Abstract
While every individual of Bemisia tabaci (Hemiptera: Aleyrodidae) harbors the primary symbiont (P-symbiont) Portiera, the infection frequencies of the six secondary symbionts (S-symbionts) including Hamiltonella, Arsenophonus, Cardinium, Wolbachia, Rickettsia and Fritschea vary greatly among different populations. To characterize the factors influencing the infection dynamics of the six S-symbionts in B. tabaci, gene-specific PCR were conducted to screen for the presence of the P-symbiont Portiera and the six S-symbionts in 61 (17 B and 44 Q biotypes) field populations collected from different plant species and locations in China. All individuals of the 61 populations hosted the P-symbiont Portiera, but none of them harbored Arsenophonus and Fritschea. The presence and infection rates of Hamiltonella, Cardinium, Rickettsia, Wolbachia and their co-infections Rickettsia + Hamiltonella (RH), Rickettsia + Cardinium (RC), Hamiltonella + Cardinium (HC) and Rickettsia + Hamiltonella + Cardinium (RHC) varied significantly among the 61 field populations; and the observed variations can be explained by biotypes, sexes, host plants and geographical locations of these field populations. Taken together, at least three factors including biotype, host plant and geographical location affect the infection dynamics of S-symbionts in B. tabaci.
Introduction
Bacteria commonly form intimate, symbiotic associations with insects. These bacterial endosymbionts occur in a diverse array of insect species and are usually passed from host to host by vertical transmission [1], [2]. The endosymbionts of insects are divided into two groups: primary symbionts (P-symbionts) and secondary symbionts (S-symbionts) [1]. P-symbionts often produce/provide essential nutrients for their hosts and thus form an obligatory mutualistic relationship with their hosts, i.e., they depend on each other for survival. Examples include the bacterial symbionts that make nutrients for their insect hosts, such as Buchnera aphidicola in aphids, Carsonella ruddii in psyllids, and Tremblaya princeps in mealybugs [1]. By contrast, S-symbionts may not be required for host survival. Nonetheless, they may play important roles in their host's nutrition [3], [4], genetic differentiation [5], adaptation to a wide range of food plants [6], defense against natural enemies [7]–[9], reproduction [10], [11], sensitivity to heat stress and other environmental factors [12].
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most destructive insect pests of numerous protected and field crops worldwide. It causes serious damage in many crops by direct feeding and by vectoring 111 plant viruses [13]. B. tabaci has long been thought to comprise morphologically indistinguishable biotypes that often differ in host range, fecundity, insecticide resistance, and/or transmission competency for begomoviruses [14], [15]. Recent studies suggest that most of these biotypes represent genetically distinct cryptic species [16]–[19]. The B biotype of the Middle East-Minor Asia 1 and the Q biotype of Mediterranean group are among the most invasive and destructive biotypes [16], [19], [20]. B. tabaci was first recorded in the late 1940s in China, but the crop damages and loses caused by this insect had not been serious until the introduction of the B biotype in the 1990s [21]. In recent years, the Q biotype of B. tabaci has invaded China [22], and in many areas, even displaced the B biotype [23].
To date, one P-symbiont and six S-symbionts have been reported from B. tabaci [1]. The P-symbiont is Portiera aleyrodidarum, which supplements nutrients that B. tabaci can not obtain sufficient amounts from a restricted diet of plant phloem [1], [24]. The six S-symbionts are Hamiltonella [25], Arsenophonus [26], Cardinium [27], Wolbachia [25], Rickettsia [28] and Fritschea [29]. These S-symbionts co-inhabit with the P-symbiont Portiera inside the bacteriocytes and are vertically transmitted via the eggs [30], [31].
The P-symbiont Portiera infects every individual of any B. tabaci populations. By contrast, the infection frequencies of the six S-symbionts vary greatly among B. tabaci populations [31]–[36]. Little, however, is known about the factors affecting the infection dynamics of S-symbionts in B. tabaci. In this study, we conducted a systematic survey of symbiotic bacteria in 61 field populations of B. tabaci collected from various crops and locations in China. The results obtained suggest that biotype, host plant species, and geographical location of B. tabaci are the major factors affecting the population dynamics of S-symbionts in B. tabaci.
