Abstract
Background
Assumptions about survival of HIV-infected children in Africa without antiretroviral therapy need to be updated to inform ongoing UNAIDS modelling of paediatric HIV epidemics among children. Improved estimates of infant survival by timing of HIV-infection (perinatally or postnatally) are thus needed.
Methodology/Principal Findings
A pooled analysis was conducted of individual data of all available intervention cohorts and randomized trials on prevention of HIV mother-to-child transmission in Africa. Studies were right-censored at the time of infant antiretroviral initiation. Overall mortality rate per 1000 child-years of follow-up was calculated by selected maternal and infant characteristics. The Kaplan-Meier method was used to estimate survival curves by child's HIV infection status and timing of HIV infection. Individual data from 12 studies were pooled, with 12,112 children of HIV-infected women. Mortality rates per 1,000 child-years follow-up were 39.3 and 381.6 for HIV-uninfected and infected children respectively. One year after acquisition of HIV infection, an estimated 26% postnatally and 52% perinatally infected children would have died; and 4% uninfected children by age 1 year. Mortality was independently associated with maternal death (adjusted hazard ratio 2.2, 95%CI 1.6–3.0), maternal CD4<350 cells/ml (1.4, 1.1–1.7), postnatal (3.1, 2.1–4.1) or peri-partum HIV-infection (12.4, 10.1–15.3).
Conclusions/Results
These results update previous work and inform future UNAIDS modelling by providing survival estimates for HIV-infected untreated African children by timing of infection. We highlight the urgent need for the prevention of peri-partum and postnatal transmission and timely assessment of HIV infection in infants to initiate antiretroviral care and support for HIV-infected children.
Introduction
Sub-Saharan Africa remains the region most heavily affected by HIV. In 2008, an estimated 1,800,000 HIV-infected children under 15 years were living in sub-Saharan Africa, and this continent accounted for 91% of new HIV infections among children [1]. While it is important to have accurate estimates of survival when children are treated with antiretroviral therapy, assumptions about survival of HIV-infected children in Africa in the absence of treatment [2], [3] need to be updated and refined to inform ongoing UNAIDS modelling of HIV epidemiology among children. For this purpose, UNAIDS convened a working group to attempt to pool data from all available clinical trials on mother-to-child HIV transmission prevention conducted in sub-Saharan Africa over the last 15 years, to reliably assess mortality rates in HIV-infected children.
More specifically, precise estimates of infant survival by timing of HIV-infection (perinatally or postnatally through breastfeeding) are urgently needed. Such estimates will provide benchmarks for assessing the impacts of paediatric HIV treatment whilst allowing for the effect of background mortality on survival post-infection of children. We here present the results based on data with consistent information on timing of acquisition of infection, both before or during delivery, and postnatally to reliably estimate infant survival by timing of HIV-infection. We update the previously conducted individual patient meta-analysis on survival in HIV-exposed children [3] which had limited statistical power especially on the timing of acquisition of infection.
Methods
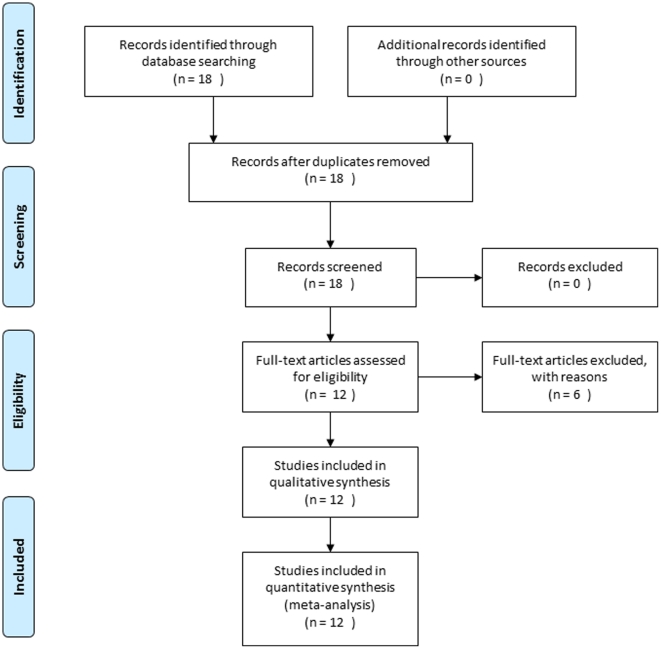
Studies eligible for this pooled analysis were clinical trials or cohort studies in sub-Saharan African countries that investigated the effect of antiretroviral-based and/or infant feeding interventions in risk reduction of HIV vertical transmission with paediatric follow-up for 18–24 months and information about infant feeding. Overall, 18 trials and cohort studies aimed at the prevention of mother-to-child transmission of HIV in African settings were eligible for this pooled analysis and contacted (Figure 1). Of these, four teams did not want to participate or could not share the data at the time of the analysis [4], [5], [6], [7], two datasets were excluded (missing key variables) [8], [9], and 12 provided the necessary data [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. The PRISMA checklist is available as supporting information; see Checklist S1.
Figure 1. The PRISMA flow chart showing the progress of trials through the review.
