Abstract
Spinal cord injury induces maladaptive synaptic transmission in the somatosensory system that results in chronic central neuropathic pain. Recent literature suggests that glial-neuronal interactions are important modulators in synaptic transmission following spinal cord injury. Neuronal hyperexcitability is one of the predominant phenomenon caused by maladaptive synaptic transmission via altered glial-neuronal interactions after spinal cord injury. In the somatosensory system, spinal inhibitory neurons counter balance the enhanced synaptic transmission from peripheral input. For a decade, the literature suggests that hypofunction of GABAergic inhibitory tone is an important factor in the enhanced synaptic transmission that often results in neuronal hyperexcitability in dorsal horn neurons following spinal cord injury. Neurons and glial cells synergistically control intracellular chloride ion gradients via modulation of chloride transporters, extracellular glutamate and GABA concentrations via uptake mechanisms. Thus, the intracellular “GABA-glutamate-glutamine cycle” is maintained for normal physiological homeostasis. However, hyperexcitable neurons and glial activation after spinal cord injury disrupts the balance of chloride ions, glutamate and GABA distribution in the spinal dorsal horn and results in chronic neuropathic pain. In this review, we address spinal cord injury induced mechanisms in hypofunction of GABAergic tone that results in chronic central neuropathic pain.
Keywords: Central neuropathic pain, GABA, Glia, Hyperexcitability, Spinal cord injury
1. Introduction
Spinal cord injury (SCI) causes central neuropathic pain (CNP) in as high as 70% of people with SCI (Beric et al., 1988; Rintala et al., 1988). In terms of clinical pain behavior, allodynia (pain behavior evoked by non-noxious stimuli) and hyperalgesia (exaggerated pain behavior evoked by noxious stimuli) characterize CNP syndromes (Merskey and Bogduk, 1994). Patients with CNP suffer from altered quality of life that negatively influences the individual and often results in depression and suicide (Siddall and Loeser, 2001; Werhagen et al., 2004). The majority of patients with SCI suffer from CNP; however, current treatment strategies are inadequate and refractive because the cellular mechanisms that provide the substrate for CNP are poorly understood (Beric et al., 1988; Davidoff et al., 1987; Hulsebosch, 2005; Siddall and Loeser, 2001).
Descending and local interneuron inhibitory pathways, such as GABAergic pathways, critically contribute to the modulation of the balance between excitatory and inhibitory tone in synaptic transmission. The GABAergic interneurons produce synaptic inhibition, and thereby prevent or inhibit CNP, via both GABAA and GABAB receptors in the spinal dorsal horn (Hwang and Yaksh, 1997; Munro et al., 2008). Pharmacological blockade of GABAA or GABAB receptor in rats (Gwak et al., 2006; Malan et al., 2002) and transgenic mice of GABAA (Knabl et al., 2008, 2009) or GABAB (Gangadharan et al., 2009) receptor demonstrate increased sensitivity to external stimuli that results in various pain conditions induced by spinal cord injury, peripheral nerve injury and inflammation. Mounting evidence indicates that SCI induces a hypofunction of GABAergic tone in the spinal dorsal horn and results in CNP (Gwak et al., 2006; Liu et al., 2004; Zhang et al., 1994). Thus, it is possible that the hypofunction of spinal GABAergic inhibitory tone in the spinal dorsal horn is a key factor in CNP after SCI (Drew et al., 2004; Liu et al., 2004).
Spinal systems are composed of both neuronal and non-neuronal cells, including astrocytes and microglia. Spinal glia cells outnumber neurons and play important roles in maintaining ionic balance, as well as glutamate and GABA concentrations in the central nervous system (Anderson and Swanson, 2000; Chesler and Kaila, 1992; Largo et al., 1996; Schlue and Deitmer, 1988). However, recent literature consistently reports that activation of astrocytes and microglia contributes significantly to CNP following SCI (Gwak et al., 2008, Gwak and Hulsebosch, 2009; Hains and Waxman, 2006). Neurons and astrocytes both contribute to GABA uptake (also released from both neurons and glia) to control extracellular concentrations of GABA (Chatton et al., 2003; Schousboe et al., 2004). Although, it is known that SCI alters neuronal and glial activity; little is known about the mechanisms underlying hypofunction of spinal GABAergic inhibitory tone following SCI. In this review, we focus on mechanisms that lead to hypofunction of GABAergic tone that contributes to CNP following SCI.
2. Central Neuropathic Pain and GABA
2.1 Central Neuropathic pain following SCI
Traumatic spinal cord injuries (SCI) directly and indirectly produce dramatic changes of neuroanatomical and neurochemical shifts that result in maladaptive synaptic circuits in the spinal dorsal horn. Upregulated glutamate receptors and ion channels, increased release of proinflammatory cytokines and reactive oxygen species (ROS), activation of glial cells and subsequent activation of intracellular downstream/upstream cascades (Crown et al., 2006; Gwak et al., 2008; 2009a; Hains et al., 2003, Leem et al., 2010; Tan et al., 2008; Zinck et al., 2007) are predominant events that lead to enhanced pain transmission following SCI.
Maladaptive synaptic circuits in the spinal dorsal horn induced by SCI, individually or synergistically, contribute to the neuronal hyperexcitability in response to mechanical, chemical and thermal stimuli. Electrophysiologically, neuronal hyperexcitability or central sensitization is characterized by the enhanced spontaneous or evoked neuronal response properties to external stimuli applied to peripheral receptive fields with lowered thresholds for the activation, increased peripheral receptive field size and increased afterdischarge activity (Drew et al., 2004; Gwak et al., 2008; Hains et al., 2003). Thus, neuronal hyperexcitability is a substrate of central neuropathic pain following SCI. SCI is categorized by below-, at-, and above-level pain based on dermatomal regions that involves neuropathic pain symptoms, such as mechanical allodynia and thermal hyperalgesia, following SCI (Siddall et al., 1997, 1999, 2002). Clinically, below-level pain is defined as pain that occurs several dermatomes caudal to the lesion. At-level pain is defined as pain that occurs at dermatomes adjacent to the lesion. Above-level pain is defined as pain that occurs several dermatomes rostral to the lesion (Siddall et al., 1997, 1999). The development of central neuropathic pain syndromes take several weeks to months after SCI, but once developed, persists for life. Consequently, the abnormal pain syndromes are important to understand for effective treatment strategies (Carlton et al., 2009; Hulsebosch et al., 2009).
Mechanically and chemically induced mammalian SCI models were developed to study the development and the maintenance of CNP after SCI. The models include; 1) spinal ischemic injury via intravascular photochemical reaction (Hao et al., 1991); 2) clip compression of the thoracic cord (Bruce et al., 2002); 3) excitotoxic injury via quisqualic acid injection into the dorsal horn (Yezierski et al., 1998); 4) spinal hemisection injury (Christensen et al., 1996; Gwak et al., 2006); 5) spinal contusion injury (Hulsebosch et al., 2000; Siddall et al., 1997). It is a worthy to note that these SCI models showed CNP that correlates with hypofunction of GABAergic inhibitory tone in the spinal dorsal horn (Drew et al., 2004; Eaton et al., 2007; Gwak et al., 2006; Xu et al., 1993). Taken together, hypofunction of GABAergic tone may provides a representative mechanism in CNP syndromes after SCI, although Polar and Todd reported the GABAergic loss is not sufficient to induce neuropathic pain following peripheral nerve injury (Polgar et al., 2003, 2005).
