors without CTNNB1 mutations, thus displaying a similar property of exclusivity seen in CRC.56–58 As is also the case with CRC, activating mutations in β-catenin and inactivating mutations of the destruction complex (APC or AXIN1) do not appear to be functionally equivalent in HCC. Zucman-Rossi et al. looked at 45 tumors and four tumor lines and compared those with activating CTNNB1mutations to those with AXIN1 mutations. They found that β-catenin-dependent transcriptional targets such as glutamine synthetase, LGR5, and glutamate transporter-1 were only upregulated in tumors with β-catenin activating mutations.59 Similarly, Hoshida et al. performed a meta-analysis of expression profiles of eight different patient cohorts and uncovered a robust classification system based on global gene expression signatures.60 Again, the subclass characterized by an experimentally-defined Wnt signature (including MYC and CYD1) was not enriched with tumors containing activating N-terminal mutations in β-catenin (often denoted in the literature as “CTNNB1 exon 3 mutations”). These studies imply that the functional consequences of Wnt/β-catenin pathway activation in HCC are distinct depending on which member of the pathway is mutated.
Chronic viral hepatitis and cirrhosis are important predisposing factors for the development of HCC.61 Interestingly, studies implicate direct roles for hepatitis B (HBV) and hepatitis C (HCV) in modulating Wnt/β-catenin signaling. The HCV core protein correlates with increased WNT1 expression in a HCC-derived cell line,62 and genes inhibitory to Wnt/β-catenin signaling are preferentially methylated in HCV-related HCC.63 HBV X protein is able to bind APC and displaces β-catenin from the destruction complex, resulting in increased Wnt/β-catenin signaling.64 Interestingly, mutations in AXIN1 correlate with HBV-associated HCC, while mutations in β-catenin correlate with non-HBV-associated tumors.65 Although correlative, these specific associations suggest a potential causal link between the manner of Wnt/β-catenin activation and the development of HCC in the context of different forms of viral hepatitis and cirrhosis.
Lessons from transgenic models of HCC
Numerous studies in mice offer direct evidence for the Wnt/β-catenin pathway in the progression of HCC (reviewed in 66). For example, various transgenic models of HCC show an accumulation of β-catenin in tumors,53, 67 with the highest occurrence in c-myc/E2F-1 transgenic mice.68 Tumors in transgenic mice that exhibit nuclear β-catenin proliferate faster and are larger than those without nuclear β-catenin.69 In contrast, forced activation of Wnt/β-catenin signaling does not usually initiate tumorigenesis. Transgenic mice overexpressing a mutant non-phosphorylated (N-terminal-truncated) and constitutively active β-catenin in the liver, kidney, and intestine (EAB/9K/ΔN131 β-catenin mice) develop hepatomegaly within three weeks of age, but no HCC prior to the mice succumbing to intestinal cancers.70 Liver-specific adenovirus-mediated expression of mutant stable β-catenin also does not result in tumorigenesis.71 Finally, while liver specific overexpression of wild type β-catenin using an albumin promoter (ALB-CTNNB1) results in hepatomegaly at high penetrance, no HCC were seen out to 24 months of age.72 In contrast to activation of Wnt/β-catenin signaling via β-catenin overexpression, activation of Wnt/β-catenin signaling via conditional Apc loss specifically in the liver (AdCre injections into Apclox/lox mice) can result in HCC.73 In addition, transgenic mice with low levels of Apc do develop HCC but not intestinal polyps.33, 34 This emphasizes the key point that the functional consequences of increased Wnt/β-catenin signaling are dependent on the particular part of the pathway that is modulated and the subsequent level of signaling. It may also be that the progression to HCC is due to loss of other functions of the tumor suppressor APC.74
Although forced activation of Wnt/β-catenin signaling by itself does not usually initiate tumorigenesis in HCC, it can act in concert with other oncogenes or disease states to promote tumor progression in mice. Introduction of Hras and mutant stable β-catenin mutations via Cre-mediated recombination (Catnblox(ex3):Tglox(pA)H-ras*) results in HCC at complete penetrance.75 Similarly, activation of mutant stable β-catenin in heterozygote Lkb1 knockouts (Catnb+/lox(ex3)Lkb1+/−) via AdCMV-cre injection leads to accelerated development of HCC.76 Mouse studies using the carcinogen diethylnitrosamine (DEN) suggest that increased Wnt/β-catenin signaling facilitates the development of HCC. In the context of DEN, transgenic mice conditionally expressing mutant stable β-catenin in the liver develop HCC at six months, while no tumors are seen in control wild-type livers.77
With regards to studies on HCC and Wnt/β-catenin signaling in mouse models, it should be noted that often these models utilize either carcinogens or forced overexpression of oncogenes in every cell of the organ to promote tumors. By contrast, most human HCC arise focally following complex processes such as steatohepatitis and cirrhosis that are not accounted for in these mouse models. The refinement of mouse models to better mirror the development of human HCC will likely clarify how Wnt/β-catenin signaling is affected by these processes, as well as the consequences of Wnt/β-catenin signaling on tumor progression in these particular contexts.
Evidence for altered Wnt/β-catenin signaling in human HCC
In addition to the high incidence of mutations in CTNNB1 and AXIN1 in patient tumors, further evidence implicates dysregulation of Wnt/β-catenin in HCC progression. Whole genome expression profiling has implicated Wnt/β-catenin signaling in HCC. Unsupervised global transcriptome analysis of HCC defines six subgroups, two of which are notable for increased Wnt/β-catenin signaling.78 Hierarchical clustering of gene expression among HCC associated with hepatitis C virus defines five subgroups, with the CTNNB1-associated group marked by significant overexpression of liver-specific Wnt/β-catenin target genes such as GLUL, LGR5, and TBX3.79 Overexpression of the Wnt receptor Frizzled-7 may contribute to pathway dysregulation in some HCC tumors.80 Some HCC tumors exhibit reduced expression of WNT11 (classically a non-canonical Wnt), which has been shown to decrease the activity of a β-catenin signaling reporter upon its overexpression in Huh-7 HCC cells.81 This finding is consistent with the reported ability of non-canonical Wnt ligands to antagonize the canonical Wnt/β-catenin pathway in other contexts,82 and is an example of this interplay in the setting of cancer.
