Abstract
Background
Little information is available on the comparative effectiveness of osteoporosis pharmacotherapies.
Objective
To compare the relative effectiveness of osteoporosis treatments to reduce nonvertebral fracture risk among older adults.
Design
Cohort study.
Setting
Enrollees in 2 statewide pharmaceutical benefit programs for persons age 65 years or older.
Patients
43 135 new recipients of oral bisphosphonates, nasal calcitonin, and raloxifene who began treatment from 2000 to 2005. The mean age was 79 years (SD, 6.9), and 96% were women.
Measurements
The primary outcome was nonvertebral fracture (hip, humerus, or radius or ulna) within 12 months of treatment initiation. Cox proportional hazard models stratified by state and adjusted for risk factors for fracture were used to compare fracture rates. Alendronate was the reference category in all analyses.
Results
A total of 1051 nonvertebral fractures were observed within 12 months (2.62 fractures per 100 person-years). No large differences in fracture risk were found between risedronate (hazard ratio [HR], 1.01 [95% CI, 0.85 to 1.21]) or raloxifene (HR, 1.18 [CI, 0.96 to 1.46]) and alendronate. However, among those with a fracture history, raloxifene recipients experienced more nonvertebral fractures within 12 months (HR, 1.78 [CI, 1.20 to 2.63]) compared with alendronate recipients. Patients who received calcitonin experienced more nonvertebral fractures than those who received alendronate (HR, 1.40, [CI, 1.20 to 1.63]). Results were similar in sensitivity analyses that examined different lengths of follow-up (6 months and 24 months), were restricted to hip fracture as the outcome, and were completed in various subgroups.
Limitation
Confounder adjustment was limited to health care utilization data, and the confidence bounds of some comparisons were too wide to rule out potential clinically important differences between agents.
Conclusion
Differences in fracture risk between risedronate or raloxifene and alendronate were small. Nasal calcitonin recipients may have a higher risk for nonvertebral fractures compared with alendronate recipients. Future studies that can better adjust for possible confounding may further clarify these relationships.
Osteoporosis is characterized by decreased bone mass and deterioration of bone tissue, resulting in reduced bone strength and increased fracture risk (1, 2). Approved therapies for osteoporosis include bisphosphonates, calcitonin, raloxifene, and teriparatide. Findings from randomized, controlled, head-to-head trials show that women who received alendronate have greater gains in bone mineral density and greater reductions in bone turnover markers within 12 and 24 months of initiation than those who received risedronate (3, 4) or raloxifene (5–7). Although bone mineral density is a strong predictor of fracture (8), differences in these surrogate markers may not translate into appreciable differences in fracture risk (9 –11). Results from observational studies suggest that risedronate may reduce the risk for nonvertebral fracture (clavicle, hip, humerus, leg, pelvis, and wrist) within 12 months more effectively than alendronate or nasal calcitonin (12, 13). To our knowledge, no studies have compared the relative effectiveness of raloxifene versus bisphosphonates or calcitonin in reducing fracture risk. Further comparative effectiveness studies may help to clarify the relative effectiveness of osteoporosis treatments (14). We completed a population-based study of new recipients of oral bisphosphonates (alendronate or risedronate), nasal calcitonin, and raloxifene to compare the relative effectiveness of these agents in reducing nonvertebral fracture risk.
Methods
Study Cohort
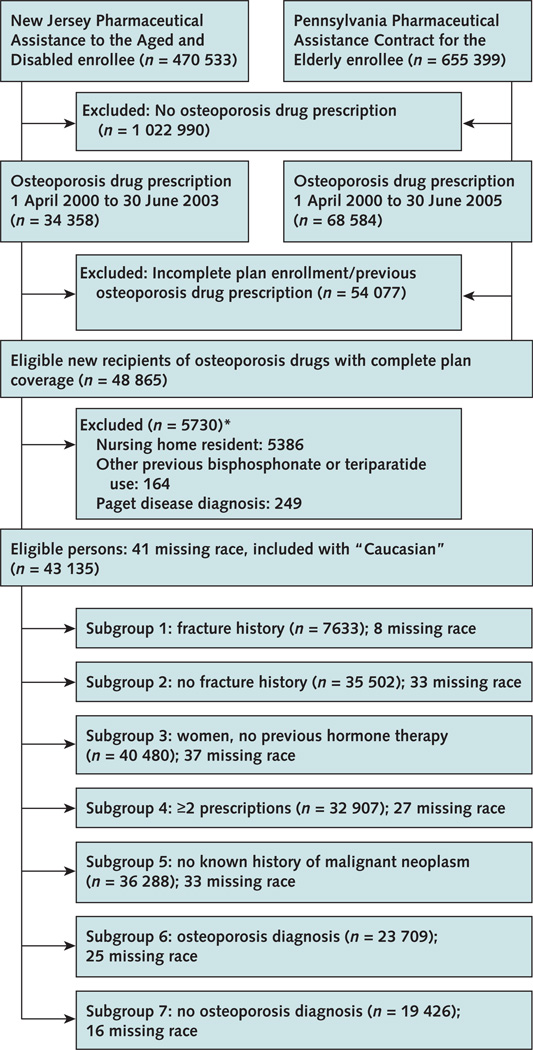
The study population comprised Medicare beneficiaries enrolled in 2 statewide pharmaceutical benefit plans: the New Jersey Pharmaceutical Assistance to the Aged and Disabled program and the Pennsylvania Pharmaceutical Assistance Contract for the Elderly. These programs provide drug coverage without restriction for low-income residents age 65 years or older with minimal copayment. Our cohort consisted of new recipients (no use of any of these agents in the previous year) of oral bisphosphonates (alendronate, 10 mg or 70 mg, or risedronate, 5 mg or 35 mg), nasal calcitonin, or raloxifene between 1 April 2000 and 30 June 2005 (Figure 1). To ensure complete plan coverage, study eligibility was limited to patients with 1 or more claims in both Medicare and their state pharmaceutical assistance plan in each of the three 6-month intervals preceding the index prescription. We excluded nursing home residents (for whom prescription data may not be complete), patients with a Medicare claim for Paget disease (International Classification of Diseases, Ninth Revision, Clinical Modification code 731.0), and patients with pharmacy claims indicating receipt of any bisphosphonate or teriparatide in the year before treatment initiation. Our data included all Medicare beneficiaries from the 2 plans that met eligibility criteria; we did not do formal sample size calculations. We restricted inclusion to the period when all drugs were available: that is, 1 April 2000 (risedronate received U.S. Food and Drug Administration approval in April 2000). At the time of analysis, we had complete Medicare data from 1 April 2000 to 31 December 2003 for New Jersey and from 1 April 2000 to 31 December 2005 for Pennsylvania.
Figure 1. Study flow diagram.
Osteoporosis drugs were oral bisphosphonates (alendronate, 10 mg or 70 mg; risedronate, 5 mg or 35 mg), nasal calcitonin, or raloxifene. *May meet >1 exclusion criterion.
Outcomes
Our primary outcome of interest was nonvertebral fracture within 12 months of treatment initiation. We defined nonvertebral fracture as a fracture of the hip, humerus, or radius or ulna by using previously validated criteria requiring diagnostic and procedural codes from Medicare claims (15). When medical records are used as the reference standard, the estimated sensitivity of each outcome is at least 90% (15). Secondary outcomes included nonvertebral fractures within 6 and 24 months of treatment initiation and hip fracture within 6, 12, and 24 months of treatment initiation.
Covariates
Patient demographic characteristics were determined at treatment initiation and other variables by medical and pharmacy claims within the year before treatment initiation. We considered covariates that were plausibly related to our fracture outcomes (16): demographic characteristics (age, sex, race), osteoporosis-related factors (such as diagnosis of osteoporosis, fracture history), relevant comorbid conditions (such as comorbidity score [17, 18]; diabetes mellitus; history of falls, syncope, and gait abnormalities; cancer; rheumatoid arthritis), drug use (such as anti-epileptics, β-blockers, benzodiazepines, glucocorticoids, hormone therapy, selective serotonin reuptake inhibitors, thiazide diuretics, number of drugs), and previous hospitalization. We also included calendar time (month and year) of the index prescription to adjust for potential secular trends in prescribing. Appendix Table 1 (available at www.annals.org) lists all variables, definitions, and coding. If a record of a specific diagnosis, procedure, or prescription was lacking, patients were coded as not having these characteristics. As a result of this coding rule, there were no participants for whom exposure, confounder, or outcome information was missing. However, race was unknown in 41 patients. These 41 missing data points were recoded as “Caucasian.”
Appendix Table 1.
Definition and Coding of Variables Included in Multinomial Logistic Regression Model Used to Create Propensity Scores*
| Variable | Definition | Coding |
|---|---|---|
| Medicare enrollment information at time of index prescription | ||
| Age | Age in years | Categorical: 1 category for each age, except that all patients ≥96 y were placed in 1 reference group (31 dummies) |
| Male sex | Male sex | Dichotomous (yes/no) |
| Race | White | Dichotomous (yes/no) |
| Date of index prescription | Month/year of index prescription | Categorical (mo/y): 1 category for each month/year (38 dummies for New Jersey, 62 dummies for Pennsylvania) |
| Medicare claims within 365 d before index osteoporosis drug prescription | ||
| Health services use/overall measure of health status | ||
| Hospitalized in previous year | Any | Dichotomous (yes/no) |
| Comorbidity score | Charlson comorbidity score (17, 18) | Ordinal (quartiles) |
| Osteoporosis-related variables | ||
| Osteoporosis | ICD-9-CM codes 733.0x | Dichotomous (yes/no) |
| Kyphosis | ICD-9-CM codes 737.1x, 737.41, 737.3x | Dichotomous (yes/no) |
| Previous fracture | ||
| Vertebral | Vertebral (ICD-9-CM codes 733.13, 805.xx) | Dichotomous (yes/no) |
| Nonvertebral | Hip (ICD-9-CM codes 820.xx, 733.14), humerus (ICD-9-CM codes 812.xx, 733.11), and/or radius or ulna (ICD-9-CM codes 813.xx, 733.12) | Dichotomous (yes/no) |
| Other | Any fracture (ICD-9-CM codes 733.1x, 800.xx–829.xx) other than vertebral or nonvertebral defined above | Dichotomous (yes/no) |
| Comorbid conditions | ||
| Alzheimer disease or other dementia | ICD-9-CM codes 290.xx, 294.xx, 330.xx, 331.xx | Dichotomous (yes/no) |
| Asthma or chronic obstructive pulmonary disease | ICD-9-CM codes 493.xx, 490, 491.xx, 492.xx, 494.xx, 496.xx, 506.4x | Dichotomous (yes/no) |
| Cataracts | ICD-9-CM codes 366.xx | Dichotomous (yes/no) |
| Crohn disease or gastroenteritis | ICD-9-CM codes 555.xx, 556.xx, 558.xx | Dichotomous (yes/no) |
| Depression | ICD-9-CM codes 293.83, 296.2x. 296.3x, 298.0x, 300.4x, 309.0x, 309.1x, 309.28, 311.xx | Dichotomous (yes/no) |
| Diabetes mellitus | $1 hospitalization discharge ICD-9-CM codes 250.xx or $2 outpatient ICD-9-CM codes 250.xx | Dichotomous (yes/no) |
| History of falls, syncope, or gait abnormality | ICD-9-CM codes E885, E885.9x, E888.xx, 780.2x, 458.0x, 781.2x, 782.3x | Dichotomous (yes/no) |
| Hyperthyroidism | ICD-9-CM codes 242.0x–242.9x | Dichotomous (yes/no) |
| Hyperparathyroidism | ICD-9-CM codes 252.0x | Dichotomous (yes/no) |
| Ischemic stroke | ICD-9-CM codes 434.xx, 436.xx | Dichotomous (yes/no) |
| Liver disease | ICD-9-CM codes 571.4x, 571.6x, 571.8x, 571.9x, 573.xx, 070.xx | Dichotomous (yes/no) |
| Malignant neoplasm | ICD-9-CM codes 140.xx–208.xx | Dichotomous (yes/no) |
| Overweight or obese | ICD-9-CM codes 278, 278.0x | Dichotomous (yes/no) |
| Parkinson disease | ICD-9-CM codes 332.xx or 333.0x, or use of antiparkinsonian drug | Dichotomous (yes/no) |
| Renal disease | ICD-9-CM codes 250.4x, 403.xx, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93 | Dichotomous (yes/no) |
| Rheumatoid arthritis | ICD-9-CM codes 714.xx | Dichotomous (yes/no) |
| Pharmacy claims within 365 d before index osteoporosis drug prescription | ||
| Number of generic drugs | Count | Ordinal (quintiles) |
| Antiepileptic | Any pharmacy claim | Dichotomous (yes/no) |
| β-Blocker | Any pharmacy claim | Dichotomous (yes/no) |
| Benzodiazepine | Any pharmacy claim | Dichotomous (yes/no) |
| Gastroprotective agent | Any pharmacy claim | Dichotomous (yes/no) |
| Glucocorticoids | Any pharmacy claim | Dichotomous (yes/no) |
| Hormone therapy | Any pharmacy claim | Dichotomous (yes/no) |
| Selective COX-2 inhibitor | Any pharmacy claim | Dichotomous (yes/no) |
| Other NSAID | Any pharmacy claim | Dichotomous (yes/no) |
| SSRI | Any pharmacy claim | Dichotomous (yes/no) |
| Non-SSRI antipsychotic | Any pharmacy claim | Dichotomous (yes/no) |
| Thiazide diuretic | Any pharmacy claim | Dichotomous (yes/no) |
| Thyroid drug | Any pharmacy claim | Dichotomous (yes/no) |
| Miscellaneous sleep agent, hypnotic, or barbiturate | Any pharmacy claim | Dichotomous (yes/no) |
COX-2 5 cyclooxygenase-2; ICD-9-CM 5 International Classification of Diseases, Ninth Revision, Clinical Modification; NSAID 5 nonsteroidal anti-inflammatory drug; SSRI 5 selective serotonin reuptake inhibitor.
Statistical Analysis
We calculated fracture rates among recipients of each drug within 6, 12, and 24 months of treatment initiation. We used Kaplan–Meier methods to plot cumulative fracture incidence and Cox proportional hazard models to compare fracture rates between agents. In our primary analysis, we considered a patient exposed to drug throughout follow-up by censoring only at date of death or end of follow-up (hereafter referred to as “intent-to-treat analysis,” an analogue of intention-to-treat analysis). We tested proportional hazard assumptions by using interaction terms between exposures and time and found no violations for the primary analysis of 12-month follow-up. However, we observed a violation resulting in an attenuated effect for raloxifene over 24 months. This observation is expected when an intent-to-treat scenario is assumed because adherence to osteoporosis pharmacotherapy is suboptimal (19, 20). Similar attenuation of effects was also observed when hip fracture was the outcome.