2.2. GABA Synthesis and Release
In the somatosensory system, GABAergic descending pathways originate from locus ceruleus (LC), nucleus raphe-magnus (NRM) and periaquctal gray (PAG) and terminate in the spinal cord (Willis and Westlund, 1997). Gamma-aminobutyric acid (GABA) is a widely distributed inhibitory neurotransmitter in the spinal cord and plays a “counter balance” role against enhanced synaptic transmission in the spinal cord as a result of glutamate-mediated excitation of neurons following SCI. GABA is produced by the decarboxylation of L-glutamate by glutamic acid decarboxylase (GAD, a rate-limiting enzyme) that is distributed in GABA interneurons and glial cells (Bu et al., 1992; Erlander et al., 1991; Kaufman et al., 1991; Mackie et al., 2003). GAD65 (65 kDa) is a membrane associated protein that produces vesicular GABA, released by exocytosis, and contributes to the rapid and focal communication to individual postsynaptic site on neurons. GAD67 (67kDa) is a cytosolic protein that produces cytosolic GABA release and contributes to both paracrine signaling and intracellular metabolites. However, neurons are not the only cells that synthesize GABA in the central nervous system. After ischemic injury, forebrain regions show increased GFAP immunoreactivity (activated astrocytes) co-labeled with GABA and GAD that indicate that glial cells also synthesize GABA, since GAD is the enzyme necessary in GABA synthesis (Bellier et al., 2000; Kozlov et al., 2006; Lin et al., 1993; Liu et al., 2007; New and Rabkin, 1998).
GABAergic neurons predominantly synapse axodendritically and axosomatically and only a small number have axoaxonic synapses. Activation of NMDA receptors and other calcium channels, largely located on neuronal dendritic or somatic membranes, trigger large influxes of calcium ions, dependent on the depolarization of the membrane and initiate subsequent Ca2+ dependent GABA release via vesicular exocytosis (Isaacson, 2001; Koch and Magnusson, 2009). Thus, the somatic and dendritic localized GABA release may produce widespread inhibition in nociceptive transmission in synaptic and extrasynaptic terminals.
2.3 GABA Receptors
GABA, released by neurons and glia, provides an inhibitory role via neighboring GABA receptors that are distributed in presynaptic and postsynaptic membranes on both neurons and glial cells (Barakat and Bordey, 2002; Malcangio and Bowery, 1996). There are three types of GABA receptors: 1) GABAA, 2) GABAB and 3) GABAC. The GABAA receptor is an ionotropic ligand-gated Cl− channel and is present throughout the spinal cord gray matter on both neurons and glial cells. Activation of GABAA receptor increases the permeability of chloride ions and hyperpolarizes postsynaptic neurons, which results in increases in the resting membrane conductance of the cell (Jensen et al., 2002; Sieghart and Sperk, 2002). The GABAB receptor is a metabotropic receptor, coupled to a G-protein, and concentrated in the superficial layers of the spinal dorsal horn on both neurons and glial cells (Albrecht et al., 1986; Bowery et al., 1980; Charles et al., 2003a,b). Activation of the GABAB receptor inhibits synaptic transmission at primary afferent terminals in the spinal cord via reduction of calcium entry at the presynatic terminal and hyperpolarization at the postsynaptic terminal through increased conductance of potassium ions (Bowery et al., 1980). The GABAC receptor is a subtype of GABAA and insensitive to benzodiazepine (Johnston, 1996; Shimada et al., 1992). Currently, the role of the ionotropic GABAc receptor in somatosensory systems is unknown but it appears to be important in cognition and neuronal processing (Johnston, 1996; Lukasiewicz et al., 1994).
2.4 Hypofunction of GABA and CNP
The evidence that hypofunction of GABAergic tone contributes to central neuropathic pain following SCI is well documented by several experimental obervations. First, pharmacological treatments that enhance GABAergic function attenuate central neuropathic pain behavior and neuronal hyperexcitability following SCI. For example, intrathecal administration of GABA attenuated mechanical allodynia and hyperexcitability of spinal dorsal horn neurons following SCI (Figure 1). The attenuation of pain behavior and neuronal hyperexcitability are mediated by both GABAA and GABAB receptors (Drew et al., 2004; Gwak et al., 2006). In addition, treatment with bicuculline (a GABAA receptor antagonist) produced neuronal hyperexcitability and pain behavior in normal rats (Drew et al., 2004). Second, immunohistochemical studies demonstrated decreased numbers of GABAergic interneurons and GAD expression in the spinal dorsal horn following SCI that correlated well with central neuropathic pain behavior (Gwak et al., 2008; Meisner et al., 2010; Zhang et al., 1994). Third, transplantation of herpes simplex virus (HSV)-mediated GAD65 and GAD67 producing gene vector or transfer of human foamy virus (HFV) mediated GAD67 gene, attenuated central neuropathic pain following SCI (Liu et al., 2004, 2008; New and Rabkin, 1998). In addition, transplantation of the human neuronal NT cell line (NT2.17), which synthesizes and releases GABA, attenuated SCI-induced mechanical allodynia and thermal hyperalgesia (Eaton et al., 2007). Taken together, these pharmacological and molecular approaches suggest that hypofunction of GABAergic tone is a substrate for the central neuropathic pain following SCI.
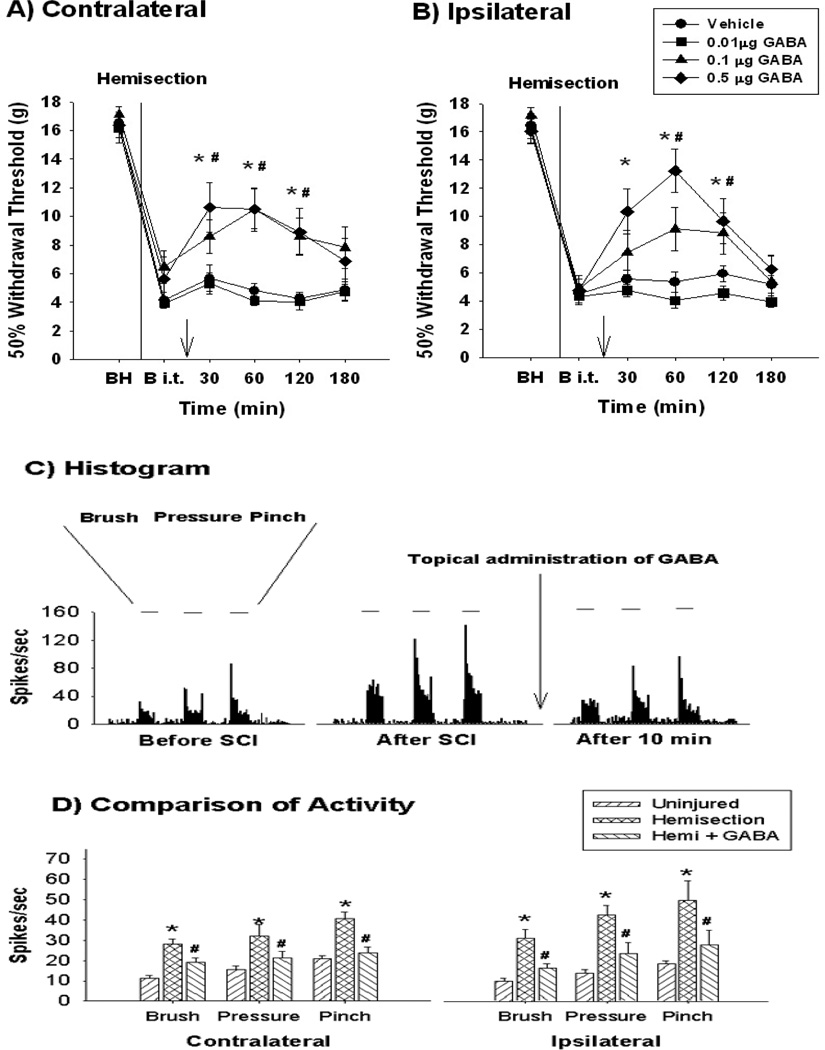
Figure 1.