Materials and Methods
B. tabaci field populations
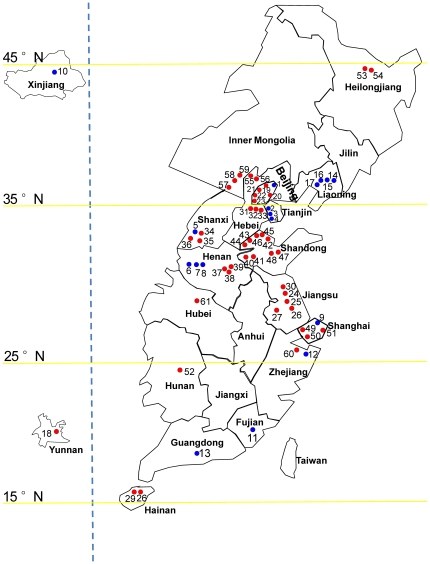
The 61 B. tabaci field populations used in this study were collected from 61 localities in 19 provinces of China in 2009 (Fig. 1). The 61 locations cover an area stretching from Hainan province (16°86′N–110°47′E) in the South to Heilongjiang province (44°83′N–132°53′E) in the North, and from Xinjiang Uygur autonomous region (40°78′N–78°58′E) in the West to Shanghai city (28°18′N–126°46′E) in the East. From each location, dozens of adults were collected alive, fixed in 95% ethanol, and stored at −20°C until analysis of symbionts. The locations, host plants, and biotypes of the 61 field populations are presented in Table S1.
Figure 1. The collection sites of the 61 field populations of B. tabaci in China in 2009.
The numbers on the map correspond to the codes of the 61 populations in Table S1. The provinces of China in which no samples were collected are not included in this map. The vertical blue dot line indicates deletion of the central provinces between the western and the eastern provinces of China. Beijing, Tianjin and Shanghai cities are disproportionally enlarged. The filled blue and red circles represent B- and Q-biotype populations, respectively.
Biotype determination
Genomic DNA was extracted from each of approximately 24 individual whiteflies (or 24 pairs) per population using the methods of Luo et al. (2002) [21]. Briefly, individual adult was homogenized in 20 µL of extraction buffer (50 mM Tris-HCl, pH 8.0, 20 mM NaCl, 1 mM EDTA, and 1% SDS) on a piece of parafilm. The extract was transferred to a microcentrifuge tube containing 2 µL of proteinase K and incubated at 60°C for 3 h. 78 µL of distilled water was added to the tube, which was incubated at 100°C for 5 min. After incubation, the extract (about 100 µL in total) was mixed well with 200 µL of absolute alcohol. The sample was then placed at −20°C for 2 h and centrifuged at 12,000 rpm for 20 min. The DNA pellets obtained were washed once with 75% ethanol, air dried, dissolved in 20 µL of TE buffer (pH 8.0), and stored at −20°C for subsequent biotyping and PCR detection of symbionts. The biotype of every individual of each population was determined by PCR amplification of mitochondrial cytochrome oxidase I (mtCOI) gene fragment using biotype-specific primers [37]. The accuracy of such PCR-based biotyping was further verified by sequencing a ∼620-bp mtCOI fragment of two individuals per population amplified using the primers C1-J-2195 and TL2-N-2819 [38].
Screening for the presence of symbionts and data analysis
Approximately 24 (or 24 pairs of) adults each of the 61 field populations (Fig. 1; Table S1) were analyzed for the presense of the P-symbiont Portiera and the S-symbionts Hamiltonella, Arsenophonus, Cardinium, Wolbachia, Rickettsia, and Fritschea (Table S1). The presence of Portiera, Hamiltonella, Arsenophonus, Cardinium, Wolbachia, Rickettsia, and Fritschea in each individual adult was determined by PCR-gel analyses of 16S rRNA (Portiera, Hamiltonella, Cardinium, and Rickettsia), 23S rRNA (Arsenophonus and Fritschea) or wsp (Wolbachia) gene using genus-specific primers (Table 1). PCR amplifications were performed in 25 µL reactions containing 2.5 µL 10×PCR Buffer (Mg2+ Plus), 2 µL dNTP mix (2.5 mM of each nucleotide), 0.5 µL of each primer (10 µM each), and 0.125 µL of TaKaRa Taq (5 U/ µL) (TaKaRa Biotechnology (Dalian) Co., Ltd). The PCR cycling conditions for detection of the seven symbionts were summarized in Table 1. The resultant PCR products were electrophoresed with the negative and positive controls of each symbiont on a 1.0% agarose gel in a 0.5×TBE buffer and visualized by ethidium bromide staining. The identities of the expected bands for each of the seven symbionts were verified by subsequent sequencing of two independent clones derived from two different whitefly individuals. As a result, whether an individual adult was infected by any of the seven symbionts was determined based on the presence of the corresponding band.
