Abstract
Background
Increased iron stores are associated with greater cardiovascular risk in post-menopausal women. Oral contraceptive pill (OCP) use decreases the volume of menstrual blood loss and increases iron stores, but the link between OCP use, iron stores, and cardiovascular risk in pre-menopausal women has not been characterized.
Study Design
We conducted a cross-sectional study of 23 healthy OCP users to determine the association between type and duration of OCP exposure, iron stores, and vascular endothelial function [flow-mediated dilation (FMD) in the brachial artery].
Results
Median duration of OCP use was 45 months. FMD in the brachial artery was significantly associated with progestin type used (estranes/gonanes vs. drospirenone) and duration of OCP use (both p<0.05) but not iron stores. In multivariate analysis, progestin type was the only independent predictor of FMD.
Conclusions
Use of OCP containing drospirenone was independently associated with greater FMD in the brachial artery, and thus a potentially more favorable cardiovascular risk profile, when compared with use of OCP containing estranes/gonanes.
Keywords: oral contraceptive pills, progestins, vascular endothelium, iron
1. Introduction
Oral contraceptive pills (OCPs) are the most common form of reversible hormonal contraception in the United States, used as the primary method of contraception by an estimated 11.6 million women. While the evidence for increased risk of venous thromboembolism in OCP users is well established, studies of the association between OCP use and risk of cardiovascular disease have yielded conflicting findings [1]. There is also limited characterization of plausible mechanisms linking administration of exogenous estrogens and progestins to increased cardiovascular risk. Prior studies have focused on pathways related to direct effects of gonadal hormones on vascular tissues, or indirect effects on lipid metabolism or coagulation pathways [1].
Another mechanism potentially linking OCP use to cardiovascular risk is the impact of these agents on iron stores. The iron hypothesis, first published by Sullivan in 1981, proposes that increased risk of cardiovascular events in post-menopausal women is causally linked to increased iron stores after cessation of menstrual blood loss [2,3]. Use of a conventional 21/7 low-dose monophasic, combination OCP is known to decrease menstrual blood loss and increase biomarkers of tissue iron stores [4,5]. Whether a link exists between OCP use, iron stores, and cardiovascular risk has not been previously reported.
The vascular endothelium is recognized to play an important role in the normal regulation of vascular function via synthesis and release of nitric oxide and other vasoactive substances [6]. Reduced bioavailability of nitric oxide associated with dysfunction of the vascular endothelium has been linked to the pathogenesis of atherosclerosis progression and increased risk of cardiovascular events [6]. Flow-mediated dilation (FMD) in the brachial artery is a validated physiological marker of vascular endothelial function that is associated with coronary circulation endothelial function and cardiovascular morbidity and mortality risk [6,7]. Prior studies have demonstrated that acute and chronic alterations in iron stores in blood donors and other healthy populations are associated with measurable changes in FMD in the brachial artery [8–10]. We hypothesized that iron stores would increase according to duration of OCP exposure and that increased iron stores would be associated with decreased FMD in the brachial artery. Accordingly, the current study was undertaken to determine the association between OCP use, biochemical markers of iron stores, and FMD in the brachial artery in healthy women without known risk factors for cardiovascular disease.
2. Materials and Methods
2.1. Study sample
Twenty-three study subjects were recruited from printed flyers placed on the New York University Langone Medical Center campus. Non-smoking healthy women between the ages of 21 and 35 years who had been taking OCPs for at least the last three months were eligible for the study. Subjects with a history of major bleeding events (including trauma, surgery, other major blood loss) within the last 2 years, blood transfusion within the last 2 years, pregnancy within the last 2 years, history of chronic disease requiring daily medication, hemoglobin <11 gm/dL, body mass index >30 kg/m2, blood pressure >140/90 mm Hg, or history of significant breakthrough bleeding on OCPs were excluded. The study protocol was approved by the Institutional Review Board of New York University Langone Medical center. All subjects provided written informed consent before participation in this study.