Attenuation of mechanical allodynia and neuronal hyperexcitability by administration of GABA. Spinal T13 hemisection injury results in bilateral mechanical allodynia on the contralateral (A, uninjured side) and the ipsilateral (B, injured side) hindlimbs compared to preinjury values before spinal hemisection (BH). On post operation day 28 (B i.t, before intrathecal administration), intrathecal 0.1 (#) and 0.5 (*) µg GABA administration significantly affects the mechanical allodynia in both hindlimbs compared to before intrathecal administration (p<0.05). Arrow reflects the time point of intrathecal administration. (C) displays the typical peri-stimulus histogram of lumbar wide dynamic range (WDR) dorsal horn neurons produced after GABA administration. After T13 hemisection, the lumbar WDR dorsal horn neurons show hyperexcitability (compare to uninjured, before SCI). Topical administration of GABA (arrow, 0.1 µg) significantly attenuated the hyperexcitability. (D) displays significant changes in evoked activity in the lumbar WDR dorsal horn neurons among the three different groups (p<0.05). The data reflect evoked activity at 30 min post injection (modified from Gwak et al., 2008).
3. Central Neuropathic Pain and Glial Activation
3.1 Glial activation after SCI
After SCI, dramatic neuroanatomical and neurochemical changes in the spinal cord are not just neuronal events. Neurons and glia (astrocytes and microglia) compose the central nervous system and paradoxically express similar receptors/ion channels and release similar transmitters, cytokines and ROS that contribute to neuropathic pain behavior and neuronal hyperexcitability following SCI (Gwak and Hulsebosch, 2010; Pineau and Lacroix, 2007; Porter and McCarthy, 1997; Wang et al., 2009; Jarvis, 2010). These neurochemical and neuroanatomical changes suggest that glial cells also modify input mediated by primary afferent fibers at synaptic clefts by neuronal-glial-neuronal interactions. Thus, neuronal function is influenced via bidirectional communication in neuronal-glial and glial-neuronal interactions (Scholz and Woolf, 2007).
Recently, the literature demonstrated that SCI directly and indirectly causes glial activation in the dorsal horn in both the early phase (less than 3 days after injury) and the chronic phase (over a month and longer) in rats (Gwak et al., 2009a; Knerlich-Lukoschus et al., 2010; Nesic et al., 2005; Ritz and Hausmann, 2008) and mice (White et al., 2010). Somatic hypertrophy, proliferation, thickened branches and activation of membrane surface markers, such as CD11b, toll-like receptors and glial fibrillary acidic protein (GFAP), are morphological characteristics of glial activation; whereas, increased release of glutamate, ROS, ATP, CGRP, interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α are physiological characteristics of glial activation, respectively (Chao et al., 1995; Lieberman et al., 1989; Priller et al., 1995; Rischke and Krieglstein, 1991; Stanley et al., 1994; Svensson et al., 1993). However, increased extracellular concentrations of glutamate, ATP, neuropeptides, IL-1, IL-6 and TNFα are all associated with sensitization of sensory circuits following SCI (Bennett et al., 2000a,b; Detloff et al., 2008). Thus, morphologically and physiologically, activated glia produce increased release of pain-mediating substances and contribute significantly to mechanisms that contribute to central neuropathic pain following SCI (Hulsebosch, 2008).
In addition, following SCI, ionic imbalances are maintained by activated glia and are important factors in both glial activation and CNP. First, high affinity glutamate transporters in astrocytes accumulate glutamate and increase uptake of K+ by Na+/K+–ATPase (Bender et al., 1998). The accumulation of K+ leads to anion channel opening, to enhance the passive influx of Cl−, K+, HCO3−, followed by H2O accumulation in the astrocytes that results in astrocytic hypertrophy. Second, SCI causes decreased expression of inwardly-rectifying potassium channels 4.1 (Kir4.1) in astrocytes and facilitates astrocytic depolarization (Olsen et al., 2010). Third, overexpression of intracellular glial ROS after SCI activates astrocytic Na+/K+/Cl− (NKCC) cotransporters and increases intracellular uptake of K+ ions with release of Na+ ions into the extracellular space (Jayakumar and Norenberg, 2010). The K+ and Na+ ionic imbalance at glial-neuronal synaptic clefts causes the reduction of K+-mediated resting potentials and initiates Na+-mediated depolarization of neuronal membranes followed by Ca2+-mediated intracellular events that result in persistent hyperexcitability of the spinal dorsal horn neurons following SCI.
3.2 Glial activation and CNP
The evidence that activated glia contribute to CNP following SCI is well documented from both in vitro and in vivo studies (Gwak et al., 2008; Gwak and Hulsebosch, 2009; Hains and Waxman, 2006; Zhao et al., 2007). Intrathecal treatments with glial cell inhibitor agents, such as minocycline (microglial inhibitor) and propentophylline (phosphodiesterase inhibitor and glial modulator), attenuated SCI-induced mechanical allodynia. In addition, pain behaviors were attenuated using regimens that began pre- and post-SCI. Electrophysiologically, treatments with minocycline or propentophylline onto the spinal surface attenuated SCI-induced evoked hyperexcitability of wide dynamic range (WDR) neurons in the spinal dorsal horn in response to mechanical stimuli applied to identified peripheral receptive fields. Immunohistochemically, SCI produced astrocytic and microglial hypertrophy in the spinal dorsal horn and restored their morphology to a quiescent state after minocycline or propentophylline treatment, respectively. Pharmacologically both minocycline and propentophylline, separately, are known to inhibit the roles of proinflammatory cytokines (Bastos et al., 2007; Padi and Kulkarni, 2008; Sweitzer et al., 2001), such as interleukins (IL-1, IL-6 and IL-8) and tumor necrosis factor (TNF)α, all which initiate activation of glutamate receptors (Zhang et al., 2008) leading to massive influx of Ca2+ ions into the intracellular space. Taken together, these data indicate that activated glia produce neuronal ligand-receptor mediated cascades from proinflammatory cytokines and glutamate that result in neuropathic pain behavior with dorsal horn neuronal hyperexcitability following SCI.
