Abstract
Platelet-rich plasma (PRP) has been clinically used as an easily prepared growth factor cocktail that can promote wound healing, angiogenesis, and tissue remodeling. However, the therapeutic effects of PRP are still controversial, due partly to the lack of optimized and standardized preparation protocols. We used whole blood (WB) samples to optimize the preparation protocols for PRP, white blood cell-containing (W-PRP), platelet-concentrated plasma (PCP), and noncoagulating platelet-derived factor concentrate (PFC). PRP and W-PRP were most efficiently collected by 10 min centrifugation in a 15-mL conical tube at 230–270 g and 70 g, respectively. To prepare PCP, platelets were precipitated by centrifugation of PRP at >2300 g, 90% of supernatant plasma was removed, and the platelets were resuspended. For preparation of noncoagulating PFC, the supernatant was replaced with one-tenth volume of saline, followed by platelet activation with thrombin. Platelet (before activation) and platelet-derived growth factor (PDGF)-BB (after activation) concentrations in PCP were approximately 20 times greater than those in WB, whereas PFC contained a 20-times greater concentration of platelets before platelet activation and a 50-times greater concentration of PDGF-BB without formation of a fibrin gel after platelet activation than WB. Surprisingly, total PDGF-BB content in the PFC was twice that of activated WB, which suggested that a substantial portion of the PDGF-BB became trapped in the fibrin glue, and replacement of plasma with saline is crucial for maximization of platelet-derived factors. As an anticoagulant, ethylene di-amine tetra-acetic acid disodium inhibited platelet aggregation more efficiently than acid citrate dextrose solution, resulting in higher nonaggregated platelet yield and final PDGF-BB content. These results increase our understanding of how to optimize and standardize preparation of platelet-derived factors at maximum concentrations.
Introduction
The preparation of platelet-rich plasma (PRP) was originally developed as a method to divide red blood cells (RBCs) and plasma from whole blood (WB) in blood transfusion hematology.1 PRP was first used for hemostasis during surgical operations and platelet transfusions for some thrombocytopenic disorders.2 It was revealed through a number of studies that various bioactive substances, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and epidermal growth factor (EGF), were discharged from the α-granules of platelets into plasma when the platelets were destroyed and activated.3,4 These growth factors are contained in wound exudates from injured subcutaneous tissue and are important in the early phase of the wound-healing process.5 Specifically, they promote stromal stem cell proliferation and angiogenesis and are regarded as key signals in tissue repair/regeneration.6–8
Autologous PRP has less safety concerns than cell-based regenerative therapies. As such, PRP is an issue of extensive research for tissue engineering and regenerative medicine.9,10 PRP has been therapeutically used to accelerate wound healing and tissue repair in dentistry since 1998,11 and the clinical application of PRP was recently expanded to other fields, including cardiac surgery,12 ophthalmology,13 oral and maxillofacial surgery,14 orthopedic surgery,15 plastic surgery,16,17 sports medicine,18 and cosmetic medicine.19,20 For therapeutic purposes, PRP extracted from WB through a single centrifugation is frequently concentrated by a second centrifugation. The first centrifugation is slow to avoid spinning down platelets, whereas the second spin is fast, so the platelets are spun down. In this study, we refer to this concentrated PRP by the second spin as platelet-concentrated plasma (PCP) to differentiate it from PRP.
Despite the increasing use of PRP or PCP therapeutically, its reported clinical effects are quite variable.21 In fact, there is even a report that concludes that it is invalid.22 Although many commercialized devices are now available for the clinical preparation of PRP/PCP, there is no standardized protocol for PRP/PCP preparation. Moreover, there have been surprisingly few scientific studies on how to optimize the preparation of human PRP/PCP. For example, there has not been a comprehensive study on how the centrifugal force exerted on WB contained in common laboratory ware affects PRP/PCP yield. There are many reasons for a lack of such studies besides the obvious difficulty of obtaining sufficient quantities of fresh human WB samples. One of the many reasons is the complexity of blood coagulation (fibrin polymerization) and platelet aggregation. Since the precise and consistent control of fibrinogen and platelets is very difficult, well-designed studies are hard to plan and implement. Another reason is that the evaluation methods for the PRP/PCP products23,24 have not been consistent among previous studies. Finally, blood components and physical properties also vary between patient samples, making standardization difficult.
The first aim of this study is to optimize the protocol for preparing human PRP and PCP. This entails the establishment of a reliable and practical method to maximize platelet yield and concentration in plasma from a relatively small volume (7.5–75 mL) of blood using common laboratory ware and equipment. To evaluate the efficiency of the protocols, we have measured the number of nonaggregated platelets and the concentration of platelet-derived growth factor-BB (PDGF-BB) in the products. The other aim is to design a protocol for the preparation of noncoagulating growth factor concentrate, because fibrin glue formation sometimes prevents the therapeutic use of PCP depending on therapeutic purposes. Here, we propose a novel and reliable method to prepare PCP at either a maximal or an adjustable concentration and noncoagulating platelet-derived factor concentrates (PFC, a noncoagulating fluid that contains platelet-derived factors at either a maximal or an adjustable concentration).