2.2. Study protocol
Studies were performed in the morning after a 12-h fast during the interval of hormone withdrawal on any of the 7 days when the subject was taking inactive pills. Subjects were instructed to avoid caffeine and exercise for 12 h before the study visit. After participants provided written informed consent, information on OCP history (type of OCP, compliance, breakthrough bleeding, duration of use), brief medical history, height and weight measurements, seated blood pressure measurements, and fingerstick hemoglobin (HemoCue® Hb 201+ Analyzer, HemoCue, Inc., Lake Forest CA) were obtained. FMD was measured in the brachial artery during supine rest with noninvasive ultrasound imaging as described below. After completion of the FMD measurements, a standardized mental arithmetic task was performed as described below and the flow-mediated dilation was repeated. After completion of the ultrasound imaging studies, 20 mL of blood was obtained from a forearm vein for laboratory studies of iron stores and vascular inflammation as described below.
2.3. Endothelial function testing
Flow-mediated, endothelium-dependent vasodilation in response to transient arterial occlusion was determined non-invasively with high resolution ultrasound imaging of the brachial artery with a high resolution 11.5 MHz linear array transducer connected to a Sonosite HeartElite plus duplex ultrasound machine (Sonosite Inc, Bothell, Washington, USA) as previously described [8]. The axial resolution of the 11.5 MHz transducer can detect changes in brachial artery diameter of <0.1 mm. Arterial diameter was determined as the internal dimension of the vessel wall, from trailing edge to leading edge of the anterior and posterior intima markings, respectively, with a validated semi-automated image analysis software program. Brachial artery blood flow velocity was determined with a 1.2-mm pulsed Doppler ultrasound sampling volume placed in the center of the image of the vessel lumen with software correction for the incident angle. Mean blood flow velocity (MBFV, cm/s) was determined by calculation of the area under the hand-traced curve of the spectral display. Brachial artery diameter and MBFV were measured at rest, and after transient arterial occlusion induced by inflation of a forearm blood pressure cuff to suprasystolic pressure for 5 min. MBFV was measured for 15 s immediately upon release of the occluding cuff; the first 5 beats after release were averaged. Brachial artery diameter was measured over a 1-cm vessel length at end-diastole 60–75 s following release of the occluding cuff; 3 diameter measurements were averaged. FMD was calculated as the % change in brachial artery diameter after cuff release compared with the resting diameter. Internal and external landmarks were used to make repeated measurements at the same vessel site. In our laboratory, mean FMD in normal adult male and female subjects, mean age 43 years, is 5.89% (95% CI 4.53%, 7.23%).
2.4. Laboratory studies
A 20-mL blood sample was obtained from a forearm vein with standard phlebotomy techniques to measure serum iron, total iron binding capacity, serum ferritin as previously described [10].
2.5. Data analysis
Continuous variables are summarized as mean±SD or median (interquartile range (IQR)) for normal and non-normal distributions, respectively (determined with Shapiro Wilk test). FMD of the brachial artery was the primary outcome variable of the study. Associations between type of OCP (based on progestin type and estrogen dose), duration of OCP use, measures of iron stores and FMD were estimated with unpaired Student’s t-test or simple linear regression. Multivariate regression was used to estimate the independent association of these predictor variables with FMD in the brachial artery (Stata Version 10.1, College Station TX). For all analyses, a two-tailed p<0.05 was used to infer statistical significance.
3. Results
3.1. Study sample characteristics
Median lifetime duration of OCP use in the study sample was 45 months (IQR 34–63 months). Median duration of the currently used type of OCP was 20 months (IQR 9–51 months). Subjects were grouped according to the progestin type of the currently used OCP (Table 1). There were no significant differences in clinical characteristics between groups.
Table 1.
Clinical Characteristics of Study Sample (Mean±SD, Median (IQR) or percent) by progestin type
| Estranes/gonanes (N=16) |
Drospirenone (N= 7) |
|
|---|---|---|
| Age (years) | 24.9±2.9 | 23.9±1.7 |
| Body mass index (kg/m2) | 21.9±2.7 | 23.3±4.2 |
| Systolic blood pressure (mm Hg) | 106.8±11.3 | 112.6±5.5 |
| Diastolic blood pressure (mm Hg) | 67±9.9 | 68.9±6.6 |
| Heart rate (min−1) | 67.2±10.7 | 77.6±6.7 |
| Hemoglobin (g/dL) | 12.8±0.8 | 12.9±0.8 |
| % Hemoglobin <12g/dl | 18.75% | 16.67% |
| Duration of lifetime OCP use (months) | 47 (40–67) | 34 (12–58) |
| Duration of most recent type of OCP use (months) | 32 (10–57) | 10 (6–34) |
| Estrogen dose (%) | ||
| Low dose (20–25 mcg) | 68.8% | 71.4% |
| High dose (>25 mcg) | 31.2% | 28.6% |
3.2. Biomarkers of iron stores
Biomarkers of iron stores in subjects grouped according to progestin content of the currently used type of OCP are shown in Table 2. Serum iron and transferrin saturation were significantly lower in subjects taking drospirenone-containing OCP when compared with that in subjects taking an estrane/gonane-containing OCP (norgestimate n=7, desogestrel n=3, levonorestrel, n=3, norethindrone n=2, norgestrel n=1). Serum ferritin levels and the proportion of subjects with evidence of nearly depleted iron stores (serum ferritin <12 ng/mL) did not differ between groups. Biomarkers of iron stores did not differ in subjects grouped according to estrogen content of the OCP, or subjects grouped by duration of OCP use (above or below median duration value, data not shown).