Table 1. PCR primers and conditions used.
| Symbiont name | Gene amplified | Primer name | Sequence (5′-3′) | Annealing temperature | Product size (kb) | References |
| Portiera | 16S Rdna | 28F | TGCAAGTCGAGCGGCATCAT | 58°C | ∼1 | [25] |
| 1098R | AAAGTTCCCGCCTTATGC GT | |||||
| Rickettsia | 16S rDNA | Rb-F | GCTCAGAACGAACGCTATC | 60°C | ∼0.9 | [28] |
| Rb-R | GAAGGAAAGCATCTCTGC | |||||
| Hamiltonella | 16S rDNA | Ham-F | TGAGTAAAGTCTGGAATCTGG | 58°C | ∼0.7 | [25] |
| Ham-R | AGTTCAAGACCGCAACCTC | |||||
| Cardinium | 16S rDNA | CFB-F | GCGGTGTAAAATGAGCGTG | 57°C | ∼0.4 | [27] |
| CFB-R | ACCTMTTCTTAACTCAAGCCT | |||||
| Wolbachia | Wsp | wsp-81F | TGGTCCAATAAGTGATGAAGAAAC | 55°C | ∼0.6 | [51] |
| wsp-691R | AAAAATTAAACGCTACTCCA | |||||
| Fritschea | 23S rDNA | U23F | GATGCCTTGGCATTGATAGGCGATGAAGGA | 60°C | ∼0.6 | [29] |
| 23SIGR | TGGCTCATCATGCAAAAGGCA | |||||
| Arsenophonus | 23S rDNA | Ars23S-1 | CGTTTGATGAATTCATAGTCAAA | 58°C | ∼0.6 | [26] |
| Ars23S-2 | GGTCCTCCAGTTAGTGTTACCCAAC |
The total numbers of tested and infected adults by the seven symbionts for each field population were recorded to calculate their infection frequencies by the formula: the number of individuals infected or co-infected by a given symbiont or a symbiont combination/the total number of individuals screened (usually n = 24) ×100%. The impacts of biotype, sex, host plant species, and geographical location on the infection frequencies of the symbionts were analyzed using the χ2 tests, followed by multiple comparisons with Bonferroni corrections. The differences between the observed and expected ( = multiplication of infection frequencies of each symbionts) frequencies of multiple infections were compared by nonparametric χ2 tests. All statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
1. Nation-wide survey of symbionts in the field populations of B. tabaci
A total of 61 field populations of B. tabaci collected from a wide range of host plants in 19 provinces of China during July-September 2009 were biotyped and tested for the diversity and infection frequencies of the P- and S-symbionts (Fig. 1 and Table S1). 44 out of the 61 populations belonged to the Q1 subclade of the Q biotype and the remaining 17 were of pure B biotype (Table S1). All individuals of the 61 field populations had the P-symbiont Portiera, regardless of their collection sites, host plants, biotype and sex of the whitefly (Table S1).
Among the 6 S-symbiont species we examined, Arsenophonus was not detected in any of the 61 field populations (data not shown). PCR-gel analysis revealed that 24.6% of the 61 populations had the expected band for Fritschea (data not shown), but subsequent cloning and sequencing of the band eluted from gels confirmed that it was either Flavobacterium or Acidobacteria, not Fritschea (data not shown). Wolbachia was extremely rare and only detected in one B biotype (Code 6 in Table S1) and one Q biotype (Code 52 in Table S1) populations. Interestingly, both populations were collected from cotton and had the same infection frequency (4.2%) by Wolbachia (Table S1), but were geographically far from each other (Fig. 1).
Rickettsia, Hamiltonella, and Cardinium were present alone or together in more than 2 field populations (Table S1). The infection frequencies of the three S-symbionts varied greatly among the 61 populations. Statistical analyses revealed that the plant species, geographical location, biotype and sex of the field populations were the four factors significantly contributing to the great variations in the diversity and infection frequency of the S-symbionts in the B. tabaci field populations.