Crosstalk between the Wnt/β-catenin pathway and other developmental signaling pathways also contributes to dysregulation of Wnt/β-catenin signaling in HCC. Several studies implicate TGF-β as an important regulator of the Wnt/β-catenin pathway83, 84 and suggest that interactions between the TGF-β and β-catenin pathways are crucial for the expression of β-catenin target genes in HCC.60 Indeed, previous observations show that the TGF-β effector Smad3 can promote the nuclear translocation of β-catenin.85 However, the actual consequence of crosstalk between the TGF-β and the Wnt/β-catenin pathways is unclear.86 Crosstalk between the β-catenin pathway and the HGF/MET pathway might also contribute to the progression of HCC. Hepatocyte growth factor (HGF) signals through the tyrosine kinase receptor, MET (reviewed in 87 and citations within). β-catenin associates with MET at the membrane in hepatocytes, a complex that might represent a large and functionally important pool of β-catenin.88 Membrane bound β-catenin dissociates from MET upon HGF treatment and translocates to the nucleus in a manner dependent on tyrosine phosphorylation. MET is overexpressed in many HCC tumors and is correlated with poor prognosis,89 while subsets of HCC patient tumors defined by a MET-induced gene expression signature show a more invasive phenotype and decreased mean survival time.90
Surrogate markers of pathway activation are variable in human HCC. Between 17–43% of patient tumors stain for nuclear β-catenin, while 57–62% of tumors show increased cytoplasmic and/or membranous staining.91–94 Conceivably, MET overexpression or interaction with other signaling pathways such as Notch (as observed for CRC) may lead to the increase in membranous β-catenin seen in many HCC tumors, although this has not been directly addressed.
The prognostic significance of mutations in CTNNB1and AXIN, the presence of detectable nuclear β-catenin, and the overexpression of Wnt/β-catenin target genes is unresolved. One study finds nuclear β-catenin expression in HCC correlates with a non-invasive phenotype and better survival.95 Curiously, tumors exhibiting nuclear β-catenin in association with an activating CTNNB1 mutation have a better 5-year survival rate than tumors exhibiting detectable nuclear β-catenin in the absence of a CTNNB1 mutation.96 Other studies find no significant link between mutant β-catenin and favorable prognosis65 or tumor size and differentiation.91 The variable results seen in studies based on immunohistochemical analysis of nuclear β-catenin suggest that as yet unexplained variations between tumor specimens may be complicating the interpretation of results. These variations may be related to either technical differences (such as with tissue fixation and processing), temporal differences relating to the duration of tumor progression between patients (since analysis is usually performed on a single time point from a patient), the genetic heterogeneity of patient populations analyzed, or some combination of multiple factors therein. As more genetic, transcriptome, and clinical pathological correlates are analyzed, we will hopefully develop more robust means of evaluating Wnt/β-catenin status in order to sub-classify tumors into clinically meaningful prognostic or predictive categories.
The function of Wnt/β-catenin signaling in human HCC
Experiments using human liver cancer lines support an important role for Wnt/β-catenin in HCC tumorigenesis and malignant behavior. Expression and nuclear accumulation of β-catenin is associated with proliferation in HA22T cells, while β-catenin knockdown reduces migration and invasion assays of these cells.97 Adenovirus-mediated gene transfer of wild type AXIN and APC into various HCC cell lines reduces Wnt/β-catenin signaling and results in growth suppression.58 On the other hand, tumor formation is accelerated in Huh7 cells with the introduction of constitutively active β-catenin.98 Corroborating these findings, injection of anti-Wnt-1 antibodies into tumors of a Huh7 xenograph model suppresses in vivo tumor growth.99 These studies provide more direct evidence that Wnt/β-catenin signaling mediates cellular phenotypes associated with cancer and suggest that targeting this pathway may be beneficial in certain types of HCC.
In summary, the manner in which the Wnt/β-catenin pathway is dysregulated in HCC has disparate functional consequences. Different mutations in the pathway drive different β-catenin-dependent gene expression, segregate separately with HBV or HCV-associated tumors, and confer differential effects on tumorigenesis in mouse models.
Pancreatic Ductal Adenocarcinoma
The role of the Wnt/β-catenin pathway in pancreatic ductal adenocarcinoma (PDAC) is less clear and somewhat controversial. This is a reflection of an evolving literature showing Wnt/β-catenin signaling has variable and sometimes paradoxical effects in the pancreas dictated by its timing, location, strength, and mechanism of activation.
Genetics of Kras and the Wnt/β-catenin pathway in PDAC
Pancreatic cancer is genetically complex with individual PDAC tumors averaging more than 60 different genetic alterations.100 Key genes mutated at high frequency in most tumors include KRAS2, CDKN2A/p16, TP53 and SMAD4/DPC4.100, 101 Although many additional genetic mutations and molecular alterations are linked to the development and/or progression of PDAC, these do not commonly include mutations in APC, AXIN1 or CTNNB1.100, 102, 103 In contrast, mutations of these key Wnt genes do occur at high frequency in rarer, histologically distinct pancreatic neoplasms, including solid-pseudopapillary neoplasms (SPNs, 90–100%), pancreatoblastomas (50–80%) and acinar carcinomas (~25%) (see 104 and references therein). Thus, while genetic mutations resulting in high levels of constitutive Wnt/β-catenin signaling define certain less common pancreatic tumors, they are not a common feature of PDAC.
Highlighting its importance as an initiating oncogenic event in PDAC tumorigenesis, pancreas-specific expression of oncogenic Kras from its endogenous allele (LSL- KrasG12D) via Pdx1- or p48-Cre driven recombination in mice results in dysplastic precursor lesions referred to as pancreatic intraepithelial neoplasia (PanIN) at high penetrance, as well as occasional PDAC after prolonged latency.105 Of note, chronic pancreatitis accelerates mPanIN-PDAC progression in the context of oncogenic Kras. In the setting of acinar cell injury and chronic inflammation, Kras drives acinar cells into a transdifferentiated ductal state, a process referred to as acinar-to-ductal metaplasia (ADM) and facilitates the further development of mPanIN and PDAC (see 106 and references therein). An important role for Wnt/β-catenin in this process will be discussed in further detail below.
Lessons from transgenic models of pancreatic cancer
Transgenic mice with pancreas-specific, constitutive Wnt/β-catenin activation elaborate variable, context-dependent phenotypes, but do not develop PanIN or PDAC (see ref 106 for detailed review). Introduction of a β-catenin stabilizing mutation in exon 3 of Ctnnb1 using a Cre-driver targeting all progenitor cells in the early embryonic pancreas (Pdx1-CreEarly; Cttnb1exon3/+) results in severe pancreatic hypoplasia due to exocrine and endocrine agenesis.107 In contrast, introduction of the identical Ctnnb1 mutation using a Cre-driver with slightly delayed expression limited to maturing acinar and endocrine cells (Pdx1-CreLate; Cttnb1exon3/+) conversely results in increased acinar proliferation without tumor formation,107 a phenotype shared by mice with disrupted Apc function (Pdx1-Cre; Apcflox/flox).108 Mice with β-catenin stabilizing mutation introduced instead by p48-driven Cre recombination (p48-Cre; Cttnb1exon3/+) also show increased acinar proliferation, but additionally develop tumors resembling SPNs109 Therefore, CTNNB1 mutations not only occur at high frequency in SPNs, but appear able to serve as an initiating event in their formation.