We developed propensity scores for each drug by using multinomial logistic regression (21). Alendronate, the most commonly prescribed osteoporosis treatment, was selected as the reference category. To account for baseline differences between New Jersey and Pennsylvania, we derived state-specific propensity scores and stratified all adjusted Cox proportional hazard models by state. Propensity score quintiles for risedronate, calcitonin, and raloxifene were included as 12 dummy variables (4 for each drug) to adjust for confounding (21–24). We summarized the balance achieved within state-specific propensity score quintiles into descriptive tables and examined the magnitude of difference for each covariate within each propensity score quintile. Preliminary examination suggested residual imbalance within some quintiles (Appendix Tables 2 and 3, available at www.annals.org). For example, within the lowest propensity score quintile for receipt of raloxifene in New Jersey (Appendix Table 2, available at www.annals.org), 76% of patients who received alendronate and 87% of patients who received raloxifene had a background prevalence of osteoporosis. Therefore, we included age groups, fracture history, race, and diagnosis of osteoporosis, in addition to propensity score quintiles, in our adjusted regression models. Analyses were performed with SAS software, version 9.1 (SAS Institute, Cary, North Carolina).
Appendix Table 2.
Propensity Score Quintiles: New Jersey*
| Covariate | Risedronate Propensity Score | Calcitonin Propensity Score | Raloxifene Propensity Score | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||||||||||||||||
| ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | ALD | CCT | ALD | CCT | ALD | CCT | ALD | CCT | ALD | CCT | ALD | RAL | ALD | RAL | ALD | RAL | ALD | RAL | ALD | RAL | |
| Mean age (SD), y | 78.0 (7.4) | 77.3 (7.5) | 77.8 (6.6) | 78.1 (6.7) | 78.2 (6.4) | 78.0 (6.9) | 77.7 (6.8) | 77.9 (7.0) | 77.9 (6.3) | 78.3 (6.1) | 73.6 (5.4) | 74.0 (5.4) | 76.6 (5.8) | 76.3 (5.9) | 78.8 (6.1) | 79.0 (6.0) | 80.0 (6.5) | 79.8 (6.9) | 82.3 (6.8) | 82.3 (7) | 81.8 (6.2) | 82.3 (5.8) | 80.5 (5.6) | 81.0 (5.5) | 78.3 (6.1) | 79.0 (6.2) | 75.8 (5.9) | 76.0 (6.1) | 72.2 (5.2) | 72.2 (5.1) |
| Men, % | 2.9 | 2.5 | 4.0 | 4.5 | 4.3 | 3.3 | 5.7 | 3.8 | 6.9 | 8.2 | 1.8 | 3.3 | 2.6 | 3.5 | 5.2 | 5.2 | 6.3 | 4.9 | 9.2 | 7.3 | 22.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| White, % | 90.2 | 88.5 | 89.3 | 89.1 | 90.6 | 89.7 | 87.3 | 91.5 | 87.4 | 85.8 | 83.5 | 87.9 | 89.0 | 88.1 | 90.9 | 88.9 | 91.3 | 91.9 | 91.9 | 91.0 | 92.4 | 95.5 | 91.9 | 90.5 | 89.5 | 88.7 | 86.6 | 86.4 | 83.9 | 84.4 |
| Year of index prescription, % | ||||||||||||||||||||||||||||||
| 2000 | 26.4 | 20.5 | 8.7 | 11.4 | 10.9 | 18.3 | 19.5 | 28.2 | 5.2 | 4.6 | 0.3 | 0.0 | 2.0 | 2.2 | 6.6 | 6.4 | 20.6 | 27.1 | 55.7 | 68.1 | 7.4 | 11.9 | 6.3 | 10.4 | 11.2 | 17.1 | 15.3 | 24.0 | 33.7 | 42.5 |
| 2001 | 37.0 | 40.5 | 58.4 | 58.5 | 62.7 | 59.9 | 13.8 | 9.9 | 0.1 | 0.0 | 22.6 | 24.2 | 41.9 | 40.4 | 47.1 | 43.8 | 43.2 | 35.9 | 26.3 | 18.5 | 27.6 | 24.3 | 37.4 | 32.6 | 39.2 | 36.7 | 42.8 | 34.5 | 36.2 | 31.7 |
| 2002 | 36.5 | 39.0 | 32.9 | 30.1 | 24.9 | 19.5 | 41.3 | 38.4 | 43.2 | 42.2 | 47.7 | 46.9 | 42.6 | 39.6 | 35.6 | 35.6 | 28.3 | 27.2 | 13.9 | 10.4 | 44.4 | 43.5 | 38.9 | 37.7 | 36.6 | 32.7 | 31.7 | 27.9 | 22.3 | 19.0 |
| 2003 | 0.1 | 0.0 | 0.0 | 0.0 | 1.5 | 2.3 | 25.4 | 23.4 | 51.6 | 53.1 | 29.4 | 28.9 | 13.5 | 17.8 | 10.8 | 14.3 | 7.9 | 9.9 | 4.1 | 3.0 | 20.6 | 20.3 | 17.4 | 19.3 | 13.0 | 13.5 | 10.2 | 13.6 | 7.8 | 6.7 |
| Age group, % | ||||||||||||||||||||||||||||||
| 65–69 y | 14.2 | 15.5 | 11.9 | 12.8 | 10.7 | 12.8 | 13.0 | 14.8 | 11.9 | 8.5 | 26.9 | 24.2 | 13.4 | 13.2 | 7.9 | 7.9 | 6.2 | 8.3 | 2.9 | 3.9 | 2.9 | 1.7 | 2.3 | 0.6 | 7.4 | 5.6 | 15.6 | 17.2 | 37.5 | 36.2 |
| 70–74 y | 21.0 | 23.5 | 21.1 | 18.9 | 17.5 | 18.3 | 21.2 | 19.1 | 18.3 | 18.4 | 32.4 | 33.3 | 21.6 | 25.1 | 16.0 | 16.2 | 14.8 | 14.0 | 10.3 | 9.6 | 9.4 | 6.2 | 11.5 | 10.1 | 19.4 | 18.0 | 27.8 | 24.7 | 33.6 | 35.1 |
| 75–79 y | 23.3 | 24.5 | 26.7 | 24.2 | 28.4 | 27.0 | 26.1 | 23.3 | 27.1 | 30.6 | 22.4 | 24.2 | 30.9 | 30.1 | 29.0 | 25.9 | 24.7 | 26.1 | 24.0 | 23.0 | 21.5 | 24.9 | 28.9 | 32.0 | 31.2 | 30.2 | 30.4 | 28.6 | 18.5 | 18.6 |
| 80–84 y | 19.2 | 18.0 | 23.4 | 26.5 | 27.2 | 25.5 | 23.5 | 24.3 | 27.9 | 26.6 | 16.6 | 15.8 | 26.4 | 23.7 | 29.0 | 31.3 | 26.3 | 23.9 | 22.7 | 23.5 | 32.7 | 32.8 | 33.8 | 29.1 | 25.4 | 27.3 | 18.0 | 20.6 | 8.2 | 8.4 |
| 85–89 y | 16.3 | 11.5 | 13.3 | 13.9 | 12.5 | 10.5 | 11.6 | 13.9 | 11.9 | 12.5 | 1.8 | 2.6 | 7.0 | 7.3 | 15.3 | 15.4 | 21.3 | 19.5 | 26.0 | 25.7 | 24.4 | 26.0 | 18.6 | 23.4 | 13.2 | 11.7 | 6.0 | 6.8 | 1.5 | 1.3 |
| ≥90 y | 2.6 | 3.5 | 1.1 | 0.8 | 0.7 | 1.4 | 1.3 | 1.2 | 0.6 | 0.3 | 0.0 | 0.0 | 0.5 | 0.4 | 1.4 | 1.6 | 2.2 | 3.0 | 3.1 | 2.2 | 2.2 | 1.1 | 1.4 | 0.6 | 1.3 | 3.2 | 1.0 | 0.7 | 0.2 | 0.2 |
| Hospitalized in previous year, % | 25.2 | 30.0 | 20.9 | 20.3 | 19.0 | 17.9 | 18.8 | 21.2 | 15.8 | 16.5 | 4.3 | 4.0 | 11.2 | 12.1 | 19.7 | 18.0 | 31.1 | 30.4 | 43.3 | 45.2 | 37.7 | 41.2 | 22.5 | 21.4 | 15.9 | 19.6 | 13.4 | 16.8 | 9.0 | 7.9 |
| Comorbidity score quartile†, % | ||||||||||||||||||||||||||||||
| 1 | 26.7 | 23.0 | 27.3 | 27.3 | 28.2 | 28.6 | 28.5 | 29.2 | 30.3 | 27.5 | 51.3 | 50.2 | 31.8 | 32.1 | 21.9 | 23.9 | 16.0 | 17.0 | 12.0 | 10.0 | 16.1 | 18.6 | 24.3 | 26.4 | 28.7 | 30.4 | 34.4 | 25.9 | 38.9 | 38.1 |
| 2 | 25.1 | 28.0 | 25.9 | 26.5 | 26.4 | 22.8 | 25.5 | 25.8 | 24.7 | 24.8 | 25.9 | 23.8 | 31.0 | 29.5 | 26.0 | 28.0 | 23.5 | 22.9 | 19.2 | 19.2 | 20.3 | 19.2 | 26.0 | 22.6 | 27.1 | 24.8 | 26.9 | 29.2 | 27.9 | 28.8 |
| 3 | 23.3 | 22.0 | 20.0 | 17.8 | 19.0 | 18.5 | 19.3 | 18.7 | 17.8 | 19.6 | 12.9 | 15.8 | 19.1 | 18.9 | 23.4 | 19.7 | 24.1 | 24.3 | 21.7 | 21.7 | 22.6 | 22.6 | 21.0 | 22.8 | 19.4 | 19.6 | 18.6 | 19.9 | 17.6 | 16.0 |
| 4 | 12.6 | 14.5 | 13.7 | 16.2 | 14.2 | 16.9 | 14.2 | 15.0 | 16.1 | 17.0 | 3.8 | 4.4 | 8.1 | 9.5 | 14.0 | 16.7 | 20.6 | 21.1 | 30.4 | 29.9 | 22.2 | 18.1 | 14.5 | 14.2 | 13.0 | 15.1 | 11.6 | 14.7 | 8.5 | 10.2 |
| Osteoporosis-related variables, % | ||||||||||||||||||||||||||||||
| Osteoporosis | 59.2 | 55.5 | 60.7 | 58.2 | 65.7 | 58.2 | 65.1 | 61.4 | 70.6 | 70.1 | 86.5 | 86.8 | 71.2 | 70.3 | 58.4 | 52.3 | 50.2 | 50.0 | 45.2 | 43.7 | 75.9 | 87.0 | 72.5 | 72.1 | 64.1 | 61.9 | 58.1 | 53.5 | 46.5 | 41.2 |
| Kyphosis | 2.7 | 2.5 | 1.8 | 1.9 | 1.9 | 1.9 | 2.7 | 2.2 | 2.1 | 2.7 | 1.2 | 1.1 | 1.8 | 2.2 | 1.8 | 2.6 | 3.2 | 2.3 | 3.7 | 3.8 | 4.2 | 7.3 | 2.2 | 2.7 | 2.1 | 0.9 | 1.2 | 1.1 | 1.1 | 1.2 |
| Previous fracture | ||||||||||||||||||||||||||||||
| Vertebral | 13.3 | 12.5 | 4.9 | 6.4 | 3.9 | 3.9 | 6.4 | 5.5 | 1.0 | 0.9 | 0.4 | 0.7 | 1.6 | 0.4 | 4.7 | 3.8 | 9.2 | 9.4 | 18.6 | 18.1 | 21.7 | 22.6 | 3.9 | 3.0 | 2.4 | 1.8 | 0.7 | 1.1 | 0.2 | 0.6 |
| Nonvertebral (hip, humerus, radius or ulna) | 9.8 | 6.0 | 5.8 | 3.9 | 2.8 | 3.5 | 5.6 | 5.6 | 1.6 | 1.7 | 5.1 | 2.6 | 4.3 | 4.4 | 5.1 | 4.4 | 5.6 | 4.8 | 6.4 | 6.6 | 8.4 | 9.6 | 6.5 | 6.5 | 4.3 | 5.4 | 3.3 | 2.5 | 3.2 | 1.4 |
| Other | 5.1 | 6.0 | 5.9 | 7.0 | 5.9 | 4.5 | 5.5 | 5.1 | 6.1 | 6.6 | 4.5 | 5.9 | 4.8 | 4.2 | 5.5 | 6.7 | 7.2 | 7.1 | 7.3 | 6.0 | 8.9 | 10.2 | 7.2 | 7.4 | 4.9 | 5.4 | 3.7 | 3.2 | 3.5 | 3.1 |
| Comorbid conditions, % | ||||||||||||||||||||||||||||||
| Alzheimer disease or other dementia | 8.3 | 11.0 | 5.8 | 5.8 | 4.5 | 4.9 | 5.8 | 6.0 | 3.5 | 3.8 | 1.1 | 1.5 | 2.8 | 1.8 | 4.9 | 7.2 | 8.2 | 7.9 | 14.2 | 13.7 | 8.2 | 7.3 | 7.3 | 8.9 | 5.2 | 5.4 | 4.0 | 4.3 | 2.8 | 3.5 |