2. Impacts of B. tabaci biotype on the infection frequency of the S-symbionts
The B- and Q-biotype field populations differed significantly in their infection frequencies by the S-symbionts. All the 17 B biotype populations possessed at least one S-symbiont, whereas 4 (i.e. 9.1%; Codes 30, 37, 49, and 56 in Table S1) out of the 44 Q biotype populations did not harbor any of the 6 S-symbionts. The infection frequency of Wolbachia was very low in both biotypes and there was no significant difference between the B- and Q- biotype individuals (Table 2). The two biotypes also had a similar percentage of Hamiltonella infection. The infection frequency of Rickettsia was significantly higher in the 17 B biotype populations (64.3% of a total of 456 B biotype individuals) than the 44 Q biotype populations (7.2% of a total of 1149 Q biotype individuals) (Table 2). By contrast, the infection frequency of Cardinium was significantly greater in the 44 Q biotype populations (16.3%) than in the 17 B biotype populations (10.8%). Co-infection of whiteflies by Wolbachia and any other S-symbionts was not found in the two biotypes. The B biotype field populations also had significantly higher co-infection frequencies by Rickettsia + Hamiltonella (RH) (40.6%), Rickettsia + Cardinium (RC) (8.6%), and Rickettsia + Hamiltonella + Cardinium (RHC) (5.5%) than the Q biotype field populations (Table 2). In the B biotype, the observed frequency of RH co-infection was significantly higher than its expected frequency, which was calculated by multiplying the infection frequencies of each symbionts. No differences were found between the observed and expected frequencies of RC, HC and RHC co-infections in the two biotypes (Table 2).
Table 2. Impacts of biotype on the infection frequency of S-symbionts in the field populations of B. tabaci (B biotype: 17 populations, 456 adults in total; Q biotype: 44 populations, 1149 adults in total)*.
| Infection Frequency (%) By | ||||||||||||
| Biotype | RHC | RH | RC | HC | R | H | C | W | ||||
| exp | obs | exp | obs | exp | obs | exp | obs | |||||
| B | 3.2a | 5.5Aa | 30.0b | 40.6Aa | 6.9a | 8.6Aa | 5.0a | 6.8Aa | 64.3A | 46.7A | 10.8B | 0.2A |
| Q | 0.5a | 0.8Ba | 3.3a | 4.2Ba | 1.2a | 1.0Ba | 7.6a | 6.7Aa | 7.2B | 46.5A | 16.3A | 0.0A |
*The frequencies in columns sharing the same upper case letter are not significantly different at P<0.05 (multiple comparisons with Bonferroni corrections). The expected (exp) co-infection frequencies of whiteflies by two or three symbionts (e.g. RH or RHC) were calculated by multiplying the infection frequencies of whiteflies by each of the two or three symbionts. The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
3. Impacts of plant species on the infection frequency of S-symbionts
Chi-square tests showed that host plant species significantly and differentially affected the infection frequencies of the B and Q biotypes B. tabaci by the S-symbionts (Tables 3 and 4). In the B biotype populations, Rickettsia was the most prevalent S-symbiont. It was detected in the B biotype whiteflies collected from all the 7 plant species (cucumber, tomato, cabbage, cotton, poinsettia, sweet potato, and bean; each representing 1 plant family) surveyed (Table S1 and 3). The infection frequency of Rickettsia was the highest in the B biotype populations collected from cotton (100%), followed by those from cucumber (73.3%), poinsettia (70.8%), and cabbage (67.7%), those from bean (41.7%) and sweet potato (33.3%), and those from tomato (12.5%) (Table 3). Hamiltonella was less prevalent than Rickettsia in the B biotype populations and was not detected in the B biotype whiteflies from sweet potato (Table 3). Chi-square tests divided the B biotype whiteflies from the 6 plants into 2 significantly different groups. The B biotype populations from poinsettia (83.3%), cotton (61.5%), cucumber (57.5%) and cabbage (50.0%) had significantly higher Hamiltonella infection than those from tomato (20.8%) and bean (8.3%) (Table 3). Cardinium was detected only in two B biotype populations, one from cucumber and another from cotton (Table S1). The infection frequency of Cardinium was significantly higher in the cucumber population than in the cotton population (Table 3). As a result, the co-infection rates of RC, HC and RHC were higher (significant for RHC and HC) in the cucumber population than in the cotton population (Table 3). RH co-infection was significantly more prevalent in the cucumber, cabbage, cotton, and poinsettia populations than in the tomato, bean and sweet potato populations. Further nonparametric χ2 tests revealed no significant differences between the observed and expected frequencies of RHC, RC and HC co-infection in the populations from all the seven plant species (Table 3).