Given oncogenic Kras is the critical initiating event for mPanIN-PDAC progression, an obvious question that arises is whether Wnt/β-catenin signaling acts cooperatively with Kras to promote pancreatic tumorigenesis. To this point, mice with both β-catenin stabilizing mutation and oncogenic Kras (p48-Cre; Cttnb1exon3/+; LSL- KrasG12D) do not develop PanIN or PDAC, but instead develop an unusual tumor histology resembling intraductal tubular neoplasm,109 a rare and indolent tumor in humans. Thus, while high constitutive Wnt/β-catenin has tumor-initiating activity and shows synergy with KRAS in colon cancer, it conversely antagonizes the formation of Kras-initiated mPanIN and PDAC in mice. This inhibition appears linked to the role of Wnt/β-catenin in promoting acinar cell regeneration following inflammation-mediated acinar cell injury, whereby Wnt/β-catenin hyperactivation opposes Kras-mediated ADM and subsequent mPanIN formation.110 Therefore, appropriate temporospatial regulation and precise levels of Wnt/β-catenin signaling are necessary for acinar-to-ductal reprogramming and subsequent PanIN-PDAC progression. However, it remains to be determined at what level endogenous Wnt/β-catenin signaling is permissive or even necessary for ADM and subsequent mPanIN-PDAC progression. This question will need to be answered in transgenic models where up or down-regulation Wnt/β-catenin signaling at different levels and specific stages of ADM-PanIN-PDAC sequence is investigated in the context of oncogenic Kras.
Evidence for altered Wnt/β-catenin signaling in human PDAC
Although Wnt/β-catenin signaling is unable to initiate PDAC in mouse models and somatic mutations of its key intracellular regulatory molecules are rare in PDAC, there is ample in vitro and in vivo evidence that Wnt/β-catenin signaling is involved in PDAC tumorigenesis. Deep sequencing demonstrates that PDAC tumors have large number of highly variable genetic alterations, but that these genetic alterations can be linked to 12 core pathways and processes shared by all tumors, including the Wnt pathway.100 Unbiased global epigenetic analysis of PDAC reveals most tumors also have corresponding changes in DNA methylation and expression status of multiple genes that regulate the Wnt pathway, suggesting epigenetic mechanisms are an alternative means of altering Wnt/β-catenin signaling in PDAC.111
Developmental signaling pathways whose activation are strongly linked to the development and/or progression of PDAC are also notable for their known or potential cross-talk with Wnt/β-catenin, including TGFβ, Notch, Hedgehog (Hh) and FGF signaling (see 112 and references therein). For example, ectopic activation of Hh signaling in pancreatic ductal cells increases Wnt/β-catenin-mediated transcriptional activity through upregulation of TCF4 expression, while elevated nuclear β-catenin expression is seen in mPanIN lesions and PDAC tumors that form in transgenic mice with combined oncogenic Kras and activated Hh signaling via ectopic expression of GLI2 (Pdx1-Cre;CLEG2;KrasG12D).113 In regards to Notch signaling, concurrent loss of Notch1 and activation of Kras in transgenic mice (Pdx1-Cre; LSL- KrasG12D; Notch1lox/lox) results in accelerated mPanIN progression and is accompanied by increased cytoplasmic β-catenin in ductal epithelium, although this change is correlative and not definitively linked to the altered phenotype in these animals.114 Dominant-negative inhibition of SMAD4 activity in the PDAC cell line PANC1 results in increased β-catenin degradation, reduced Wnt/β-catenin signaling activity and inhibition of tumorigenicity in vivo.115 Thus, mutations in SMAD4, which occur in large subset of patients with PDAC and are associated with worse prognosis in PDAC,116 may also serve as an important determinant of Wnt/β-catenin activity.
Surrogate markers of elevated Wnt/β-catenin signaling are frequently seen in PDAC. However, these surrogates must be viewed cautiously as they are both correlative and not definitive indicators of pathway activation. A comprehensive gene expression microarray study of bulk and microdissected PDAC and normal pancreas samples shows that a large subset of PDAC tumors have higher expression of AXIN2, a widely accepted universal target of Wnt transcriptional activation.117 While increased expression of AXIN2 or other gene targets commonly viewed as Wnt/β-catenin transcriptional targets (i.e., CCND1, MMP7, RARG, etc.)113 are circumstantial evidence of pathway activation in PDAC, a comprehensive set of validated Wnt/β-catenin-specific target genes has yet to be delineated in PDAC.
Positive IHC expression of nuclear and/or cytoplasmic β-catenin is reported in anywhere from 4–65% of human PDAC tumors102, 113, 118–121 and up to 40% of pancreatic intraductal papillary mucinous neoplasms.122 Positive nuclear β-catenin expression is also reported in advanced (but not early) PanIN lesions in humans and at later stages of mPanIN progression in the LSL-Kras mouse model,113, 121 possibly representing a transition point at which elevated Wnt/β-catenin signaling ceases to inhibit tumor progression. Wide disparities in reported nuclear and cytoplasmic β-catenin have mostly been attributed to variations in technique and/or interpretation. However, these differences may also reflect functionally relevant variations in the strength or duration of Wnt/β-catenin signaling across the full spectrum of human PDACs. Some smaller retrospective studies report alterations in β-catenin IHC that correlate with tumor differentiation,119, 123 metastasis,124, 125 or patient survival,125, 126 although other reports fail to find a statistical correlation between β-catenin IHC and clinical outcomes. It is worth noting that IHC may underestimate functionally relevant low-to-moderate levels of Wnt/β-catenin signaling in PDAC. The detection of nuclear β-catenin has been largely optimized and interpreted in the context of tumors with classical mutations leading to constitutive pathway hyperactivation. Illustrating this point, β-catenin-dependent transcriptional reporter assays detect low to moderate Wnt/β-catenin transcriptional activity across a majority of human PDAC tumor lines in vitro,113, 121, 127 but at levels 5–20 fold lower than colon cancer lines carrying mutations in APC, CTNNB1 or AXIN1. Moving forward, the adoption of alternative surrogates of Wnt/β-catenin signaling may be necessary to best define its activity and relevance in PDAC clinical samples.