| Asthma or COPD | 24.0 | 30.0 | 22.9 | 19.8 | 19.8 | 20.0 | 21.3 | 20.7 | 17.3 | 18.9 | 12.0 | 13.2 | 16.5 | 18.0 | 23.0 | 22.3 | 26.6 | 26.4 | 32.4 | 30.3 | 30.6 | 29.4 | 21.9 | 19.0 | 20.2 | 21.4 | 17.4 | 19.1 | 14.7 | 15.9 |
| Cataracts | 42.2 | 34.5 | 38.7 | 37.0 | 36.7 | 36.6 | 36.7 | 39.1 | 36.3 | 36.7 | 37.8 | 38.5 | 39.4 | 42.0 | 39.4 | 40.7 | 36.1 | 37.2 | 37.8 | 35.7 | 32.1 | 26.6 | 38.2 | 35.9 | 38.6 | 39.6 | 40.9 | 43.8 | 41.6 | 41.0 |
| Crohn disease or gastroenteritis | 3.7 | 6.5 | 4.3 | 5.0 | 5.2 | 6.4 | 5.6 | 6.0 | 6.8 | 7.1 | 2.4 | 5.9 | 4.2 | 5.3 | 5.5 | 6.1 | 6.3 | 6.4 | 8.2 | 8.8 | 5.0 | 9.6 | 5.1 | 5.6 | 4.3 | 4.1 | 5.2 | 5.5 | 5.9 | 6.7 |
| Depression | 8.1 | 11.0 | 8.5 | 8.9 | 9.2 | 9.3 | 8.9 | 10.3 | 8.4 | 9.1 | 3.2 | 4.0 | 6.0 | 6.6 | 7.7 | 8.7 | 12.5 | 13.0 | 16.9 | 17.4 | 6.9 | 7.3 | 8.7 | 9.2 | 8.6 | 10.1 | 9.1 | 10.4 | 9.9 | 10.8 |
| Diabetes mellitus | 18.3 | 20.5 | 22.2 | 27.3 | 26.4 | 28.6 | 23.8 | 23.0 | 29.0 | 29.5 | 17.5 | 14.3 | 21.7 | 24.6 | 26.2 | 25.6 | 28.0 | 27.8 | 27.6 | 31.5 | 22.7 | 24.9 | 22.2 | 22.8 | 24.1 | 26.4 | 24.2 | 24.9 | 26.2 | 26.8 |
| History of falls, syncope, or gait abnormality | 18.1 | 18.5 | 15.5 | 18.9 | 13.3 | 15.4 | 15.4 | 15.4 | 14.1 | 14.0 | 6.1 | 6.6 | 9.5 | 9.2 | 15.3 | 16.2 | 22.2 | 20.4 | 28.6 | 30.6 | 24.4 | 26.0 | 16.9 | 19.9 | 13.0 | 13.3 | 11.3 | 13.6 | 9.7 | 9.4 |
| Hyperthyroidism | 5.2 | 6.5 | 3.7 | 3.1 | 3.3 | 1.9 | 4.6 | 4.6 | 3.2 | 2.7 | 5.5 | 3.7 | 4.1 | 3.3 | 3.4 | 2.1 | 3.6 | 3.7 | 2.9 | 3.7 | 3.6 | 4.0 | 4.4 | 5.6 | 4.6 | 3.2 | 3.8 | 3.2 | 3.4 | 3.0 |
| Hyperparathyroidism | 0.5 | 1.0 | 0.5 | 0.8 | 1.6 | 1.2 | 1.8 | 0.8 | 1.6 | 2.3 | 1.6 | 0.7 | 1.3 | 0.4 | 0.9 | 1.6 | 1.1 | 1.0 | 0.8 | 1.1 | 1.8 | 2.3 | 1.8 | 1.8 | 1.2 | 1.1 | 0.6 | 0.7 | 0.2 | 0.2 |
| Ischemic stroke | 4.9 | 5.0 | 5.5 | 8.1 | 4.7 | 4.1 | 5.8 | 5.0 | 5.0 | 5.3 | 1.8 | 1.5 | 4.0 | 3.1 | 4.6 | 4.8 | 7.2 | 7.6 | 10.4 | 10.4 | 8.2 | 4.5 | 6.0 | 7.7 | 5.1 | 4.3 | 3.7 | 6.8 | 2.3 | 2.1 |
| Liver disease | 4.8 | 4.0 | 4.8 | 6.1 | 4.4 | 5.1 | 4.5 | 5.0 | 4.9 | 4.8 | 2.4 | 2.2 | 4.3 | 4.8 | 4.9 | 4.4 | 5.8 | 5.4 | 7.0 | 7.7 | 6.2 | 8.5 | 5.3 | 3.9 | 4.5 | 5.2 | 4.0 | 3.8 | 3.3 | 3.8 |
| Malignant neoplasm | 16.0 | 14.0 | 16.4 | 15.0 | 18.6 | 19.8 | 17.3 | 16.3 | 19.5 | 20.3 | 14.7 | 16.1 | 17.0 | 16.5 | 19.4 | 18.2 | 18.6 | 18.0 | 18.2 | 18.8 | 23.9 | 19.2 | 18.1 | 16.6 | 17.3 | 12.8 | 14.0 | 18.8 | 13.3 | 13.8 |
| Overweight or obese | 2.3 | 2.0 | 3.1 | 2.5 | 3.3 | 3.9 | 2.6 | 2.1 | 2.7 | 3.5 | 2.8 | 1.8 | 2.9 | 2.2 | 2.4 | 2.6 | 2.9 | 3.2 | 3.3 | 3.7 | 2.4 | 4.0 | 2.0 | 1.8 | 2.4 | 2.7 | 2.9 | 3.8 | 4.8 | 4.1 |
| Parkinson disease | 0.9 | 2.5 | 1.4 | 2.2 | 1.6 | 0.4 | 1.3 | 1.2 | 1.7 | 1.9 | 0.6 | 0.7 | 0.8 | 0.4 | 1.4 | 1.1 | 2.4 | 2.6 | 2.0 | 2.2 | 2.1 | 1.7 | 1.5 | 1.5 | 0.9 | 0.7 | 1.3 | 1.3 | 0.9 | 1.4 |
| Renal disease | 1.6 | 1.5 | 1.8 | 2.2 | 0.8 | 0.8 | 1.3 | 1.6 | 0.7 | 1.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.7 | 0.3 | 1.6 | 2.1 | 4.9 | 5.6 | 2.6 | 2.3 | 1.1 | 3.0 | 1.3 | 1.1 | 0.6 | 0.5 | 0.5 | 0.4 |
| Rheumatoid arthritis | 4.5 | 6.5 | 6.9 | 5.0 | 8.0 | 6.6 | 7.6 | 7.2 | 8.1 | 9.6 | 4.1 | 5.5 | 6.8 | 6.4 | 7.7 | 6.6 | 8.3 | 8.5 | 9.0 | 9.5 | 11.3 | 10.2 | 7.9 | 6.8 | 5.8 | 6.5 | 5.4 | 5.5 | 4.3 | 5.3 |
| Drug use | ||||||||||||||||||||||||||||||
| Quintile of number of generic drugs used, % | ||||||||||||||||||||||||||||||
| 1 | 48.4 | 50.5 | 20.2 | 24.5 | 8.0 | 6.6 | 25.6 | 19.0 | 2.5 | 2.0 | 33.1 | 37.7 | 25.9 | 24.6 | 20.1 | 17.0 | 13.4 | 10.8 | 7.7 | 6.0 | 7.1 | 4.0 | 11.2 | 11.3 | 20.5 | 20.9 | 31.2 | 24.3 | 39.3 | 34.2 |
| 2 | 16.9 | 16.5 | 24.6 | 23.1 | 18.4 | 16.3 | 19.9 | 21.6 | 20.8 | 15.8 | 28.3 | 25.3 | 24.9 | 24.6 | 19.3 | 16.2 | 14.0 | 15.0 | 9.9 | 9.1 | 17.3 | 17.5 | 21.5 | 20.2 | 21.9 | 18.5 | 20.2 | 19.1 | 19.8 | 18.5 |
| 3 | 13.4 | 10.5 | 22.3 | 21.2 | 32.8 | 30.7 | 22.5 | 21.7 | 34.6 | 35.7 | 25.6 | 20.5 | 26.7 | 26.6 | 25.5 | 26.2 | 23.1 | 25.2 | 22.7 | 20.2 | 34.8 | 40.1 | 31.8 | 28.8 | 24.3 | 23.6 | 17.2 | 19.1 | 14.6 | 14.7 |
| 4 | 10.7 | 8.0 | 16.7 | 13.9 | 20.3 | 22.8 | 14.8 | 19.0 | 21.6 | 21.3 | 8.9 | 9.5 | 13.5 | 12.5 | 18.9 | 20.8 | 22.5 | 21.8 | 23.1 | 25.7 | 18.5 | 16.4 | 19.3 | 20.5 | 16.4 | 16.9 | 15.7 | 18.8 | 13.4 | 16.3 |
| 5 | 10.6 | 14.5 | 16.2 | 17.3 | 20.5 | 23.7 | 17.2 | 18.8 | 20.5 | 25.2 | 4.0 | 7.0 | 9.0 | 11.6 | 16.1 | 19.7 | 27.0 | 27.2 | 36.5 | 39.1 | 22.2 | 22.0 | 16.3 | 19.3 | 16.8 | 20.0 | 15.7 | 18.6 | 13.0 | 16.4 |
| Type, % | ||||||||||||||||||||||||||||||
| Antiepileptic | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 0.2 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| β-Blocker | 8.5 | 8.0 | 10.6 | 8.6 | 10.2 | 10.1 | 8.5 | 9.3 | 10.8 | 10.3 | 9.9 | 5.9 | 8.9 | 8.4 | 8.9 | 9.0 | 10.3 | 11.7 | 11.3 | 10.9 | 12.7 | 13.6 | 11.8 | 14.5 | 10.0 | 11.5 | 7.8 | 5.9 | 5.9 | 5.1 |
| Benzodiazepine | 2.0 | 2.5 | 3.0 | 4.7 | 3.7 | 3.3 | 3.4 | 3.9 | 3.2 | 3.4 | 1.2 | 0.7 | 2.3 | 3.7 | 3.4 | 3.8 | 4.6 | 3.8 | 4.6 | 5.6 | 1.8 | 1.7 | 2.5 | 1.2 | 2.7 | 4.1 | 3.7 | 3.9 | 5.0 | 5.9 |
| Gastroprotective drug | 4.3 | 4.0 | 5.0 | 5.6 | 5.9 | 9.7 | 4.7 | 6.5 | 6.4 | 6.7 | 0.8 | 0.7 | 2.6 | 5.3 | 5.0 | 6.6 | 8.4 | 9.3 | 12.1 | 13.5 | 3.4 | 4.5 | 3.8 | 3.0 | 4.9 | 6.1 | 5.9 | 8.8 | 8.9 | 11.0 |
| Glucocorticoids | 2.7 | 3.0 | 2.9 | 1.7 | 1.9 | 1.9 | 2.5 | 2.6 | 1.5 | 2.0 | 0.2 | 1.1 | 1.3 | 1.5 | 2.6 | 2.0 | 3.8 | 3.3 | 4.7 | 4.5 | 4.7 | 2.8 | 3.0 | 4.5 | 2.0 | 2.3 | 0.8 | 0.5 | 0.7 | 0.7 |
| Hormone therapy | 0.5 | 1.0 | 1.2 | 0.8 | 1.2 | 2.1 | 1.3 | 1.4 | 1.8 | 1.7 | 1.5 | 1.5 | 1.4 | 1.3 | 1.4 | 1.1 | 1.0 | 1.7 | 0.5 | 0.7 | 0.2 | 0.6 | 0.4 | 0.0 | 0.8 | 0.7 | 1.2 | 1.3 | 3.7 | 4.9 |
| Selective COX-2 inhibitor | 4.3 | 4.0 | 6.6 | 4.5 | 6.5 | 5.3 | 5.6 | 5.8 | 5.7 | 6.3 | 8.0 | 8.8 | 6.0 | 4.8 | 4.9 | 4.8 | 5.0 | 5.6 | 4.2 | 3.3 | 6.1 | 5.1 | 5.6 | 5.9 | 6.5 | 5.4 | 6.1 | 5.9 | 4.3 | 4.1 |
| Other NSAID | 2.4 | 1.5 | 2.9 | 1.9 | 3.0 | 3.5 | 2.8 | 2.1 | 1.9 | 2.7 | 2.9 | 2.6 | 2.5 | 2.4 | 2.5 | 2.5 | 2.4 | 1.8 | 3.0 | 3.5 | 2.4 | 4.0 | 3.1 | 1.2 | 2.8 | 3.2 | 2.7 | 2.7 | 2.1 | 2.6 |
| SSRI | 1.4 | 0.0 | 1.5 | 1.7 | 2.6 | 3.3 | 1.3 | 2.2 | 2.3 | 2.1 | 0.6 | 1.1 | 1.0 | 1.1 | 2.2 | 1.8 | 3.3 | 3.0 | 2.4 | 3.3 | 1.2 | 0.0 | 1.9 | 2.1 | 2.5 | 2.9 | 1.9 | 1.8 | 1.7 | 2.4 |
| Non-SSRI antipsychotic | 2.3 | 3.0 | 1.9 | 1.9 | 2.7 | 1.9 | 1.5 | 2.0 | 1.6 | 2.1 | 0.9 | 1.1 | 1.8 | 2.6 | 1.7 | 2.0 | 3.1 | 3.0 | 3.2 | 3.0 | 1.1 | 0.6 | 2.0 | 2.4 | 2.3 | 1.6 | 2.4 | 2.7 | 2.5 | 3.0 |
| Thiazide diuretic | 2.2 | 1.0 | 1.4 | 2.5 | 0.7 | 0.6 | 1.6 | 1.4 | 1.1 | 0.8 | 0.8 | 0.0 | 1.3 | 1.5 | 1.5 | 1.5 | 1.6 | 1.1 | 2.0 | 2.0 | 4.3 | 2.3 | 1.1 | 1.8 | 0.8 | 0.9 | 0.4 | 0.4 | 0.1 | 0.1 |
| Thyroid drug | 3.6 | 5.5 | 5.3 | 6.1 | 7.5 | 6.4 | 5.9 | 6.4 | 8.9 | 9.2 | 5.0 | 5.9 | 5.9 | 5.5 | 7.3 | 7.5 | 6.4 | 6.5 | 6.3 | 7.0 | 4.9 | 6.2 | 6.6 | 7.1 | 7.1 | 8.1 | 6.3 | 6.8 | 5.7 | 5.2 |
| Miscellaneous sleep agent, hypnotic agent, or barbiturate |
1.2 | 1.0 | 1.7 | 1.1 | 1.3 | 0.8 | 1.5 | 1.3 | 1.0 | 1.8 | 0.7 | 0.0 | 1.2 | 1.1 | 1.3 | 1.8 | 1.8 | 1.8 | 1.9 | 2.2 | 0.8 | 0.6 | 1.3 | 0.6 | 1.1 | 0.7 | 1.8 | 2.5 | 1.8 | 1.8 |
ALD = alendronate; CCT = calcitonin; COPD = chronic obstructive pulmonary disease; COX-2 = cyclooxygenase-2; NSAID = nonsteroidal anti-inflammatory drug; RAL = raloxifene; RSD = risedronate; SSRI = selective serotonin reuptake inhibitor.