Table 3. Impacts of host plant on the diversity and infection frequency of S-symbionts in the field populations of B biotype B. tabaci *.
| Infection Frequency (%) By | ||||||||||||
| Host plant | RHC | RH | RC | HC | R | H | C | W | ||||
| exp | obs | exp | obs | exp | obs | exp | obs | |||||
| Cucumber | 14.0a | 18.3Aa | 42.1a | 51.7Aa | 24.4a | 25.0Aa | 19.1a | 23.3Aa | 73.3B | 57.5A | 33.3A | 0.0A |
| Tomato | 0.0a | 0.0Ba | 2.6a | 6.9Ba | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 12.5E | 20.8B | 0.0C | 0.0A |
| Cabbage | 0.0a | 0.0Ba | 33.9a | 45.8Aa | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 67.7BC | 50.0A | 0.0C | 0.0A |
| Cotton | 5.8a | 3.1Ba | 61.5a | 60.4Aa | 9.4a | 9.4Aa | 5.8a | 3.1Ba | 100.0A | 61.5A | 9.4B | 1.0A |
| Poinsettia | 0.0a | 0.0Ba | 59.0a | 62.5Aa | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 70.8BC | 83.3A | 0.0C | 0.0A |
| Sweet potato | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 33.3DE | 0.0B | 0.0C | 0.0A |
| Bean | 0.0a | 0.0Ba | 3.5a | 4.2Ba | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 41.7CD | 8.3B | 0.0C | 0.0A |
*The frequencies in columns sharing the same upper case letter are not significantly different at P<0.0024 (multiple comparisons with Bonferroni corrections). The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
Table 4. Impacts of host plant on the diversity and infection frequency of S-symbionts in the field populations of Q biotype B. tabaci *.
| Infection Frequency (%) By | ||||||||||||
| Host plant | RHC | RH | RC | HC | R | H | C | W | ||||
| exp | obs | Exp | obs | exp | obs | exp | obs | |||||
| Cucumber | 0.0a | 0.0Aa | 0.4a | 0.0Ca | 0.3a | 0.0Aa | 1.8a | 1.9Ba | 2.3C | 16.4CD | 11.2BC | 0.0A |
| Towel gourd | 2.0a | 4.4Aa | 9.1a | 13.0Ba | 5.7a | 4.4Aa | 7.6a | 17.4Aa | 26.1AB | 34.8BCD | 21.7AB | 0.0A |
| Squash | 0.0a | 0.0Aa | 0.0a | 0.0Ca | 0.0a | 0.0Aa | 0.0a | 0.0Ba | 4.2BC | 0.0D | 0.0D | 0.0A |
| Tomato | 0.0a | 0.0Aa | 0.0a | 0.0Ca | 0.0a | 0.0Aa | 5.7a | 7.3ABa | 0.0C | 32.3BCD | 17.7BC | 0.0A |
| Pepper | 0.0a | 0.0Aa | 0.0a | 0.0Ca | 0.0a | 0.0Aa | 31.8a | 19.4Ab | 0.0C | 61.1AB | 52.1A | 0.0A |
| Eggplant | 0.7a | 1.5Aa | 5.4a | 6.0Ba | 1.3a | 2.1Aa | 6.9a | 6.0ABa | 10.1B | 53.6AB | 12.8BC | 0.0A |
| Cotton | 0.0a | 0.0Aa | 5.7a | 5.4BCa | 0.1a | 0.0Aa | 0.3a | 0.6Ba | 11.3B | 50.6AB | 0.6D | 0.6A |
| Japanese hop | 0.1a | 1.4Aa | 1.1a | 1.4BCa | 0.1a | 1.4Aa | 6.4a | 6.9ABa | 1.4C | 76.4A | 8.4BCD | 0.0A |
| Poinsettia | 0.0a | 0.0Aa | 0.0a | 0.0Ca | 0.0a | 0.0Aa | 24.3a | 0.0Bb | 0.0C | 83.3A | 29.2AB | 0.0A |
| Gerbera | 16.4a | 8.3Aa | 65.6a | 62.5Aa | 18.8a | 12.5Aa | 21.9a | 20.8Aa | 75.0A | 87.5A | 25.0AB | 0.0A |
| Bean | 0.0a | 0.0Aa | 0.0a | 0.0Ca | 0.0a | 0.0Aa | 5.2a | 8.3ABa | 0.0C | 41.7ABC | 12.5BCD | 0.0A |
*The frequencies in columns sharing the same upper case letter are not significantly different at P<0.00091 (multiple comparisons with Bonferroni corrections). The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
Unlike in the B biotype populations, Rickettsia was the least prevalent S-symbiont in the Q biotype whiteflies (Table S1 and Table 4). It was found in the Q biotype populations from gerbera, Japanese hop, cotton, egg plant, squash, towel gourd, and cucumber, but not from tomato, pepper, poinsettia, and bean (Table 4). Rickettsia infection was the highest in the Q biotype whiteflies from Gerbera (75.0%), followed by those from towel gourd (26.1%), those from cotton (11.3%) and eggplant (10.1%), and those from squash (4.2%), cucumber (2.3%) and Japanese hop (1.4%). Hamiltonella and Cardinium were more prevalent and detected in the Q biotype populations from all the 11 plants but squash. Q biotype whiteflies from gerbera (87.5%), poinsettia (83.3%) and Japanese hop (76.4%) had the highest rate of Hamiltonella infection, followed by those from pepper (61.1%), eggplant (53.6%), cotton (50.6%), and bean (41.7%), those from towel gourd (34.8%) and tomato (32.3%), and those from cucumber (16.4%) and squash (0.0%). For Cardinium, the pepper populations (52.1%) had the highest infection frequency, whereas the squash (0.0%) and cotton (0.6%) populations had the lowest infection (Table 4). The plant populations between the two groups had a more or less similar level of Cardinium infection. Further, Q whiteflies from gerbera and towel gourd had a greater frequency of RH (significant), RC and RHC co-infection than those from other plants (Table 4). For HC co-infection, Q whiteflies from gerbera, pepper and towel gourd had the greatest frequency, followed by those from bean, tomato, Japanese hop, and eggplant, and those from the remaining plants. Nonparametric χ2 tests showed no significant differences between the observed and expected frequencies of RH, RC and RHC co-infections in the populations from all the seven plant species (Table 4). The observed frequencies of HC co-infection were significantly lower than its expected frequencies in the pepper and poinsettia populations (Table 4).