The function of Wnt/β-catenin signaling in human PDAC
Apart from surrogate markers, studies directly addressing Wnt/β-catenin and its effects on in vitro and in vivo tumorigenesis offer the most compelling evidence of its importance in PDAC. The direct inhibition Wnt/β-catenin signaling by dominant-negative LEF1 or siRNA/shRNA knockdown of β-catenin suppresses human PDAC cell line growth and survival in vitro.113, 121 Accumulating evidence in the literature further suggests that Wnt/β-catenin signaling in PDAC is functionally deregulated by various cellular and molecular events that do not autonomously hyperactivate Wnt/β-catenin, but instead modulate existing levels of autocrine or paracrine Wnt signaling. SULF-1 and SULF-2, novel sulfatases that act on heparin sulfate proteoglycans (HSPGs), are overexpressed in human PDAC and are able to potentiate Wnt signaling and in vitro and in vivo cancer cell growth in PDAC cell lines with autocrine Wnt activity.127 Interestingly, SULF-2 may also enhance Wnt/β-catenin signaling in HCC.128 GATA6 is overexpressed in PanIN and PDAC and promotes cell line growth in soft agar and mouse xenografts, a function linked to its repression of the secreted Wnt inhibitor DKK1 and which corresponds with increased levels of Wnt activation.129 Intracellularly, the increased expression of ATDC in PDAC correlates with increased Wnt/β-catenin transcription and promotes in vitro and in vivo tumor growth and metastasis in a β-catenin-dependent fashion. These are linked to ATDC’s direct interaction with DVL2 and other members of the destruction complex.121
Wnt signaling also appears to be involved in the critical interplay between PDAC cells and their surrounding stromal environment. Increased nuclear and cytoplasmic expression of β-catenin is seen in PDAC cell lines when they are cultured on type I collagen130 or placed in an organotypic culture model in combination with an immortalized pancreatic stellate cell line.120 Further work demonstrates that retinoic acid treatment is able to induce pancreatic stellate cell quiescence and reduce paracrine-mediated Wnt signaling activity through increased secretion of sFRP4, which is associated with corresponding tumor growth inhibition and apoptosis in a transgenic mouse model of pancreatic cancer.131 Additional extracellular and intracellular modulators of autocrine or paracrine-mediated Wnt/β-catenin are likely to be uncovered over time.
In summary, our present understanding of Wnt/β-catenin signaling and PDAC is incomplete and possibly flawed, as it has often been studied in the non-physiologically relevant context of constant and high levels of pathway activation. Developmental and cancer models indicate Wnt/β-catenin dictates diverse phenotypic outcomes in the pancreas that are based on varying context and levels of activation. Although canonical activating mutations are uncommon, Wnt/β-catenin signaling can be dysregulated in PDAC through a variety of mechanisms that modulate existing levels of autocrine or paracrine Wnt activation. While this dysregulation is more subtle and nuanced than that seen in CRC or HCC, it is also evident that these changes have meaningful phenotypic effects on PDAC tumorigenesis. Unlike colon cancer, the manner in which Wnt/β-catenin signaling is activated and readily modulated in PDAC also may imply that PDAC may be more amenable to genetic or pharmacological targeting of Wnt/β-catenin as clinical therapy.
Conclusions from examining Wnt/β-catenin signaling across CRC, HCC, and PDAC
To summarize, there are significant similarities and differences in the regulation and function of Wnt/β-catenin signaling between CRC, HCC and PDAC (Table 1). What are some of the major conclusions that can be drawn from the comparison of Wnt/β-catenin signaling in these three tumors of the gastrointestinal tract? First, while markers of deregulated Wnt/β-catenin signaling in patient tumors (i.e., somatic mutations that activate the pathway, detection of nuclear β-catenin, etc.) are traditionally viewed as strong evidence for the role of the pathway in tumor initiation and/or progression in CRC, this view does not accurately reflect the pathway and its relevance in HCC and PDAC. Second, the timing of Wnt/β-catenin signaling dysregulation is crucial for determining whether pathway activation increases or inhibits tumorigenesis (as described in PDAC). Third, different cancers are preferentially driven by different levels of pathway activation (as described with CRC and HCC). Furthermore, the different mechanisms of pathway dysregulation (CTNNB1 vs. APC mutations for example) result in different tumor phenotypes. While Wnt/β-catenin pathway activation may be linked to the development of cancer, in some instances it may also define a subset of tumors with less aggressive clinical behavior (as described in some studies with HCC). Finally, the existing linear model of Wnt/β-catenin signaling with its transcriptional activation of known target genes is too simplistic. In particular, a linear model does not readily account for the variable presence and actions of known (or unknown) transcriptional co-repressors or activators and their isoforms, as well as the influence of epigenetic regulatory mechanisms on target gene accessibility. Moreover, we are only just beginning to define the consequences of crosstalk with other signaling pathways, as well as the actions of a variety of other molecular perturbations capable of modulating the signaling pathway. It is reasonable to expect that these various factors may be responsible for unexpected divergent outcomes that occur within and across tumor types.
Table 1.
| CRC | HCC | PDAC | REFERENCES | |
|---|---|---|---|---|
| % of human tumors containing mutations in key members of Wnt/β-catenin pathway | 90% of CRC | 18–44% of HCC | Rare in PDAC | reviewed in 17,18 52–54, 56–58 100,102,103 |
| % of human tumors with β-catenin staining | 58–80% nuclear 100% membrane and cytoplasmic |
17–43% nuclear 57–62% membrane and cytoplasmic |
4–11% nuclear 35–65% cytoplasmic |
20–22 91–94 102,113,118–121 |
| Wnt/β-catenin pathway members mutated most often | 80% APC 10% β-catenin |
3–44% β-catenin 5–25% AXIN1 Not APC |
Not seen in PDAC | reviewed in 18 52–54,56–58 100,102,103 |
| Effects of Wnt/β-catenin hyperactivation in murine tumor models | Initiating event in tumorigenesis and is sufficient for tumor formation (adenomas not carcinomas) | Not sufficient for malignant transformation, but promotes tumorigenesis | Antagonizes Kras-initiated mPanIN and PDAC | reviewed in 17,18 70–72 107,109,110 |
| Necessity of temporal regulation of Wnt/β-catenin pathway in tumorigenesis | No | No | Yes | reviewed in 17,18 70–72 107,109,110 |
| Crosstalk with other signaling pathways (not an exhaustive list) | Notch, Hedgehog | TGF-β, HGF/MET | TGF-β, Hedgehog, Notch |
48–50 60,86,88 113–115 |
These collective observations on Wnt/β-catenin signaling across different cancer models suggest that the concept of pathway homeostasis, defined as a steady-state level of pathway activation, offers perhaps a more nuanced and accurate view of signaling in the cell compared to the classical view of Wnt/β-catenin signaling being defined as either “on” or “off”. While the term homeostasis (traditionally defined as some type of maintenance of internal stability) may seem paradoxical in the setting of an inherently unstable environments such as a cancer cell, it is clear from experimental studies that both increasing or decreasing the level of Wnt/β-catenin signaling can have functionally significant consequences that are difficult to predict based on existing linear models of cell signaling that do not account for the complex and dynamic mechanisms of feedback inhibition and feed-forward activation. Studies on CRC, HCC and PDAC also implicate the presence of a complicated and dynamic network of pathway crosstalk during tumor progression that has profound consequences for the homeostatic maintenance of Wnt/β-catenin signaling. The continued refinement of both transgenic mouse models and cell culture-based models that address these aspects of tumor progression will help to further clarify these issues.