Table 2.
Biomarkers of iron stores (mean±SD or median (IQR)) by progestin type
| Estranes/Gonanes | Drospiredone | Reference Value* |
p-value | |
|---|---|---|---|---|
| Serum iron (mcg/dL) | 96±36 | 65±20 | 49–158 | 0.04 |
| Total iron binding capacity (mcg/dL) | 508±28 | 486±27 | 463–535 | 0.10 |
| Transferrin saturation (%) | 19±6 | 13±4 | 10–30 | 0.04 |
| Serum ferritin (ng/mL) | 15 (10–25) | 23 (10–44) | 8–45 | 0.54 |
| % Serum ferritin <12 ng/mL | 38% | 29% | - | 0.68 |
Reference values are based on 5th-95th percentiles derived from our study sample of pre-menopausal females taking OCPs.
3.3. Flow-mediated dilation in the brachial artery
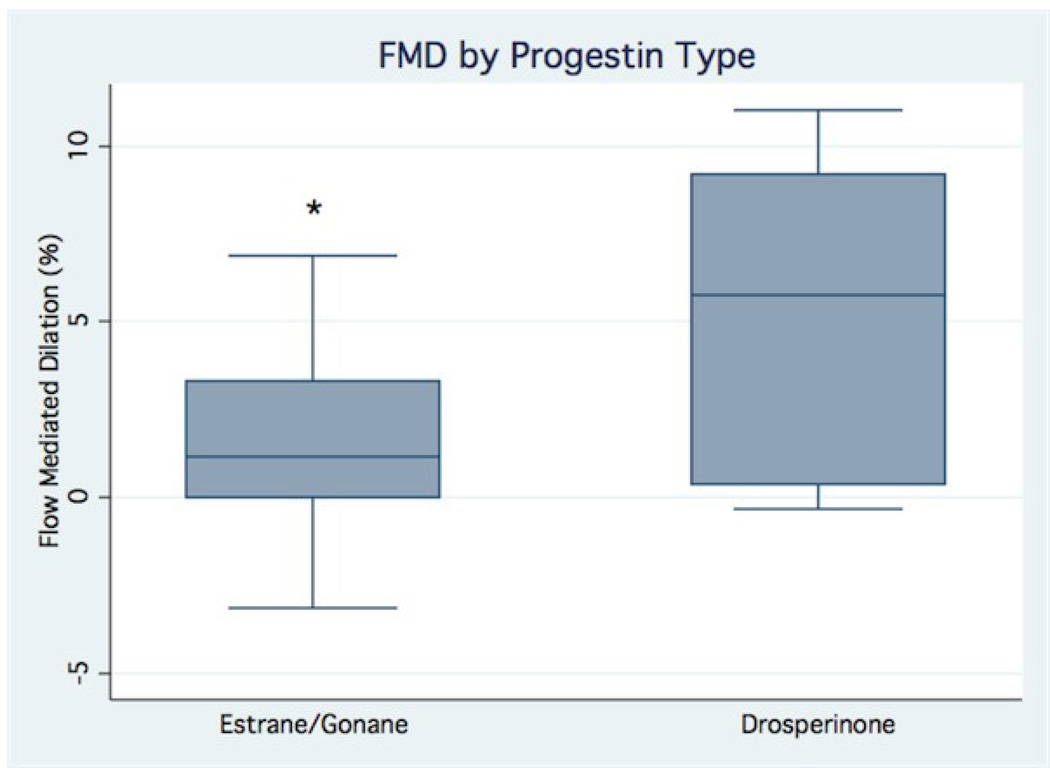
The mean resting brachial artery diameter was 2.62±0.23 mm and did not differ among subjects taking OCPs containing estranes/gonanes and subjects taking OCPs containing drospirenone (2.62±0.20 mm vs. 2.63±0.25 mm, p=0.93). The mean resting FMD in the brachial artery of subjects taking estrane/gonane-containing OCP was significantly less than that of subjects taking drospirenone-containing OCP (1.5±2.8% vs. 5.6±4.4%, p=0.01, Fig. 1). The mean resting FMD in the brachial artery of subjects taking OCPs with higher-dose estrogen tended to be lower than that of subjects taking OCPs with lower-dose estrogen (higher dose 30–35 mcg: 0.3±3.5% vs. lower dose 20–25 mcg: 3.7±3.6%, p=0.057). In univariate analysis, flow-mediated dilation in the brachial artery was inversely associated with duration of OCP use (r= −0.44, p=0.039), but not with any of the biomarkers of iron stores (all p>0.20, data not shown). A multivariate model that included predictor variables of OCP type based on progestin type and estrogen dose, duration of OCP use, and potential confounding variables (age and body mass index) accounted for more than half of the variability observed in FMD in the brachial artery (R2=0.58, p=0.016). The estimate from the multivariate model demonstrated little change from the univariate estimate of the effects of progestin type on FMD in the brachial artery and remained statistically significant (mean difference between groups 3.76%, p=0.021), suggesting that this predictor variable is independent of the other variables in the model. The estimate from the multivariate model also demonstrated little change from the univariate estimate of the effects of estrogen content on FMD in the brachial artery (mean difference between groups 2.97%, p=0.057), but the effect remained non-significant, suggesting that estrogen content was not independent of the other variables in the model. The estimate from the multivariate model for the association between duration of OCP use and FMD was no longer statistically significant (partial correlation coefficient −0.32, p=0.17) suggesting that age was a likely confounder for this association.
Fig. 1.
Box plots showing the distribution of brachial artery flow-mediated dilation (FMD) values in subjects grouped by progestin type (estrane/gonane vs. drospirenone). Box plots indicate median value (within box), interquartile range (upper and lower limits of box), values adjacent to 1.5 × IQR (box whiskers) and if present, individual data points outside this range. The mean brachial artery FMD of subjects taking estranes/gonanes (1.5±2.8%, N= 15) was significantly lower than that of subjects taking drospirenone (5.6±4.4%, N= 7). * indicates p<0.05 vs. drospirenone group.