4. Impacts of geographical location on the infection frequency of S-symbionts
To test the effects of geographical location on the infection and co-infection of the 4 S-symbionts, we divided the collection sites into three geographical regions: 15°N–25°N, 25°N–35°N and 35°N–45°N (Fig. 1). The B biotype populations collected from the three regions had a similar level of Rickettsia and Hamiltonella infection frequency (Table 5). By contrast, Cardinium and Wolbachia were found only in the 35°N–45°N region (18.6%, significantly greater than 0.0% in the other two regions) and the 25°N–35°N region (0.7%, not significantly different from 0.0% in the two other regions), respectively. Consequently, RC, HC, and RHC co-infections of the B biotype whiteflies existed only in the 35°N–45°N region; the corresponding co-infection frequency was significantly greater than 0.0% in the two southern regions. In contrast, RH co-infection was prevalent in all the regions, but no significant difference in co-infection frequency existed between the three regions (Table 5). In addition, the observed co-infection frequencies of HC and RHC in the 35°N–45°N region and RH in the 25°N–35°N and 35°N–45°N regions were significantly greater than their expected frequencies.
Table 5. Impacts of geographical location on the diversity and infection frequency of S-symbionts in the field populations of B biotype B. tabaci *.
| Infection Frequency (%) By | |||||||||||||
| Location | RHC | RH | RC | HC | R | H | C | W | |||||
| exp | obs | Exp | obs | exp | obs | exp | obs | ||||||
| 15°N–25°N | 0.0a | 0.0Ba | 21.7a | 31.3Aa | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 52.1A | 41.7A | 0.0B | 0.0A | |
| 25°N–35°N | 0.0a | 0.0Ba | 36.4b | 46.5Aa | 0.0a | 0.0Ba | 0.0a | 0.0Ba | 64.6A | 56.3A | 0.0B | 0.7A | |
| 35°N–45°N | 5.2b | 9.5Aa | 28.1b | 34.9Aa | 12.3a | 14.8Aa | 7.9b | 11.7Aa | 66.3A | 42.4A | 18.6A | 0.0A | |
*The frequencies in columns sharing the same upper case letter are not significantly different at P<0.017 (multiple comparisons with Bonferroni corrections). The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
In the Q biotype whiteflies, Rickettsia, Hamiltonella and Cardinium were present in the three geographical regions, whereas Wolbachia (1.1%) was detected only in the 15°N–25°N region (Table 6). Hamiltonella was prevalent in the three geographical regions, with no significant difference in the infection rate among the three regions (χ2 = 2.821, P = 0.244). The infection rate of Rickettsia increased significantly from the North to the South. The infection rate of Cardinium was the highest in the 35°N–45°N region, followed by the 15°N–25°N region, and the 25°N–35°N region (Table 6). Double or triple co-infection rate of Q biotype whiteflies by the three S-symbionts ranged from 0.4% to 5.9% across the three regions, with no significant difference. There were no significant differences between the observed and expected frequencies of RH, RC and RHC in all the three regions. But the observed frequency of HC in the 35°N–45°N region was significantly lower than its expected frequency (Table 6).