Future Perspectives in Wnt-Based Therapies
Over the past two decades, a growing number of bioactive compounds ranging from small molecules to targeted antibodies have proven effective at activating and inhibiting the Wnt/β-catenin pathway in experimental settings, including in model developmental organisms (inhibitors are summarized in Figure 4).2 Despite this progress, drugs specifically designed to target Wnt/β-catenin signaling have been slow to transition into the clinic. Efforts to therapeutically target Wnt/β-catenin signaling have focused largely on inhibitors, based on the classical model of tumor promotion by Wnt/β-catenin in CRC and certain other cancers. While recently-identified inhibitors of Wnt/β-catenin signaling such as XAV939 132 and IWP-2 133 exhibit impressive inhibition of the pathway in experimental systems, their pharmacokinetic profiles have prevented their use in in vivo preclinical models. To date, the only inhibitors of Wnt/β-catenin signaling that have progressed to early stage clinical trials are the compounds IGC-001 (now optimized as CWP 231A by Choongwae Pharmaceuticals),134 CWP232291, and PRI-724 (clinicaltrials.gov).
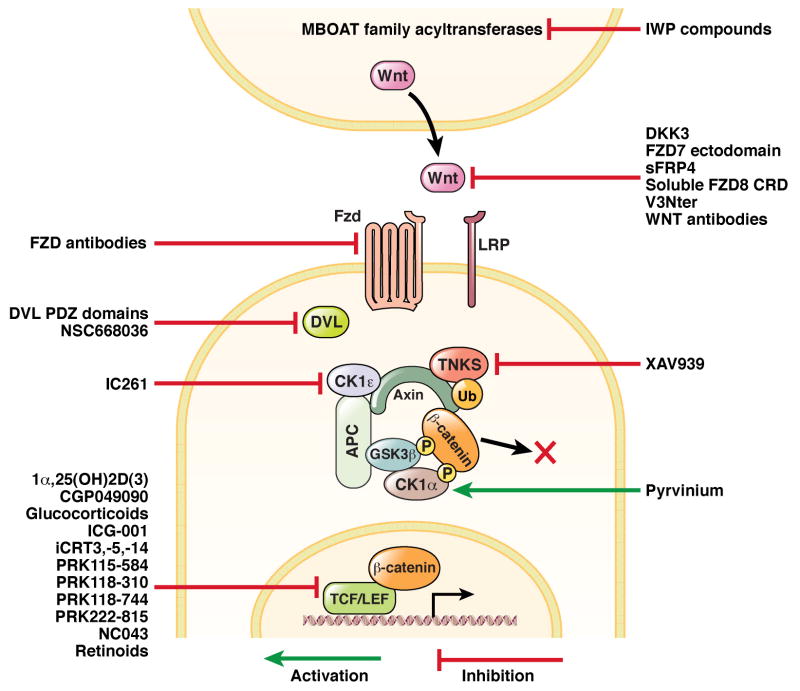
Figure 4.
Schematic illustrating numerous small molecules and biological compounds identified in the literature that inhibit Wnt/β-catenin signaling at various points in the pathway.
While inhibiting Wnt/β-catenin signaling should be technically achievable, several questions about its efficacy and potential toxicities remain unanswered. The implication of Wnt/β-catenin signaling in the maintenance of stem cell pluripotency and lineage specification in normal tissues throughout the human body raises concerns that any effort to systemically inhibit the pathway could have unwanted consequences.135 The heterogeneity of Wnt/β-catenin signaling activity observed in both normal tissues and within tumors also complicates efforts to predict the biological outcome of targeting the pathway. In addition, the potential for inhibiting Wnt/β-catenin signaling could be largely determined by the manner in which the pathway is dysregulated in cancer. For instance, it may be difficult inhibit the pathway in cancers (i.e., CRC) with cell-autonomous, constitutive, hyperactivating mutations. In contrast, other tumors where the pathway is dysregulated through changes in levels of signaling initiated by Wnt ligand (i.e., PDAC) may be more responsive to therapeutic modulation.
Although activation of Wnt/β-catenin signaling in the setting of cancer runs counter to established dogma,136 the transgenic cancer models presented in this review highlight instances in which forced activation of the pathway might be an appropriate strategy based on disease context and timing. In regards to such an approach, lithium chloride is a clinically experienced compound that represents a classic activator of Wnt/β-catenin signaling through its inhibition of GSK3. However, its narrow therapeutic index and significant off-target effects would presumably limit its widespread usage as a pathway activator in patients. Additional other patient-experienced compounds, including some in widespread clinical use, also demonstrate activity as enhancers of Wnt/β-catenin signaling,137 although further research is needed to establish whether their biological effects can be wholly or partially attributed to their ability to activate Wnt/β-catenin signaling.
In summary, therapeutic targeting of Wnt/β-catenin signaling is an attractive and technically achievable goal, but must be pursued with an appreciation for the complex nature of Wnt/β-catenin pathway regulation and function both within and across different tumor types. In particular, the successful deployment of a Wnt-targeted therapy will likely depend on the development and optimization of clinical biomarkers that accurately detect the variable states and biological activities of Wnt/β-catenin signaling across a full spectrum of patient tumors in order to individually tailor therapy.
Acknowledgments
Grant support: For D.W.D., American Association for Cancer Reseach/Pancreatic Action Network Career Development Award; for A.J.C., 1K08CA128565 from NIH/NCI.