Paediatric HIV infection was defined as a positive plasma HIV-1 DNA/RNA PCR at any age. Children with a positive HIV-1 diagnostic test result before 4–6 weeks of age (depending on the timing of collection of blood samples in each study) were considered to be HIV-infected in peri-partum [22], [23]. This includes infants infected in utero and DNA/RNA positive on the day of birth. Children with a negative DNA/RNA PCR from a sample obtained at or after 4–6 weeks of age who subsequently acquired infection were considered to be HIV-infected postnatally [22], [23]. Children with positive test results at or after 4–6 weeks of age but with either no previous negative test result, or last negative test result before 4–6 weeks were considered infected with unknown timing of infection [22], [23].
Antiretroviral treatment became available to children in the Mashi trial on the first of October 2002, so follow-up was rightly censored at this point. Infant antiretroviral treatment was not available during the time of the other trials. Maternal antiretroviral treatment was available in the Mitra Plus study only.
Baseline characteristics were described for each study included in the pooled analysis. Overall mortality as a rate per 1000 child-years of follow-up was calculated by selected maternal and infant characteristics. The Kaplan-Meier method was used to estimate survival curves among HIV-infected children: children infected in peri-partum, infected in postnatal, and infected with unknown timing of infection. Estimated time of diagnosis of HIV-infection for those infected postnatally was taken as mid-way between the last HIV-negative test and the first HIV-positive test. The survival of children infected peri-partum or with unknown timing was estimated from birth. Studies were right-censored at the time of infant antiretroviral initiation. We used random-effects Weibull regression models to estimate mortality hazard ratios accounting for heterogeneity between trials and cohorts. Adjusted hazard ratios were obtained, both overall and for HIV-uninfected and HIV-infected children separately. The multivariable models included the following variables: region of origin (South Africa, East Africa, West Africa), sex (boys, girls, unknown), maternal vital status (time-varying: alive, dead), antenatal maternal CD4 count in cells/ml (≥350, <350, unknown), breastfeeding practice in the first year of life (ever, never), and child HIV-infection status (uninfected, determined infected in peri-partum, infected postnatally, infected with unknown timing). Age at time of infection was allowed for in the model.
Results
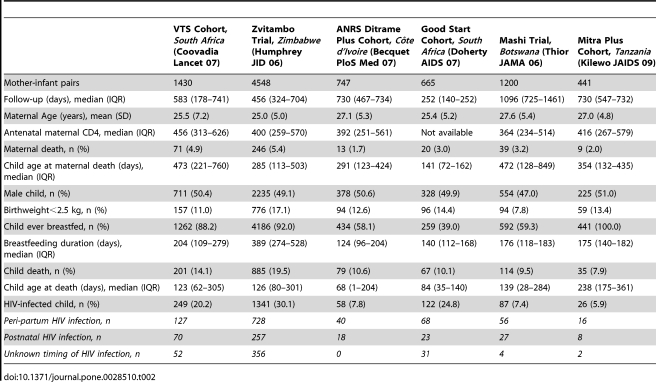
The characteristics of the datasets included in the first step analysis are detailed in Table 1 and Table 2. Overall 12,112 infants born to HIV-infected mothers were included. Children with unknown HIV status were excluded from the analysis (n = 639). Analysis was therefore conducted among uninfected children (n = 8,964) and HIV-infected (n = 2,509, HIV infection acquired perinatally, through breastfeeding, or with unknown timing).
Table 1. Characteristics of the mother-infant pairs included in the pooled analysis (first generation studies, before year 2000).
| ANRSa Ditrame Trial, Côte d'Ivoire, Burkina Faso (Dabis Lancet 99) | ANRSb Ditrame Trial, Burkina Faso, Côte d'Ivoire (Msellati Sex Transm Infect 99) | Nairobi Trial, Kenya (Nduati Lancet 01) | Petra Trial, Tanzania, South Africa, Uganda (Petra Group Lancet 02) | Retro-CI Trial, Côte d'Ivoire (Wiktor Lancet 99) | Vitamin A Trial, South Africa (Coutsoudis Lancet 99) | |
| Mother-infant pairs | 401 | 104 | 197 | 1458 | 261 | 660 |
| Follow-up (days), median (IQR) | 550 (303–639) | 542 (293–589) | 729 (332–849) | 548 (388–554) | 730 (290–1008) | 300 (128–456) |
| Maternal Age (years), mean (SD) | 25.6 (5.3) | 24.5 (5.0) | 23.9 (4.4) | 27.0 (8.1) | 26.4 (5.2) | 26.2 (4.7) |
| Antenatal maternal CD4, median (IQR) | 541 (359–741) | 524 (384–768) | 392 (262–530) | 449 (295–630) | 549 (354–726) | 440 (311–597) |
| Maternal death, n (%) | 18 (4.5) | 9 (8.7) | 17 (8.6) | 61 (4.2) | 9 (3.4) | 5 (0.8) |
| Child age at maternal death (days), median (IQR) | 274 (80–561) | 342 (97–379) | 240 (171–413) | 341 (234–441) | 142 (128–319) | 229 (13–232) |
| Male child, n (%) | 207 (51.6) | 49 (47.2) | 96 (48.7) | 726 (49.9) | 131(50.2) | 317 (50.3) |
| Birthweight<2.5 kg, n (%) | 62 (15.5) | 18 (17.3) | 13 (6.6) | 96 (6.6) | 27 (10.3) | 74 (11.2) |
| Child ever breastfed, n (%) | 401 (100.0) | 104 (100.0) | 197 (100.0) | 1397 (95.8) | 261 (100.0) | 452 (68.5) |
| Breastfeeding duration (days), median (IQR) | 274 (183–295) | 342 (231–484) | 398 (152–548) | 212 (69–420) | 458 (336–559) | 90 (30–278) |
| Child death, n (%) | 88 (22.0) | 26 (25.0) | 45 (22.8) | 184 (12.6) | 39 (14.9) | 49 (7.4) |
| Child age at death (days), median (IQR) | 202 (88–429) | 175 (54–505) | 247 (122–366) | 171 (82–293) | 101 (4–339) | 86 (36–153) |
| HIV-infected child, n (%) | 89 (24.0) | 14 (16.5) | 59 (30.9) | 253 (18.1) | 68 (27.2) | 143 (22.8) |
| Peri-partum HIV infection, n | 38 | 1 | 27 | 110 | 41 | 111 |
| Postnatal HIVinfection, n | 22 | 5 | 19 | 87 | 23 | 22 |
| Unknown timing of HIV infection, n | 29 | 8 | 13 | 56 | 4 | 10 |
Table 2. Characteristics of the mother-infant pairs included in the pooled analysis (second generation studies, after year 2000).