It is well known that SCI produces high concentrations of extracellular glutamate at both neuronal-neuronal and neuronal-glial cell appositions (Xu et al., 2004). Because neurons and glial cells express similar receptors and ion channels, glial activation may trigger similar intracellular cascades as those that occur in neurons. Briefly, after SCI, high concentration of glutamate at neuronal-glial clefts activate astrocytic and microglial glutamate receptors, both ionotropic and metabotropic, with subsequent membrane depolarization that triggers huge influxes of Na+ and Ca2+ ions into cells. In turn, elevated Ca2+ concentrations in astrocytes and microglia initiate activation of mitogen-activated protein kinase (MAPK, p38-MAPK and ERK) and phospholipase A2 (PLA2) that result in the modulation of target protein expression or phosphorylation of membrane receptor and ion channels via activation of transcription factors, such as NF-κB or pCREB (Crown et al., 2006; Gwak et al., 2009b). Finally, activated glial cells release glutamate, ATP, proinflammatory cytokines, prostaglandins (PGs) as well as both reactive oxygen (ROS) and nitrogen species (NOS) into the extracellular space. These pain mediating substances, released by activated glia, provide for continued production of reactants that contribute to intracellular downstream biochemical pathways that provide an intracellular feed forward mechanism for continued phosphorylation/activation of receptors and ion channels that ensures persistent neuronal hyperexcitability (Detloff et al., 2008; Hulsebosch et al., 2009; Keane et al., 2006; Scholz and Woolf, 2007, Vallejo et al., 2010). Additionally, it is important to note that chronically activated astrocytes lead to permanent blood spinal cord barrier breakdown that ensure continued immune cell infiltration and feed forward continued activation of both astrocytes and microglia (Nesic et al., 2005). These pharmacological and neuroanatomical changes in glial cells also result in enhanced release of transmitters, proinflammatory cytokines, ROS and chemokines (Johnstone et al., 1999). These substances continue to provide changes in the activation state of receptors, for example all three general groups of glutamate receptors, such as NMDA, kainate/AMPA and metabotropic, are involved in CNP after SCI (Bennett et al., 2000a; Mills et al., 2001), and in the activation state of ion channels, for example Nav1.3 (Hains et al., 2003) in neuronal membranes. Taken together, these data suggest that glial activation is a key factor in developing and maintaining central neuropathic pain following SCI (Gwak and Hulsebosch, 2010).
4. Glial Modulation of GABAergic Function
Neurons and glia cells control extracellular GABA concentrations via “GABA-glutamate-glutamine cycle”. Neurons and glial cells both transport GABA from extracellular to intracellular spaces via high affinity GABA transporters (Borden, 1996; Guastella et al., 1990; Nelson, 1998). Once GABA is transported intracellularly, GABA is converted to glutamate by GABA transaminase followed by conversion to glutamine by glutamine synthase. Glutamine freely passes to neurons and serves as a substrate for the production of either glutamate or GABA. Thus, release of GABA from GABAergic neurons and/or glial cells into the extracellular space, and reuptake of extracellular GABA by neurons and activated glia is a major mechanism for the control of GABA synthesis via concentration dependent feedback mechanisms of the “GABA-glutamate-glutamine cycle” (Struzynska and Sulkowski, 2004; Schousboe et al., 1993). However, the mechanism of GABAergic hypofunction in the spinal dorsal horn following SCI is unclear. The following discussion proposes four lines of hypotheses that result in hypofunction of GABA systems following SCI.
4.1 Decreased number of GABA neurons
It is well documented that SCI produces high extracellular concentrations of cytotoxic agents, such as glutamate, inflammatory cytokines and ROS. Subsequently, neural cells (both neurons and glial cells) undergo a secondary wave of cell loss that include the loss of GABAergic interneurons in the spinal dorsal horn (Keane et al., 2006; Lee et al., 2008; Rafati et al., 2008; Sah, et al., 2002; Song et al., 2001). The balance of apoptosis and anti-apoptosis activators are critically important to cell death. However, SCI increases the apoptotic gene activator, NK-κB p65/p50 and decreases the anti-apoptotic gene activator, c-Rel, in neurons. NK-κB p65/p50 produces inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) that contributes to cytotoxocity of neurons via oxidative stress, such as lipid peroxidation, protein S-thiolation and nucleic acid degradation and inflammation. Thus, SCI produces apoptotic gene activators and subsequent increases in ROS and inflammation that results in the death of GABAergic neurons in lamina I-III (Bao and Liu, 2003; Rafati et al., 2008; Sah et al., 2002; Sharma and Sjoguist, 2002), which predominantly receive nociceptive input from primary afferent Aδ and unmyelinated C fibers. In addition, proinflammatory cytokines and ROS, released by activated glial cells, activate TNFR1 and initiate activation of cysteine proteinase family, such as caspases 3 and 8, that results in apoptosis of neurons, including GABA interneurons in the spinal dorsal horn (Di Santo et al., 1996; Gavilán, et al., 2007; Kim et al., 2001).
Recently Meisner et al. reported that SCI causes caspase-3 activation in the spinal superficial dorsal horn, resulting in selective losses of GABA neurons as well as decreased expression of GAD65 and GAD67 in a central neuropathic pain animal model (Meisner et al., 2010). The loss of GABAergic inhibitory tone may produce facilitation of sub-threshold primary afferent Aδ and unmyelinated C fibers to nociceptive transmission (Lu et al., 2008). Normally, sub-threshold primary afferent input is not sufficient to cause action potentials in post-synaptic neural membranes. The propagation of signal rapidly decays during transmission due to the weak property of the input and the presence of GABAergic inhibition in the spinal dorsal horn (Takazawa and MacDermott, 2010). However, the loss of GABAergic tone after SCI results in the temporal and the spatial summation of primary afferent transmission and produces generation of action potentials in the post-synaptic membranes that result in enhanced nociceptive transmission in second order neurons in the spinal dorsal horn following SCI.
4.2 Downregulation of GAD expression
Several lines of evidence suggest that SCI results in neuropathic pain behaviors that appear to be related to downregulation of GAD expression in the spinal dorsal horn (Gwak et al., 2008; Liu et al., 2004, Meisner et al., 2010). Gwak et al. reported that SCI produced downregulation of GAD65 expression, mechanical allodynia and neuronal hyperexcitability in the spinal dorsal horn. However, inhibition of glial activation prevented downregulation of GAD65 expression as well as attenuation of mechanical allodynia and hyperexcitability of spinal dorsal horn neurons (Gwak et al., 2008). In addition, peripherally delivered HSV-based vector, encoding GAD67, attenuated mechanical allodynia and thermal hyperalgesia via decreased spinal calcitonin gene-related peptide (CGRP) expression following SCI (Liu et al., 2004). These studies suggest that SCI produces neuropathic pain by downregulation of GAD65 and GAD67 expression in the spinal dorsal horn.
Although the literature suggests that downregulation of GAD expression occurs in the spinal dorsal horn following SCI, it is not known which mechanisms account for the GAD downregulation. One speculation is that the death of GABAergic neurons (see 4.1, above) cause the loss of GAD expression following SCI. The other speculation is that GAD downregulation occurs without the death of GABAergic neurons following SCI. After SCI, activated primary afferent fibers stimulate GABAergic interneurons and subsequently initiate release of GABA into the extracellular space to prevent glutamate mediated excitotoxicity (Diaz-Ruiz et al., 2007; Liu and McAdoo, 1993). Elevation of extracellular GABA activates neighboring GABA receptors on second order neurons that result in inhibitory roles of neurons in the somatosensory circuit. However, GABA transporters on neurons and activated glial cells initiate uptake of GABA from the extracellular space. This results in high concentrations of glutamate and GABA intracellularly. In addition, glutamate and other pain stimulating agents, such as proinflammatory cytokines, ROS and ATP, initiate activation of intracellular downstream events and modulate specific gene expression that result in downregulation of GAD expression (Figure 2).