4. Discussion
The current study demonstrates that use of drospirenone-containing OCPs is associated with significantly decreased serum iron and transferrin saturation and significantly increased FMD in the brachial artery when compared with use of estrane/gonane-containing OCPs in healthy young women. The findings do not support our a priori hypothesis that decreased menstrual blood loss during oral contraceptive use would be associated with a time-dependent increase in iron stores and consequent impairment of FMD in the brachial artery. On the contrary, the findings of our multivariate analysis indicate that type of progestin exposure, rather than duration of exposure or changes in body iron stores, is the strongest independent predictor of FMD response in OCP users.
The effects of OCP on vascular function are influenced by the type and dose of estrogen and progestins in the preparation. Stimulation of estrogen receptors in vascular tissues is associated with increased synthesis and release of nitric oxide [11]. Clinical studies have consistently demonstrated that estrogen augments endothelium-dependent nitric oxide mediated vasodilation in pre- and post-menopausal women [12–15].
In contrast to the consistent body of evidence demonstrating favorable effects of estrogens on vascular endothelial function, existing reports on the effects of progestins on vascular endothelial function have yielded conflicting findings. In many but not all studies, progestins are associated with attenuation of the effects of estrogen on endothelial production of nitric oxide [12,16–19]. Detrimental effects of synthetic progestins on endothelial function have been attributed to direct androgen receptor signaling effects in endothelial cells, and indirect effects related to changes in lipid profile and/or levels of sex hormone binding globulin during therapy [1,20,21]. Drospirenone is pharmacologically distinct from estrane and gonane progestins as it is derived from aldosterone rather than testosterone, and has distinct mineralocorticoid receptor-binding and anti-androgen properties [22]. In the current study, use of drospirenone-containing OCP was independently associated with increased FMD of the brachial artery when compared to use of estrane/gonane-containing OCPs. This observation is consistent with previous reports that drospirenone increases expression and activity of endothelial nitric oxide synthase in cultured human vein umbilical cells and that drospirenone does not antagonize estrogen-dependent effects on FMD in the brachial artery in healthy pre-menopausal women [23,24]. A favorable impact of drospirenone on endothelium-mediated vasodilation is also consistent with previous work demonstrating salutary effects of mineralocorticoid receptor blockade with spironolactone and eplerenone on nitric oxide-mediated dilation in experimental models and patients with cardiovascular disease [25,26].