Materials and Methods
Collection and preparation of plasma and serum
This study was conducted with the informed consent of volunteer subjects, and the experimental protocol was approved by our institutional review board. Nine healthy donors, five men and four women, whose ages ranged from 29 to 49, were enrolled in this study. All donors were not on any medication, including aspirin and other nonsteroidal anti-inflammatory drugs, during the 2 weeks before this study. WB (40–72 mL) was drawn by venipuncture, collected, and divided into conical tubes (15 mL; BD Falcon) that contained either 1 mg/mL ethylene di-amine tetra-acetic acid disodium (EDTA; Wako Pure Chemical Industries, Ltd.) or acid citrate dextrose solution (ACD; Terumo; at ratio of 10:1.5) as an anticoagulant. The ACD solution consists of 2.2 w/v% sodium citrate hydrate, 0.8 w/v% citric acid hydrate, and 2.2 w/v% glucose. WB was obtained three to twelve times from each donor.
To isolate plasma, tubes were centrifuged at various centrifugation parameters (30, 50, 70, 90, 120, 150, 190, 230, 270, 320, 370, 430, 620, 840, 1010, 1600, 2330 g, 10 min, 20°C) in a refrigerated centrifuge (Kubota 5900; Kubota Co.). The plasma was gently transferred to an empty tube, and its volume and hematological values were measured as described next.
The obtained plasma (the PRP) was centrifuged again at a higher spin (1010–2330 g, 10 min, 20°C) to precipitate the platelets at the bottom of the tube. After the second spin, samples were divided into two parts; the PCP (the lower one-third or one-tenth) and the platelet-poor plasma (PPP, the remaining upper portion; Fig. 1). Hematological values of both parts were measured as described next. The samples were activated by adding thrombin, permitted to clot at 37°C, and then centrifuged at 2330 g for 10 min at 4°C. The PDGF-BB concentration of the clear supernatant was measured as described next. WB plasma samples for PDGF-BB measurement were prepared by centrifuging WB at 2330 g for 10 min at 4°C after adding thrombin (500 unit per sample), without using an anticoagulant, and collecting the supernatant plasma.
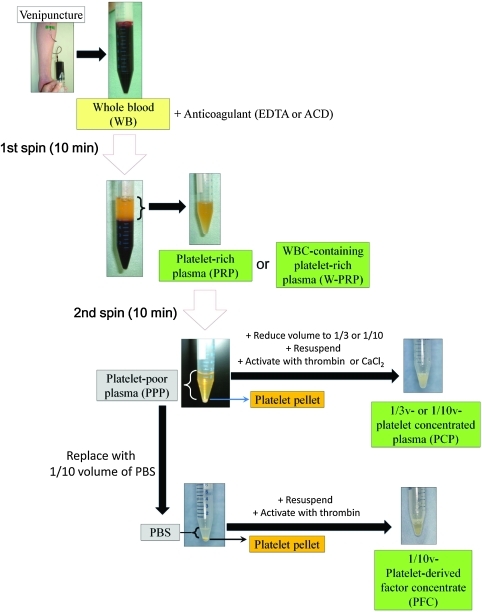
FIG. 1.
Flow chart for the preparation process of platelet-rich plasma (PRP)-related products. Whole blood (WB) was drawn from donors by venipuncture and collected into conical tubes that contained an anticoagulant. The PRP or white blood cell-containing PRP (W-PRP) was isolated by the first spin for 10 min. PRP was centrifuged again for 10 min to spin down the platelets. The platelet-poor plasma (PPP) layer was partially removed to create platelet-concentrated plasma (PCP) or replaced with one-tenth volume of phosphate-buffered saline (PBS) for noncoagulating platelet-derived factor concentrate (PFC). After the platelets were resuspended, thrombin was added for platelet activation. Color images available online at www.liebertonline.com/tec
In an attempt to eliminate fibrinogen (the PFC experiment), all supernatant (PPP) was removed after the second spin. The remaining platelet pellet was resuspended in an appropriate volume of phosphate-buffered saline (PBS), and the platelets were activated by adding thrombin (500 unit per sample; Thrombin oral/topical 5000 units-BENESIS®; Benesis Corp.). Then, the PDGF-BB concentration of the sample was measured. The preparation methods of each product are summarized in Figure 1.
Hematological analysis
A small portion of each sample (WB, PRP, PPP, and PCP) was used for hematological analyses. Numbers of RBCs, white blood cells (WBCs), platelets, and other hematological parameters were measured with a multi automatic blood corpuscle analyzer (XS-1000i; Sysmex Co.).