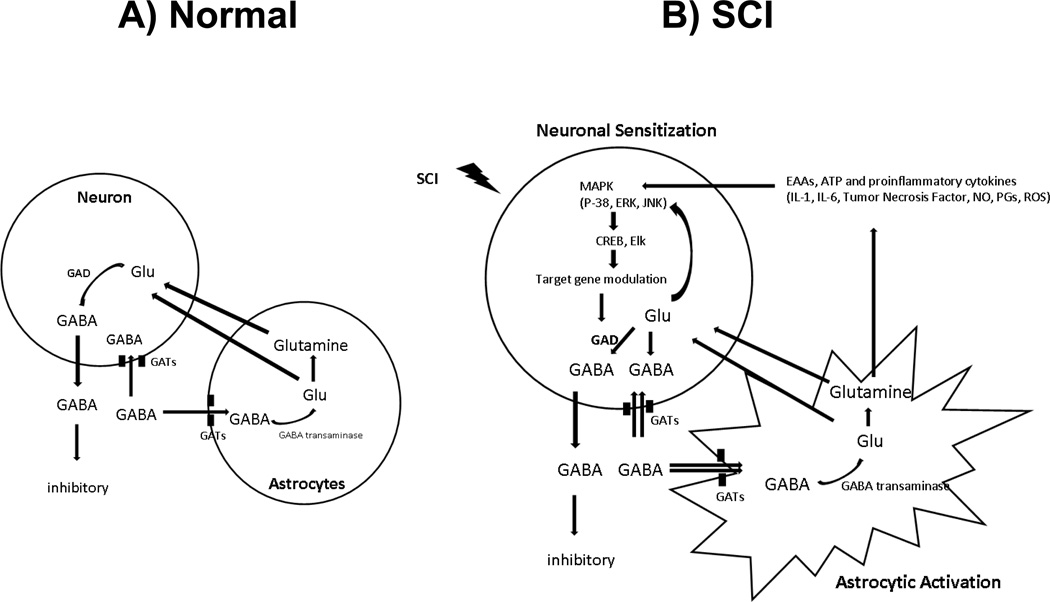
Figure 2.
Diagrams of inter- and intracellular mechanisms for the control of GABA following SCI. (A) In the normal condition, GABA concentrations are controlled by neuronal and glial GABA transporters and are dependent on the glutamate-GABA cycle. (B) After spinal cord injury, activated primary afferent fibers release huge concentration of GABA to prevent glutamate excitotoxicity. Elevation of extracellular GABA activates GABA transporters on neurons and activated glial cells. Once GABA is taken up, GABA is converted to glutamate and glutamine, which serves as a source of glutamate in neurons. This results in high concentrations of glutamate and GABA. In addition, glutamate and other pain stimulating agents, such as proinflammatory cytokines, ROS and ATP, released by activated glial cells, initiate activation of intracellular downstream events and modulate specific gene expression that result in downregulation of GAD expression. This positive feed forward cycle “GABA-glutamate-glutamine” contributes to hypofunction of GABA and persistent neuronal hyperexcitability, and plays a key role as a mechanism for neuropathic pain after SCI. Abbreviations; EAAs : excitatory amino acids, CREB and Elk : transcriptional factors, GAD : glutamic acid decarboxylase, GATs : GABA transporters, IL-1 : interleukin-1, IL-6 : interleukin-6, MAPK : mitogen activated protein kinase, NO : nitric oxide, PGs : prostaglandins, ROS : reactive oxygen species, SCI : spinal cord injury, TNF : tumor necrosis factor.
Glutamate transporters, which control extracellular glutamate concentrations, are an important consideration for the control of GABA concentrations. High concentrations of glutamate produce cytotoxic events in neurons, glia and other cells following SCI; thus uptake by transporters is critical for maintaining extracellular homeostasis of glutamate concentrations (Danbolt, 2001; Xu et al., 2004). GABA uptake by GABA transporters and glutamate uptake synergistically combine to provide appropriate concentrations of extracellular glutamate and GABA, providing substrates for GABA synthesis via modulation of GAD expression (Bröer et al., 2004; Struzynska and Sulkowski, 2004).
4.3. Decreased extracellular GABA concentration
Neurons and glia modulate extracellular GABA concentrations by GABA uptake via GABA transporters (GATs) (Borden et al., 1996; Guastella et al., 1990; Schousboe et al., 2003). GAT is divided into five different transporters: a vesicular GABA transporter (VGAT) and four Na+-dependent transporters; GAT1, GAT2, GAT3 (high affinity GABA transporters) and BGT1 (low affinity GABA transporter). GAT1-3 transporters are particularly dense in the synaptic cleft and serve to keep the extracellular neurotransmitter concentrations below neurotoxic levels and also serve to terminate the synaptic action of GABA as a neurotransmitter. BGT1 is particularly dense extrasynaptically on both neurons and glial cells (Nelson, 1998; Schousboe et al., 2004). GATs rapidly remove GABA and control the extracellular GABA concentrations (Figure 2).
Overproduction of GATs in neurons or in glial cells in pathophysiological conditions facilitate GABA uptake and result in a loss of GABAergic tone in spinal circuits, called disinhibtion, and results in hyperexcitability of neurons. For example, genetically enhanced or decreased GAT1 expression resulted in hyperalgesic or hypoalgesic conditions, respectively (Hu et al., 2003; Xu et al., 2008). Pharmacological blockade of GAT1 produced attenuation of pain behavior; treatment with GAT1 inhibitors (NO-711 and tiagabine) produced attenuation of spontaneous and evoked pain behaviors (Hu et al., 2003; Todorov et al., 2005). These data suggest that GATs contribute to nociceptive transmission in the spinal dorsal horn.
4.4. Abnormal Cation-Chloride cotransporters
The chloride gradient in the neural membrane is critically important to GABAA receptor mediated inhibitory function because the GABAA receptor is anion-permeable (Kaila, 1994). Two chloride tranporters control intracellular Cl− concentration in the spinal cord; Na+-K+-Cl− cotransporter 1 (NKCC1) transports Cl− into the cell and 2) K+-Cl− cotransporter 2 (KCC2) transports Cl− into the extracellular space (Misgeld et al., 1986; Payne et al., 2003).
SCI upregulates NKCC1 whereas KCC2 is downregulated in the spinal cord, which correlates with allodynia and hyperalgesia (Cramer et al., 2008; Hasbargen et al., 2010). The upregulation of NKCC1 and downregulation of KCC2 produce high concentrations of intracellular Cl− that facilitate efflux of Cl− when GABAA receptor is activated (opened state), and thereby generates depolarization. In axonal membranes, the final efflux of Cl− generates small amplitude (subthreshold for action potential) depolarizations, such as primary afferent depolarizations (PADs) (Eccles et al., 1963). This depolarization interrupts orthodromic propagation of action potentials from peripherally originated sources and decreases the release of excitatory neurotransmitters, called presynaptic inhibition. However, upregulation of NKCC1 generates sufficient depolarization a to surpass threshold and produces action potentials which increases the release of excitatory transmitters, resulting in enhanced nociceptive transmission (Garcia-Nicas, et al., 2006; Laird et al., 2004). Downregulation of KCC2 produces increased intracellular Cl− concentrations. Enhances GABAA receptor activation (opened state) and triggers massive efflux of Cl−, generating depolarization of membranes. Finally, GABAAR-mediated membrane depolarizations cause activation of cation channels, such as voltage-dependent Na+ and Ca2+ channels that generate excitation of neurons (Aptel et al., 2007). Taken together, the increased intracellular concentrations of Cl− contribute to the enhanced nociceptive transmission, rather than inhibition, when GABAA receptor is activated after SCI (Figure 3).