Table 6. Impacts of geographical location on the diversity and infection frequency of S-symbionts in the field populations of Q biotype B. tabaci *.
| Infection Frequency (%) By | ||||||||||||
| Location | RHC | RH | RC | HC | R | H | C | W | ||||
| exp | obs | exp | obs | exp | obs | exp | obs | |||||
| 15°N–25°N | 1.1a | 1.1Aa | 8.4a | 4.2Aa | 2.5a | 1.1Aa | 5.3a | 4.2Aa | 20.0A | 42.1A | 12.6B | 1.1A |
| 25°N–35°N | 0.2a | 0.4Aa | 3.9a | 4.5Aa | 0.3a | 0.6Aa | 2.2a | 2.4Aa | 7.5B | 52.2A | 4.3C | 0.0B |
| 35°N–45°N | 0.3a | 1.4Aa | 0.7a | 2.0Aa | 0.7a | 1.4Aa | 12.6a | 5.9Ab | 2.0C | 36.6A | 34.4A | 0.0B |
*The frequencies in columns sharing the same upper case letter are not significantly different at P<0.017 (multiple comparisons with Bonferroni corrections). The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
5. Impacts of sex on the infection frequency of S-symbionts
Male and female adults of 2 B- (Codes 1 and 2 in Table S1) and 4 Q-biotype (Codes 19, 20, 22, and 23 in Table S1) field populations were separately screened for the presence of S-symbionts. χ2 tests indicated that sex significantly affected the infection frequency of Hamiltonella, but not Rickettsia or Cardinium (Table 7). The average infection frequency of Hamiltonella was significantly higher in females (88.9%) than in males (12.5%) (χ2 = 165.046, P<0.0001). The co-infection frequencies of RHC, RH, and HC, but not RC, were also significantly higher in females than in males (P<0.0001). No significant differences existed between the observed and expected co-infection frequencies of female and male whiteflies by RH, RC, and RHC. The observed frequency of HC was significantly lower than its expected frequency in female, but not male whiteflies (Table 7).
Table 7. Impacts of sex on the diversity and infection frequency of S-symbionts in the 6 field populations (population codes 1, 2, 19, 20, 22, and 23 in Table S1) of B. tabaci*.
| Infection Frequency (%) By | |||||||||||
| Sex | RHC | RH | RC | HC | R | H | C | ||||
| Exp | obs | exp | obs | exp | obs | exp | obs | ||||
| ♀ | 17.8a | 17.4Aa | 30.2a | 33.3Aa | 20.1a | 17.4Aa | 52.5a | 29.2Ab | 34.0A | 88.9A | 59.0A |
| ♂ | 2.4a | 3.5Ba | 4.1a | 6.3Ba | 19.5a | 14.6Aa | 7.5a | 3.5Ba | 32.6A | 12.5B | 59.7A |
• The frequencies in columns sharing the same upper case letter are not significantly different at P<0.05 (multiple comparisons with Bonferroni corrections). The expected (exp) and observed (obs) co-infection frequencies that share the same lower case letter are not significantly different at P<0.05 (nonparametric tests χ2).
Discussion
Several recent studies have showed a 100% infection frequency of B. tabaci by the P-symbionat Portiera, but a 0–100% infection frequency by any of the six S-symbionts (Hamiltonella, Arsenophonus, Cardinium, Wolbachia, Rickettsia and Fritschea), depending on the populations tested [31]–[36]. To reveal the key factors that govern the infection dynamics of whiteflies by the six S-symbionts, we surveyed 61 field populations of (24 individuals or 24 pairs each) for the presence of the P-symbiont Portiera and the six S-symbionts. Consistent with the aforementioned studies, all individuals of the 61 field populations harbored the P-symbiont Portiera. Arsenophonus and Fritschea were not present in any of the 61 populations, whereas the presence and infection frequencies of Hamiltonella, Cardinium, Wolbachia and Rickettsia varied greatly among the 61 populations.
Our data from the 61 (17 B biotype and 44 Q biotype) field populations and all the prior studies [31]–[36] demonstrate that biotype or genetic group of B. tabaci is a key factor determining the infection dynamics of the six S-symbionts. However, there are noticeable discrepancies in the S-symbiont-host biotype associations or linkage disequilibrium revealed by these studies. For example, Chiel et al. (2007) and Gueguen et al. (2010) reported that the B biotype only harbored Hamiltonella (100%) and Rickettsia (64.0%), the Q1 subclade of the Q biotype only harbored Hamiltonella (close to 100.0%), Cardinium and Wolbachia, the Q2 subclade only harbored Rickettsia (74.0%), Wolbachia (33.0%) and Arsenophonus (87.0%), and the Q3 subclade only harbored Rickettsia (about 30.0%) and Arsenophonus (about 97%). By contrast, all of the six S-symbionts but Fritschea were detected in biotypes B and Q (i.e. Q1) in China during 2005 to 2009 [36], and the Q biotype in Croatia [31] hosted Hamiltonella, Rickettsia, Wolbachia and Cardinium, but not Arsenophonus and Fritschea.
