Abstract
Background
Nitric oxide (NO)-dependent vasodilation is impaired in middle cerebral arteries (MCas) from Dahl salt-sensitive (SS) rats that are fed normal salt (NS) diet, due to low plasma renin activity and chronic exposure to low plasma angiotensin II (aNG II) levels. NO-dependent vasodilator responses are rescued in MCas from Ren1-BN congenic rats, which have a 2.0 Mbp portion of Brown Norway (BN) chromosome 13 containing the renin gene introgressed onto the Dahl SS genetic background.
Methods
Vascular superoxide levels were measured with dihydroethidium (DHE) fluorescence in basilar arteries from 10- to 14-week-old, male Dahl SS and Ren1-BN congenic rats that fed NS diet. Nicotinamide adenine dinucleotide phosphate (NaDPH) oxidase and xanthine oxidase (XO) activity were also measured in cerebral artery tissue homogenates. Expression of the superoxide dismutase (SOD) enzymes was evaluated via western blotting in cerebral arteries from the two rat strains.
results
Superoxide levels were significantly higher in basilar arteries from Dahl SS rats compared to Ren1-BN congenic rats. NaDPH oxidase and XO activity were similar between the two rat strains. Cu/Zn SOD expression was significantly higher in cerebral arteries from Ren1-BN congenic rats vs. those from Dahl SS rats. The expression of Mn-SOD was similar in cerebral arteries from both strains.
conclusions
These findings suggest that introgressing the BN renin allele onto the Dahl SS genetic background to restore normal activity of the renin-angiotensin system (RaS) protects NO-dependent vascular relaxation in cerebral arteries by increasing the expression of Cu/Zn SOD and lowering vascular superoxide levels.
Keywords: angiotensin II, blood pressure, cerebral vasculature, hypertension, renin gene, superoxide dismutase
Elevated superoxide levels in the vasculature reduce the bio-availability of nitric oxide (NO) and cause endothelial dysfunction, which can lead to the onset and progression of cardiovascular disease.1 Vascular tone in the larger arteries of the cerebral circulation is strongly influenced by NO under resting conditions.2 Therefore, increased levels of superoxide can result in the loss of NO-dependent vasodilation, cause vasoconstriction, and adversely affect blood flow regulation in the brain.3
Sprague-Dawley (SD) rats that are fed a short-term high salt (HS) diet have severe endothelial dysfunction and increased levels of reactive oxygen species in multiple vascular beds.4-6 Accumulating evidence has shown that the endothelial dysfunction are that occurs in middle cerebral arteries (MCAs) of SD rats that are fed a HS-diet is a consequence of salt-induced suppression of the renin-angiotensin system (RAS), because chronic infusion of a subpressor dose of angiotensin II (ANG II) (5 ng/ kg/ min) sufficient to return plasma levels of ANG II to normal salt (NS) control values also restores normal vascular relaxation mechanisms in MCAs and prevents downregulation of the crucial antioxidant enzyme Cu/Zn superoxide dismutase (SOD).4,7
The Dahl salt-sensitive (SS) rat is a well-accepted animal model of salt -sensitive, low-renin hypertension similar to that of commonly observed in the African American population.8 When maintained on a NS diet, the Dahl SS rat is normotensive, but similar to HS-fed SD rats, has vascular dysfunction caused by chronically low activity of the RAS because intravenous infusion of a subpressor dose of ANG II restores endothelial function to normal.9,10 Chronically treating Dahl SS rats with the SOD mimetic tempol also restores the vasodilator response to acetylcholine (ACh) in isolated MCAs.11 The Ren1-BN congenic rat (which has the Brown Norway (BN) renin allele introgressed onto the Dahl SS genetic background through marker-assisted breeding) provides a unique animal model to test the relationships between suppressed RAS activity, vascular oxidative stress, and endothelial dysfunction in the large cerebral arteries because previous studies have shown that introgressing the renin allele from the BN rat onto the Dahl SS genetic background not only restores normal activity of the RAS10,12 but also restores NO-dependent vasodilatation in MCAs of the congenic animals.11
The goal of this study was to determine whether superoxide levels are lower in cerebral arteries of Ren1-BN congenic rats compared to those of parental Dahl SS rats. To determine the potential causes of increased vascular superoxide levels in cerebral arteries of the Dahl SS rat, we also evaluated the contributions of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and xanthine oxidase (XO) to vascular superoxide production, and the relative expression of SOD enzymes in the larger cerebral arteries (MCAs, posterior cerebral arteries, basilar arteries, and arteries from the Circle of Willis) of Dahl SS and Ren1-BN congenic rats.