Quantification of PDGF-BB
Among representative growth factors derived from activated platelets such as PDGF, EGF, and TGF-β, PDGF-BB was selected as an index of platelet aggregation (activation) in plasma, because PDGF-BB is not contained in plasma and, thus, its concentration well correlates with how much platelets are activated. PDGF-BB was measured using an anti-human enzyme-linked immunosorbent assay kit (Quantikine; R&D Systems) according to the manufacturer's instructions. A microplate reader (Model 550; Bio-Rad Laboratories) was used to measure the absorbance of each well at 450 nm, and a standard curve was generated to determine growth factor concentrations (in picograms per milliliter). A small portion of each sample (WB, PRP, PPP, PCP, and PFC) was used for the quantification of PDGF-BB after an appropriate dilution.
Quantification of fibrinogen in plasma
Fibrinogen content in plasma was measured by HemosIL® Fibrinogen-C XL on an automated coagulation analyzer (ACL TOP; Instrumentation Laboratory).
Statistical analysis
Results were described as mean±standard deviation. Comparisons between two groups were performed with the unpaired Students' t-test. Comparisons of more than two groups were done by analysis of variance with the Bonferroni correction. Statistical significance was defined as p<0.05.
Results
Collected blood components after the first spin
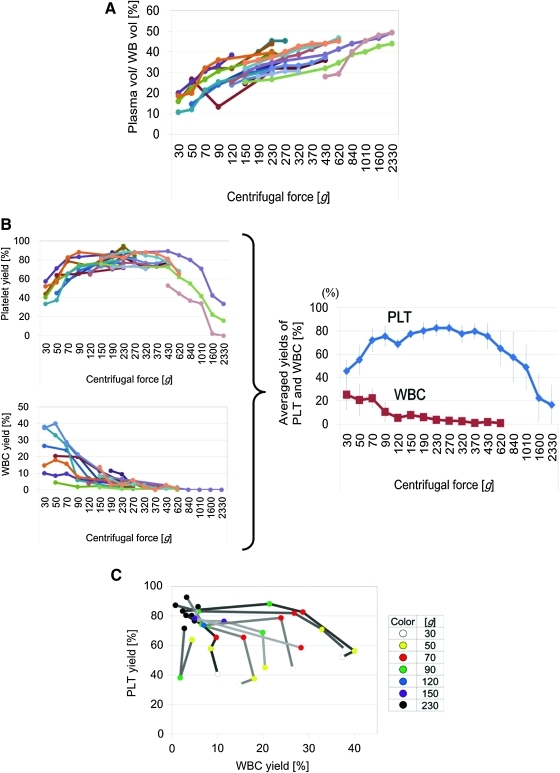
WB samples (40–72 mL) that were collected with EDTA as the anticoagulant were divided into 7.5-mL aliquots in 15-mL conical tubes and centrifuged for 10 min at centrifugal forces ranging from 30 to 2330 g. The volume of the collected plasma increased based on the centrifugal force, as larger volumes were collected at higher centrifugal forces. The volumes collected ranged from 1.22±0.268 mL (16.27%±3.58%) at 30 g to 3.57±0.231 mL (47.56%±3.08%) at 2330 g (Fig. 2A).
FIG. 2.
Differential centrifugations for isolating PRP or W-PRP. WB was centrifuged for 10 min at various centrifugal forces to isolate plasma. *p<0.05. (A) Collected plasma volume relative to WB volume. The collected plasma volume increased with increasing centrifugal force and peaked at up to 50% of the WB volume at 2330 g (n=20). (B) Platelet (PLT) and white blood cell (WBC) yield in collected plasma relative to that in WB. PLT yield peaked at between 190 and 320 g (n=15), whereas WBC yield decreased with as centrifugal force increased (n=16). The right figure shows the averaged data for PLT (red) and WBC (blue) yields. Values represent means±standard deviation. (C) Two-dimensional data of PLT and WBC collection rates at various centrifugal forces. PLT yield is relatively consistent, whereas WBC yield widely varies among donors. Yields (%) of PLT and WBC relative to WB were plotted on the x-axis and y-axis, respectively. Each centrifugal force is expressed in different colors (n=8). Color images available online at www.liebertonline.com/tec
The platelet collection rate increased as the centrifugal force increased from 30 to 190 g, peaked at 190 to 320 g, and progressively decreased from 320 to 2330 g (Fig. 2B, left, above). Although the best centrifugal force slightly differed among donors, the averaged data suggested that a centrifugation force of 230–270 g would be the most likely to produce the maximum platelet yield from a WB sample in a 10 min spin (Fig. 2B, right). Thus, we concluded that our optimized protocol for PRP preparation is a single centrifugation of WB at 230–270 g for 10 min. This protocol collected ≥80% of the platelets in a WB sample. The optimized PRP contained a low number of WBCs (4.1%–5.8% of WB). Thus, it can be also referred to as WBC-poor PRP.
The WBC collection rate decreased based on centrifugal force, as smaller percentages were detected in plasma after centrifugation at higher centrifugal forces. These percentages ranged from 25.3%±12.8% at 30 g to 0.875%±0.66% at 620 g (Fig. 2B, left, below). Almost all WBCs appeared to be precipitated when the centrifugation force was ≥840 g.