Abbreviations used in this paper
- ADM
acinar-to-ductal metaplasia
- AES
amino terminal enhancer of split
- APC
adenomatous polyposis coli
- ATDC
ataxia telangiectasia group-D complementing
- CRC
colorectal carcinoma
- CREB
cyclic AMP response element-binding protein
- CS1
casein kinase-1
- DEN
diethylnitrosamine, FGF, fibroblast growth factor
- FGF
fibroblast growth factor
- FZD
frizzled
- GI
gastrointestinal
- GSK3
glycogen synthase kinase-3
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- Hh
hedgehog
- HSPG
heparin sulfate proteoglycan
- IHC
immunohistochemistry
- LRP
low-density lipoprotein receptor-related protein
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- sFRP
secreted frizzled related protein
- Smo
smoothened
- SPN
solid-pseudopapillary neoplasm
- TCF/LEF
T-cell factor/lymphoid enhancer factor
- TGF
transforming growth factor
Footnotes
No conflicts of interest exist.
Author contributions: All authors contributed to the drafting and critical revisions of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bryan D. White, Science and Technology Program University of Washington Bothell Bothell, WA, USA
Andy J. Chien, Department of Medicine, Division of Dermatology Institute for Stem Cell and Regenerative Medicine University of Washington School of Medicine Seattle, WA, USA
David W. Dawson, Department of Pathology and Laboratory Medicine Jonsson Comprehensive Cancer Center The David Geffen School of Medicine at UCLA Los Angeles, CA, USA
References
- 1.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–27. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien AJ, Moon RT. WNTS and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–57. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon RT, Kohn AD, De Ferrari GV, et al. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–77. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 6.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005:cm1. 27. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 7.Kimelman D, Xu W. Beta-Catenin Destruction Complex: Insights and Questions From a Structural Perspective. Oncogene. 2006;25:7482–91. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 8.Rijsewijk F, Schuermann M, Wagenaar E, et al. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–57. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 9.Yost C, Torres M, Miller JR, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 10.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–14. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishita M, Enomoto M, Yamagata K, et al. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–54. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Major MB, Camp ND, Berndt JD, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–6. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 13.Major MB, Roberts BS, Berndt JD, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008;1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- 14.Shitashige M, Hirohashi S, Yamada T. Wnt signaling inside the nucleus. Cancer science. 2008;99:631–7. doi: 10.1111/j.1349-7006.2007.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. Journal of Pathology, The. 2009:307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 16.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes & development. 2005;19:877–90. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 17.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science (New York, NY) 2005;307:1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 18.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 19.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Current opinion in cell biology. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Martensson A, Oberg A, Jung A, et al. Beta-catenin expression in relation to genetic instability and prognosis in colorectal cancer. Oncology reports. 2007;17:447–52. [PubMed] [Google Scholar]
- 21.Wanitsuwan W. Overall expression of beta-catenin outperforms its nuclear accumulation in predicting outcomes of colorectal cancers. World Journal of Gastroenterology. 2008;14:6052. doi: 10.3748/wjg.14.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elzagheid A, Buhmeida A, Korkeila E, et al. Nuclear beta-catenin expression as a prognostic factor in advanced colorectal carcinoma. World journal of gastroenterology: WJG. 2008;14:3866–71. doi: 10.3748/wjg.14.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparks AB, Morin PJ, Vogelstein B, et al. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–4. [PubMed] [Google Scholar]
- 24.Samowitz WS, Powers MD, Spirio LN, et al. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999;59:1442–4. [PubMed] [Google Scholar]
- 25.Johnson V, Volikos E, Halford SE, et al. Exon 3 beta-catenin mutations are specifically associated with colorectal carcinomas in hereditary non-polyposis colorectal cancer syndrome. Gut. 2005;54:264–7. doi: 10.1136/gut.2004.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholer-Dahirel A, Schlabach MR, Loo A, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/{beta}-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:17135–40. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mologni L, Dekhil H, Ceccon M, et al. Colorectal tumors are effectively eradicated by combined inhibition of {beta}-catenin, KRAS, and the oncogenic transcription factor ITF2. Cancer Res. 2010;70:7253–63. doi: 10.1158/0008-5472.CAN-10-1108. [DOI] [PubMed] [Google Scholar]
- 28.Hanson CA, Miller JR. Non-traditional roles for the Adenomatous Polyposis Coli (APC) tumor suppressor protein. Gene. 2005;361:1–12. doi: 10.1016/j.gene.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Fodde R, Kuipers J, Rosenberg C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–8. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan KB, Burds AA, Swedlow JR, et al. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–32. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 31.Albuquerque C, Breukel C, van der Luijt R, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–60. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar C, Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol. 2004;48:377–86. doi: 10.1387/ijdb.041807cg. [DOI] [PubMed] [Google Scholar]
- 33.Gaspar C, Franken P, Molenaar L, et al. A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis. PLoS Genet. 2009;5:e1000547. doi: 10.1371/journal.pgen.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchert M, Athineos D, Abud HE, et al. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS genetics. 2010;6:e1000816. doi: 10.1371/journal.pgen.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–98. doi: 10.1053/j.gastro.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 36.Janssen KP, Alberici P, Fsihi H, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Kohno H, Suzuki R, et al. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118:25–34. doi: 10.1002/ijc.21282. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen L, De Sousa E, Melo F, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 39.Brabletz T, Jung A, Hermann K, et al. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194:701–4. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 40.de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–7. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 41.Carmon KS, Gong X, Lin Q, et al. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:11452–7. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glinka A, Dolde C, Kirsch N, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/betacatenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–61. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazanskaya O, Glinka A, del Barco Barrantes I, et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–34. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Kim KA, Kakitani M, Zhao J, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–9. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 45.Merlos-Suarez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell stem cell. 2011;8:511–24. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi H, Ishii H, Nishida N, et al. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol. 2011;18:1166–74. doi: 10.1245/s10434-010-1373-9. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Sakamoto M, Fujii G, et al. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37:528–33. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]