Methods
General procedures
The experiments in this study were performed in male Dahl SS and Ren1-BN congenic rats weighing 300-400 g. The Ren1-BN rat was derived through marker-assisted breeding between the SS-13BN/Mcwi consomic rat strain and the Dahl SS (Dahl SS/JHsd/Mcwi) parental strain. The 2.0 Mbp portion of BN chromosome 13 that was introgressed onto the Dahl SS genetic background is located between the microsatellite markers D13hmgc41-D13hmgc23 and includes the BN renin allele. All animals were maintained on a normal salt (NS; 0.4% NaCl) diet (Dyets, Bethlehem, PA) since weaning, with water to drink ad libitum. An additional group of Dahl SS rats were given the SOD mimetic tempol (15 mg/kg/day) in their drinking water for 7 days.
Isolated vessel preparation and vasodilator stimuli
On the day of the experiment, animals were anesthetized with an intraperitoneal injection of pentobarbital sodium. MCAs were isolated from the ventral surface of the brain and cannulated with glass micropipettes in a heated chamber perfused with physiological salt solution as previously described.13 After a 1 h equilibration period, MCAs were pressurized to 80 mm Hg and their response to cumulative addition of acetylcholine (ACh; 10-10-10-5 mol/l) to the tissue bath was assessed. To evaluate the potential role of SOD activity in determining responses to ACh in MCAs of Dahl SS and Ren1-BN rats, the SOD inhibitor diethyldithiocarbamate (DETC, 1 mmol/l) was added to the perfusate and superfusate 20 min before adding ACh.
Evaluation of vascular superoxide levels
Vascular superoxide levels were assessed in cross-sections of the basilar artery using dihydroethidium (DHE) fluorescence as previously described.13 The basilar artery is slightly larger than the MCA and can more easily be cleaned of connective tissue, which minimizes mechanical damage and allows for better crosssectioning of the vessel. Mayhan14 has shown that ACh-mediated dilation of the basilar artery is NO-dependent, making it an appropriate surrogate vessel for the MCA.
On the day of the experiment, basilar arteries were isolated and incubated for 1 h in physiological salt solution heated to 37 °C. The arteries were then incubated with 5 μmol/l DHE for 15 min, cut into 10 μm transverse sections and imaged with a Nikon Eclipse TS100 (Nikon, Tokyo, Japan) microscope equipped with a ×20 objective, a 540 nm excitation filter, a 605 nm emission filter (Chroma Technology Corp., Bellows Falls, VT) and a QImaging Retiga-2000R digital camera (Surrey, British Columbia, Canada). Multiple images of each artery were taken and quantified using ImageJ software. The amount of fluorescence in each basilar artery ring was quantified by subtracting the background fluorescence of each image from the brightness value of the freehand-selected ring section as previously described.13
Western blot analysis for pro- and antioxidant enzymes
To evaluate the expression of antioxidant enzymes in cerebral arteries, western blots were performed with pooled samples of basilar arteries, arteries isolated from the Circle of Willis, and arteries just downstream from the Circle of Willis, including the MCA and posterior cerebral arteries. After homogenizing the arteries, 5 μg of protein were loaded onto a 4-20% Biorad Criterion precast gel (Bio-Rad Laboratories, Hercules, CA) for separation by electrophoresis. Following electrophoretic separation, the protein was transferred onto a nitrocellulose membrane and the membranes were incubated overnight with the primary antibodies for Cu/Zn SOD (1:10,000 dilution; Assay Designs, Ann Arbor, MI), Mn-SOD (1:25,000 dilution; Assay Designs) and β-actin (1:25,000 dilution; Sigma Aldrich, St. Louis, MO) in 5% nonfat dry milk. The next day, the membranes were incubated with the secondary antibodies in 5 % nonfat dry milk for 2 h and protein bands were visualized using the SuperSignal West pico chemiluminescent substrate (Thermo Fisher Scientific, Rockford, IL). Protein bands were quantified using the UNSCAN-IT software (Silk Scientific, Orem, UT) and final expression was normalized to β-actin.