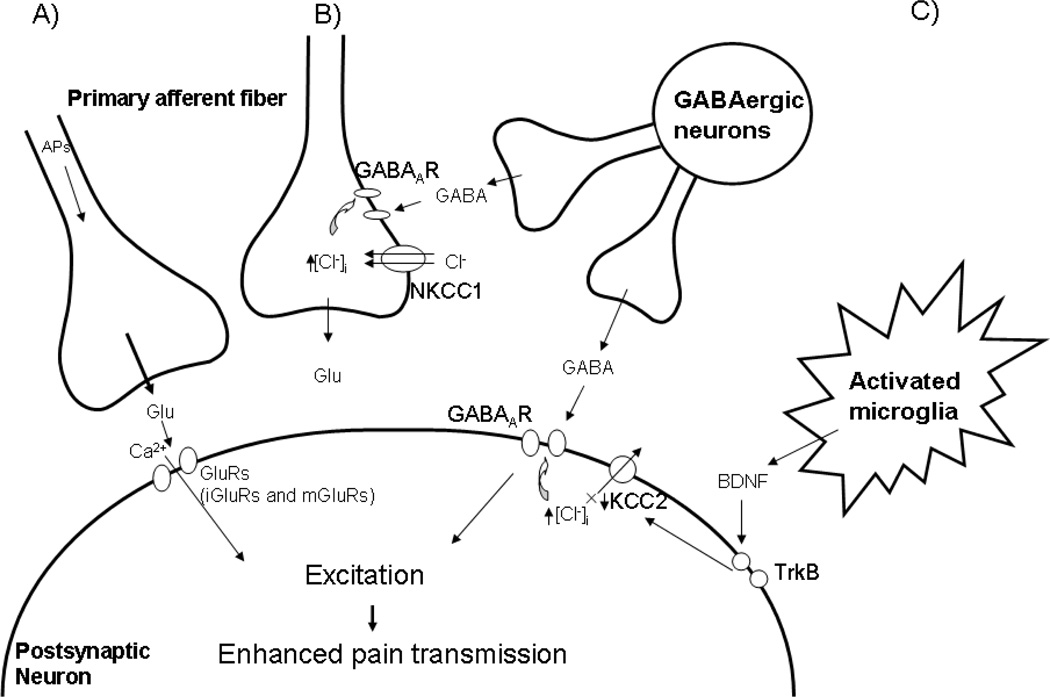
Figure 3.
GABAergic excitation facilitates pain transmission in the spinal dorsal horn neurons following SCI. (A) After SCI, primary afferent fibers release excitatory neurotransmitters, such as glutamate, and initiate activation of glutamate (ionotropic and metabotropic) receptors. Massive influx of calcium ions trigger intracellular downstream pathways and contribute to neuronal excitation that results in enhanced pain transmission. (B) After SCI, upregulated NKCC1 induces influx of Cl− into the cell that results in increased intracellular Cl−. Activation (opened state) of the GABAA receptor facilitates efflux of Cl−. Due to the Cl- gradient, the final net of Cl− is efflux, producing membrane depolarizarion and enhancing the release of excitatory neurotransmitters, such as glutamate. (C) BDNF, released by activated microglia after SCI, activates trkB and contributes to the downregulation of KCC2. Inhibition of Cl− efflux produces increased intracellular Cl− concentrations. However, activation (opened state) of GABAA receptor facilitates a massive efflux of Cl− and produces membrane depolarizarion that results in activation of voltage-dependent cation channels, such as Na+ and Ca2+ channels. This leads to neuronal hyperexcitability and neuropathic pain. Abbreviations; Glu : glutamate, iGluR : ionotropic glutamate receptor, mGluR : metabotropic glutamate receptor, BDNF : brain-derived neurotropic factor, NKCC1 : Na+-K+-Cl− cotransporter 1, KCC2 : K+-Cl− cotransporter 2, APs : action potentials, TrkB : tyrosine kinase receptor.
The contribution of glial cells on the modulation of the chloride gradient is not clear. However, ATP-stimulated microglia release brain-derived neurotropic factor (BDNF) and contributes to the shift of anion equilibrium potential (Eanion) via activation of tyrosine kinase receptor (TrkB). This Eanion shift generates depolarizing rather than hyperpolarizing, which correlates with decreased paw withdrawal thresholds (Coull et al., 2003, 2005). In SCI, inhibition of microglial activation by minocycline produces decreased BDNF release, attenuation of neuropathic pain behavior and decreased neuronal hyperexcitability (Hains and Waxman, 2006). Taken together, microglia modulate chloride gradients that result in neuronal hyperexcitability and enhanced nociceptive transmission in spinal circuits that result in CNP following SCI (Lu et al., 2008).
5. Conclusion
Spinal cord injury causes maladaptive synaptic circuits and produces central neuropathic pain. Neuronal cells are not the only neural cells that mediate pain mechanisms; activated glial cells also actively contribute to pain mechanisms. Neurons and glial cells express similar receptors and ion channels as well as release similar transmitters, cytokines and ROS; all known as key candidates for pain transmission.
Neurons and glial cells synergistically control intracellular chloride ion gradients via modulation of chloride transporters, extracellular glutamate and GABA concentrations via uptake mechanisms and the intracellular “GABA-glutamate-glutamine cycle” to maintain normal physiological homeostasis. However, hyperexcitable neurons and glial activation after spinal cord injury disrupt the balance of chloride ions, glutamate and GABA distribution and results in neuronal hyperexcitability and chronic neuropathic pain. Thus, tight coupling of neuronal-glial and glial-neuronal interactions make major contributions to the loss of GABAergic inhibitory tone following SCI. Selective pharmacological inhibition of glial activation or molecular approaches that increase GABA synthesis are possible therapeutic intervention strategies to alleviate persistent central neuropathic pain in patients following SCI.
Highlights.