- 48.Kwon C, Cheng P, King IN, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nature Cell Biology. 2011;13:1–9. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arimura S, Matsunaga A, Kitamura T, et al. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology. 2009;137:629–38. doi: 10.1053/j.gastro.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 50.van den Brink GR, Bleuming Sa, Hardwick JCH, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nature genetics. 2004;36:277–82. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 51.Zender L, Villanueva A, Tovar V, et al. Cancer gene discovery in hepatocellular carcinoma. Journal of hepatology. 2010;52:921–9. doi: 10.1016/j.jhep.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyoshi Y, Iwao K, Nagasawa Y, et al. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998;58:2524–7. [PubMed] [Google Scholar]
- 53.de La Coste A, Romagnolo B, Billuart P, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8847–51. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui J, Zhou X, Liu Y, et al. Wnt signaling in hepatocellular carcinoma: analysis of mutation and expression of beta-catenin, T-cell factor-4 and glycogen synthase kinase 3-beta genes. Journal of gastroenterology and hepatology. 2003;18:280–7. doi: 10.1046/j.1440-1746.2003.02973.x. [DOI] [PubMed] [Google Scholar]
- 55.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi K, Roberts LR, Aderca IN, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–71. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y-D, Park C-H, Kim H-S, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2008;23:110–8. doi: 10.1111/j.1440-1746.2007.05250.x. [DOI] [PubMed] [Google Scholar]
- 58.Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nature genetics. 2000;24:245–50. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 59.Zucman-Rossi J, Benhamouche S, Godard C, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–80. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 60.Hoshida Y, Nijman SMB, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer research. 2009;69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–46. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 62.Fukutomi T, Zhou Y, Kawai S, et al. Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression. Hepatology (Baltimore, Md) 2005;41:1096–105. doi: 10.1002/hep.20668. [DOI] [PubMed] [Google Scholar]
- 63.Deng Y-B, Nagae G, Midorikawa Y, et al. Identification of genes preferentially methylated in hepatitis C virus-related hepatocellular carcinoma. Cancer science. 2010;101:1501–10. doi: 10.1111/j.1349-7006.2010.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh A, Kim H-S, Lim S-O, et al. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer letters. 2011;300:162–72. doi: 10.1016/j.canlet.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 65.Puig PL, Legoix P, Bluteau O, et al. Genetic Alterations Associated With Hepatocellular Carcinomas Define Distinct Pathways of Hepatocarcinogenesis. Gastroenterology. 2001:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 66.Nejak-Bowen KN, Monga SPS. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Seminars in cancer biology. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvisi DF, Factor VM, Loi R, et al. Activation of β-Catenin during Hepatocarcinogenesis in Transgenic Mouse Models: Relationship to Phenotype and Tumor Grade Activation of β-Catenin during Hepatocarcinogenesis in Transgenic Mouse Models: Relationship to Phenotype and Tumor Grade. Cancer Research. 2001:2085–2091. [PubMed] [Google Scholar]
- 68.Calvisi DF, Factor VM, Ladu S, et al. Disruption of β-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology. 2004;126:1374–1386. doi: 10.1053/j.gastro.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Calvisi DF, Conner Ea, Ladu S, et al. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. Journal of hepatology. 2005;42:842–9. doi: 10.1016/j.jhep.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Cadoret A, Ovejero C, Saadi-kheddouci S. Hepatomegaly in Transgenic Mice Expressing an Oncogenic Form of β-Catenin Hepatomegaly in Transgenic Mice Expressing an Oncogenic Form of β-Catenin 1. Cancer. 2001:3245–3249. [PubMed] [Google Scholar]
- 71.Harada N, Miyoshi H, Murai N. Lack of Tumorigenesis in the Mouse Liver after Adenovirus-mediated Expression of a Dominant Stable Mutant of β-Catenin Lack of Tumorigenesis in the Mouse Liver after Adenovirus-mediated Expression of. Cancer. 2002:1971–1977. [PubMed] [Google Scholar]
- 72.Tan X, Apte U, Micsenyi A, et al. Epidermal Growth Factor Receptor: A Novel Target of the Wnt/β-Catenin Pathway in Liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colnot S, Decaens T, Niwa-Kawakita M, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17216–21. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fodde R. The multiple functions of tumour suppressors: it’s all in APC. Nat Cell Biol. 2003;5:190–2. doi: 10.1038/ncb0303-190. [DOI] [PubMed] [Google Scholar]
- 75.Harada N. Hepatocarcinogenesis in Mice with -Catenin and Ha-Ras Gene Mutations. Cancer research. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 76.Miyoshi H, Deguchi A, Nakau M, et al. Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1+/− mice. Cancer science. 2009;100:2046–53. doi: 10.1111/j.1349-7006.2009.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nejak-Bowen KN, Thompson MD, Singh S, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology (Baltimore, Md) 2010;51:1603–13. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyault S, Rickman DS, de Reynies A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology (Baltimore, Md) 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 79.Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–88. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merle P, de la Monte S, Kim M, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Toyama T, Lee HC, Koga H, et al. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Molecular cancer research: MCR. 2010;8:254–65. doi: 10.1158/1541-7786.MCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stoick-Cooper CL, Weidinger G, Riehle KJ, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 83.Zulehner G, Mikula M, Schneller D, et al. Nuclear beta-catenin induces an early liver progenitor phenotype in hepatocellular carcinoma and promotes tumor recurrence. The American journal of pathology. 2010;176:472–81. doi: 10.2353/ajpath.2010.090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer ANM, Fuchs E, Mikula M, et al. PDGF essentially links TGF-beta signaling to nuclear betacatenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395–405. doi: 10.1038/sj.onc.1210121. [DOI] [PubMed] [Google Scholar]
- 85.Jian H, Shen X, Liu I, et al. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–74. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ceballos MP, Parody JP, Alvarez MDL, et al. Interferon-α2b and transforming growth factor-β1 treatments on HCC cell lines: Are Wnt/β-catenin pathway and Smads signaling connected in hepatocellular carcinoma? Biochemical pharmacology. 2011:1–10. doi: 10.1016/j.bcp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nature reviews Molecular cell biology. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 88.Monga SPS, Mars WM, Pediaditakis P, et al. Hepatocyte Growth Factor Induces Wntindependent Nuclear Translocation of β-Catenin after Met- β-Catenin Dissociation in Hepatocytes. Cell. 2002:2064–2071. [PubMed] [Google Scholar]
- 89.Ueki T, Fujimoto J, Suzuki T, et al. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology (Baltimore, Md) 1997;25:862–6. doi: 10.1002/hep.510250413. [DOI] [PubMed] [Google Scholar]
- 90.Kaposi-novak P, Lee J-s, Gomez-quiroz L, et al. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. Gene Expression Patterns. 2006:116. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 92.Inagawa S, Itabashi M, Adachi S, et al. Expression and Prognostic Roles of β-Catenin in Hepatocellular Carcinoma: Correlation with Tumor Progression and Postoperative Survival Expression and Prognostic Roles of β-Catenin in Hepatocellular Carcinoma: Correlation with Tumor Progression and Postoperative Survival. Clinical Cancer Research. 2002:450–456. [PubMed] [Google Scholar]