Pro-oxidant enzyme contributions to superoxide production
To determine the contribution of NADPH oxidase and XO to total vascular superoxide production in cerebral arteries of Dahl SS and Ren1-BN congenic rats, cerebral artery tissue homogenates were loaded onto a 96-well plate in the presence of DHE (10 μmol/l) as previously described by Taylor et al.15 On the day of the experiment, basilar arteries and the arteries of the Circle of Willis were removed from the ventral surface of the brain and cleaned of connective tissue and blood. The vessels were homogenized and 5 μg of protein (total volume 40 μl) were loaded into individual wells of a 96-well plate with salmon testes DNA (0.5 mg/ml) ± the NADPH oxidase inhibitor diphenyleneiodonium (DPI; 500 μmol/l) or the XO inhibitor oxypurinol (500 μmol/l). Phosphate buffered saline was added to the wells designated as controls. After a 15 min equilibration period, DHE (10 μmol/l) was added to individual wells, and the 96-well plate was read on a Tecan Spectraflour Plus plate reader (Tecan, Salzburg, Austria) at 37 °C for 1 h at 5 min kinetic intervals. The increase in oxyethidium fluorescence was read at an excitation of 485 nm and an emission of 570 nm. All data are expressed as a percent of control value.
Statistical analysis
All data are presented as mean value ± s.e.m. The differences between multiple groups were assessed using one-way analysis of variance. Differences between individual means following analysis of variance were evaluated using a post hoc Holm-Sidak test. For comparisons between two groups, an unpaired Student’s t-test was used to evaluate significance. A P value of <0.05 was considered statistically significant for all groups.
results
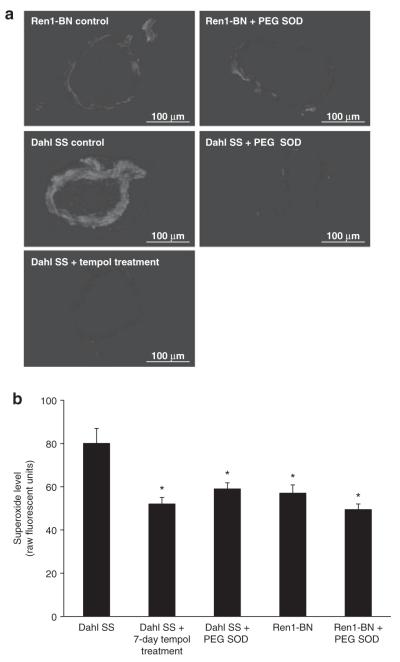
Vascular superoxide levels assessed by DHE fluorescence were significantly lower in basilar arteries from Ren1-BN congenic rats vs. those from Dahl SS rats (Figure 1). Chronic antioxidant treatment with tempol or preincubation of arteries from Dahl SS rats with polyethylene glycol-SOD reduced vascular superoxide to levels similar to those observed in Ren1-BN rats, while preincubation of arteries from Ren1-BN rats with polyethylene glycol-SOD had no effect on vascular superoxide levels.
Figure 1.
Superoxide levels in SS and Ren-1 BN rats. (a) Representative fluorescent images of basilar artery cross-sections (10 μm thickness) from Dahl salt-sensitive (SS) and Ren1-Brown Norway (BN) rats ± 15 min preincubation with 100 U/ml polyethylene glycol (PEG) superoxide dismutase (SOD) in the presence of 5 μmol/l dihydroethidium (DHE). a separate group of Dahl SS rats was treated with tempol (15 mg/kg/day) for 7 days. Images were taken at ×20 magnification. Bar = 100 μm. (b) Vascular superoxide levels quantified as fluorescence units in DHE-treated basilar artery cross-sections from Dahl SS (n = 5) and Ren1-BN (n = 6) rats ± 100 U/ml PEG SOD (n = 6, 5 respectively). a separate group of Dahl SS rats was treated with tempol for 7 days (n = 8). Data are expressed as mean value (raw fluorescence units) ± s.e.m. *Significant difference (P < 0.05) all groups vs. Dahl SS.