The collection rate data for WBCs and platelets were plotted on the x- and y- axis, respectively, and analyzed (Fig. 2C). According to the plotted data, we concluded that our optimized protocol for producing WBC-containing PRP (W-PRP; see discussion for details) is a single centrifugation of WB (7.5 mL) at 70 g for 10 min, as this was the method in which both WBCs and platelets were efficiently collected (10%–35% and 60%–80%, respectively [red plots in Fig. 2C]).
Collected blood components after the second spin
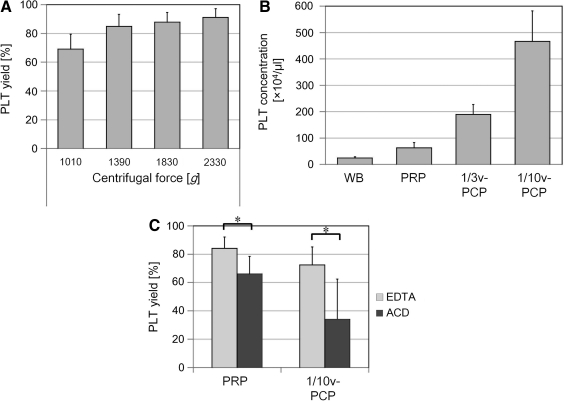
The second hard spin is needed to convert PRP into PCP (by the reduction of volume and the condensation of nonaggregated platelets). The platelets in PRP were precipitated by the second spin. A larger amount of platelets were precipitated by a higher centrifugal force. The precipitated pellets contained 69.0%±10.5%, 84.9%±8.39%, 87.8%±6.85%, and 91.2%±6.05% of the platelets contained in the original PRP after a 10 min centrifugation at 1010, 1390, 1830, and 2330 g, respectively (Fig. 3A). Thus, a higher centrifugal force was recommended for the second spin.
FIG. 3.
Measurement of PLTs after centrifugation of PRP. PRP obtained by the first spin for 10 min at 270 g was centrifuged again to prepare PCP. *p<0.05. (A) PLT yield in PCP after the second spin for 10 min at various centrifugal forces relative to PRP (n=5). (B) PLT concentration in WB, PRP, and one-third and one-tenth volume of PCP (1/3v-PCP, 1/10v-PCP; n=11). PLTs in PRP were further concentrated by the second spin and the reduction of plasma volume in PCP. (C) Comparison of PLT yield (%) in PRP and 1/10v-PCP between two anticoagulants. PRP and 1/10v-PCP were prepared with the same protocol with either ethylene di-amine tetra-acetic acid (EDTA) or acid citrate dextrose (ACD) as the anticoagulant (n=10). EDTA showed significantly higher inhibitory effects on PLT aggregation than ACD.
After the second spin, we prepared two kinds of PCP by reducing the volume by different amounts; one-third volumetric PCP (1/3v-PCP) and one-tenth volumetric PCP (1/10v-PCP) were prepared by removing the upper two-thirds or nine-tenths of the PPP supernatant, respectively, before the precipitated platelets were resupended. The platelet concentrations (×104/μL) were 24.5±4.79 for WB, 62.7±14.8 for PRP, 189.6±37.3 for 1/3v-PCP, and 466.7±5.27 for 1/10v-PCP (Fig. 3B). Thus, the platelet concentration was elevated by up to 7.4 times that of PRP by the 1/10v-PCP preparation.
Comparison of the effects of the anticoagulants EDTA and ACD on platelet yield in PRP and PCP
To analyze the beneficial effects of the anticoagulants EDTA and ACD on PRP preparation, the PRP platelet collection rates were compared at the optimized centrifugation parameters (230–270 g, 10 min; n=11). The PRP and 1/10v-PCP nonaggregated platelet collection rates were significantly higher when EDTA rather than ACD was used as the anticoagulant (Fig. 3C). Thus, EDTA was apparently superior to ACD in preventing blood coagulation and platelet aggregation. The preservation of nonaggregated platelets is critical in maximizing the concentration of platelets and platelet-derived factors.
Concentration and total yield of nonaggregated platelets in PCP and PFC before thrombin activation
The 1/10v-PFC was prepared by replacing the supernatant (PPP) with one-tenth volume of PBS after the second spin, followed by the activation of platelets by thrombin (Fig. 1). The platelet concentration (×104/μL) of the 1/10v-PFC before platelet activation was 415±50.1, a concentration comparable to that of 1/10v-PCP (427.3±21.1) and much higher than that of WB and optimized PRP (20.2±2.26 and 51.4±7.15, respectively; Fig. 4A). Significant differences were found between WB and PRP, PRP and 1/10v-PCP, and PRP and 1/10v-PFC.
FIG. 4.