- 93.Tien LT, Ito M, Nakao M, et al. Expression of β-catenin in hepatocellular carcinoma. Journal of Gastroenterology. 2005;11:2398–2401. doi: 10.3748/wjg.v11.i16.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nhieu JT, Renard CA, Wei Y, et al. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–10. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mao TL, Chu JS, Jeng YM, et al. Expression of mutant nuclear beta-catenin correlates with noninvasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. The Journal of pathology. 2001;193:95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 96.Hsu HC, Jeng YM, Mao TL, et al. Beta-catenin mutations are associated with a subset of lowstage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. The American journal of pathology. 2000;157:763–70. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lai TY, Su CC, Kuo WW, et al. beta-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep. 2011;26:415–22. doi: 10.3892/or.2011.1323. [DOI] [PubMed] [Google Scholar]
- 98.Wege H, Heim D, Lutgehetmann M, et al. Forced Activation of {beta}-Catenin Signaling Supports the Transformation of hTERT-Immortalized Human Fetal Hepatocytes. Molecular cancer research: MCR. 2011;9:1222–31. doi: 10.1158/1541-7786.MCR-10-0474. [DOI] [PubMed] [Google Scholar]
- 99.Wei W, Chua M-S, Grepper S, et al. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Molecular cancer. 2009;8:76. doi: 10.1186/1476-4598-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hong SM, Park JY, Hruban RH, et al. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med. 135:716–27. doi: 10.5858/2010-0566-ra.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng G, Germinaro M, Micsenyi A, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–89. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerdes B, Ramaswamy A, Simon B, et al. Analysis of beta-catenin gene mutations in pancreatic tumors. Digestion. 1999;60:544–8. doi: 10.1159/000007704. [DOI] [PubMed] [Google Scholar]
- 104.Hruban RH, Bishop Pittman M, Klimstra DS. Tumors of the Pancreas. Washington D.C: ARP Press; 2007. [Google Scholar]
- 105.Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb Symp Quant Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- 106.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–95. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heiser PW, Lau J, Taketo MM, et al. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133:2023–32. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- 108.Strom A, Bonal C, Ashery-Padan R, et al. Unique mechanisms of growth regulation and tumor suppression upon Apc inactivation in the pancreas. Development. 2007;134:2719–25. doi: 10.1242/dev.02875. [DOI] [PubMed] [Google Scholar]
- 109.Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morris JPt, Cano DA, Sekine S, et al. beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010 doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vincent A, Omura N, Hong SM, et al. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin Cancer Res. 2011;17:4341–54. doi: 10.1158/1078-0432.CCR-10-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rhim AD, Stanger BZ. Molecular biology of pancreatic ductal adenocarcinoma progression: aberrant activation of developmental pathways. Prog Mol Biol Transl Sci. 2010;97:41–78. doi: 10.1016/B978-0-12-385233-5.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasca di Magliano M, Biankin AV, Heiser PW, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanlon L, Avila JL, Demarest RM, et al. Notch1 functions as a tumor suppressor in a model of Kras- induced pancreatic ductal adenocarcinoma. Cancer Res. 70:4280–6. doi: 10.1158/0008-5472.CAN-09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero D, Iglesias M, Vary CP, et al. Functional blockade of Smad4 leads to a decrease in betacatenin levels and signaling activity in human pancreatic carcinoma cells. Carcinogenesis. 2008;29:1070–6. doi: 10.1093/carcin/bgn054. [DOI] [PubMed] [Google Scholar]
- 116.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–9. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lowe AW, Olsen M, Hao Y, et al. Gene expression patterns in pancreatic tumors, cells and tissues. PLoS ONE. 2007;2:e323. doi: 10.1371/journal.pone.0000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao D, Maitra A, Saavedra JA, et al. Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: additional evidence of different genetic pathways. Mod Pathol. 2005;18:752–61. doi: 10.1038/modpathol.3800363. [DOI] [PubMed] [Google Scholar]
- 119.Lowy AM, Fenoglio-Preiser C, Kim OJ, et al. Dysregulation of beta-catenin expression correlates with tumor differentiation in pancreatic duct adenocarcinoma. Ann Surg Oncol. 2003;10:284–90. doi: 10.1245/aso.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 120.Froeling FE, Mirza TA, Feakins RM, et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636–48. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L, Heidt DG, Lee CJ, et al. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15:207–19. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chetty R, Serra S, Salahshor S, et al. Expression of Wnt-signaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol. 2006;37:212–7. doi: 10.1016/j.humpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 123.Joo YE, Rew JS, Park CS, et al. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology. 2002;2:129–37. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 124.Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Expression patterns of alpha-, beta- and gamma-catenin in pancreatic cancer: correlation with E-cadherin expression, pathological features and prognosis. Anticancer Res. 2001;21:4127–34. [PubMed] [Google Scholar]
- 125.Li YJ, Wei ZM, Meng YX, et al. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–23. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qiao Q, Ramadani M, Gansauge S, et al. Reduced membranous and ectopic cytoplasmic expression of beta -catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int J Cancer. 2001;95:194–7. doi: 10.1002/1097-0215(20010520)95:3<194::aid-ijc1033>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 127.Nawroth R, van Zante A, Cervantes S, et al. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS ONE. 2007;2:e392. doi: 10.1371/journal.pone.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lai J-P, Oseini AM, Moser CD, et al. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology (Baltimore, Md) 2010;52:1680–9. doi: 10.1002/hep.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong Y, Wang Z, Fu B, et al. GATA6 Activates Wnt Signaling in Pancreatic Cancer by Negatively Regulating the Wnt Antagonist Dickkopf-1. PLoS ONE. 2011;6:e22129. doi: 10.1371/journal.pone.0022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koenig A, Mueller C, Hasel C, et al. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–71. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 131.Froeling FE, Feig C, Chelala C, et al. Retinoic Acid-Induced Pancreatic Stellate Cell Quiescence Reduces Paracrine Wnt-beta-Catenin Signaling to Slow Tumor Progression. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 132.Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 133.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–7. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miki T, Yasuda SY, Kahn M. Wnt/beta-catenin Signaling in Embryonic Stem Cell Self-renewal and Somatic Cell Reprogramming. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9275-1. [DOI] [PubMed] [Google Scholar]
- 136.Lucero OM, Dawson DW, Moon RT, et al. A re-evaluation of the “oncogenic” nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep. 2010;12:314–8. doi: 10.1007/s11912-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Biechele T, Camp N, Fass D, et al. Chemical-genetic screen identifies riluzole as an enhancer of Wnt/β-catenin signaling in melanoma. Chemistry and Biology. 2010;17:1177–82. doi: 10.1016/j.chembiol.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]