Overall, 1,363 children acquired infection perinatally with median time of first positive DNA/RNA test at 19 days (interquartile range: 1–42 days), 581 children were negative at or after 4 weeks of age and acquired infection through breastfeeding at a median 24 weeks of age (interquartile range: 7–39 weeks), and 565 children were infected with unknown timing of infection and had a first positive DNA/RNA test at 80 days in median (interquartile range: 51–168 days).
Crude mortality rates per 1,000 child-years of follow-up were 39.3 and 381.6 for HIV-uninfected and HIV-infected children, respectively. Mortality rate per 1000 child-years of follow-up by selected maternal and infant characteristics are detailed in Table 3.
Table 3. Mortality rate per 1000 child-years of follow-up by selected maternal and infant characteristics (n = 12,112).
| Number (n = 12,112) | Deaths (n = 1,812) | Mortality rate per 1000 child-years of follow-up | |
| Location | |||
| Southern Africa | 9270 (76.5) | 1384 | 105.8 |
| Eastern Africa | 1329 (11.0) | 196 | 100.1 |
| Western Africa | 1513 (12.5) | 232 | 100.0 |
| Maternal PMTCT ARV | |||
| None | 6634 (54.8) | 1179 | 149.2 |
| sdNVP | 1657 (13.7) | 223 | 99.3 |
| 2 or 3 drugs regimen | 3821 (31.5) | 410 | 56.8 |
| Infant PMTCT ARV | |||
| None | 6892 (46.9) | 1227 | 141.9 |
| sdNVP +/− ZDV/3TC | 5220 (53.1) | 585 | 67.1 |
| Maternal CD4 count | |||
| <350 | 3973 (32.8) | 796 | 135.2 |
| ≥350 | 6605 (54.5) | 776 | 77.2 |
| Unknown | 1534 (12.7) | 240 | 167.7 |
| Maternal vital status | |||
| Mothers known alive | 11595 (95.7) | 1598 | 95.6 |
| Mothers known dead | 517 (4.3) | 214 | 332.3 |
| Sex of the infant | |||
| Girls | 5957 (49.2) | 883 | 103.5 |
| Boys | 6072 (50.1) | 918 | 104.3 |
| Unknown | 83 (0.7) | 11 | - |
| Infant birthweight | |||
| <2.5 kg | 1566 (12.9) | 445 | 232.4 |
| ≥2.5 kg | 10372 (85.6) | 1333 | 87.3 |
| Unknown | 174 (1.5) | 34 | - |
| Breastfeeding status | |||
| Never | 2126 (17.5) | 310 | 113.9 |
| Ever | 9986 (82.5) | 1502 | 102.6 |
| Child infection status | |||
| HIV-uninfected | 8964 (74.0) | 562 | 39.3 |
| HIV-infected | 2509 (20.7) | 1096 | 381.6 |
| Unknown | 639 (5.3) | 154 | - |
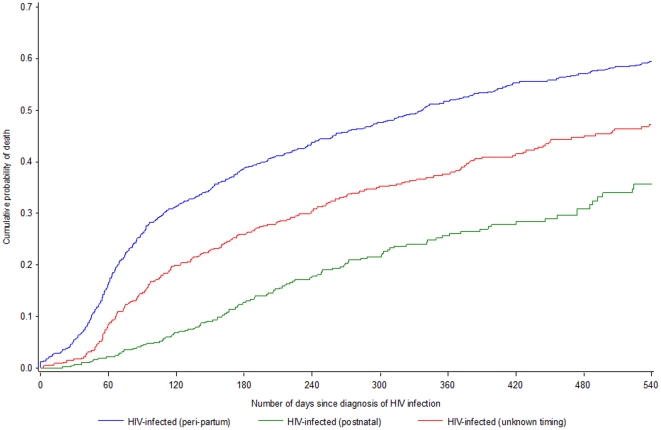
Mortality was estimated for HIV-infected children by timing of transmission; initial time was the estimated date of acquisition of HIV-infection (Figure 2). Overall, 12 months post-acquisition of infection, an estimated 52% of children with peripartum infection (95% confidence interval [CI]: 49–55%) and 26% (95% CI: 22–31%) of those with postnatal infection died. Among children with unknown timing, an estimated 38% (95% CI: 22–31%) died compared with an estimated 4% of uninfected children who died by age 1 year. Detailed probabilities are available as supporting information; see Table S1.