Measurement of platelets and platelet-derived growth factor (PDGF)-BB in the preparation process of PCP and PFC. PCP and PFC were prepared using an optimized protocol. The number of PLTs was counted before PLT activation with thrombin, whereas the PDGF-BB protein level was measured after PLT activation. *p<0.05. (A) Comparison of PLT concentration in WB, PRP, 1/10v-PCP, and 1/10v-PFC obtained from a single sample (n=6). It was shown that PLTs were highly concentrated in 1/10v-PCP and 1/10v-PFC compared with PRP. (B) PLT yield relative to WB in PRP, 1/10v-PCP, and 1/10v-PFC obtained from a single sample (n=6). (C) Comparison of PDGF-BB concentration in WB, PRP, 1/10v-PCP, and 1/10v-PFC obtained from a single sample (n=6). PDGF-BB concentration differs between each product and was highest (nearly 50 times of WB) in 1/10v-PFC. (D) Total PDGF-BB content in WB, PRP, 1/10v-PCP, and 1/10v-PFC obtained from a single sample (n=6). Surprisingly, the total PDGF content in 1/10v-PFC was larger than the amount obtained from activated WB.
The platelet collection rates of PCP (74.8%±13.1%) and PFC (72.1%±10.4%) were similar, but both were significantly lower than that of PRP (88.7±6.2; Fig. 4B). This showed that some of the platelets were lost or aggregated in the process of the second spin, but not in the replacement of PPP with PBS. However, 1/10v-PFC still contained more than 70% of platelets. Thus, the loss of platelets in the second spin was deemed to be very small and acceptable.
Concentration and total amount of PDGF-BB in PCP and PFC after thrombin activation
The PDGF-BB concentration (ng/mL) of 1/10v-PFC after platelet activation was 157.9±16.0, much higher than that of WB, PRP, and 1/10v-PCP (3.05±0.76, 6.79±1.33, and 52.7±20.8, respectively; Fig. 4C). Significant differences were detected between WB and PRP, PRP and 1/10v-PCP, and 1/10v-PCP and 1/10v-PFC. Interestingly, PFC showed a significantly higher PDGF-BB concentration than PCP, even though the PFC platelet concentration was similar to PCP before thrombin activation.
The total amount of PDGF-BB in each sample was calculated by multiplying the PDGF-BB concentration (ng/mL) by its volume (mL; Fig. 4D). As expected, the total amount of PDGF-BB was highly correlated to platelet yield before activation in WB, PRP, and 1/10v-PCP. However, the 1/10v-PFC showed a remarkably high total amount of PDGF-BB compared with the other products (even higher than WB), even though its platelet yield was comparable to 1/10v-PCP.
Comparison of the effects of the anticoagulants EDTA and ACD on PDGF-BB concentration
The 1/10v-PCP and 1/10v-PFC were prepared with either EDTA or ACD as the anticoagulant, and PDGF-BB concentrations were compared. The PDGF-BB concentrations (ng/mL) were 4.30±2.75 (WB), 64.9±3.00 (1/10v-PCP), and 166.5±41.1 (1/10v-PFC) when EDTA was used as an anticoagulant, whereas they were 3.37±2.11 (WB), 68.5±5.14 (1/10v-PCP), and 52.8±2.79 (1/10v-PFC) when ACD was used (Fig. 5A). The PDGF-BB concentration in 1/10v-PFC was significantly higher when EDTA rather than ACD was used as the anticoagulant. In the PPP replacement process after the second spin for PFC preparation, the platelet sediment was easily scattered by pipetting when EDTA was used, but not when ACD was used. This is probably because ACD induced a less complete inhibition of aggregation than EDTA, and platelets were not fully recovered during the process of PPP replacement and the second spin. Another explanation may be an inhibitory effect of ACD on intracellular signaling (leading to granule release of platelets) by lowering platelet pH.
FIG. 5.
Measurement of PDGF-BB and fibrinogen in PFC. PFC was prepared by replacing supernatant PPP with PBS after the second spin. *p<0.05. (A) Comparison of two anticoagulants in regards to PDGF-BB concentration in 1/10v-PFC. Either EDTA or ACD was used as the anticoagulant for preparation of 1/10v-PFC with the rest of the protocol being the same (n=10). EDTA showed significantly higher inhibitory effects on platelet aggregation than ACD. (B) Comparison of fibrinogen concentration between 1/10v-PCP and 1/10v-PFC (n=3). Plasma replacement with PBS effectively reduced the fibrinogen concentration.
Quantification of fibrinogen in PCP and PFC
To ascertain the effect of PPP replacement on fibrinogen removal, fibrinogen in 1/10v-PCP and 1/10v-PFC was measured (n=3). Fibrinogen levels in 1/10v-PCP were significantly higher (240.3±16.4 mg/dL) than that in 1/10v-PFC (37.0±5.2 mg/dL; Fig. 5B), suggesting that the replacement of fibrinogen-containing PPP with PBS successfully removed most, but not 100%, of the fibrinogen.