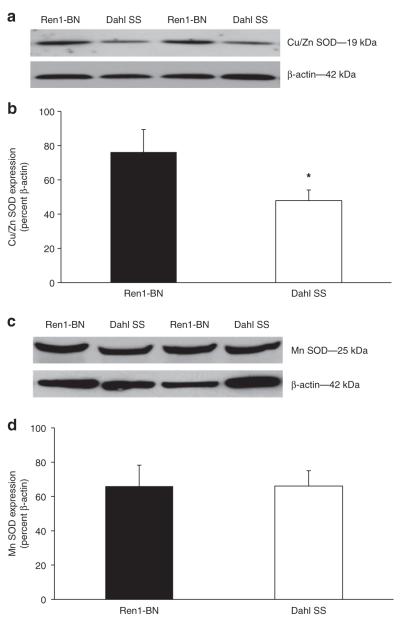
There were no significant differences in NADPH oxidase or XO activity in cerebral arteries from Dahl SS or Ren1-BN rats, as evaluated by measuring vascular superoxide production with DHE ± the NADPH oxidase inhibitor diphenyleneiodonium or the XO inhibitor oxypurinol (Figure 2). However, expression of Cu/Zn SOD was significantly higher in cerebral arteries from Ren1-BN congenic rats compared to arteries from Dahl SS rats (Figure 3a-b). Mn-SOD expression was similar in the two strains (Figure 3c-d).
Figure 2.
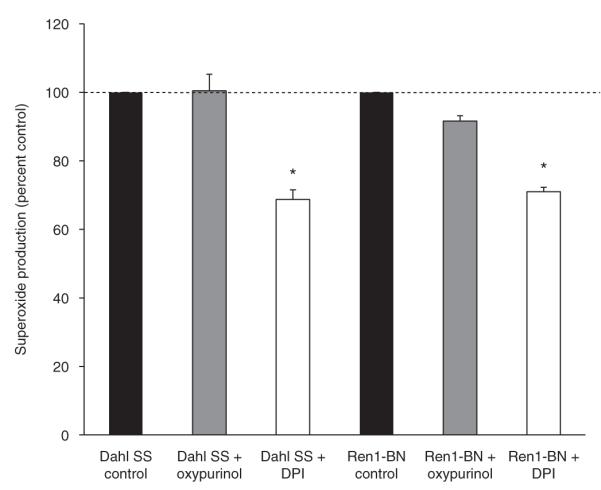
Nicotinamide adenine dinucleotide phosphate (NaD(P)H) oxidase activity (n = 6) and xanthine oxidase (n = 3) activity were not significantly different in cerebral artery tissue homogenates from Dahl salt-sensitive (SS) and Ren1-Brown Norway (BN) rats as evaluated by dihydroethidium (10 μmol/l) fluorescence. Preincubation of the cerebral artery tissue homogenates with the NaD(P)H oxidase inhibitor diphenyleneiodonium (DPI) reduced superoxide levels significantly from control values (P < 0.05). The percent reduction of superoxide levels between strains was not significantly different. Data are expressed as mean percent of control ± s.e.m. *Significant difference from control (P < 0.05).
Figure 3.
Cu/Zn superoxide dismutase (SOD) and Mn-SOD expression in cerebral arteries of Ren1-Brown Norway (BN) (n = 8) and Dahl SS (n =7) rats. (a) Representative western blots of Cu/Zn SOD. (b) Summarized data are normalized to β-actin and expressed as mean ± s.e.m. Cu/Zn SOD expression was significantly greater in cerebral arteries from Ren1-BN rats compared to Dahl salt-sensitive (SS) rats. (c) Representative western blots of Mn-SOD. (d) Summarized data are normalized to β-actin and expressed as mean ± s.e.m. There were no significant differences in Mn-SOD expression in cerebral arteries of Ren1-BN and Dahl SS rats. *Significant difference (P < 0.05) Ren1-BN vs. Dahl SS.