-
*
Mammalian SCI models were developed to study CNP after SCI
-
*
SCI produces hypofunction of GABAergic tone
-
*
SCI directly and indirectly causes glial activation
-
*
Neurons and glial cells control GABA concentrations
-
*
Glial inhibition and increases in GABAergic tone are strategies to alleviate CNP
Acknowledgements
This research is supported by Liddell Grant, West and Dunn Foundations and NIH NS11255 and NS39161 and Army PRO43199, Mission Connect, a project of the TIRR Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
No conflict of interest
References
- Albrecht J, Pearce B, Murphy S. Evidence for an interaction between GABAB and glutamate receptors in astrocytes as revealed by changes in Ca2+ flux. Eur. J. Pharmacol. 1986;125:463–464. doi: 10.1016/0014-2999(86)90805-8. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Aptel H, Hilaire C, Pieraut S, Boukhaddaoui H, Mallie S, Valmier J, Scamps F. The Cav3.2/alpha1H T-type Ca2+ current is a molecular determinant of excitatory effects of GABA in adult sensory neurons. Mol. Cell. Neurosci. 2007;36:293–303. doi: 10.1016/j.mcn.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces apoptotic cell death and activates caspase-3. Neuroscience. 2003;116:59–70. doi: 10.1016/s0306-4522(02)00571-7. [DOI] [PubMed] [Google Scholar]
- Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J. Neurophysiol. 2002;88:1407–1419. doi: 10.1152/jn.2002.88.3.1407. [DOI] [PubMed] [Google Scholar]
- Bastos LF, Merlo LA, Rocha LT, Coelho MM. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur. J. Pharmacol. 2007;576:171–179. doi: 10.1016/j.ejphar.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Bellier JP, Sacchettoni S, Prod'hon C, Perret-Liaudet A, Belin MF, Jacquemont B. Glutamic acid decarboxylase-expressing astrocytes exhibit enhanced energetic metabolism and increase PC12 cell survival under glucose deprivation. J. Neurochem. 2000;75:56–64. doi: 10.1046/j.1471-4159.2000.0750056.x. [DOI] [PubMed] [Google Scholar]
- Bender AS, Schousboe A, Reichelt W, Norenberg MD. Ionic mechanisms in glutamate-induced astrocyte swelling: role of K+ influx. J. Neurosci. Res. 1998;52:307–321. doi: 10.1002/(SICI)1097-4547(19980501)52:3<307::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000a;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP(8-37) in a rodent model of chronic central pain. Pain. 2000b;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Beric A, Dimitrijevic MR, Lindblom U. Central dysesthesia syndrome in spinal cord injury in patients. Pain. 1988;34:109–116. doi: 10.1016/0304-3959(88)90155-8. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bröer A, Deitmer JW, Bröer S. Astroglial glutamine transport by system N is upregulated by glutamate. Glia. 2004;48:298–310. doi: 10.1002/glia.20081. [DOI] [PubMed] [Google Scholar]
- Bruce JC, Oatway MA, Weaver KC. Chronic pain after clip-compression injury of the rat spinal cord. Exp. Neurol. 2002;178:33–48. doi: 10.1006/exnr.2002.8026. [DOI] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ. Two human glutamate decarboxylases: 65-kDa GAD and 67-kDa GAD are each encoded by a single gene. Proc. Natl. Acad. Sci. 1992;89:2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Crit. Rev. Neurobiol. 1995;9:189–205. [PubMed] [Google Scholar]
- Charles KJ, Deuchars J, Davies CH, Pangalos MN. GABAB receptor subunit expression in glia. Mol. Cell. Neurosci. 2003a;24:214–223. doi: 10.1016/s1044-7431(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Calver AR, Jourdain S, Pangalos MN. Distribution of a GABAB-like receptor protein in the rat central nervous system. Brain Res. 2003b;989:135–146. doi: 10.1016/s0006-8993(03)03163-9. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: Implication for brain imaging of inhibitory transmission. Proc. Natl. Acad. Sci. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol. Pain. 2008;4:36–44. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davidoff G, Roth E, Guarracini M, Sliwa J, Yarkony G. Function-limiting dysesthetic pain syndrome among traumatic spinal cord injury patients: a cross-sectional study. Pain. 1987;29:39–48. doi: 10.1016/0304-3959(87)90176-X. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo E, Foddi MC, Ricciardi-Castagnoli P, Mennini T, Ghezzi P. DHEAS inhibits TNF production in monocytes, astrocytes and microglial cells. Neuroimmunomodulation. 1996;3:285–288. doi: 10.1159/000097282. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Salgado-Ceballos H, Montes S, Maldonado V, Tristan L, Alcaraz-Zubeldia M, Ríos C. Acute alterations of glutamate, glutamine, GABA, and other amino acids after spinal cord contusion in rats. Neurochem. Res. 2007;32:57–63. doi: 10.1007/s11064-006-9225-5. [DOI] [PubMed] [Google Scholar]
- Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Wolfe SQ, Martinez M, Hernandez M, Furst C, Huang J, Frydel BR, Gómez-Marín O. Subarachnoid transplant of a human neuronal cell line attenuates chronic allodynia and hyperalgesia after excitotoxic spinal cord injury in the rat. J. Pain. 2007;8:33–50. doi: 10.1016/j.jpain.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J. Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Gangadharan V, Agarwal N, Brugger S, Tegeder I, Bettler B, Kuner R, Kurejova M. Conditional gene deletion reveals functional redundancy of GABAB receptors in peripheral nociceptors in vivo. Mol. Pain. 2009;19(5):68–79. doi: 10.1186/1744-8069-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird JM, Cervero F. GABAA-Receptor blockade reverses the injury-induced sensitization of nociceptor-specific (NS) neurons in the spinal dorsal horn of the rat. J. Neurophysiol. 2006;96(2):661–670. doi: 10.1152/jn.00377.2006. [DOI] [PubMed] [Google Scholar]
- Gavilán MP, Revilla E, Pintado C, Castaño A, Vizuete ML, Moreno-González I, Baglietto-Vargas D, Sánchez-Varo R, Vitorica J, Gutiérrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J. Neurochem. 2007;103:984–996. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Gwak SY, Hulsebosch EC. “Gliopathy” Maintains Persistent Hyperexcitability of Spinal Dorsal Horn Neurons after Spinal Cord Injury: Substrate of Central Neuropathic Pain. In: Costa A, Villalba E, editors. Horizons in Neuroscience Research. Volume I. New York: Nova Science Publishers; 2010. pp. 195–224. [Google Scholar]
- Gwak YS, Unabia GC, Auerbach AJ, Kang JH, Hulsebosch CE. Spatial and Temporal Activation of Astrocytes and Microglia in the Dorsal Horn following Spinal Cord Injury in Rat. SFN Abstract. 2009a 170.10/X12. [Google Scholar]
- Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp. Neurol. 2009b;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J. Neurotrauma. 2006;23:1111–1124. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JX, Xu XJ, Aldskogious H, Seiger A, Wiesenfield-Hallin Z. Allodynia-like effects in rat after ischemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991;45:175–185. doi: 10.1016/0304-3959(91)90186-2. [DOI] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann. N. Y. Acad. Sci. 2010;1198:168–172. doi: 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- Hu JH, Yang N, Ma YH, Zhou XG, Jiang J, Duan SH, Mei ZT, Fei J, Guo LH. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. J. Neurosci. Res. 2003;73:565–572. doi: 10.1002/jnr.10677. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. From discovery to clinical trials: treatment strategies for central neuropathic pain after spinal cord injury. Curr. Pharm. Des. 2005;11:1411–1420. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp. Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1207–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic gamma-aminobuyric acid (GABA) release in the rat olfactory bulb. Proc. Natl. Acad. Sci. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab. Brain Dis. 2010;25:31–38. doi: 10.1007/s11011-010-9180-3. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Timmermann DB, Johansen TH, Schousboe A, Varming T, Ahring PK. The beta subunit determines the ion selectivity of the GABAA receptor. J. Biol. Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- Johnston GA. GABAc receptors: relatively simple transmitter -gated ion channels? Trends Pharmacol. Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J. Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous sytem. Prog. Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane RW, Davis AR, Dietrich WD. Inflammatory and apoptotic signaling after spinal cord injury. J. Neurotrauma. 2006;23:335–344. doi: 10.1089/neu.2006.23.335. [DOI] [PubMed] [Google Scholar]
- Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J. Neurosci. 2001;21:6617–6625. doi: 10.1523/JNEUROSCI.21-17-06617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;17:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Chemokine expression in the white matter spinal cord precursor niche after force-defined spinal cord contusion injuries in adult rats. Glia. 2010;58:916–931. doi: 10.1002/glia.20974. [DOI] [PubMed] [Google Scholar]