Figure 2. Estimated 18-month unadjusted mortality for HIV-infected children since acquisition of HIV infection (n = 2,509).
Adjusted mortality hazard ratios (aHR) with 95% CI by selected characteristics were estimated in regression analyses among HIV-infected and uninfected children (n = 11,473). With South Africa as reference, mortality was significantly higher in East Africa, aHR 1.41 (1.14–1.74), but not West Africa, aHR 1.20 (0.88–1.63). Mortality did not differ significantly by sex: with boys as reference, aHR in girls was 1.02 (0.76–1.36). When the mother died during follow-up, children were more than twice likely to die (aHR 2.21 (1.63–3.00)) than when mothers survived. With mothers with CD4≥350 cells/ml as reference, children born to women with CD4<350 (aHR 1.36 (1.08–1.71)) or to women with unknown CD4 (aHR 1.30 (0.72–2.37)) were at significantly increased risk of dying. Children who had never been breastfed were at more than double the risk of dying than ever breastfed children (aHR 2.21 (1.62–3.01)). Mortality was substantially and significantly associated with child HIV infection status: compared to uninfected children, the aHRs were 12.45 (10.15–15.27) for perinatally infected children, 3.08 (2.29–4.14) for children infected through breastfeeding and 7.21 (5.53–9.39) for children with unknown timing of infection.
Discussion
Mortality of children born to HIV-infected mothers was assessed on a large dataset from three different regions in sub-Saharan Africa. Overall mortality of HIV-infected children was dramatically elevated, irrespective of timing of acquisition of infection. However, among HIV-infected children, survival from acquisition of infection in postnatally infected children was higher than in those with infection acquired around delivery: the 18-month post-infection mortality risks were 36% (30%–42%) and 60% (57%–63%), respectively. Most these children had been included in research studies offering relatively high standard of care compared to less supported field areas; a closed clinical follow-up adapted to the child's age with free provision of care was provided to these children. Hence, we assume the survival estimates presented here were higher than what would be expected in more operational field settings. Our results are in accordance with previous findings of high mortality in vertically-infected children [24]: more than half of these children will have died by age two years. While the previously conducted meta-analysis on the subject had limited statistical power to assess infant mortality according to the timing of acquisition of HIV infection, we here present more reliable estimates on this matter.
Our analysis strategy implied that the survival was assessed from the estimated timing of infection in order to assess survival after infection for perinatally and postnatally infected children. This estimate would thus need to be interpreted in the light of the selection that occurs with children at risk of postnatal infection by definition being those who survived without infection the neonatal period which is one of high mortality risk. On the other hand, perinatally infected children will have to survive this risky period with the infection as an added exposure. However, there is no evidence of an increased neonatal mortality risk associated with perinatal infection [25]. Further, complementary analyses on the same dataset showed that differences seen in the survival of perinatally or postnatally infected children cannot be explained by differences in background mortality, which is much higher in the neonatal period [26]. We suggest that our findings are explained by the immunological immaturity, and thus reduced virological control, of the fetus and new-born at the time of acquisition of infection whereas postnatal acquisition of infection in infants who are more immunologically mature would be associated with a greater chance of survival [27].
We further also confirm the impact of both maternal health, as measured by her HIV immunological progression, and vital status on infant survival. Two-year infant mortality adjusted risk was one third higher among women with ante-partum CD4 count <350 cells/ml than among those with mothers a CD4 count above this threshold; and it was two times higher when the mother had died during the two years post-partum than when the mother was alive two years after birth. Lifelong antiretroviral treatment was not available to women followed-up in all studies selected for this pooled analysis but one. By lack of statistical power, it was therefore not possible to assess the impact of maternal antiretroviral treatment on the survival of children. Bearing in mind that maternal health is an important confounder of child survival, it would be crucial to investigate whether there is a positive impact of such a maternal treatment on infant survival. As maternal antiretroviral treatment keeps an infected mother alive, there would be an indirect effect of reducing child mortality in both her HIV-infected and uninfected children.
We present estimates of risk of mortality in children born to HIV-infected mothers in Africa, on the basis of a large dataset with wide variations in the characteristics of the patients included in the meta-analysis. The individual meta-analysis and adjustments in the model account for some of these effects. We acknowledge that there may have been confounding factors that had not been taken into account by our design, but we consider that the most important ones were controlled for. Additionally, the science in the prevention of mother-to-child transmission of HIV has changed since the time of the studies included in this meta-analysis. Expanding the indication of use of potent antiretroviral drug combinations to all pregnant, delivering, and breast-feeding HIV-infected women is now seen as an unprecedented opportunity to radically reduce the burden of paediatric AIDS [28]. The implication of this on the predicted trends in child mortality will need to be carefully assessed in future studies.