Addition of the SOD inhibitor DETC to the perfusate and superfusate had no effect on resting vascular tone in isolated MCAs from either Ren1-BN or Dahl SS rats (Ren1-BN control diameter = 145 ± 3.5 μm vs. Ren1-BN + DETC diameter = 138 ± 5.3 μm; Dahl SS control diameter = 146 ± 5.4 μm vs. Dahl SS + DETC diameter = 146 ± 6.8 μm). However, DETC addition eliminated the dilation of MCAs from Ren1-BN congenic rats to ACh, compared to previously reported responses by Durand et al.11 (Figure 4). These findings support the hypothesis that Cu/Zn SOD is important in maintaining NO-mediated vascular relaxation in MCAs from the Ren1-BN rat.
Figure 4.
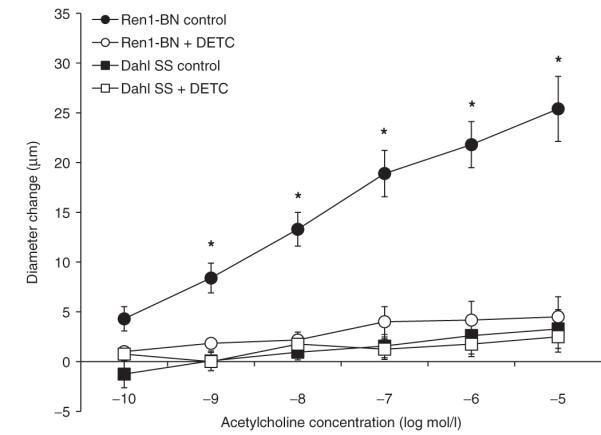
Response of isolated middle cerebral arteries of Ren1-Brown Norway (BN) (n = 10) and Dahl salt-sensitive (SS) (n = 9) rats to acetylcholine (10-10-10-5 mol/l) ± the superoxide dismutase (SOD) inhibitor diethyldithiocarbamate (DETC; 1 mmol/l; n = 6, 4 respectively) in the perfusate and superfusate. *Significant difference Ren1-BN control vs. all groups (P < 0.05). Data are expressed as mean change in diameter from baseline (μm) ± s.e.m. Due to the limited availability of Dahl SS and Ren1-BN rats, the control responses are replotted from Durand et al.11
discussion
As previously reported,11 MCAs from Ren1-BN rats dilate in a NO-dependent manner to ACh whereas MCAs from Dahl SS rats do not. Additionally, the expression and activation of endothelial NO synthase are similar in the large cerebral arteries from the two rat strains,11 suggesting that the point of impairment in MCAs from Dahl SS rats lies downstream of NO synthase activation. This finding is in accordance with numerous studies from our laboratory4-6 and others16-19 showing that HS-diet suppresses the RAS, increases vascular oxidant stress, and compromises vascular relaxation mechanisms in multiple vascular beds of normotensive rats.
However, those studies have reached varying conclusions regarding the cellular source of the increased reactive oxygen species (ROS) formation. For example, increased activity of NADPH oxidase contributes to increased superoxide production in both mesenteric resistance arteries5 and skeletal muscle arterioles17 from SD rats that fed a HS-diet. However, reduced expression and activity of antioxidant enzymes have been observed in cerebral arteries7 and skeletal muscle arterioles16 from HS-fed rats, indicating that the increase in vascular superoxide levels may not be solely due to an increased production of superoxide, but may also reflect a reduced ability to scavenge ROS from the vasculature.
There is indisputable evidence in the literature that elevations of ANG II levels above the normal physiological range lead to increased activity of NADPH oxidase and contribute to cardiovascular disease by causing excessive ROS generation.20,21 Paradoxically, HS-diet, which is accompanied by suppression of the RAS, also causes endothelial dysfunction via NADPH oxidase-mediated ROS production in mesenteric resistance arteries5 and skeletal muscle arterioles.17 Because NADPH oxidase activity is 10-90-fold higher in cerebral arteries vs. arteries from the systemic vasculature,22 the physiological importance of NADPH oxidase-derived ROS must be considered in the pathogenesis of cerebrovascular dysfunction. As shown in Figure 2, the relative amount of NADPH oxidase-derived superoxide was similar in the cerebral vasculature of the Dahl SS rat compared to the Ren1-BN congenic rat, suggesting that the increased superoxide levels observed in basilar arteries from Dahl SS rats are not directly related to increased NADPH oxidase activity.