Discussion
The specific gravity differs among the components of blood. RBCs are the heaviest (1.095), followed by WBCs (1.063–1.085), whereas platelets (1.032) are the lightest. The specific gravity of WB is 1.055–1.060 in men and 1.050–1.056 in women, plasma's specific gravity is 1.025–1.029, and the specific gravity of serum is 1.024–1.028.25 Each component has been isolated by various centrifugation protocols, but cell contamination after separation cannot be avoided because of the components' slightly overlapping specific gravities,26 a fact further confirmed by this study. Thus, the use of WB is the only manner to use 100% of the platelets in a sample. However, this study clearly indicated the limitation of using WB, as a substantial portion of the total PDGF-BB was lost in the WB sample. This was presumably due to some PDGF-BB becoming trapped within the fibrin gel formed by coagulation (Fig. 4D).
For the optimization of the preparation protocol for a small volume of PFC, we selected some of the most readily available laboratory ware (15-mL and 1.5-mL conical tubes) to hold the sample during centrifugation and a spin time (10 min) short enough to be acceptable in clinical settings. Although the higher centrifugal force definitely leads to greater plasma recovery, it also leads to more platelets pelleting down out of the plasma and into the sediment layer. In this study, we found that the optimal gravity for maximum recovery of platelets varied between individuals likely because the specific gravity of blood differs between people due to individual differences in the number and composition of blood cells. This suggests that even physical conditions may affect the optimal centrifugal condition. Therefore, ideally, the optimal protocol would be personalized on a case-by-case basis. However, to establish a simple protocol, we concluded that 230–270 g and 70 g were the best centrifugal forces for PRP and W-PRP preparation, respectively, when a 10-min spin in a 15-mL conical tube was used. The optimal separation protocol differs based on many conditional parameters such as the size and shape of the container used, the time of spin, and the anticoagulant used. More than 30 years ago, Slichter and Harker27 reported that approximately 86% of platelets could be recovered by the use of a soft plastic bag of 250–450 mL with ACD as the anticoagulant. They used a first centrifugation of 9 min at 1000 g to harvest PRP, which was further centrifuged at 3000 g for 20 min to concentrate the platelets.
WBC contamination changes the growth factor profile in PRP. Hence, PRP that contained a substantial amount of WBCs was proposed as another therapeutic tool.28,29 Although their therapeutic values have yet to be established, terms, such as W-PRP, leukocyte (or WBC)-rich (or poor) PRP,28,29 and leucocyte- and platelet-rich plasma,23 have become used in the field. On centrifugation of WB, WBCs are concentrated in a white layer known as the buffy coat that is located above the RBC layer. Platelets are mostly concentrated right on the top of the buffy coat. W-PRP contains part of the buffy coat, but the platelet yield and obtained plasma volume are smaller than those of PRP.
PRP was separated from WB by the first slow spin. Since our PRP volume (2.63±0.32 mL) was approximately one-third the initial volume of WB (7.5 mL), the platelet concentration of PRP was twice to thrice that of WB (Fig. 3B). By reducing the volume of PRP while avoiding the activation (aggregation) of platelets, PCP was obtained through a second high spin. The platelet concentration of PCP depended on the volume reduction; the platelet concentration of 1/10v-PCP was more than 7 times more concentrated than that of PRP and nearly 20 times more concentrated than that of WB.
There was a low level of platelet loss at every step in preparation (Figs. 3A and 4B). For all steps except the first spin, this is thought to be mainly due to the aggregation of platelets. Platelet aggregation and activation result in the reduction of platelet count and the release of platelet-derived factors into the solution.16 Therefore, to obtain an ample, nonactivated platelet yield and a highly concentrated PFC, it is crucial to minimize platelet aggregation in the preparation process. In addition, blood coagulation (fibrin polymerization) is not easy to control. Fibrin glue formation reduces the fluid portion of plasma and increases the difficulty in standardizing PRP and PCP preparation. Thus, the role of anticoagulants to avoid coagulation and platelet aggregation is vital in this process.30
There are five major anti-coagulants used in clinical laboratories: heparin, sodium citrate, sodium fluoride, ACD, and EDTA, and all besides heparin chelate Ca2+ to some degree. ACD is a combination of citric acid and dextran and is usually used in the protocols of commercially available PRP preparation systems. Since dextrose has RBC protecting effects, ACD is frequently used to preserve blood for transfusions. EDTA is a stronger chelator than ACD30 and is used for general blood cell count. After a preliminary study, we selected ACD and EDTA as the anticoagulants to be used for PRP preparation in this study. Our results showed that the use of EDTA leads to a larger yield of platelets in PRP and 1/10v-PCP (Fig. 3C) and a higher concentration of PDGF-BB in 1/10v-PFC (Fig. 5A) than the use of ACD, indicating that EDTA had significantly larger inhibitory effects on coagulation and platelet aggregation.