Our findings draw attention to the importance of comprehensive care for HIV-infected women. More specifically, our results stress the urgency of providing optimal antiretroviral treatment to women who need it for their own health (e.g. with ante-partum CD4 count <350 cell/ml according to the newly revised WHO guidelines [29]) to also improve survival of their (infected and uninfected) children. Maternal antiretroviral treatment will radically reduce the burden of paediatric HIV worldwide, and contribute to the improvement of survival of children born to HIV-infected women in improving maternal health and considerably lowering the risk of maternal death [30]. Moreover, there is still an urgent need for the effective prevention of mother-to-child transmission of HIV through breastfeeding: even postnatally infected children are three-times more likely to die than uninfected children. These findings also highlight the urgent need for the early assessment of HIV infection in HIV-exposed children to allow the timely initiation of antiretroviral care and support for HIV-infected children. Finally, these revised estimates of infant survival by timing of acquisition of HIV infection will now be used by UNAIDS to improve forecasts of the future of the HIV pandemic [31].
Supporting Information
PRISMA checklist.
(DOC)
Probability with 95%CI of 18-month unadjusted mortality for HIV-infected children since acquisition of HIV infection (n = 2,509).
(DOCX)
Acknowledgments
Composition of the UNAIDS Child survival group
Coordination. Renaud Becquet, François Dabis (INSERM, Unit 897, Bordeaux, France); Milly Marston, Basia Zaba (London School of Hygiene and Tropical Medicine, London, UK); Marie-Louise Newell (Africa Centre for Health and Population Studies, University of KwaZulu Natal, South Africa); Peter Ghys (UNAIDS, Epidemiology and Analysis Division, Geneva, Switzerland).
Data management. Sophie Karcher, Renaud Becquet (INSERM, Unit 897, Bordeaux, France).
Representatives of the participating studies. Larry Moulton (Zvitambo trial, Zimbabwe); Anna Coutsoudis (Vitamin A trial, South Africa); Glenda Gray (Petra trial, Tanzania-SA-Uganda); Charles Kilewo (Mitra cohort, Tanzania); Jerry Coovadia (VTS cohort, South Africa); Valériane Leroy (ANRSa trial, Côte d'Ivoire); Max Essex (Mashi trial, Botswana); Stephan Wiktor (Retro-Ci trial, Côte d'Ivoire); Didier Ekouevi (Ditrame Plus cohort, Côte d'Ivoire); Ruth Nduati (Nairobi trial, Kenya); Debra Jackson (Good Start cohort, South Africa); Philippe Msellati (ANRSb trial, Burkina Faso).
Investigators and collaborators of the participating studies
ANRSa Trial, Côte d'Ivoire & Burkina Faso. Investigators: François Dabis, Philippe Msellati, Nicolas Meda, Christiane Welffens-Ekra, Bruno You, Olivier Manigart, Valériane Leroy, Arlette Simonon, Michel Cartoux, Patrice Combe, Amadou Ouangré, Rosa Ramon, Odette Ky-Zerbo, Crépin Montcho, Roger Salamon, Christine Rouzioux, Philippe Van de Perre, Laurent Mandelbrot.
Other investigators: L Dequae-Merchadou, R Lassalle (Bordeaux Coordination Unit); A Bazie, A M Cassel Beraud, B Dao, L Gautier-Charpentier, F D Ky, B Nacro, O Sanou, I Sombié, F Tall, S Tiendrebeogo, Y Traore, D Valea, S Yaro (Bobo-Dioulasso Centre); and D Bonnard, R Camara, M Dosso, N Elenga, G Gourvellec, J B Kottan, R Likikouet, V Noba, M Timité, I Viho.
ANRSb Trial, Burkina Faso & Côte d'Ivoire. Biostatistics: R Lassalle, V Leroy, R Salamon.
Epidemiology: M Cartoux, F Dabis (coordinator of the ANRS 049 trial/DITRAME programme), N Meda (coordinator of Bobo-Dioulasso Center), P Msellati (coordinator of Abidjan Center), R Ramon.
Gynaecology-obstetrics: A Bazié, B Dao, R Likikouet, L Mandelbrot (principal investigator), C Welffens-Ekra (principal investigator).
Microbiology: D Bonard, P Combe, M Dosso, L Gautier-Charpentier, F D Ky, A Ouangré, T Ouassa, O Sanou, F Sylla-Koko, Y Traore, P Van de Perre.
Molecular biology: A M Cassel-Beraud, J B Kottan, OManigart, CMontcho, C Rouzioux, A Simonon, D Valea, B You.
Paediatrics: R Camara, N Elenga, B Nacro, F Tall, M Timité.
Trial monitoring: G Gourvellec, O Ky-Zerbo, V Noba, I Sombié, S Tiendrebeogo, I Viho, S Yaro.
