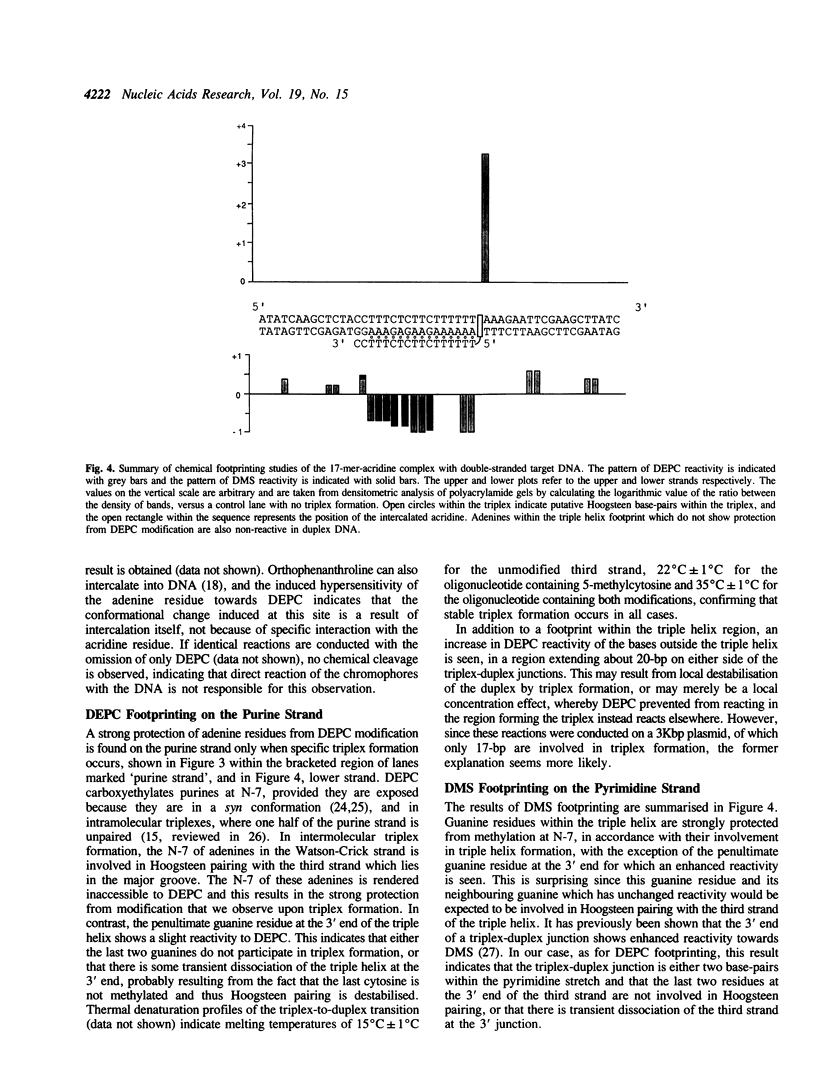
Abstract
Using site-specific intercalation directed by intermolecular triplex formation, the conformation of an intercalation site in DNA was examined by footprinting with the purine-specific (A much greater than G) reagent diethylpyrocarbonate. Site specific intercalation was achieved by covalently linking an intercalator to the 5' end of a homopyrimidine oligodeoxynucleotide, which bound to a homopurinehomopyrimidine stretch in a recombinant plasmid via intermolecular triplex formation. This directs intercalation to a single site in 3kb of DNA at the 5' triplex-duplex junction. Footprinting with diethylpyrocarbonate and dimethylsulphate revealed strong protection from modification of adenine residues within the triple-helix in concordance with their Hoogsteen pairing with the third strand, and a strong hypersensitivity to diethylpyrocarbonate at the first adenine of the duplex. This result indicates that intercalation at this site induces a conformational change at the 5' triplex-duplex junction. Furthermore, the same diethlypyrocarbonate hypersensitivity was observed with an unmodified triple-strand forming oligonucleotide and a range of intercalating molecules present in solution. Thus the 5' triplex-duplex junction is a strong binding site for some intercalating molecules and the junction undergoes a conformational change which is sensitive to diethylpyrocarbonate upon insertion of the planar aromatic chromophore. This conformational change can be used to direct a single-strand cut in duplex DNA to a defined site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier D. A., Griffin J. A., Wells R. D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J Biol Chem. 1988 May 25;263(15):7397–7405. [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Barbier C., Chassignol M., Thuong N. T., Hélène C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Hélène C. Sequence-specific recognition of the major groove of DNA by oligodeoxynucleotides via triple helix formation. Footprinting studies. Nucleic Acids Res. 1988 Dec 23;16(24):11431–11440. doi: 10.1093/nar/16.24.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. E., van der Marel G. A., van Boom J. H., Feigon J. Unstable Hoogsteen base pairs adjacent to echinomycin binding sites within a DNA duplex. Proc Natl Acad Sci U S A. 1989 May;86(9):3006–3010. doi: 10.1073/pnas.86.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990 Jan 11;18(1):157–161. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen C., Nielsen P. E. Detection of intercalation-induced changes in DNA structure by reaction with diethyl pyrocarbonate or potassium permanganate. Evidence against the induction of Hoogsteen base pairing by echinomycin. FEBS Lett. 1988 Apr 11;231(1):172–176. doi: 10.1016/0014-5793(88)80725-7. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985 Oct;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J., Morgan A. R. Poly(pyrimidine) . poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984 Aug 24;12(16):6603–6614. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D., Cantor C. R. A stable complex between homopyrimidine oligomers and the homologous regions of duplex DNAs. Nucleic Acids Res. 1988 Mar 25;16(5):2165–2178. doi: 10.1093/nar/16.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Seela F., Waring M. J. Echinomycin-induced hypersensitivity to osmium tetroxide of DNA fragments incapable of forming Hoogsteen base pairs. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9687–9691. doi: 10.1073/pnas.86.24.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Waring M. J. Chemical probes reveal no evidence of Hoogsteen base pairing in complexes formed between echinomycin and DNA in solution. J Mol Recognit. 1988 Jun;1(3):138–151. doi: 10.1002/jmr.300010307. [DOI] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Neidle S., Abraham Z. Structural and sequence-dependent aspects of drug intercalation into nucleic acids. CRC Crit Rev Biochem. 1984;17(1):73–121. doi: 10.3109/10409238409110270. [DOI] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Portugal J., Fox K. R., McLean M. J., Richenberg J. L., Waring M. J. Diethyl pyrocarbonate can detect a modified DNA structure induced by the binding of quinoxaline antibiotics. Nucleic Acids Res. 1988 May 11;16(9):3655–3670. doi: 10.1093/nar/16.9.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Ughetto G., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986 Jun 6;232(4755):1255–1258. doi: 10.1126/science.3704650. [DOI] [PubMed] [Google Scholar]
- Slama-Schwok A., Jazwinski J., Béré A., Montenay-Garestier T., Rougée M., Hélène C., Lehn J. M. Interactions of the dimethyldiazaperopyrenium dication with nucleic acids. 1. Binding to nucleic acid components and to single-stranded polynucleotides and photocleavage of single-stranded oligonucleotides. Biochemistry. 1989 Apr 18;28(8):3227–3234. doi: 10.1021/bi00434a017. [DOI] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]