Previous studies have demonstrated a time-dependent increase in biomarkers of iron stores among OCP users when compared with non-OCP users [4,5]. Increased iron stores are thought to be attributable primarily to reduced menstrual blood loss, but the effects of gonadal hormones on expression of iron regulatory proteins may also influence iron metabolism [27]. Our study did not include a control group of non-OCP users, and we did not find an association between duration of OCP exposure and biomarkers of iron stores in our study sample. This unanticipated finding may be partly attributable to the known limitations of the use of serum biomarkers for assessment of tissue iron stores. Although we excluded subjects with known bleeding or blood transfusion events, it is possible that unmeasured confounders related to occult blood loss and/or dietary iron intake may have contributed to our findings.
The iron hypothesis proposes that reduction in tissue iron stores is associated with reduced risk of heart disease [2,3]. Support for the iron hypothesis was originally based on epidemiological observations of increased cardiovascular risk in post-menopausal women and women with hysterectomy without oophorectomy [28]. Subsequent studies have demonstrated that iron is a pro-oxidant factor that is linked to vascular dysfunction in experimental models and human endothelial cells [29,30]. In studies in human subjects, acute and chronic reductions in iron stores and/or iron bioavailability are associated with increased brachial artery FMD [8,9]. In a randomized prospective trial in patients with peripheral vascular disease, reduction of iron stores with serial phlebotomy was associated with reduction in risk of coronary artery events [31]. We found that drospirenone-containing OCP use was associated with decreased serum iron and decreased transferrin saturation when compared with estrane/gonane-containing OCP use. However, none of the markers of tissue iron stores used in this study were significantly associated with brachial artery FMD. Biomarkers of iron stores were unexpectedly low in our study sample with a median duration of exposure to OCP of 45 months [5]. Since the narrow range of biomarkers of iron stores in our study sample reduced power to detect an association with brachial artery FMD, investigations in larger populations are needed to further test this hypothesis.
There are several caveats to consider in interpretation of the study findings. We chose to classify OCP based on broad pharmacological properties but acknowledge that there are important pharmacological differences among individual agents in the estrane and gonane classes as well as substantial variability in pharmacokinetics within individuals that were not measured in this study [20,21,32]. Starting and ending dates and types of past OCP use were obtained through patient interview and may have been subject to recall misclassification. Brachial artery FMD measurements were made during the interval of hormone withdrawal, but differences in half-lives of the progestins in the OCPs could have confounded our results [32]. We did not test nitroglycerin-mediated vasodilation in our study subjects so cannot determine whether the observed differences in brachial artery FMD were due to increased nitric oxide bioavailabilty and/or vascular smooth muscle responsiveness. However, in previous studies, gonadal hormones have not been observed to have a direct effect on nitric oxide responsiveness in vascular smooth muscle [17,19].
Brachial artery FMD is known to be associated with coronary artery vascular endothelial function and clinical outcomes in cardiovascular disease populations [6,7]. The mean brachial artery FMD in the young healthy women in our study sample exposed to estrane/gonane-containing OCPs was below the normal range of values for our laboratory and was comparable to previous values from our laboratory obtained from much older male and female subjects (mean age 61 years) with multiple cardiac risk factors and 10-year Framingham risk score of 5% [8]. Further investigation is warranted to determine if the cardiovascular safety profile of drospirenone-containing OCP differs from that of estrane/gonane-containing OCP. Additionally, although we were unable to demonstrate a link between iron stores and vascular function in our study sample, further investigation of the long-term effects of OCP use on iron metabolism, and the cardiovascular safety profile of newer extended cycle formulations that greatly reduce or nearly eliminate menstrual blood loss is warranted [33].