Nairobi Trial, Kenya. Ruth Nduati, Barbra A Richardson, Grace John, Dorothy Mbori-Ngacha, Anthony Mwatha, Jeckoniah Ndinya-Achola, Job Bwayo, Francis E Onyango, Joan Kreiss.
Petra Trial, Tanzania - South Africa – Uganda. Trial management committee
J M A Lange (chair), J Saba (study coordinator), G Gray, J McIntyre, F Mmiro, Ch Ndugwa, J Moodley, H M Coovadia, D Moodley, Ch Kilewo, A Massawe, P Okong, P Kituuka, H von Briesen, J Goudsmit, G Biberfeld, F Mhalu, K Karlson, M Guliano, S Declich, S Clapp, G Haverkamp, G J Weverling, D Cooper, A Grulich, D Bray, J Perriens. Representatives of People Living with HIV: F Ngobeni, G Baguma, S Kyambadde.
Participating clinical centres
Uganda: Mulago Hospital, Mulago, Kampala (F Mmiro, Ch Ndugwa, P Musoke, C Nakabito, P Bakaki, I Kalyesbula, M Lutajumwa, S Mmiro, R Kato, R Byenkya, S Kabenge, R Mulira, R Bagenda, J Matavu, F Kikonyogo, E Kawuki, B Nkoyoyo, N Matovu, C Mukasa, C Dukar, M Mubiru, D Bagenda, K Khantaway); St Francis Hospital, Nsambya, Kampala (P Okong, P Kituuka, K Kayanja, S Kateera, B Sam, B Sabrina, M Ravera, E Omobono, M Magoni).
Tanzania: Muhimbili General Hospital, Dar es Salaam (Ch Kilewo, A Massawe, F Mhalu, E Urassa, F Kalokola, F Phillip, M Giattas, F Temu, K Kallanga, A Mkumbukwa, E Lugaiya, S Nyoni, S Haule, E Hilbayuded, N Kavugha, I Kayombo, E Lyamuya, E Mbena, V Msangi, C Lema, H Matimbwa, I Semali, K Karlsson).
South Africa: Chris Hani Baragwanath Hospital, Johannesburg (S Johnson, A Violari, L Connell, G Nelson, J Moetlo, A Makhofola, B Jivkov, F Ngobeni, M Kunene, G Ngakane, G Tshabalala, W Saba, P Khela, N Radebe); King Edward VII Hospital, Durban (J Moodley, H M Coovadia, D Moodley K Naidoo, M Adhikari, T Moniwa, D Moholo, I Mtshali, C Ngubane, A Mlaba, N Mkhize, C Sibiya, L Shozi, T Ngubane, V Mkhize, L Madurai, V Gopaul, L Thaver, G Swart, J Thomas).
Trial coordination, central data management and statistical analysis
Switzerland, Geneva, UNAIDS (J Saba, J Perriens); and International Antiviral Therapy Evaluation Center (J M A Lange, G Haverkamp, S Clapp, H Huisman, P Hollak, L Hendriks), and Department of Clinical Epidemiology and Biostatistics (G J Weverling), Academic Medical Centre, Amsterdam, Netherlands.
Laboratories
Germany, Frankfurt: Georg-Speyer-Haus (A de Amorim-Nink, H Klunker, L Kurunci, H von Briesen, A Simon). Sweden, Stockholm: Swedish Institute for Infectious Disease Control and Microbiology and Tumorbiology Center Karolinska Institute (G Biberfeld, K Karlsson, E Olausson Hansson, A Östborn). Netherlands, Amsterdam: Academic Medical Centre–Department of Human Retrovirology (J Goudsmit, S Jurriaans, E de Rooy, M Bakker, N Romp).
Retro-CI Trial, Côte d'Ivoire
Stefan Z Wiktor, Ehounou Ekpini, John M Karon, John Nkengasong, Chantal Maurice, Sibailly T Severin, Thierry H Roels, Moise K Kouassi, Eve M Lackritz, Issa-Malick Coulibaly, Alan E Greenberg.
Vitamin A Trial, South Africa
Investigators: Anna Coutsoudis, Kubendran Pillay, Louise Kuhn, Elizabeth Spooner, Wei-Yann Tsai and Hoosen M. Coovadia.
Additional members: Gill Sinclair, Anne Mburu, Nolwandle Mngqundaniso, Kerry Uebel, Ingrid Coetzee, Ken Annamalai, Trevor Doorasamy, Ugene Govender, Juana Willumsen, Nigel Rollins, Jagidesa Moodley and Daya Moodley.
VTS Cohort, South Africa
Study investigators: Ruth Bland, Hoosen Coovadia (principal investigator), Anna Coutsoudis, Marie-Louise Newell, Nigel Rollins.
Steering Committee: Janet Darbyshire (chair), Nono Simelela (South African National Department of Health), Victoria Sithole (Community Advisory Board) and the study investigators.
Data management: Cookie Govender, Londiwe Mthethwa and team.
Clinical team: Thembi Blose, Nqobile Mkhwanazi, Dumo Mkwanazi and team.
Field team: Zanele Fakude, Samukelisiwe Dube and team.
Laboratory team: Johannes Viljoen, Natalie Graham, Siva Davaviah and team.