When platelets are activated by thrombin, PDGF-BB and other platelet-derived factors such as EGF and TGF-β are released from aggregated platelets. A fibrin gel was formed in PRP and PCP by thrombin, and, consequently, the fluid portion of plasma was reduced. Clotting was not seen in PFC, because fibrinogen had been mostly removed by plasma replacement with PBS (Fig. 5B). Unexpectedly, the PDGF-BB concentration was much higher (nearly three times) in 1/10v-PFC than in 1/10v-PCP (Fig. 4C), even though the platelet concentrations of the two were similar (Fig. 4A). Moreover, surprisingly, the calculated total PDGF-BB amount in 1/10v-PFC was much larger than that of WB (Fig. 4D), even though the platelet yield of 1/10v-PFC was only 70% of WB (Fig. 4B). The total PDGF-BB amount in PRP and PCP was smaller than that of WB and almost in direct proportion to platelet yield of each product (Fig. 4B, D). These results indicated that the PDGF-BB released from platelets was partially lost in WB, PRP, and PCP, but not PFC. The presence of fibrinogen and the formation of fibrin clots after thrombin stimulation are suggested to be the reasons for the partial loss of PDGF-BB; a substantial amount of released PDGF-BB was trapped in fibrin glue and that the maximum amount of PDGF-BB cannot be obtained even by the direct activation of WB.
In the clinical use of PRP, fibrinogen in the PRP frequently leads to uncontrollable coagulation and platelet activation. Although fibrin gel may be useful as a controlled-release carrier of growth factors31 or as a filler injectable,32 the formed fibrin gel reduces the final product volume, hinders the easy injection of PRP, and is not preferable in many clinical situations. In this study, we have established easy-to-perform methods that use common laboratory ware and equipment for the preparation of PRP, W-PRP, PCP, and a highly concentrated solution of platelet derived factors (summarized in Fig. 6). The PDGF-BB concentration in 1/10v-PFC was 50 times higher than that of WB and highly exceeded (nearly 5 times) previous reports.4 Our results showed that the removal of fibrinogen from plasma is crucial to obtain the maximum amount of platelet-derived factors and that the replacement of PPP with PBS after a strong spin is an easy and efficient method to remove fibrinogen.
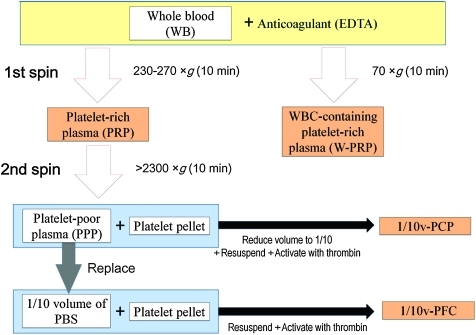
FIG. 6.
Conclusive preparation protocol for PRP-related products. Our optimized protocols for PRP-related products are as follows. WB collected using EDTA as an anticoagulant is centrifuged for 10 min at 230–270 g or 70 g in a 15-mL conical tube for the isolation of PRP and WBC-containing PRP, respectively. PRP is further centrifuged for 10 min at 2300 g (or higher) for the precipitation of platelets. For one-tenth volume of PCP (1/10v-PCP), nine-tenths volume of the supernatant PPP is removed, and the platelet pellet is resupended, followed by platelet activation with thrombin. For one-tenth volume of PFC (1/10v-PFC), all PPP is removed, one-tenth volume of PBS is added, and the platelet pellet is then resupended, followed by platelet activation with thrombin. Color images available online at www.liebertonline.com/tec
Platelet-derived factors are primarily released in the first phase of wound healing upon injury/hemorrhage. The injection of platelet-derived factor into a tissue appears to trigger the wound healing process without any actual wounding.5,33 It is thought that PRP induces local tissue remodeling and angiogenesis by activating tissue-resident progenitor/stem cells and may also recruit bone-marrow-derived progenitor/stem cells.33 For the clinical application of PRP-related products, a lot of questions remain to be answered, such as which clinical situations can benefit from the use of these products and what are the appropriate/effective concentrations and quantities for each product to be used in each therapeutic situation.34 A high concentration of platelet-derived factors may be crucial in inducing detectable therapeutic effects, and our established protocol may help further the advancement of therapies with PRP-related products as well as standardization of PRP-related products for comparative basic and clinical research.
Acknowledgments
The authors thank Ayako Kurata for technical assistance. This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT; contact grant number: B-21592283).
Disclosure Statement
No competing financial interests exist.