Quartile 1 = score, 0; quartile 2 = score, 1; quartile 3 = score, 2; quartile 4 = score ≥3.
Appendix Table 3.
Propensity Score Quintiles: Pennsylvania*
| Covariate | Risedronate Propensity Score | Calcitonin Propensity Score | Raloxifene Propensity Score | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||||||||||||||||
| ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | ALD | CCT | ALD | CCT | ALD | CCT | ALD | CCT | ALD | CCT | ALD | RAL | ALD | RAL | ALD | RAL | ALD | RAL | ALD | RAL | |
| Mean age (SD), y | 79.8 (7.1) | 80.2 (6.9) | 78.8 (6.4) | 79.3 (6.5) | 79.1 (6.9) | 79.6 (7.2) | 78.4 (6.7) | 78.2 (6.8) | 77.9 (6.3) | 78.3 (6.4) | 74.5 (5.5) | 74.7 (5.5) | 77.4 (5.8) | 77.3 (5.8) | 79.0 (6.0) | 79.4 (6.2) | 80.9 (6.2) | 80.8 (6.3) | 83.8 (6.6) | 84.3 (6.6) | 82.1 (7.1) | 83.6 (6.4) | 80.4 (6.4) | 81.2 (6.0) | 79.2 (6.0) | 79.3 (6.0) | 77.3 (5.8) | 77.6 (6.0) | 74.7 (5.8) | 74.7 (6.0) |
| Men, % | 5.6 | 7.1 | 3.3 | 3.4 | 6.0 | 5.5 | 7.3 | 4.9 | 2.9 | 2.9 | 3.3 | 2.2 | 4.1 | 2.9 | 4.7 | 5.4 | 6.0 | 6.1 | 7.7 | 6.0 | 24.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| White, % | 94.0 | 93.4 | 96.6 | 96.1 | 92.9 | 94.5 | 92.9 | 93.9 | 96.3 | 97.1 | 89.9 | 94.8 | 94.3 | 95.6 | 95.9 | 95.0 | 96.1 | 96.2 | 97.8 | 98.1 | 92.7 | 93.9 | 93.7 | 94.0 | 95.1 | 93.9 | 95.3 | 96.8 | 96.5 | 96.9 |
| Year of index prescription, % | ||||||||||||||||||||||||||||||
| 2000 | 22.9 | 21.4 | 14.0 | 24.1 | 22.5 | 24.1 | 0.6 | 0.5 | 0.0 | 0.0 | 0.1 | 0.4 | 2.4 | 3.1 | 7.2 | 9.1 | 18.7 | 22.8 | 41.5 | 52.8 | 3.0 | 2.7 | 2.1 | 4.5 | 4.9 | 7.9 | 10.3 | 17.7 | 44.9 | 54.0 |
| 2001 | 42.1 | 47.9 | 70.5 | 62.2 | 9.9 | 6.1 | 0.0 | 0.0 | 0.0 | 0.0 | 7.9 | 8.9 | 25.3 | 20.8 | 35.5 | 28.7 | 36.2 | 30.1 | 27.7 | 20.5 | 14.8 | 15.0 | 26.7 | 23.7 | 34.1 | 27.5 | 35.6 | 31.6 | 19.4 | 11.5 |
| 2002 | 34.8 | 30.7 | 14.9 | 12.8 | 34.5 | 34.7 | 16.7 | 17.0 | 3.6 | 3.3 | 13.1 | 13.0 | 23.3 | 21.6 | 25.7 | 24.0 | 22.8 | 20.9 | 18.0 | 15.0 | 10.8 | 5.4 | 16.1 | 15.6 | 23.4 | 21.6 | 29.7 | 24.9 | 23.8 | 22.4 |
| 2003 | 0.1 | 0.0 | 0.3 | 0.5 | 18.7 | 21.4 | 30.6 | 33.5 | 29.3 | 30.6 | 20.8 | 21.9 | 19.4 | 22.0 | 14.5 | 20.0 | 11.4 | 12.6 | 6.5 | 6.5 | 10.9 | 9.5 | 15.5 | 18.0 | 20.8 | 24.3 | 17.2 | 18.2 | 9.9 | 10.5 |
| 2004 | 0.0 | 0.0 | 0.2 | 0.2 | 6.8 | 7.5 | 26.8 | 26.1 | 48.6 | 49.2 | 35.3 | 31.9 | 17.8 | 21.6 | 10.6 | 11.9 | 6.8 | 8.8 | 4.2 | 3.8 | 31.1 | 36.1 | 25.2 | 25.2 | 12.5 | 14.6 | 6.5 | 6.5 | 1.7 | 1.7 |
| 2005 | 0.0 | 0.0 | 0.1 | 0.3 | 7.6 | 6.4 | 25.4 | 23.0 | 18.5 | 16.9 | 22.8 | 24.1 | 11.8 | 11.0 | 6.5 | 6.3 | 4.0 | 4.7 | 2.0 | 1.4 | 29.4 | 31.3 | 14.4 | 12.9 | 4.4 | 4.1 | 0.8 | 1.0 | 0.1 | 0.0 |
| Age group, % | ||||||||||||||||||||||||||||||
| 65–69 y | 9.1 | 6.8 | 8.0 | 8.1 | 10.6 | 10.8 | 11.2 | 12.0 | 10.3 | 8.9 | 21.6 | 19.3 | 10.9 | 10.6 | 7.3 | 7.0 | 4.7 | 5.0 | 1.9 | 2.1 | 4.8 | 0.7 | 5.0 | 3.0 | 6.9 | 5.6 | 10.6 | 10.4 | 23.3 | 24.8 |
| 70–74 y | 15.2 | 15.6 | 18.8 | 17.8 | 17.1 | 15.3 | 19.8 | 19.6 | 19.7 | 19.5 | 29.4 | 30.7 | 20.8 | 22.0 | 17.4 | 16.9 | 11.9 | 12.3 | 7.9 | 6.3 | 11.0 | 7.5 | 14.9 | 12.0 | 15.5 | 17.3 | 22.8 | 20.6 | 27.4 | 26.8 |
| 75–79 y | 23.0 | 22.2 | 27.5 | 22.8 | 24.4 | 23.8 | 23.7 | 24.5 | 29.8 | 29.0 | 30.7 | 29.3 | 30.9 | 31.8 | 26.3 | 24.3 | 21.6 | 22.6 | 16.0 | 14.2 | 21.3 | 21.8 | 24.6 | 23.7 | 28.5 | 27.5 | 28.7 | 29.7 | 25.1 | 22.6 |
| 80–84 y | 24.6 | 27.9 | 26.7 | 28.9 | 24.1 | 23.3 | 24.4 | 24.3 | 25.7 | 25.4 | 14.2 | 18.5 | 26.3 | 22.5 | 29.1 | 30.7 | 30.6 | 30.2 | 25.6 | 25.2 | 23.3 | 21.8 | 27.1 | 29.1 | 28.8 | 30.0 | 26.3 | 25.9 | 19.3 | 20.3 |
| 85–89 y | 18.8 | 18.4 | 15.0 | 17.6 | 16.6 | 17.8 | 16.5 | 14.9 | 12.5 | 14.4 | 4.0 | 2.2 | 10.4 | 12.7 | 17.1 | 16.6 | 24.3 | 23.0 | 27.6 | 28.6 | 24.6 | 30.6 | 21.2 | 24.9 | 17.3 | 16.0 | 10.6 | 10.7 | 4.5 | 5.1 |
| ≥90 y | 2.7 | 2.7 | 1.0 | 1.5 | 2.7 | 3.0 | 1.7 | 1.2 | 0.2 | 0.5 | 0.1 | 0.0 | 0.3 | 0.4 | 1.3 | 1.8 | 2.7 | 2.1 | 4.9 | 5.4 | 3.6 | 4.8 | 3.1 | 2.1 | 1.0 | 1.6 | 0.4 | 1.0 | 0.0 | 0.0 |
| Hospitalized in previous year, % | 36.0 | 42.5 | 17.9 | 18.4 | 31.7 | 33.1 | 21.4 | 23.2 | 11.2 | 9.4 | 5.1 | 5.6 | 11.5 | 11.0 | 21.3 | 21.2 | 35.6 | 36.9 | 54.0 | 55.9 | 40.3 | 40.1 | 28.7 | 33.0 | 21.7 | 23.2 | 15.5 | 19.5 | 10.2 | 8.5 |
| Comorbidity score quartile†, % | ||||||||||||||||||||||||||||||
| 1 | 30.6 | 26.6 | 31.5 | 30.2 | 30.1 | 27.3 | 39.8 | 38.5 | 31.0 | 32.4 | 49.1 | 49.6 | 42.5 | 41.0 | 30.8 | 29.5 | 22.4 | 23.0 | 11.5 | 10.9 | 15.3 | 17.7 | 26.6 | 23.4 | 29.5 | 30.2 | 42.1 | 39.0 | 51.8 | 50.4 |
| 2 | 22.3 | 23.8 | 25.5 | 25.4 | 21.7 | 21.0 | 21.2 | 21.5 | 22.9 | 21.2 | 25.1 | 25.9 | 23.8 | 22.0 | 24.3 | 21.6 | 20.9 | 21.9 | 18.6 | 18.5 | 18.1 | 13.6 | 21.2 | 22.8 | 24.8 | 25.5 | 25.2 | 25.4 | 24.8 | 24.8 |
| 3 | 16.4 | 14.8 | 20.4 | 21.2 | 18.0 | 18.7 | 14.5 | 15.2 | 23.1 | 22.7 | 16.0 | 14.4 | 17.2 | 17.9 | 19.5 | 20.3 | 19.9 | 19.6 | 20.4 | 20.4 | 22.4 | 23.8 | 21.1 | 19.2 | 19.3 | 18.7 | 16.4 | 16.1 | 12.6 | 13.8 |
| 4 | 19.6 | 21.1 | 10.9 | 11.0 | 18.1 | 20.6 | 14.2 | 14.2 | 10.2 | 10.4 | 4.0 | 4.4 | 7.8 | 8.7 | 13.5 | 14.6 | 21.5 | 21.1 | 30.9 | 32.2 | 26.0 | 23.1 | 17.2 | 20.7 | 14.8 | 14.9 | 8.3 | 10.9 | 5.3 | 4.9 |
| Osteoporosis-related variables, % | ||||||||||||||||||||||||||||||
| Osteoporosis | 40.2 | 37.8 | 60.4 | 53.3 | 50.8 | 49.8 | 41.1 | 43.7 | 67.5 | 65.8 | 64.3 | 60.4 | 54.7 | 54.5 | 49.6 | 48.4 | 46.3 | 45.5 | 42.3 | 41.5 | 60.6 | 64.6 | 56.5 | 58.6 | 55.9 | 61.9 | 46.6 | 42.8 | 39.0 | 34.2 |
| Kyphosis | 2.1 | 2.2 | 2.1 | 3.2 | 2.5 | 2.9 | 2.6 | 2.9 | 3.2 | 2.9 | 1.5 | 2.6 | 1.3 | 2.5 | 2.1 | 2.8 | 3.3 | 3.0 | 5.0 | 5.2 | 4.5 | 2.7 | 3.2 | 3.9 | 1.9 | 3.4 | 1.6 | 1.8 | 1.1 | 0.8 |
| Previous fracture | ||||||||||||||||||||||||||||||
| Vertebral | 13.3 | 15.1 | 4.4 | 6.1 | 10.9 | 12.1 | 5.4 | 6.2 | 2.1 | 2.2 | 0.3 | 0.0 | 0.9 | 1.2 | 3.8 | 4.8 | 10.0 | 12.8 | 27.2 | 28.6 | 16.7 | 23.8 | 9.6 | 9.9 | 5.1 | 7.7 | 2.8 | 2.9 | 1.1 | 0.6 |
| Nonvertebral (hip, humerus, radius or ulna) | 9.6 | 10.7 | 4.4 | 4.4 | 8.7 | 8.1 | 5.8 | 4.7 | 2.3 | 2.3 | 3.2 | 1.9 | 4.8 | 4.0 | 5.8 | 5.1 | 8.3 | 8.1 | 10.0 | 9.3 | 10.9 | 15.0 | 9.7 | 6.6 | 5.1 | 5.4 | 3.1 | 2.9 | 1.5 | 1.3 |
| Other | 5.0 | 4.1 | 5.4 | 5.3 | 6.2 | 5.3 | 4.9 | 5.4 | 5.5 | 5.7 | 3.5 | 1.9 | 4.7 | 5.4 | 5.6 | 5.0 | 7.0 | 6.6 | 6.7 | 6.8 | 8.0 | 7.5 | 7.9 | 9.3 | 5.9 | 4.5 | 2.4 | 3.4 | 2.4 | 2.0 |
| Comorbid conditions, % | ||||||||||||||||||||||||||||||
| Alzheimer disease or other dementia | 7.8 | 9.3 | 5.2 | 7.6 | 6.4 | 8.3 | 5.9 | 4.9 | 5.0 | 5.8 | 1.2 | 1.9 | 3.2 | 1.7 | 4.7 | 6.7 | 9.1 | 9.6 | 14.8 | 16.4 | 11.0 | 8.8 | 8.0 | 13.2 | 5.1 | 5.6 | 3.6 | 4.0 | 2.1 | 1.8 |
| Asthma or COPD | 19.7 | 26.0 | 20.3 | 19.9 | 21.0 | 21.6 | 16.9 | 18.4 | 19.5 | 18.6 | 11.6 | 11.9 | 14.2 | 14.5 | 19.6 | 21.2 | 25.7 | 25.7 | 29.8 | 31.0 | 28.8 | 23.8 | 19.8 | 23.1 | 20.0 | 18.9 | 15.5 | 18.7 | 12.5 | 12.3 |
| Cataracts | 35.1 | 34.8 | 36.2 | 36.0 | 35.1 | 35.5 | 32.6 | 32.1 | 34.1 | 32.3 | 34.3 | 29.6 | 34.5 | 34.5 | 35.6 | 35.1 | 34.3 | 34.0 | 34.5 | 33.9 | 35.6 | 25.9 | 34.4 | 35.4 | 35.5 | 35.4 | 35.6 | 33.7 | 31.7 | 35.1 |