Reactive oxygen species generated from XO contribute to endothelial dysfunction in mesenteric arteries and skeletal muscle resistance arteries of HS-fed SD rats,5,17 suggesting a possible link between suppressed RAS activity and increased XO activity. However, XO did not significantly contribute to the overall superoxide production in cerebral arteries from either Dahl SS rats or Ren1-BN congenic rats. Taken together with the observations that NADPH oxidase activity is not increased in cerebral arteries from Dahl SS rats compared to Ren1-BN congenic rats, and that the expression and activation of endothelial NO synthase are similar in cerebral arteries of the two strains,11 it seems unlikely that oxidant stress in the cerebral vasculature of normotensive Dahl SS rats is due to an overproduction of superoxide from XO, NADPH oxidase or uncoupled endothelial NO synthase.
Another potential cause of elevated vascular superoxide levels is reduced expression or activity of antioxidant enzymes. If antioxidant enzyme expression or activity is reduced, vascular superoxide levels will increase, even in the absence of an increase in superoxide production. For example, Cu/Zn SOD-deficient mice have increased vascular superoxide and peroxynitrite levels, endothelial dysfunction, augmented vasoconstrictor responses, and increased myogenic tone.23,24 Reductions in SOD expression and activity have been associated with increased vascular levels of ROS and subsequent vascular dysfunction in animal models of cardiovascular disease7,16,25-28 and in humans with hypertension and coronary artery disease.29-31 Separate studies by Sathiyapriya et al.32 and Chrysuhoou et al.33 have shown that patients classified as “prehypertensive\p="\ have lower total antioxidant capacity and elevated levels of ROS compared to their normotensive counterparts, suggesting that reductions in antioxidant enzyme capacity may play a role in the progression of hypertension and cardiovascular disease.
In the rat, extracellular SOD has little physiological activity in the vasculature.34 Therefore, Cu/Zn SOD accounts for the overwhelming majority of total SOD activity in this species.35 A recent study by McEwen et al.7 showed that decreased expression of Cu/Zn SOD in the cerebral vasculature of SD rats that fed a HS-diet could be prevented by low-dose ANG II infusion to restore plasma ANG II levels to NS control values. As shown in Figure 3, Cu/Zn SOD expression is also significantly lower in cerebral arteries from Dahl SS rats compared to those from Ren1-BN congenic rats. This finding is particularly significant in light of a previous study by Didion et al.36 showing that endogenous Cu/Zn SOD activity is vital both in controlling vascular superoxide levels in larger cerebral arteries, and in preserving NO-mediated vasodilator responses in the cerebral microcirculation.
Mn-SOD expression is greater in cerebral arteries than in systemic arteries,37 suggesting that Mn-SOD may play an important physiological role in regulating superoxide levels in the cerebral vasculature. In the present study, Mn-SOD expression was similar in cerebral arteries from Ren1-BN rats vs. those of Dahl SS rats. The latter finding is consistent with preliminary studies from our laboratory (data not shown) showing that Mn-SOD expression in multiple vascular beds does not change with elevated dietary salt intake in SD rats.