References
- 1.Flatow F.A., Jr. Freireich E.J. The increased effectiveness of platelet concentrates prepared in acidified plasma. Blood. 1966;27:449. [PubMed] [Google Scholar]
- 2.Boldt J. von Bormann B. Kling D. Jacobi M. Moosdorf R. Hempelmann G. Preoperative plasmapheresis in patients undergoing cardiac surgery procedures. Anesthesiology. 1990;72:282. doi: 10.1097/00000542-199002000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Weibrich G. Kleis W.K. Hafner G. Hitzler W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 4.Eppley B.L. Woodell J.E. Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 5.Aiba-Kojima E. Tsuno N.H. Inoue K. Matsumoto D. Shigeura T. Sato T. Suga H. Kato H. Nagase T. Gonda K. Koshima I. Takahashi K. Yoshimura K. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen. 2007;15:511. doi: 10.1111/j.1524-475X.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M. Zhang Z. Ni A. Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mimeault M. Batra S.K. Recent advances on skin-resident stem/progenitor cell functions in skin regeneration, aging and cancers and novel anti-aging and cancer therapies. J Cell Mol Med. 2010;14:116. doi: 10.1111/j.1582-4934.2009.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucarelli E. Beccheroni A. Donati D. Sangiorgi L. Cenacchi A. Del Vento A.M. Meotti C. Bertoja A.Z. Giardino R. Fornasari P.M. Mercuri M. Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 9.Anitua E. Andia I. Ardanza B. Nurden P. Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 10.Niemeyer P. Fechner K. Milz S. Richter W. Suedkamp N.P. Mehlhorn A.T. Pearce S. Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 11.Marx R.E. Carlson E.R. Eichstaedt R.M. Schimmele S.R. Strauss J.E. Georgeff K.R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 12.Khalafi R.S. Bradford D.W. Wilson M.G. Topical application of autologous blood products during surgical closure following a coronary artery bypass graft. Eur J Cardiothorac Surg. 2008;34:360. doi: 10.1016/j.ejcts.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Alio J.L. Abad M. Artola A. Rodriguez-Prats J.L. Pastor S. Ruiz-Colecha J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007;114:1286. doi: 10.1016/j.ophtha.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Nikolidakis D. Jansen J.A. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14:249. doi: 10.1089/ten.teb.2008.0062. [DOI] [PubMed] [Google Scholar]
- 15.Savarino L. Cenni E. Tarabusi C. Dallari D. Stagni C. Cenacchi A. Fornasari P.M. Giunti A. Baldini N. Evaluation of bone healing enhancement by lyophilized bone grafts supplemented with platelet gel: a standardized methodology in patients with tibial osteotomy for genu varus. J Biomed Mater Res B Appl Biomater. 2006;76:364. doi: 10.1002/jbm.b.30375. [DOI] [PubMed] [Google Scholar]
- 16.Eppley B.L. Pietrzak W.S. Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 17.Pallua N. Wolter T. Markowicz M. Platelet-rich plasma in burns. Burns. 2010;36:4. doi: 10.1016/j.burns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Foster T.E. Puskas B.L. Mandelbaum B.R. Gerhardt M.B. Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E. Sánchez M. Nurden A.T. Nurden P. Orive G. Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Man D. Plosker H. Winland-Brown J.E. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 21.Freymiller E.G. Aghaloo T.L. Platelet-rich plasma: ready or not? J Oral Maxillofac Surg. 2004;62:484. doi: 10.1016/j.joms.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Coombes B.K. Bisset L. Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 23.Dohan Ehrenfest D.M. Rasmusson L. Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Anitua E. Sánchez M. Orive G. Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Plötner R. Handbuch der gesamter Hämatologie, Band 11/2. zweiter Halbband, Műnchen, Berlin: Urban & Schwanzenberg; 1960. p. 254. [Google Scholar]
- 26.Kurita M. Aiba-Kojima E. Shigeura T. Matsumoto D. Suga H. Inoue K. Eto H. Kato H. Aoi N. Yoshimura K. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008;122:438. doi: 10.1097/PRS.0b013e31817d618d. [DOI] [PubMed] [Google Scholar]
- 27.Slichter S.J. Harker L.A. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976;34:395. doi: 10.1111/j.1365-2141.1976.tb03586.x. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann R. Reske S. Metzler P. Schlegel A. Ringwald J. Eckstein R. Preparation of highly concentrated and white cell-poor platelet-rich plasma by plateletpheresis. Vox Sang. 2008;95:20. doi: 10.1111/j.1423-0410.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 29.Castillo T.N. Pouliot M.A. Kim H.J. Dragoo J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 30.McShine R.L. Sibinga S. Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12:277. doi: 10.1111/j.1365-2257.1990.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 31.Catelas I. Dwyer J.F. Helgerson S. Controlled release of bioactive transforming growth factor beta-1 from fibrin gels in vitro. Tissue Eng Part C Methods. 2008;14:119. doi: 10.1089/ten.tec.2007.0262. [DOI] [PubMed] [Google Scholar]
- 32.Hoben G. Schmidt V.J. Bannasch H. Horch R.E. Tissue augmentation with fibrin sealant and cultured fibroblasts: a preliminary study. Aesthetic Plast Surg. 2011 doi: 10.1007/S00266-011-9724-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Eto H. Suga H. Aoi N. Kato H. Araki J. Doi K. Higashino T. Yoshimura K. Adipose injury-associated factors activate adipose stem/stromal cells, induce neoangiogenesis, and mitigate hypoxia in ischemic tissues. Am J Pathol. 2011;178:2322. doi: 10.1016/j.ajpath.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Zapata M.J. Martí-Carvajal A. Solà I. Bolibar I. Angel Expósito J. Rodriguez L. García J. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion. 2009;49:44. doi: 10.1111/j.1537-2995.2008.01945.x. [DOI] [PubMed] [Google Scholar]