| Crohn disease or gastroenteritis | 5.3 | 5.2 | 3.6 | 7.3 | 4.1 | 4.6 | 3.4 | 3.7 | 3.4 | 2.5 | 1.7 | 1.5 | 2.1 | 3.1 | 3.7 | 4.5 | 5.7 | 6.3 | 7.8 | 7.8 | 3.5 | 4.1 | 4.5 | 4.5 | 3.5 | 6.3 | 4.2 | 4.5 | 4.1 | 3.7 |
| Depression | 10.9 | 14.0 | 9.3 | 9.0 | 11.2 | 11.0 | 8.2 | 9.7 | 11.1 | 12.1 | 4.4 | 5.2 | 6.4 | 6.9 | 10.1 | 9.9 | 13.2 | 14.5 | 19.2 | 20.3 | 11.7 | 17.0 | 10.5 | 13.8 | 10.5 | 12.4 | 10.0 | 8.5 | 7.4 | 8.0 |
| Diabetes mellitus | 20.6 | 22.7 | 21.9 | 23.9 | 22.7 | 24.8 | 20.7 | 22.2 | 26.4 | 24.5 | 17.8 | 18.5 | 19.9 | 21.4 | 22.5 | 21.9 | 25.5 | 25.5 | 27.9 | 28.7 | 27.1 | 25.9 | 22.6 | 26.4 | 23.5 | 22.1 | 20.6 | 20.9 | 17.4 | 19.3 |
| History of falls, syncope, or gait abnormality | 22.4 | 24.4 | 13.9 | 15.3 | 23.3 | 24.1 | 15.9 | 16.2 | 11.8 | 11.0 | 6.5 | 4.8 | 11.0 | 11.8 | 15.9 | 15.2 | 24.4 | 26.2 | 34.5 | 35.0 | 31.3 | 34.0 | 22.4 | 24.0 | 15.8 | 18.2 | 10.2 | 10.5 | 6.2 | 5.1 |
| Hyperthyroidism | 2.9 | 2.2 | 3.6 | 3.7 | 2.9 | 3.7 | 3.1 | 2.1 | 3.8 | 4.5 | 2.1 | 2.2 | 3.4 | 2.5 | 3.3 | 3.0 | 3.8 | 3.8 | 3.9 | 4.7 | 4.8 | 5.4 | 4.7 | 3.0 | 2.8 | 2.9 | 2.4 | 3.2 | 1.5 | 1.6 |
| Hyperparathyroidism | 0.3 | 0.3 | 0.7 | 0.8 | 1.5 | 0.6 | 0.5 | 0.6 | 1.9 | 2.5 | 0.8 | 1.5 | 1.3 | 0.8 | 0.7 | 1.4 | 0.7 | 0.7 | 1.3 | 0.8 | 2.0 | 2.0 | 1.1 | 1.2 | 0.9 | 0.7 | 0.5 | 0.2 | 0.2 | 0.5 |
| Ischemic stroke | 8.1 | 8.2 | 4.0 | 5.8 | 7.4 | 7.4 | 5.1 | 4.5 | 3.5 | 2.8 | 3.1 | 2.6 | 3.7 | 3.5 | 5.2 | 6.4 | 6.7 | 7.2 | 10.8 | 9.7 | 10.2 | 8.8 | 6.7 | 7.2 | 5.1 | 5.2 | 3.4 | 4.6 | 2.1 | 1.3 |
| Liver disease | 2.3 | 1.6 | 1.8 | 1.9 | 2.4 | 1.8 | 2.0 | 2.3 | 2.5 | 3.7 | 2.0 | 1.1 | 1.5 | 3.3 | 2.3 | 2.7 | 2.6 | 2.5 | 2.9 | 2.4 | 2.0 | 5.4 | 1.6 | 3.0 | 2.2 | 1.8 | 2.2 | 2.4 | 3.2 | 2.7 |
| Malignant neoplasm | 13.8 | 13.7 | 15.1 | 12.9 | 14.5 | 15.2 | 12.6 | 12.5 | 16.7 | 18.0 | 11.1 | 13.0 | 12.9 | 12.5 | 15.3 | 16.1 | 16.7 | 14.6 | 17.4 | 18.2 | 21.1 | 25.2 | 16.1 | 15.3 | 15.3 | 12.4 | 11.1 | 10.7 | 8.1 | 9.5 |
| Overweight or obese | 2.5 | 3.0 | 1.6 | 1.0 | 3.0 | 3.9 | 2.7 | 2.6 | 1.7 | 1.4 | 1.0 | 0.0 | 2.1 | 1.7 | 2.5 | 2.1 | 2.7 | 3.6 | 3.3 | 3.2 | 2.7 | 6.8 | 3.2 | 3.6 | 2.2 | 2.3 | 1.8 | 1.3 | 1.4 | 1.2 |
| Parkinson disease | 1.5 | 3.0 | 1.2 | 1.6 | 2.0 | 1.6 | 1.2 | 1.6 | 1.6 | 1.5 | 0.4 | 0.7 | 1.0 | 0.6 | 1.0 | 1.4 | 1.7 | 2.0 | 4.2 | 4.8 | 2.5 | 2.0 | 1.8 | 3.0 | 1.5 | 1.1 | 0.8 | 1.4 | 0.6 | 0.7 |
| Renal disease | 1.5 | 1.9 | 1.0 | 1.6 | 2.1 | 1.5 | 1.6 | 1.7 | 0.7 | 1.0 | 0.4 | 0.4 | 0.5 | 2.1 | 1.1 | 0.9 | 1.9 | 1.6 | 3.6 | 3.5 | 2.5 | 2.7 | 1.2 | 1.2 | 1.1 | 1.6 | 1.1 | 1.0 | 0.8 | 0.9 |
| Rheumatoid arthritis | 5.0 | 3.0 | 5.8 | 6.5 | 5.5 | 4.3 | 4.4 | 3.8 | 5.5 | 6.4 | 3.8 | 2.6 | 5.1 | 4.6 | 5.0 | 5.0 | 6.5 | 5.5 | 6.0 | 6.1 | 9.8 | 7.5 | 7.4 | 6.9 | 4.6 | 3.4 | 2.4 | 2.7 | 1.6 | 2.3 |
| Drug use | ||||||||||||||||||||||||||||||
| Quintile of number of generic drugs used, % | ||||||||||||||||||||||||||||||
| 1 | 14.3 | 12.6 | 21.1 | 17.9 | 17.1 | 15.1 | 16.1 | 15.5 | 23.1 | 23.7 | 33.8 | 32.2 | 21.9 | 20.2 | 14.2 | 15.1 | 11.6 | 9.9 | 7.0 | 5.9 | 13.4 | 16.3 | 17.3 | 14.7 | 18.9 | 16.2 | 20.1 | 20.3 | 22.5 | 20.9 |
| 2 | 27.3 | 23.6 | 25.6 | 26.0 | 26.8 | 24.6 | 29.8 | 28.6 | 23.7 | 21.9 | 33.8 | 29.6 | 31.8 | 27.0 | 25.9 | 26.0 | 21.5 | 21.7 | 17.1 | 16.9 | 24.6 | 23.8 | 26.6 | 22.8 | 28.0 | 24.3 | 26.3 | 24.9 | 28.0 | 28.5 |
| 3 | 16.5 | 18.1 | 16.4 | 16.5 | 14.6 | 14.3 | 15.2 | 15.0 | 15.0 | 15.7 | 13.9 | 14.1 | 15.1 | 17.5 | 19.4 | 16.5 | 15.0 | 16.2 | 13.8 | 12.9 | 13.8 | 8.8 | 14.2 | 15.6 | 15.8 | 17.1 | 15.9 | 16.0 | 18.6 | 17.5 |
| 4 | 21.6 | 23.0 | 21.5 | 23.3 | 21.8 | 22.7 | 21.7 | 22.7 | 22.3 | 22.0 | 14.3 | 18.9 | 20.2 | 23.3 | 23.1 | 23.7 | 25.7 | 25.0 | 27.2 | 26.2 | 23.6 | 21.1 | 20.9 | 23.4 | 21.1 | 22.1 | 22.5 | 23.5 | 20.6 | 21.4 |
| 5 | 20.3 | 22.7 | 15.5 | 16.3 | 19.6 | 23.3 | 17.2 | 18.2 | 15.8 | 16.7 | 4.2 | 5.2 | 11.0 | 11.9 | 17.3 | 18.8 | 26.3 | 27.2 | 34.9 | 38.1 | 24.6 | 29.9 | 21.0 | 23.4 | 16.3 | 20.3 | 15.2 | 15.3 | 10.2 | 11.6 |
| Type, % | ||||||||||||||||||||||||||||||
| Antiepileptic | 0.2 | 0.0 | 0.2 | 0.3 | 0.3 | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.0 | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 0.3 | 0.0 | 0.1 | 0.3 |
| β-Blocker | 17.3 | 14.5 | 13.3 | 12.4 | 15.7 | 16.2 | 17.7 | 16.7 | 10.6 | 10.5 | 13.1 | 13.3 | 14.8 | 12.9 | 16.7 | 12.5 | 15.5 | 14.5 | 14.3 | 16.3 | 16.8 | 12.9 | 16.6 | 18.0 | 16.0 | 14.2 | 13.6 | 13.9 | 11.1 | 9.9 |
| Benzodiazepine | 6.2 | 8.2 | 4.1 | 5.7 | 4.8 | 4.4 | 4.4 | 4.3 | 3.3 | 2.3 | 4.0 | 3.3 | 4.7 | 4.6 | 4.2 | 5.5 | 5.1 | 4.6 | 5.1 | 5.0 | 3.6 | 4.8 | 5.0 | 5.7 | 4.2 | 4.3 | 5.2 | 5.8 | 4.8 | 3.7 |
| Gastroprotective drug | 6.7 | 6.3 | 6.4 | 6.5 | 6.1 | 8.1 | 5.2 | 6.2 | 6.8 | 8.4 | 2.8 | 7.0 | 4.7 | 6.9 | 6.5 | 7.2 | 8.0 | 8.2 | 10.5 | 11.1 | 4.0 | 2.7 | 3.9 | 3.9 | 5.7 | 9.0 | 6.9 | 8.0 | 11.2 | 12.4 |
| Glucocorticoids | 2.5 | 2.5 | 2.0 | 1.1 | 1.9 | 2.2 | 2.0 | 2.0 | 1.6 | 1.4 | 2.2 | 1.5 | 2.1 | 1.7 | 2.3 | 2.3 | 1.3 | 2.3 | 2.0 | 1.5 | 3.4 | 2.7 | 2.5 | 2.4 | 1.6 | 0.9 | 1.4 | 1.1 | 1.0 | 1.3 |
| Hormone therapy | 2.9 | 1.6 | 1.8 | 2.1 | 3.3 | 3.1 | 1.5 | 2.2 | 0.6 | 0.5 | 4.5 | 5.2 | 2.1 | 2.3 | 1.3 | 1.1 | 1.0 | 1.0 | 0.6 | 0.4 | 0.1 | 0.0 | 0.1 | 0.0 | 0.6 | 0.7 | 1.1 | 0.8 | 9.0 | 11.0 |
| Selective COX-2 inhibitor | 4.6 | 3.8 | 5.4 | 5.2 | 4.2 | 4.0 | 3.7 | 3.6 | 4.3 | 4.2 | 4.9 | 3.7 | 4.5 | 5.4 | 4.2 | 4.1 | 4.4 | 4.1 | 4.2 | 3.5 | 5.3 | 3.4 | 4.3 | 4.2 | 4.8 | 3.6 | 4.9 | 4.2 | 2.7 | 3.5 |
| Other NSAID | 3.3 | 4.7 | 2.1 | 1.8 | 2.9 | 2.4 | 1.7 | 1.7 | 1.1 | 0.7 | 2.6 | 2.2 | 2.4 | 2.1 | 1.8 | 1.8 | 2.1 | 2.0 | 2.1 | 2.2 | 0.9 | 0.0 | 1.7 | 2.1 | 2.3 | 0.9 | 2.4 | 2.2 | 4.0 | 4.4 |
| SSRI | 1.9 | 1.9 | 2.4 | 1.9 | 2.1 | 2.4 | 2.0 | 1.7 | 3.7 | 4.5 | 1.8 | 1.1 | 1.7 | 2.1 | 2.3 | 2.7 | 2.9 | 3.3 | 3.7 | 3.5 | 3.9 | 5.4 | 3.1 | 2.4 | 2.1 | 2.3 | 1.5 | 2.6 | 1.4 | 0.8 |
| Non-SSRI antipsychotic | 3.5 | 3.8 | 3.1 | 3.1 | 3.2 | 3.4 | 3.3 | 3.7 | 2.5 | 2.5 | 2.2 | 1.9 | 2.8 | 2.3 | 3.2 | 2.4 | 3.6 | 4.3 | 3.9 | 5.0 | 1.8 | 3.4 | 2.6 | 3.0 | 2.6 | 3.6 | 3.9 | 3.8 | 4.8 | 4.8 |
| Thiazide diuretic | 1.1 | 0.8 | 0.8 | 2.1 | 1.0 | 0.5 | 1.7 | 1.1 | 1.3 | 1.6 | 1.7 | 1.9 | 1.3 | 1.5 | 1.0 | 0.9 | 1.1 | 1.0 | 0.5 | 0.6 | 1.3 | 2.0 | 1.3 | 0.6 | 1.0 | 0.7 | 1.6 | 1.4 | 0.6 | 1.1 |
| Thyroid drug | 8.9 | 10.4 | 12.1 | 12.3 | 10.3 | 11.3 | 10.6 | 10.4 | 14.8 | 15.3 | 10.6 | 11.5 | 11.5 | 11.6 | 11.4 | 14.2 | 11.9 | 11.3 | 11.2 | 11.1 | 10.7 | 15.0 | 11.2 | 11.7 | 12.3 | 11.7 | 11.8 | 12.5 | 10.4 | 9.5 |
| Miscellaneous sleep agent, hypnotic agent, or barbiturate |
1.6 | 1.1 | 1.0 | 1.5 | 1.5 | 0.7 | 1.1 | 1.1 | 0.7 | 0.9 | 0.7 | 1.1 | 0.7 | 1.0 | 1.2 | 0.7 | 1.4 | 1.5 | 2.1 | 1.8 | 2.4 | 2.0 | 1.7 | 1.2 | 0.9 | 1.6 | 0.5 | 0.3 | 0.3 | 0.3 |
ALD 5 alendronate; CCT 5 calcitonin; COPD 5 chronic obstructive pulmonary disease; COX-2 5 cyclooxygenase-2; NSAID 5 nonsteroidal anti-inflammatory drug; RAL 5 raloxifene; RSD 5 risedronate; SSRI 5 selective serotonin reuptake inhibitor.