In summary, the results of this study and others11,38 indicate that introgression of the BN renin allele onto the Dahl SS genetic background restores normal vascular relaxation in MCAs by reducing vascular superoxide levels. The elevation in vascular oxidant stress in cerebral arteries of Dahl SS rats appears to result from suppressed RAS activity and downregulation of the crucial antioxidant enzyme Cu/Zn SOD because restoring normal regulation of the RAS12 by introgressing the BN renin allele onto the Dahl SS genetic background increases Cu/Zn SOD expression. Overall, the results of this study suggest a potential mechanism by which normal levels of plasma ANG II preserve vascular function in arteries of the cerebral circulation. This finding is particularly significant given the established relationship between endothelial dysfunction and cardiovascular disease1 and clinical studies showing that RAS activity tends to be suppressed both in hypertensive patients who are salt-sensitive39 and in normotensive subjects who are salt-sensitive and at an increased risk of developing cardiovascular disease.40
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 2.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 4.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–390. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 6.Weber DS, Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol Heart Circ Physiol. 2000;278:H500–H506. doi: 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 7.McEwen ST, Schmidt JR, Somberg L, Cruz Lde L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation. 2009;16:220–234. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weir MR, Saunders E. Pharmacologic management of systemic hypertension in blacks. Am J Cardiol. 1988;61:46H–52H. doi: 10.1016/0002-9149(88)91105-8. [DOI] [PubMed] [Google Scholar]
- 9.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–691. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 10.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 11.Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol. 2010;299:H1865–H1874. doi: 10.1152/ajpheart.00700.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics. 2008;33:33–40. doi: 10.1152/physiolgenomics.00150.2007. [DOI] [PubMed] [Google Scholar]
- 13.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1-7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2010;299:H1024–H1033. doi: 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. Am J Physiol. 1990;259:H1455–H1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- 15.Taylor NE, Glocka P, Liang M, Cowley AW., Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 16.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 17.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2002;282:H395–H402. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 18.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 19.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1550–R1556. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 21.Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 22.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 23.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 24.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Gender differences in myogenic tone in superoxide dismutase knockout mouse: animal model of oxidative stress. Am J Physiol Heart Circ Physiol. 2004;287:H40–H45. doi: 10.1152/ajpheart.01179.2003. [DOI] [PubMed] [Google Scholar]
- 25.Yelinova VI, Khramtsov VV, Markel AL. Manifestation of oxidative stress in the pathogenesis of arterial hypertension in ISIAH rats. Biochem Biophys Res Commun. 1999;263:450–453. doi: 10.1006/bbrc.1999.1396. [DOI] [PubMed] [Google Scholar]
- 26.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 27.Cabell KS, Ma L, Johnson P. Effects of antihypertensive drugs on rat tissue antioxidant enzyme activities and lipid peroxidation levels. Biochem Pharmacol. 1997;54:133–141. doi: 10.1016/s0006-2952(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 28.Fukai T, Galis ZS, Meng XP, Parthasarathy S, Harrison DG. Vascular expression of extracellular superoxide dismutase in atherosclerosis. J Clin Invest. 1998;101:2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buczynski A, Wachowicz B, Kedziora-Kornatowska K, Tkaczewski W, Kedziora J. Changes in antioxidant enzymes activities, aggregability and malonyldialdehyde concentration in blood platelets from patients with coronary heart disease. Atherosclerosis. 1993;100:223–228. doi: 10.1016/0021-9150(93)90208-c. [DOI] [PubMed] [Google Scholar]
- 30.Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16:1267–1271. doi: 10.1097/00004872-199816090-00007. [DOI] [PubMed] [Google Scholar]
- 31.Landmesser U, Merten R, Spiekermann S, Büttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 32.Sathiyapriya V, Nandeesha H, Bobby Z, Selvaraj N, Pavithran P. Perturbation of oxidant-antioxidant status in non-obese prehypertensive male subjects. J Hum Hypertens. 2007;21:176–178. doi: 10.1038/sj.jhh.1002121. [DOI] [PubMed] [Google Scholar]
- 33.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Economou M, Papadimitriou L, Stefanadis C. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: the ATTICA study. Atherosclerosis. 2007;192:169–176. doi: 10.1016/j.atherosclerosis.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988;255:223–228. [PMC free article] [PubMed] [Google Scholar]
- 35.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol. 2001;281:H1697–H1703. doi: 10.1152/ajpheart.2001.281.4.H1697. [DOI] [PubMed] [Google Scholar]
- 37.Napoli C, Witztum JL, de Nigris F, Palumbo G, D’Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999;99:2003–2010. doi: 10.1161/01.cir.99.15.2003. [DOI] [PubMed] [Google Scholar]
- 38.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol. 2004;287:H957–H962. doi: 10.1152/ajpheart.01087.2003. [DOI] [PubMed] [Google Scholar]
- 39.Ishibashi K, Oshima T, Matsuura H, Watanabe M, Ishida M, Ishida T, Ozono R, Kajiyama G, Kanbe M. Effects of age and sex on sodium chloride sensitivity: association with plasma renin activity. Clin Nephrol. 1994;42:376–380. [PubMed] [Google Scholar]
- 40.Overlack A, Ruppert M, Kolloch R, Göbel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension. 1993;22:331–338. doi: 10.1161/01.hyp.22.3.331. [DOI] [PubMed] [Google Scholar]