- Koch U, Magnusson A. Unconventional GABA release: mechanisms and function. Curr. Opin. Neurobiol. 2009;19:305–310. doi: 10.1016/j.conb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. 2006;103:10058–10063. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, García-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 2004;361:200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Somjen GG, Martín, del. Río R, Herreras O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival. J. Neurosci. 1996;16:1219–1229. doi: 10.1523/JNEUROSCI.16-03-01219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Furmanski O, Castellanos DA, Daniels LA, Hama AT, Sagen J. Prolonged nociceptive responses to hind paw formalin injection in rats with a spinal cord injury. Neurosci. Lett. 2008;439:212–215. doi: 10.1016/j.neulet.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Kim HK, Hulsebosch CE, Gwak YS. Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp. Neurol. 2010;224:321–324. doi: 10.1016/j.expneurol.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Polsky K, Matesic DF. Expression of gamma-aminobutyric acid immunoreactivity in reactive astrocytes after ischemia-induced injury in the adult forebrain. Brain Res. 1993;600:1–8. doi: 10.1016/0006-8993(93)90394-3. [DOI] [PubMed] [Google Scholar]
- Liu D, McAdoo DJ. An experimental model combining microdialysis with electrophysiology, histology, and neurochemistry for exploring mechanisms of secondary damage in spinal cord injury: effects of potassium. J. Neurotrauma. 1993;10:349–362. doi: 10.1089/neu.1993.10.349. [DOI] [PubMed] [Google Scholar]
- Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, Fink DJ. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol. Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu Z, Cao X, Cao Z, Xue L, Zhu F, He X, Li W. Recombinant human foamy virus, a novel vector for neurological disorders gene therapy, drives production of GAD in cultured astrocytes. Mol. Ther. 2007;15:1834–1841. doi: 10.1038/sj.mt.6300224. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, Xue L, Miao L, He X, Li W. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci. Lett. 2008;432:13–18. doi: 10.1016/j.neulet.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J. Physiol. 2008;586:5701–5715. doi: 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J. Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie M, Hughes DI, Maxwell DJ, Tillakaratne NJ, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience. 2003;119:461–472. doi: 10.1016/s0306-4522(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol. Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J. Neurotrauma. 2010;27:729–737. doi: 10.1089/neu.2009.1166. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of Chronic Pain. Seattle: IASP Press; 1994. [Google Scholar]
- Mills CD, Xu GY, McAdoo DJ, Hulsebosch CE. Involvement of metabotropic glutamate receptors in excitatory amino acid and GABA release following spinal cord injury in rat. J. Neurochem. 2001;79:835–848. doi: 10.1046/j.1471-4159.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Deisz RA, Dodt HU, Lux HD. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986;13(232):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EØ, Larsen JS, Ahring PK, Mirza NR. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- Nelson N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 1998;71:1785–803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Perez-Polo R. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- New KC, Rabkin SD. GABA synthesis in astrocytes after infection with defective herpes simplex virus vectors expressing glutamic acid decarboxylase 65 or 67. J. Neurochem. 1998;71 doi: 10.1046/j.1471-4159.1998.71062304.x. 2304-1312. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain. 2010;133:1013–1025. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008;601:79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Riddell JS, Maxwell DL, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycerinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Artham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J. Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic O, Perez-Polo JR. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J. Neurosci. Res. 2008;86:566–580. doi: 10.1002/jnr.21508. [DOI] [PubMed] [Google Scholar]
- Rintala DH, Loubser PG, Castro J, Hart KA, Fuhrer MJ. Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity and relationship with impairment, disability, handicap, and subjective well-being. Arch. Phys. Med. Rehabil. 1988;79:604–614. doi: 10.1016/s0003-9993(98)90032-6. [DOI] [PubMed] [Google Scholar]
- Rischke R, Krieglstein JJ. Postischemic neuronal damage causes astroglial activation and increase in local cerebral glucose utilization of rat hippocampus. Cereb. Blood. Flow. Metab. 1991;11:106–113. doi: 10.1038/jcbfm.1991.12. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Sah R, Galeffi F, Ahrens R, Jordan G, Scwartz-Bloom RD. Modulation of the GABAA-gated chloride channel by reactive oxygen species. J. Neurochem. 2002;80:383–391. doi: 10.1046/j.0022-3042.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- Schlue WR, Deitmer JW. Ionic mechanisms of intracellular pH regulation in the nervous system. Ciba Found. Symp. 1988;139:47–69. doi: 10.1002/9780470513699.ch4. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L. Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev. Neurosci. 1993;15:359–366. doi: 10.1159/000111356. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Larsson OM, White HS. GABA transporters as drug targets for modulation of GABAergic activity. Biochem. Pharmacol. 2004;68:1557–1563. doi: 10.1016/j.bcp.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Sjoquist PO. A new antioxidant compound H-290/51 modulate glutamate and GABA immunoreactivity in the rat spinal cord following trauma. Amino Acids. 2002;23:261–272. doi: 10.1007/s00726-001-0137-z. [DOI] [PubMed] [Google Scholar]
- Shimada S, Cutting G, Uhl GR. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol. Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Yezierski RP, Loeser JD. Taxonomy and epidemiology of spinal cord injury pain. In: Yezierski RP, B KJ, editors. Spinal Cord Injury Pain: Assessment, Mechanisms, Management Vol 23. Seattle, WA: IASP Press; 2002. pp. 9–24. [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Song G, Cechvala C, Resnick DK, Dempsey RJ, Rao VL. GeneChip analysis after acute spinal cord injury in rat. J. Neurochem. 2001;79:804–815. doi: 10.1046/j.1471-4159.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, Nelson SJ, Griffin WS. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer's disease. J. Neuropathol. Exp. Neurol. 1994;53:231–238. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Struzyńska L, Sulkowski G. Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J. Inorg. Biochem. 2004;98:951–958. doi: 10.1016/j.jinorgbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Svensson M, Eriksson NP, Aldskogius H. Evidence for activation of astrocytes via reactive microglial cells following hypoglossal nerve transection. J. Neurosci. Res. 1993;35:373–381. doi: 10.1002/jnr.490350404. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J. Pharmacol. Exp. Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci. 2010;1198:153–158. doi: 10.1111/j.1749-6632.2010.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, Hains BC. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J. Neurosci. 2008;28:13173–13183. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov AA, Kolchev CB, Todorov AB. Tiagabine and gabapentin for the management of chronic pain. Clin. J. Pain. 2005;21:358–361. doi: 10.1097/01.ajp.0000110637.14355.77. [DOI] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- Wang CC, Fang KM, Yang CS, Tzeng SF. Reactive oxygen species-induced cell death of rat primary astrocytes through mitochondria-mediated mechanism. J. Cell Biochem. 2009;107:933–943. doi: 10.1002/jcb.22196. [DOI] [PubMed] [Google Scholar]
- Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury-relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665–673. doi: 10.1038/sj.sc.3101641. [DOI] [PubMed] [Google Scholar]
- White RE, McTigue DM, Jakeman LB. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J. Comp. Neurol. 2010;518:1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Ye Z, Hulsebosch CE, McAdoo DJ. Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Exp. Neurol. 2004;187:329–336. doi: 10.1016/j.expneurol.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Xu YF, Cai YQ, Cai GQ, Jiang J, Sheng ZJ, Wang ZG, Fei J. Hypoalgesia in mice lacking GABA transporter subtype 1. J. Neurosci. Res. 2008;86:465–470. doi: 10.1002/jnr.21499. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Seiger A, Wiesenfeld-Hallin Z. Systemic excitatory amino acid receptor antagonists of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and of the N-methyl-D-aspartate (NMDA) receptor relieve mechanical hypersensitivity after transient spinal cord ischemia in rats. J. Pharmacol. Exp. Ther. 1993;267:140–144. [PubMed] [Google Scholar]
- Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- Zhang AL, Hao JX, Seiger A, Xu XJ, Wiesenfeld-Hallin Z, Grant G, Aldskogius H. Decreased GABA immunoreactivity in spinal cord dorsal horn neurons after transient spinal cord ischemic in the rat. Brain Res. 1994;656:187–190. doi: 10.1016/0006-8993(94)91383-8. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J. Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]