Quartile 1 5 score, 0; quartile 2 5 score, 1; quartile 3 5 score, 2; quartile 4 5 score ≥3.
We used sensitivity analysis to examine the robustness of our findings. First, we examined outcomes assuming an “on-treatment” scenario. We censored patients on the first day of switching agents, losing drug plan eligibility, entering a nursing home, or discontinuing drug therapy (last date covered by drug plus 15 days, allowing for 30-day gaps between prescriptions), on the day of death, or at the end of follow-up (12 months, 31 December 2003 [New Jersey], or 31 December 2005 [Pennsylvania]). Second, we extended the days that patients received therapy in our on-treatment scenario to the last date covered by drug plus 90 days. Third, we examined several different subgroups: history of any fracture (International Classification of Diseases, Ninth Revision, Clinical Modification codes 733.1x and 800.xx through 829.xx) within the year before treatment initiation, no fracture history, at least 2 consecutive prescriptions of their index drug, no known history of malignant neoplasm, osteoporosis diagnosis, no diagnosis of osteoporosis, and women with no previous hormone therapy. Fourth, given that the main risk factors for fracture measurable in our data set are previous fracture and age, we compared fracture rates stratified by fracture risk group (defined by fracture history and median age of our study cohort). Finally, we assessed the extent of unmeasured confounding required to explain our primary study findings of difference in fracture risk between drug exposures by using the rule-out method (25). In applying the rule-out method (Microsoft Excel [Microsoft, Redmond, Washington] file, available at www.drugepi.org [25]), we allowed the relationship between the possible unmeasured confounder and fracture risk (relative risk) to vary from 1 to 10 and the prevalence of this possible unmeasured confounder to vary from 10% to 50% (25).
The Partners HealthCare Institutional Review Board approved this project. Data use agreements are in place from the Centers for Medicare & Medicaid Services, New Jersey Pharmaceutical Assistance to the Aged and Disabled program, and Pennsylvania Pharmaceutical Assistance Contract for the Elderly.
Role of the Funding Source
This study had no external funding source. The funding organizations supporting authors did not participate in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Results
Study Cohort
We identified 48 865 new recipients of alendronate, nasal calcitonin, raloxifene, and risedronate from 1 April 2000 to 30 June 2003 (New Jersey) and 30 June 2005 (Pennsylvania). After excluding 5730 of these 48 865 new recipients, the final study cohort included 43 135 patients (Figure 1). Table 1 shows cohort characteristics, stratified by agent received. The mean age of our cohort was 78.7 years (SD, 6.9; median, 79 years), and 96% were women. Overall, alendronate and risedronate recipients were similar in age, osteoporosis diagnosis, and comorbid condition; calcitonin recipients were older (mean age 80.7 years; SD, 7.1) and had a higher prevalence of vertebral fractures and comorbid conditions; and raloxifene recipients were younger (mean age 76.9 years; SD, 6.7) and had a lower prevalence of fractures and comorbid conditions. However, both calcitonin and raloxifene recipients had a lower prevalence of osteoporosis documented in Medicare claims in the year before treatment initiation than alendronate or risedronate recipients.
Table 1.
Cohort Characteristics*
| Characteristic | Alendronate Recipients (n = 21 007) |
Risedronate Recipients (n = 8718) |
Calcitonin Recipients (n = 8372) |
Raloxifene Recipients (n = 5038) |
|---|---|---|---|---|
| Mean age (SD), y | 78.4 (6.7) | 78.5 (6.7) | 80.7 (7.1) | 76.9 (6.7) |
| Mean generic drugs used (SD), n | 8.1 (5.8) | 8.9 (5.7) | 10.2 (6.6) | 8.1 (6.0) |
| Mean comorbidity score (SD) | 1.7 (1.8) | 1.7 (1.8) | 2.3 (2.1) | 1.5 (1.7) |
| Median physician visits (25th, 75th percentile), n | 8 (5, 13) | 8 (5, 13) | 9 (5, 14) | 8 (5, 12) |
| Hospitalized in the previous year, % | 21.9 | 20.4 | 34.6 | 17.5 |
| White, % | 92.1 | 92.9 | 93.9 | 91.8 |
| Osteoporosis-related variables, % | ||||
| Osteoporosis | 57.5 | 57.0 | 49.2 | 50.4 |
| Previous fracture | ||||
| Vertebral | 6.6 | 5.6 | 13.7 | 3.9 |
| Nonvertebral (hip, humerus, radius or ulna) | 5.7 | 4.4 | 6.4 | 3.8 |
| Other | 5.5 | 5.6 | 6.1 | 4.3 |
| Comorbid conditions, % | ||||
| Alzheimer disease or other dementia | 5.9 | 6.0 | 10.0 | 4.9 |
| Asthma or chronic obstructive pulmonary disease | 20.3 | 19.9 | 25.3 | 18.0 |
| Cataracts | 36.3 | 34.8 | 35.6 | 36.9 |
| Crohn disease or gastroenteritis | 4.5 | 4.8 | 6.5 | 5.2 |
| Depression | 9.4 | 10.5 | 13.9 | 10.1 |
| Diabetes mellitus | 23.0 | 24.9 | 26.4 | 23.4 |
| History of falls, syncope, or gait abnormality | 16.4 | 16.0 | 23.7 | 13.2 |
| Hyperthyroidism | 3.6 | 3.4 | 3.6 | 3.0 |
| Hyperparathyroidism | 1.0 | 1.4 | 1.0 | 0.7 |
| Ischemic stroke | 5.4 | 5.0 | 7.4 | 4.2 |
| Liver disease | 3.3 | 3.5 | 4.0 | 3.5 |
| Malignant neoplasm | 15.9 | 16.2 | 16.9 | 13.7 |
| Overweight or obese | 2.5 | 2.5 | 2.9 | 2.7 |
| Parkinson disease | 1.4 | 1.6 | 2.4 | 1.3 |
| Renal disease | 1.3 | 1.4 | 2.5 | 1.1 |
| Rheumatoid arthritis | 6.0 | 6.0 | 6.6 | 4.8 |
| Medication use, % | ||||
| Antiepileptic | 0.2 | 0.2 | 0.1 | 0.1 |
| β-Blocker | 12.5 | 12.3 | 12.7 | 10.7 |
| Benzodiazepine | 3.9 | 3.9 | 4.6 | 4.4 |
| Gastroprotective agents | 5.8 | 7.2 | 9.2 | 8.6 |
| Glucocorticoids | 2.1 | 1.9 | 2.4 | 1.5 |
| Hormone therapy | 1.6 | 1.7 | 1.2 | 3.6 |
| Selective COX-2 inhibitor | 5.1 | 4.6 | 4.3 | 4.4 |
| Other NSAID | 2.4 | 2.0 | 2.4 | 2.7 |
| SSRI | 2.1 | 2.6 | 2.8 | 2.0 |
| Non-SSRI antipsychotic | 2.6 | 2.8 | 3.4 | 3.3 |
| Thiazide diuretic | 1.3 | 1.2 | 1.2 | 0.9 |
| Thyroid drug | 8.9 | 10.7 | 9.5 | 8.9 |
| Miscellaneous sleep agent, hypnotic, or barbiturate | 1.2 | 1.1 | 1.6 | 1.1 |
Characteristics identified by Medicare and pharmacy benefit claims within the 12 mo before treatment initiation.
COX-2 = cyclooxygenase-2; NSAID = nonsteroidal anti-inflammatory drug; SSRI = selective serotonin reuptake inhibitor.
Comparative Fracture Risk
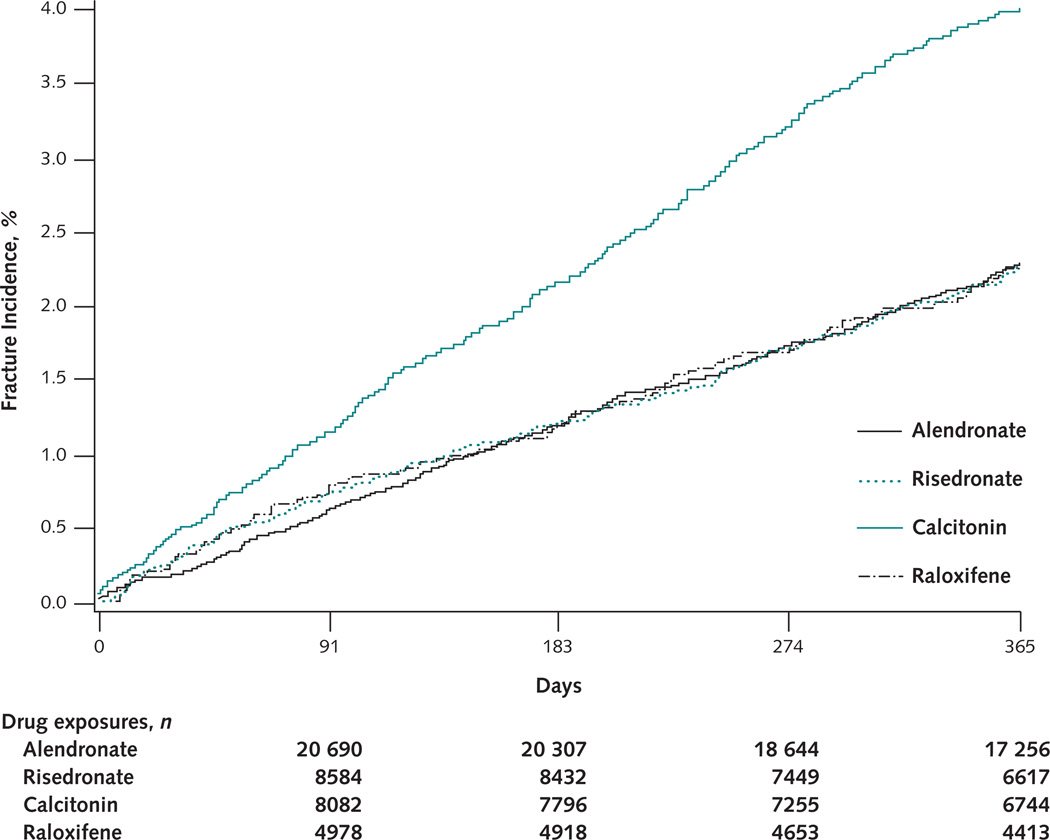
The cumulative fracture incidence for alendronate, risedronate, and raloxifene overlapped during 12 months of therapy (Figure 2). However, the cumulative fracture incidence was higher among calcitonin recipients from treatment initiation.
Figure 2. Cumulative incidence of nonvertebral fractures within 12 months of treatment initiation, by drug.
In our primary adjusted analysis, we found no large difference in nonvertebral fracture risk within 12 months between risedronate (adjusted hazard ratio [HR], 1.01 [95% CI, 0.85 to 1.21]) or raloxifene (HR, 1.18 [CI, 0.96 to 1.46]) and alendronate (Table 2). However, calcitonin recipients experienced more nonvertebral fractures than did alendronate recipients (HR, 1.40 [CI, 1.20 to 1.63]). Results were similar in secondary analyses that adjusted only for propensity score quintiles, without additional covariates (data not shown).
Table 2.
Nonvertebral Fracture Rates and Relative Effectiveness of Agents in Reducing Fracture Risk Compared with Alendronate
| Time Point and Agent | Participants with Fracture, n |
Fracture Rate per 100 Person-Years of Follow-up* |
Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| Unadjusted | P Value | Adjusted† | P Value | |||
| 6 mo | ||||||
| Alendronate | 240 | 2.36 | 1.00 (reference) | – | 1.00 (reference) | – |
| Risedronate | 103 | 2.44 | 1.03 (0.82–1.30) | 0.78 | 1.07 (0.85–1.36) | 0.56 |
| Calcitonin | 169 | 4.24 | 1.80 (1.48–2.19) | <0.001 | 1.42 (1.16–1.74) | <0.001 |
| Raloxifene | 59 | 2.40 | 1.02 (0.77–1.36) | 0.89 | 1.18 (0.88–1.58) | 0.26 |
| 12 mo | ||||||
| Alendronate | 448 | 2.28 | 1.00 (reference) | – | 1.00 (reference) | – |
| Risedronate | 183 | 2.28 | 1.00 (0.84–1.19) | 1.00 | 1.01 (0.85–1.21) | 0.88 |
| Calcitonin | 309 | 4.03 | 1.77 (1.53–2.05) | <0.001 | 1.40 (1.20–1.63) | <0.001 |
| Raloxifene | 111 | 2.31 | 1.01 (0.82–1.25) | 0.90 | 1.18 (0.96–1.46) | 0.121 |
| 24 mo | ||||||
| Alendronate | 814 | 2.39 | 1.00 (reference) | – | 1.00 (reference) | – |
| Risedronate | 300 | 2.30 | 0.96 (0.84–1.09) | 0.52 | 0.96 (0.84–1.11) | 0.56 |
| Calcitonin | 524 | 3.90 | 1.63 (1.46–1.82) | <0.001 | 1.28 (1.14–1.43) | <0.001 |
| Raloxifene | 182 | 2.10 | 0.88 (0.75–1.03) | 0.112 | 1.00 (0.85–1.18) | 1.00 |
Patients were censored on the date of death or end of follow-up (6, 12, or 24 months; 31 December 2003 [New Jersey]; or 31 December 2005 [Pennsylvania]).
Adjusted for propensity score quintiles as 12 dummy variables (4 for each drug) in Cox proportional hazard models and covariates (age, race, diagnosis of osteoporosis, previous vertebral fracture, and previous nonvertebral fracture), stratified by state.
Results were consistent when nonvertebral fracture rates at 6 and 24 months were compared (Table 2), as well as when hip fracture rates at 6, 12, and 24 months were compared. With 498 hip fractures within 12 months, we found no large difference in fracture risk between risedronate (HR, 0.92 [CI, 0.70 to 1.20]) or raloxifene (HR, 1.07 [CI, 0.77 to 1.49]) and alendronate. Patients who received calcitonin experienced more hip fractures than patients who received alendronate (HR, 1.54 [CI, 1.25 to 1.90]).