In conclusion, our findings indicate that progestin type is the strongest independent predictor of brachial artery FMD in healthy women without known risk factors for cardiovascular disease taking OCP. Further investigations are needed to determine the underlying mechanisms and whether variations in brachial artery FMD are linked to differences in cardiovascular safety profile of OCP preparations.
Acknowledgment
Supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol. 2009;53:221–231. doi: 10.1016/j.jacc.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JL. Is stored iron safe? J Lab Clin Med. 2004;144:280–284. doi: 10.1016/j.lab.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Frassinelli-Gunderson EP, Margen S, Brown JR. Iron stores in users of oral contraceptive agents. Am J Clin Nutr. 1985;41:703–712. doi: 10.1093/ajcn/41.4.703. [DOI] [PubMed] [Google Scholar]
- 5.Milman N, Kirchhoff M, Jorgensen T. Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann Hematol. 1992;65:96–102. doi: 10.1007/BF01698138. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 7.Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol. 2005;25:1577–1583. doi: 10.1161/01.ATV.0000174126.28201.61. [DOI] [PubMed] [Google Scholar]
- 9.Zheng H, Dimayuga C, Hudaihed A, Katz SD. Effect of dexrazoxane on homocysteine-induced endothelial dysfunction in normal subjects. Arterioscler Thromb Vasc Biol. 2002;22:E15–E18. doi: 10.1161/01.atv.0000023187.25914.5b. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Huang X, Zhang Q, Katz SD. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int. 2006;69:679–684. doi: 10.1038/sj.ki.5000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnal JF, Fontaine C, Billon-Gales A, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 12.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol. 2007;292:H2874–H2880. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]
- 13.Herrington DM, Espeland MA, Crouse JR, 3rd, et al. Estrogen replacement and brachial artery flow-mediated vasodilation in older women. Arterioscler Thromb Vasc Biol. 2001;21:1955–1961. doi: 10.1161/hq1201.100241. [DOI] [PubMed] [Google Scholar]
- 14.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 15.Virdis A, Ghiadoni L, Pinto S, et al. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 16.Koh KK, Jin DK, Yang SH, et al. Vascular effects of synthetic or natural progestagen combined with conjugated equine estrogen in healthy postmenopausal women. Circulation. 2001;103:1961–1966. doi: 10.1161/01.cir.103.15.1961. [DOI] [PubMed] [Google Scholar]
- 17.Virdis A, Pinto S, Versari D, et al. Effect of oral contraceptives on endothelial function in the peripheral microcirculation of healthy women. J Hypertens. 2003;21:2275–2280. doi: 10.1097/00004872-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol. 2008;294:H1630–H1637. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Ethinyl estradiol-to-desogestrel ratio impacts endothelial function in young women. Contraception. 2009;79:41–49. doi: 10.1016/j.contraception.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–283. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 Suppl 3:4–12. doi: 10.1080/13697130500330382. [DOI] [PubMed] [Google Scholar]
- 23.Simoncini T, Fu XD, Caruso A, et al. Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors. Hum Reprod. 2007;22:2325–2334. doi: 10.1093/humrep/dem109. [DOI] [PubMed] [Google Scholar]
- 24.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. A combined oral contraceptive containing 30 mcg ethinyl estradiol and 3.0 mg drospirenone does not impair endothelium-dependent vasodilation. Contraception. 82:366–372. doi: 10.1016/j.contraception.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 26.Imanishi T, Ikejima H, Tsujioka H, et al. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51:734–741. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
- 27.Stuckey R, Aldridge T, Lim FL, et al. Induction of iron homeostasis genes during estrogen-induced uterine growth and differentiation. Mol Cell Endocrinol. 2006;253:22–29. doi: 10.1016/j.mce.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 28.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89:157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 29.Araujo JA, Romano EL, Brito BE, et al. Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1172–1180. doi: 10.1161/01.atv.15.8.1172. [DOI] [PubMed] [Google Scholar]
- 30.Zweier JL, Broderick R, Kuppusamy P, Thompson-Gorman S, Lutty GA. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]
- 31.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007;297:603–610. doi: 10.1001/jama.297.6.603. [DOI] [PubMed] [Google Scholar]
- 32.Goldzieher JW, Stanczyk FZ. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008;78:4–9. doi: 10.1016/j.contraception.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89–96. doi: 10.1016/s0010-7824(03)00141-0. [DOI] [PubMed] [Google Scholar]