Zvitambo Trial, Zimbabwe
Investigators: Peter J. Iliff, Ellen G. Piwoz, Naume V. Tavengwa, Clare D. Zunguzac, Edmore T. Marinda, Kusum J. Nathoo, Lawrence H. Moulton, Brian J. Ward, Jean H. Humphrey.
Additional members: Henry Chidawanyika, John Hargrove, Florence Majo, Kuda Mutasa, Mary Ndhlovu, Robert Ntozini and Phillipa Rambanepasi (ZVITAMBO); Agnes Mahomva (AIDS and TB Unit, Ministry of Health and Child Welfare, Zimbabwe); Lucie Malaba (Faculty of Science, University of Zimbabwe); Michael Mbizvo, Partson Zvandasara and Lynn Zijenah (University of Zimbabwe College of Health Sciences); Lidia Propper and Andrea Ruff (The Johns Hopkins Bloomberg School of Public Health, Department of International Health).
ANRS Ditrame Plus Cohort, Côte d'Ivoire
Principal Investigators: Francois Dabis, Valériane Leroy, Marguerite Timite-Konan, Christiane Welffens-Ekra.
Coordination in Abidjan: Laurence Bequet, Didier K. Ekouevi, Besigin Tonwe-Gold, Ida Viho.
Methodology, biostatistics and data management: Gérard Allou, Renaud Becquet, Katia Castetbon, Laurence Dequae-Merchadou, Charlotte Sakarovitch, Dominique Touchard.
Clinical team: Clarisse Amani-Bosse, Ignace Ayekoe, Gédéon Bedikou, Nacoumba Coulibaly, Christine Danel, Patricia Fassinou, Apollinaire Horo, Ruffin Likikouet, Hassan Toure. Laboratory team: André Inwoley, Francois Rouet, Ramata Touré.
Psycho-social team: Hortense Aka-Dago, Alphonse Sihé.
Social sciences team: Hélène Agbo, Hermann Brou, Annabel Desgrées-du-Lou , Annick Tijou-Traoré, Benjamin Zanou.
Good Start Cohort, South Africa
Mark Colvin, Mickey Chopra, Tanya Doherty, Debra Jackson, Jonathan Levin, Juana Willumsen, Ameena Goga, Pravi Moodley.
MASHI Trial, Botswana (Trial Registration: clinicaltrials.gov Identifier: NCT00197587).
Investigators: Ibou Thior, Shahin Lockman, Laura M. Smeaton, Roger L. Shapiro, Carolyn Wester, S. Jody Heymann, MD, Peter B. Gilbert, Lisa Stevens, Trevor Peter, PhD, Soyeon Kim, Erik van Widenfelt, Claire Moffat, Patrick Ndase, Peter Arimi, Poloko Kebaabetswe, Patson Mazonde, Joseph Makhema, Kenneth McIntosh, Vladimir Novitsky, Tun-Hou Lee, Richard Marlink, Stephen Lagakos, Max Essex.
Collaborators: Drs C. Anude and J. Chanda, study physicians; L. Makori, nursing diploma, study nurse; J. B. Moorad, T. A. Modise, T. Moyo, and M. Malamba, nursing and midwifery diplomas, study nurses; D. Arbi and K. Koloi, nursing diplomas, nurse recruiters; L. Dube and T. Mmolotsi, health education diplomas, health educators and recruiters; S. Babitseng and D. Mere, nursing diplomas, recruiters (Molepolole site); Dr J. Boyle, study physician; J. Magetse, V. Modikwa, and M. Tsuro, nursing and midwifery diplomas, study nurses; T. Sekoto, family nurse practitioner diploma, study nurse; L. Garebatho, nursing diploma, study nurse; M. Sesinyi and K. Kelebalekgosi, health educator diplomas, health educators and recruiters (Mochudi site); Dr Z. Tedla, study physician; G. Mayondi, K. Sebinang, J. Setswalo, nursing and midwifery diplomas, study nurses; N. Makubate, community health nursing and midwifery diploma, study nurse; L. Tsalaile, MSc nursing education, study nurse; B. Tsule, nursing diploma, study nurse; I. Thebeetsile, nursing diploma, nurse recruiter; I. Leteane and O. Makgabana, health education diplomas, health educators and recruiters (Lobatse Site); Drs. M. Mogodi, A. Owor, I. Hove, and A. Asmelash, study physicians; T. Kakhu, P. Ramalepa, and J. Lubinda, nursing and midwifery diplomas, study nurses; S. Ndebele, F. Modise, C. Bohule, K. Motshabi, and M. Ntshimane, nursing diplomas, nurse recruiters; (Gaborone site).
MITRA Plus Cohort, Tanzania
Investigators: Charles Kilewo, Katarina Karlsson, Matilda Ngarina, Augustine Massawe, Eligius Lyamuya, Andrew Swai, Rosina Lipyoga, Fred Mhalu, Gunnel Biberfeld.
Physicians: G. Msemo, B. Mohamed, J. Method, J. Yuda, E. Naburi.
Nurses: A. Mkumbukwa, E. Rugaiya, N. Makundi, A. Temu.
Laboratory Technologists: E. Mbena, D. Kalovya, V. Msangi, E Olausson-Hansson and A. Östborn.