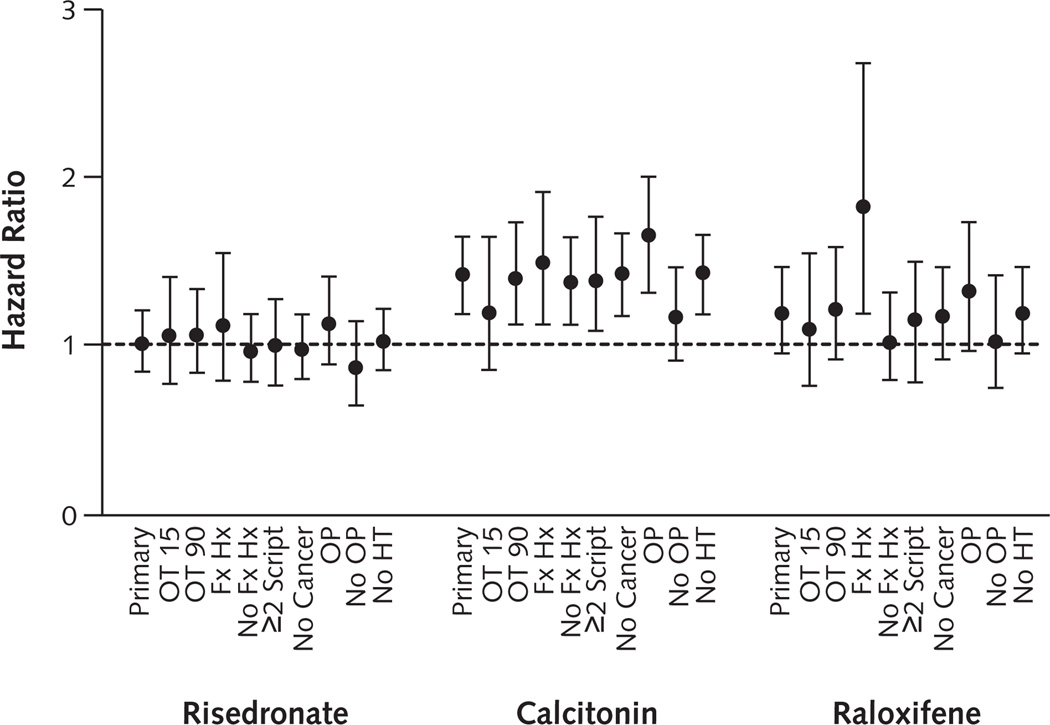
Figure 3 shows similar results across sensitivity analyses. Fracture rates among patients who received risedronate and alendronate were consistently similar, and patients who received calcitonin had consistently higher fracture rates than alendronate recipients. However, when we restricted analyses to participants with previous fracture, we observed more nonvertebral fractures among raloxifene recipients (HR, 1.78 [CI, 1.20 to 2.63]) than alendronate recipients. Fracture risk among raloxifene recipients compared with alendronate recipients was also high among the subgroup with a previous diagnosis of osteoporosis (HR, 1.30 [CI, 0.98 to 1.71]).
Figure 3. Nonvertebral fracture risk within 12 months of treatment initiation compared with alendronate.
Hazard ratios and 95% CIs (error bars) were calculated by using Cox proportional hazard models stratified by state and adjusted for propensity score quintiles, age, race, diagnosis of osteoporosis, and fracture history (previous nonvertebral and vertebral fracture). The intent-to-treat analysis (Primary) was censored on the date of death or end of follow-up (12 months after treatment initiation, 31 December 2003 [New Jersey], or 31 December 2005 [Pennsylvania]). OT 15 = patients receiving treatment who were censored on the first day of switching agents, losing drug plan eligibility, entering a nursing home, or discontinuing use of the drug (last date covered by drug plan plus 15 days, allowing for 30-day gaps between prescriptions) on the date of death or end of follow-up; OT 90 = patients receiving treatment who were censored as for OT 15, except that follow-up was extended to 90 d after drug discontinuation; Fx Hx = patients with a history of any fracture within 12 mo before treatment initiation; No Fx Hx = patients with no known history of fracture within 12 mo before treatment initiation; ≥2 Script = patients who filled ≥2 consecutive prescriptions of their index drug, excluding those who lost drug plan eligibility, entered a nursing home, died, had a nonvertebral fracture, or switched agents within the first 30 d; No Cancer = patients with no diagnosis of malignant neoplasm within 12 months before drug initiation; OP = patients with a medical claim for osteoporosis diagnosis within 12 mo before drug initiation; No OP = patients with no medical claim for osteoporosis diagnosis within 12 mo before treatment initiation; No HT = women with no history of hormone therapy within 12 mo before treatment initiation.
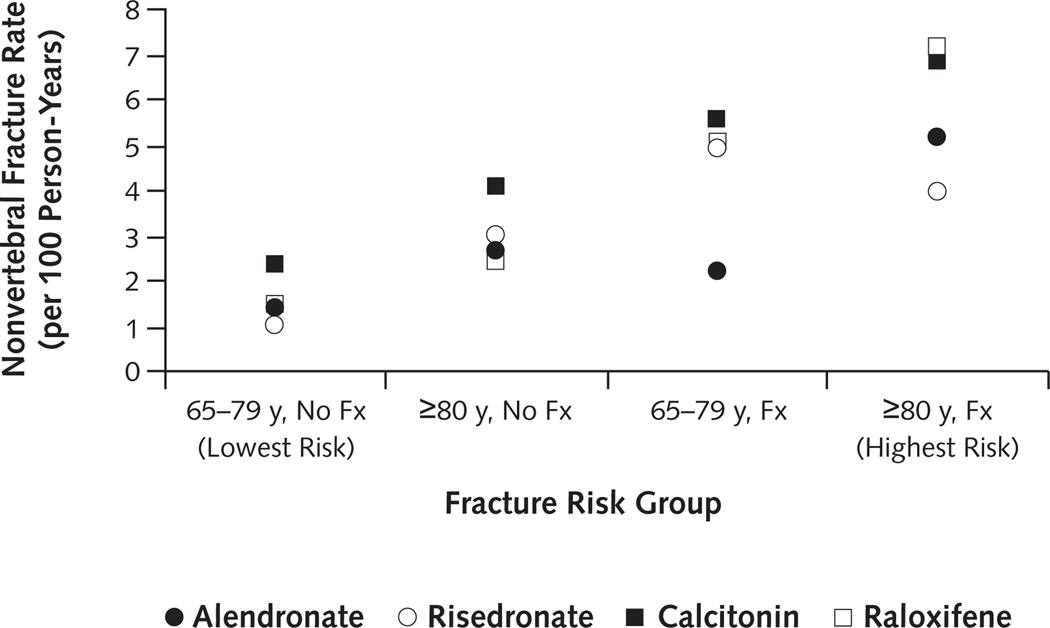
Overall nonvertebral fracture rates within 12 months increased across risk groups from 1.51 per 100 person-years (CI, 1.34 to 1.69 per 100 person-years) among those age 65 to 79 years with no fracture history to 3.04 per 100 person-years (CI, 2.76 to 3.34 per 100 person-years) among those age 80 years or older with no fracture history, then from 3.71 per 100 person-years (CI, 3.06 to 4.46 per 100 person-years) among patients age 65 to 79 years with fracture history to 5.64 per 100 person-years (CI, 4.92 to 6.44 per 100 person-years) among patients age 80 years or older. Similar patterns were seen by drug, with rates generally increasing across risk groups (Figure 4).
Figure 4. Nonvertebral fracture rates within 12 months, by fracture risk group and drug.
Age cut-off was determined by the median cohort age (79 y). Fracture history was identified by Medicare claims within 12 months before treatment initiation. 65–79 y, No Fx = patients age 65 to 79 years without fracture history (lowest risk); ≥80 y, No Fx = patients age ≥80 years without fracture history; 65–79 y, Fx = patients age 65 to 79 years with fracture history; ≥80 y, Fx = patients age ≥80 years with fracture history (highest risk).
Finally, using the rule-out method, we determined that a very strong risk factor for nonvertebral fracture must be unmeasured and imbalanced across treatment groups to explain the observed association (HR, 1.40) between alendronate and calcitonin if the 2 agents did not differ in nonvertebral fracture risk. For example, with an overall prevalence of 50% in the population, an unmeasured confounder for nonvertebral fracture with a magnitude of 2.5 (relative risk, 2.5) would require more than a 6-fold difference in prevalence between drug exposure groups (odds ratio, 6.4) to attenuate our observed hazard ratio of 1.40 (for example, 10% of alendronate recipients versus >60% of calcitonin recipients). Nonetheless, with our observed lower CI bound of 1.20, a 2.5-fold (odds ratio, 2.5) difference in the prevalence of the risk factor would be required to eliminate statistical significance.
Discussion
We found little difference in nonvertebral fracture rates among new recipients of alendronate and risedronate, regardless of the duration of observation (6, 12, or 24 months since treatment initiation), assumptions underlying the analysis (on-treatment or intent-to-treat), or subgroup considered. Our results contrast with findings from other observational studies that document risedronate as more effective than alendronate in preventing nonvertebral fractures (12, 13). Our findings are also somewhat surprising because randomized, controlled trials (RCTs) show that alendronate improves bone mineral density and reduces bone turnover markers better than risedronate (3, 4). Previous observational studies comparing bisphosphonates included preventive doses of alendronate that are less effective than treatment doses (26). We restricted our study to new recipients of pharmacotherapies approved for osteoporosis treatment. We also studied nonvertebral fracture sites most commonly associated with osteoporosis: hip, humerus, and radius or ulna (rather than also including clavicle, leg, and pelvis) (12, 13). These methodological differences in study design may partially explain the differences between our findings of equivalent fracture prevention between bisphosphonates, compared with previous observational studies suggesting that risedronate is more effective than alendronate in preventing nonvertebral fractures.
We did an English-language search of MEDLINE through December 2007 to identify relevant large head-to-head trials and large comparative observational studies with fracture outcomes. To our knowledge, FACT (Fosamax Actonel Comparison Trial) is the only head-to-head trial comparing alendronate and risedronate (3, 4). However, FACT was underpowered to examine fracture outcomes and examined surrogate markers of efficacy. By randomly assigning 1053 postmenopausal women (mean age, 64.5 years) with low bone mineral density to receive weekly alendronate or risedronate, FACT controlled for both measured and unmeasured confounding. However, FACT also excluded important candidate groups for pharmacotherapy with bisphosphonates, such as men, and women with previous hormone or long-term glucocorticoid therapy. Randomized, controlled trials establish drug efficacy within defined patient populations that are often not representative of those who may benefit from pharmacotherapy or of how the agents are used in practice (for example, adherence to drug regimen, and calcium or vitamin D supplementation) (27). In contrast, health care claims data reflect routine practice for large and representative populations (28). Therefore, observational studies play an important role in examining drug effectiveness among those treated. However, observational studies are also susceptible to confounding. Although alendronate and risedronate recipients in our study were similar according to measured covariates, we cannot rule out possible differences due to unmeasured variables, such as bone mineral density, risk for falls, family history, or nonprescription preventive therapies. Nonetheless, our findings are robust, with consistent results across all sensitivity analyses considered.
We also found no large differences between the relative effectiveness of raloxifene versus alendronate in reducing nonvertebral fracture risk. However, confidence bounds were large, and we therefore cannot rule out potential clinically important differences between these agents. Previous RCTs have found greater improvements in surrogate end points (bone mineral density and bone turnover markers) with alendronate versus raloxifene (5–7), and although alendronate has proven efficacy in reducing nonvertebral fracture risk versus placebo, no such evidence of benefit has been reported with raloxifene (14, 26, 29). Raloxifene recipients in our study were younger and seemed to be healthier than alendronate recipients on the basis of measured variables. Although balance between measured variables improved within propensity score quintiles (Appendix Tables 2 and 3, available at www.annals.org), we were limited to information contained in health care utilization databases. Our inability to adjust for baseline bone mineral density may be particularly problematic. The efficacy of bisphosphonates in reducing nonvertebral fracture risk is established among persons with a bone mineral density T-score less than −2.5. However, the National Osteoporosis Foundation recommends that treatment be considered at a T-score less than −2.0, and in the presence of other risk factors, at a T-score less than −1.5 (2). It is therefore possible that a high proportion of recipients have a bone mineral density higher than that for which bisphosphonates are documented to be effective. We found that in the subgroups most likely to have low bone mineral density at treatment initiation (fracture history and diagnosis of osteoporosis), risk for nonvertebral fracture was higher among raloxifene recipients than alendronate recipients. Therefore, our data are somewhat consistent with those from placebo-controlled trials completed among patients with low bone mass, which found that alendronate (but not raloxifene) prevents nonvertebral fractures (14, 26, 29). Further comparative studies between bisphosphonates and raloxifene may help to strengthen and clarify our findings.
We found more fractures among patients treated with calcitonin versus alendronate. This finding contrasts with that of a previous observational study suggesting no large difference between calcitonin and alendronate recipients (12). However, given that data from RCTs to support calcitonin in reducing nonvertebral fracture risk (14, 26, 30) are lacking, our finding is expected. On the basis of measured variables, calcitonin recipients in our study had higher background risk for fractures than alendronate recipients; the curves plotting cumulative fracture incidence diverged immediately after treatment initiation. Therefore, unmeasured confounding may be exaggerating the differences in observed fracture rates between calcitonin and alendronate.
We did a sensitivity analysis to assess the extent of residual confounding required to explain our finding that calcitonin recipients had a 40% higher risk for nonvertebral fracture than did alendronate recipients (25). These analyses suggested that our findings are unlikely to be entirely due to unmeasured confounding. Bone mineral density is the most important risk factor for fracture that was not included in our analysis. The relative risk for hip fracture is estimated to be 2.5 at age 65 years among persons with osteoporosis (T-score <−2.5) compared with those with higher bone mineral density (31). On the basis of measured variables, alendronate recipients were younger and had fewer documented fractures in the previous year compared with calcitonin recipients. These observed differences also suggest that a higher proportion of calcitonin recipients may have osteoporosis as measured by dual-energy x-ray absorptiometry. However, a 6-fold difference in the prevalence of osteoporosis would be required to attenuate our 1.40 observed hazard ratio (for example, dual-energy x-ray absorptiometry–documented osteoporosis among 10% of alendronate recipients versus >60% of calcitonin recipients). It is therefore unlikely that our observed difference in fracture risk between calcitonin and alendronate is completely due to unmeasured confounding; rather, it is more likely that a true difference in the effectiveness of these agents in reducing fracture risk exists. However, the observed imbalance between calcitonin and alendronate would only need to be 2.5-fold higher to move the lower bound of the 95% CI toward the null (for example, 30% of alendronate recipients had osteoporosis documented by dual-energy x-ray absorptiometry vs. 75% of calcitonin recipients). Regardless, given that we focused on alendronate doses that were approved for treating osteoporosis, it is unlikely that the difference in osteoporosis prevalence between calcitonin and alendronate was 2.5-fold or more.
Nonetheless, calcitonin may be prescribed for acute pain after fracture, and the risk for recurrent fracture is highest immediately after a fracture (32, 33). Although we adjusted for fracture history as defined by Medicare claims within the year before treatment initiation, we could not distinguish between prevalent and incident vertebral fractures. We also documented a higher background prevalence of vertebral fracture among calcitonin recipients. Therefore, if patients more often received calcitonin for acute pain associated with incident fractures, residual confounding by indication may exist. Given that we could not adjust for some major possible confounding factors, such as bone mineral density, nonprescription prevention therapies, vitamin D levels, incident versus prevalent vertebral fractures, and risk for falls, we advise using caution when interpreting our findings.
Future studies that are better able to adjust for potential unmeasured confounding may help to clarify the extent of difference in fracture prevention among osteoporosis therapies. An emerging methodological approach through propensity score calibration may be useful to adjust for unmeasured confounding, provided that a good validation data source is available (34 –37). For example, a data source among new recipients of these agents that includes important covariates not available in claims data—such as bone mineral density at treatment initiation, family history, nonprescription preventive therapies, frailty, risk for falls, and incident versus prevalent previous fractures—may be used to adjust for differences in baseline covariates between drug therapies.
In addition to those already mentioned, our study has 3 further limitations. First, the study database was limited to claims data to assess fracture outcomes and may have misclassified some fractures. However, we minimized the potential for misclassification (information bias) by studying fractures at the hip, humerus, and radius or ulna with validated fracture codes (diagnostic and procedural codes) that have an estimated sensitivity of at least 90% (15). There is also no reason to believe that differential misclassification of fractures between treatment agents occurred.
Second, we used an intent-to-treat scenario to complete our primary analysis and thus assumed that patients were exposed to drugs throughout follow-up. Randomized, controlled trials ensure a minimum level of adherence in an effort to establish biological effects. However, adherence to osteoporosis pharmacotherapy is much lower in practice (19, 20) and is linked to fracture risk. The more at risk patients perceive themselves to be (38, 39) and the more effective they perceive treatment to be (40, 41), the more likely they will be willing to initiate and adhere to therapy. On-treatment analysis may thus be subject to information bias. If alendronate versus raloxifene recipients, for example, have lower bone mineral density, they may persist with therapy longer, but they are also at higher risk for fracture (patient persistence with therapy is differential and linked to fracture risk). On the other hand, the efficacy of alendronate persists long after discontinuing therapy (42). Therefore, in the real-world setting, in which patients may not completely adhere to therapy, patient persistence with therapy is differential on the basis of fracture risk, and persistence of drug effects may differ between therapies, the intent-to-treat analysis yields the most valid results. We also found similar results in on-treatment analyses.
Third, the study cohort was limited to low-income persons with complete drug coverage residing in 2 states. Thus, our results may not be generalizable to all recipients of these agents, particularly if adherence to treatment differs among those with different drug coverage. However, our cohort of frail persons age 65 years or older is typical of patients requiring pharmacotherapy to reduce fracture risk and provides real-world comparative effectiveness data among patients with complete drug coverage. Our large cohort of new recipients also permitted us to examine results in several subgroups, demonstrating that our results are robust.
The early termination of a trial that was designed to examine fracture outcomes between osteoporosis therapies (because a sufficient number of treatment-naive women could not be recruited [7]) indicates that evidence from RCTs comparing medications is unlikely to become available. In the absence of RCT evidence, observational data provide a complementary source of information that compares drug effectiveness when prescribed in clinical practice (27). Our large observational study of persons age 65 years or older who received drug treatment for osteoporosis identified no difference in the effectiveness of bisphosphonates (risedronate versus alendronate) in preventing nonvertebral fractures. We also documented no large differences in fracture risk among raloxifene compared with alendronate. However, confidence bounds were wide and thus do not rule out potentially important clinical differences. Although we found a 40% higher risk for nonvertebral fractures among nasal calcitonin recipients than alendronate recipients, future studies that can better adjust for potential residual confounding may clarify our results.
Context
Few studies have evaluated the relative effectiveness of drug therapies for osteoporosis.
Contribution
This study compared nonvertebral fractures that occurred within 1 year of initiating osteoporosis pharmacotherapy among 43 135 enrollees in 2 statewide pharmaceutical benefit programs. Differences in fracture risk between adults prescribed risedronate or raloxifene and those prescribed alendronate were small. Fracture risk seemed to be higher with calcitonin than alendronate.
Caution
Wide confidence bounds around risk estimates did not rule out potentially important differences between some agents. No adherence data were available, and the ability to account for confounders was limited.
Implication
There probably is no single clearly superior drug therapy for osteoporosis.
—The Editors
Acknowledgment
The authors thank Raisa Levin, MS, for preparing the study data for analysis.
Grant Support: By grant K25 AG027400 from the National Institute on Aging (Dr. Brookhart), a Canadian Institutes of Health Research Post-Doctoral Fellowship (Dr. Cadarette), grants K24 AR02123 and P60 AR47782 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr. Katz), grants from the Arthritis Foundation and grants R21 AG027066 and P60 AR47782 from the National Institutes of Health (Dr. Solomon), and grant RO1 AG023178 from the National Institute on Aging (Dr. Stürmer).
Footnotes
Potential Financial Conflicts of Interest: Grants received: M.A. Brookhart (Amgen), D.H. Solomon (Merck). Grants pending: M.A. Brookhart (Amgen).
Reproducible Research Statement: Study protocol, statistical code, and data set: Not available.
Author Contributions: Conception and design: S.M. Cadarette, M.A. Brookhart, T. Stürmer, D.H. Solomon.
Analysis and interpretation of the data: S.M. Cadarette, J.N. Katz, M.A. Brookhart, T. Stürmer, M.R. Stedman, D.H. Solomon.
Drafting of the article: S.M. Cadarette, T. Stürmer, D.H. Solomon.
Critical revision of the article for important intellectual content: S.M. Cadarette, J.N. Katz, M.A. Brookhart, T. Stürmer, M.R. Stedman, D.H. Solomon.
Final approval of the article: S.M. Cadarette, J.N. Katz, M.A. Brookhart, T. Stürmer, M.R. Stedman, D.H. Solomon.
Provision of study materials or patients: D.H. Solomon.
Statistical expertise: M.A. Brookhart, T. Stürmer, M.R. Stedman.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [PMID: 8506892] [DOI] [PubMed] [Google Scholar]
- 2.National Osteoporosis Foundation. Physician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2003. [on 30 March 2004]. Accessed at www.nof.org/physguide.html. [Google Scholar]
- 3.Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, et al. Fosamax Actonel Comparison Trial Investigators. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [PMID: 15619680] [DOI] [PubMed] [Google Scholar]
- 4.Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, et al. Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab. 2006;91:2631–2637. doi: 10.1210/jc.2005-2602. [PMID: 16636120] [DOI] [PubMed] [Google Scholar]
- 5.Luckey M, Kagan R, Greenspan S, Bone H, Kiel RD, Simon J, et al. Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause. 2004;11:405–415. doi: 10.1097/01.gme.0000119981.77837.1f. [PMID: 15243278] [DOI] [PubMed] [Google Scholar]
- 6.Sambrook PN, Geusens P, Ribot C, Solimano JA, Ferrer-Barriendos J, Gaines K, et al. Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: results of EFFECT (Efficacy of FOSAMAX versus EVISTA Comparison Trial) International. J Intern Med. 2004;255:503–511. doi: 10.1111/j.1365-2796.2004.01317.x. [PMID: 15049885] [DOI] [PubMed] [Google Scholar]
- 7.Recker RR, Kendler D, Recknor CP, Rooney TW, Lewiecki EM, Utian WH, et al. Comparative effects of raloxifene and alendronate on fracture outcomes in postmenopausal women with low bone mass. Bone. 2007;40:843–851. doi: 10.1016/j.bone.2006.11.001. [PMID: 17182297] [DOI] [PubMed] [Google Scholar]
- 8.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [PMID: 8634613] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts NB, Geusens P, Barton IP, Felsenberg D. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005;20:2097–2104. doi: 10.1359/JBMR.050814. [PMID: 16294263] [DOI] [PubMed] [Google Scholar]
- 10.Allen MR, Iwata K, Sato M, Burr DB. Raloxifene enhances vertebral mechanical properties independent of bone density. Bone. 2006;39:1130–1135. doi: 10.1016/j.bone.2006.05.007. [PMID: 16814622] [DOI] [PubMed] [Google Scholar]
- 11.Seeman E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone. 2007;41:308–317. doi: 10.1016/j.bone.2007.06.010. [PMID: 17644058] [DOI] [PubMed] [Google Scholar]
- 12.Watts NB, Worley K, Solis A, Doyle J, Sheer R. Comparison of risedronate to alendronate and calcitonin for early reduction of nonvertebral fracture risk: results from a managed care administrative claims database. J Manag Care Pharm. 2004;10:142–151. doi: 10.18553/jmcp.2004.10.2.142. [PMID: 15032563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18:25–34. doi: 10.1007/s00198-006-0274-z. [PMID: 17106785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [PMID: 18087050] [DOI] [PubMed] [Google Scholar]
- 15.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [PMID: 1619449] [DOI] [PubMed] [Google Scholar]
- 16.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [PMID: 16624967] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Wang PS, Avorn J, Maclure M, Levin R, Glynn RJ. Consistency of performance ranking of comorbidity adjustment scores in Canadian and U.S. utilization data. J Gen Intern Med. 2004;19:444–450. doi: 10.1111/j.1525-1497.2004.30109.x. [PMID: 15109342] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081-90 [PMID 8410092] [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [PMID: 17308956] [DOI] [PubMed] [Google Scholar]
- 20.Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–2419. doi: 10.1001/archinte.165.20.2414. [PMID: 16287772] [DOI] [PubMed] [Google Scholar]
- 21.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–810. [Google Scholar]
- 22.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [PMID: 16611199] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–447. doi: 10.1016/j.jclinepi.2005.07.004. [PMID: 16632131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai K, King G, Stuart EA. Misunderstandings among experimentalists and observationalists about causal inference. J R Stat Soc [Ser A] 2008;171:481–502. [Google Scholar]
- 25.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [PMID: 16447304] [DOI] [PubMed] [Google Scholar]
- 26.Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–578. doi: 10.1210/er.2001-9002. [PMID: 12202472] [DOI] [PubMed] [Google Scholar]
- 27.Lindsay R. Beyond clinical trials: the importance of large databases in evaluating differences in the effectiveness of bisphosphonate therapy in postmenopausal osteoporosis. Bone. 2007;40:S32–S35. [Google Scholar]
- 28.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [PMID: 15862718] [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, et al. Effects of raloxifene on fracture risk in postmenopausal women: the raloxifene use for the heart trial. J Bone Miner Res. 2008;23:112–120. doi: 10.1359/jbmr.070904. [PMID: 17892376] [DOI] [PubMed] [Google Scholar]
- 30.Chesnut CH, 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [PMID: 10996576] [DOI] [PubMed] [Google Scholar]
- 31.Black DM, Palermo L, Grima DT. Developing better economic models of osteoporosis: considerations for the calculation of the relative risk of fracture. Value Health. 2006;1:54–58. doi: 10.1111/j.1524-4733.2006.00081.x. [PMID: 16441525] [DOI] [PubMed] [Google Scholar]
- 32.Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–179. doi: 10.1007/s00198-003-1514-0. [PMID: 14691617] [DOI] [PubMed] [Google Scholar]
- 33.Schousboe JT, Fink HA, Lui LY, Taylor BC, Ensrud KE. Association between prior non-spine non-hip fractures or prevalent radiographic vertebral deformities known to be at least 10 years old and incident hip fracture. J Bone Miner Res. 2006;21:1557–1564. doi: 10.1359/jbmr.060711. [PMID: 16995810] [DOI] [PubMed] [Google Scholar]
- 34.Stürmer T, Schneeweiss S, Avorn J, Glynn RJ. Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. Am J Epidemiol. 2005;162:279–289. doi: 10.1093/aje/kwi192. [PMID: 15987725] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stürmer T, Schneeweiss S, Rothman KJ, Avorn J, Glynn RJ. Performance of propensity score calibration—a simulation study. Am J Epidemiol. 2007;165:1110–1118. doi: 10.1093/aje/kwm074. [PMID: 17395595] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stürmer T, Glynn RJ, Rothman KJ, Avorn J, Schneeweiss S. Adjustments for unmeasured confounders in pharmacoepidemiologic database studies using external information. Med Care. 2007;45:S158–S165. doi: 10.1097/MLR.0b013e318070c045. [PMID: 17909375] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiébaut AC, Freedman LS, Carroll RJ, Kipnis V. Is it necessary to correct for measurement error in nutritional epidemiology? [Editorial] Ann Intern Med. 2007;146:65–67. doi: 10.7326/0003-4819-146-1-200701020-00012. [PMID: 17200225] [DOI] [PubMed] [Google Scholar]
- 38.Cadarette SM, Gignac MA, Jaglal SB, Beaton DE, Hawker GA. Access to osteoporosis treatment is critically linked to access to dual-energy x-ray absorptiometry testing. Med Care. 2007;45:896–901. doi: 10.1097/MLR.0b013e318054689f. [PMID: 17712261] [DOI] [PubMed] [Google Scholar]
- 39.Fitt NS, Mitchell SL, Cranney A, Gulenchyn K, Huang M, Tugwell P. Influence of bone densitometry results on the treatment of osteoporosis. CMAJ. 2001;164:777–781. [PMID: 11276543] [PMC free article] [PubMed] [Google Scholar]
- 40.Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, et al. Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92:1296–1304. doi: 10.1210/jc.2006-1526. [PMID: 17244788] [DOI] [PubMed] [Google Scholar]
- 41.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:1117–1123. doi: 10.1210/jc.2003-030501. [PMID: 15001596] [DOI] [PubMed] [Google Scholar]
- 42.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [PMID: 17190893] [DOI] [PubMed] [Google Scholar]