Secretary: C. Lema.
Footnotes
Competing Interests: Co-author Marie-Louise Newell is an Academic Editor for PLoS ONE. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials..
Funding: This pooled analysis was funded by the Epidemiology and Analysis Division of UNAIDS (Geneva, Switzerland). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. AIDS epidemic update (UNAIDS website. 2009. Available: http://www.unaids.org/en/dataanalysis/epidemiology/2009aidsepidemicupdate. Accessed 2011 December 7)
- 2.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, et al. Effects of Early, Abrupt Weaning for HIV-free Survival of Children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 6.Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, et al. Short postexposure prophylaxis in newborn babies to reduce mother-to-child transmission of HIV-1: NVAZ randomised clinical trial. Lancet. 2003;362:1171–1177. doi: 10.1016/S0140-6736(03)14538-2. [DOI] [PubMed] [Google Scholar]
- 7.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaillard P, Mwanyumba F, Verhofstede C, Claeys P, Chohan V, et al. Vaginal lavage with chlorhexidine during labour to reduce mother-to-child HIV transmission: clinical trial in Mombasa, Kenya. Aids. 2001;15:389–396. doi: 10.1097/00002030-200102160-00012. [DOI] [PubMed] [Google Scholar]
- 9.Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, et al. A Multicenter Randomized Controlled Trial of Nevirapine Versus a Combination of Zidovudine and Lamivudine to Reduce Intrapartum and Early Postpartum Mother-to-Child Transmission of Human Immunodeficiency Virus Type 1. J Infect Dis. 2003;187:725–735. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 10.Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, et al. Two-year morbidity–mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d'Ivoire. PLoS Medicine. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding: the first six months of life. Lancet. 2007;369:1607–1616. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 12.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 13.Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 15.Jackson DJ, Chopra M, Doherty TM, Colvin MS, Levin JB, et al. Operational effectiveness and 36 week HIV-free survival in the South African programme to prevent mother-to-child transmission of HIV-1. Aids. 2007;21:509–516. doi: 10.1097/QAD.0b013e32801424d2. [DOI] [PubMed] [Google Scholar]
- 16.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 17.Msellati P, Meda N, Leroy V, Likikouet R, Van de Perre P, et al. Safety and acceptability of vaginal disinfection with benzalkonium chloride in HIV infected pregnant women in west Africa: ANRS 049b phase II randomized, double blinded placebo controlled trial. DITRAME Study Group. Sex Transm Infect. 1999;75:420–425. doi: 10.1136/sti.75.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. Jama. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 19.Petra study team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 20.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. Jama. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 21.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 22.Alioum A, Cortina-Borja M, Dabis F, Dequae-Merchadou L, Haverkamp G, et al. Estimating the efficacy of interventions to prevent mother-to-child transmission of human immunodeficiency virus in breastfeeding populations: comparing statistical methods. Am J Epidemiol. 2003;158:596–605. doi: 10.1093/aje/kwg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alioum A, Dabis F, Dequae-Merchadou L, Haverkamp G, Hudgens M, et al. Estimating the efficacy of interventions to prevent mother-to-child transmission of HIV in breast-feeding populations: development of a consensus methodology. Stat Med. 2001;20:3539–3556. doi: 10.1002/sim.1076. [DOI] [PubMed] [Google Scholar]
- 24.Newell ML, Brahmbhatt H, Ghys PD. Child mortality and HIV infection in Africa: a review. AIDS. 2004;18:S27–S34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rollins NC, Coovadia HM, Bland RM, Coutsoudis A, Bennish ML, et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007;44:321–328. doi: 10.1097/QAI.0b013e31802ea4b0. [DOI] [PubMed] [Google Scholar]
- 26.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty R. HIV-1 infection in children: a clinical and immunologic overview. Curr HIV Res. 2005;3:31–41. doi: 10.2174/1570162052773022. [DOI] [PubMed] [Google Scholar]
- 28.Becquet R, Ekouevi DK, Arrive E, Stringer J, Meda N, et al. Universal antiretroviral therapy for pregnant and breast-feeding HIV-infected women: towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis. 2009;49:1936–1945. doi: 10.1086/648446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Recommendations for Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. 2010. Guidelines on Care, Treatment and Support for Women Living with HIV/AIDS and their Children in Resource-Constrained Settings (WHO website. Available: http://www.who.int/hiv/pub/mtct/guidelines/en/. Accessed 2011 December 7) [PubMed]
- 30.Ndirangu J, Newell ML, Tanser F, Herbst AJ, Bland R. Decline in early life mortality in a high HIV prevalence rural area of South Africa: evidence of HIV prevention or treatment impact? Aids. 2010;24:593–602. doi: 10.1097/QAD.0b013e328335cff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stover J, Johnson P, Hallett T, Marston M, Becquet R, et al. The Spectrum projection package: improvements in estimating incidence by age and sex, mother-to-child transmission, HIV progression in children and double orphans. Sex Transm Infect. 2010;86(Suppl 2):ii16–21. doi: 10.1136/sti.2010.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Probability with 95%CI of 18-month unadjusted mortality for HIV-infected children since acquisition of HIV infection (n = 2,509).
(DOCX)