One explanation for the above discrepancies is that there are other important factors that have not been characterized but significantly affect the infection dynamics of the S-symbionts in B. tabaci. That the female adults of 2 B- (Codes 1 and 2 in Table S1) and 4 Q-biotype (Codes 19, 20, 22, and 23 in Table S1) field populations had significantly higher infection frequencies of Hamiltonella, RHC, RH, and HC than did their male adults (Table 7) suggests that sex of the hosts could be another factor impacting the infection dynamics of B. tabaci by the 6 S-symbionts. However, this female-biased infection rate is probably not a real infection difference between male and female adults, but a matter of a combination of lower symbiont titer in males and lower detection sensitivity of our PCR-gel analysis technique. Single whiteflies are small and ethidium bromide used in this study is not the most sensitive DNA stain. It is well known that symbiont infections are not easily detectable in arthropods [39]. Moreover, it is females, not males, that transmit the symbionts to their offspring, and males are smaller than females. Therefore, it makes some sense that the titer of symbionts in males would be lower and thus would be more difficult to be detected by our PCR-gel analysis technique. Another less likely explanation for the pattern of lower frequencies of symbiont infections in males would be male-killing effects of Hamiltonella, RHC, RH, and HC. The fact that the male-killing symbiont identified in the ladybird Cheilomenes sexmaculata shares the closest similarity to Hamiltonella defensa (98% nucleotide identity) of whiteflies [40] implicates that Hamiltonella of whiteflies could be a male-killing symbiont. More experiments are necessary to resolve these two possibilities.
Our survey data of the 61 field population collected from three different regions in China (15°N–25°N, 25°N–35°N, and 35°N–45°N) suggest that geographical location is another factor affecting the infection frequency of the S-symbionts in B. tabaci. The impacts of geographical locations are best exemplified by infection of the B biotype by Cardinium and Wolbachia only in the 35°N–45°N and the 25°N–35°N regions respectively (Table 5), significantly greater infection of the Q biotype by Cardinium in the 35°N–45°N region, and the increase of Rickettsia infection with decreasing latitude in the Q biotype (Table 6). This is consistent with the results of Ahmad et al. (2010), who showed that the infection frequency of Wolbachia in B. tabaci was related to country (location).
Our survey data of the 61 field populations collected from 7 (B biotype) or 11 (Q biotype) different plant species also indicate that host plant affects the population dynamics of the S-symbionts in B. tabaci. This is because the infection frequencies of Rickettsia, Cardinium, RH, and HC were all significantly different among the B and Q biotype field populations collected from different plant species (Table 3 and 4). This agrees with the findings of Chiel et al. (2007), who reported that Rickettsia and Arsenophonus had a significantly higher infection frequency in the Q populations collected from sage than those from all other host plants.
In summary, at least three factors including biotype or genetic group, host plant and geographical location affect the infection dynamics of S-symbionts in B. tabaci. Two of the three factors—host plants [6], [41]–[45] and collection sites [42], [46]—as well as temperature [46]–[48] are known to affect the infection frequency of S-symbionts in other insects. Nonetheless, the host plant- and/or location-symbiont association patterns revealed from the field populations could be caused, at least partially, by factors other than host plants or latitude, particularly population inertia and stochasticity. In theory, these genetic (biotype, sex) and environmental factors (host plant and geographical location) could change the horizontal transfer frequency, vertical transmission fidelity, and/or relative fitness of these S-symbionts on B. tabaci. Further experiments are required to address how the four factors affect the population dynamics of the six maternally transmitted S-symbionts in B. tabaci.
This study showed that co-infections with Rickettsia and Hamiltonella are more common than expected in natural populations of B biotype B. tabaci (Table 2–3, 5). In theory, there are a number of possible mechanisms that can facilitate such endosymbiont co-infections. For example, the co-infecting endosymbionts may additively confer fitness advantages on the host, either by positively affecting different fitness parameters or by favoring overlapping fitness parameters in a synergistic way [49]. However, the co-infections by HC are significantly rarer than expected in natural populations of B- and Q-biotype B. tabaci (Table 4, 6–7), suggesting antagonistic interactions between the S-symbionts upon co-infection, possibly because of competition for limited resource and niche in the same host individual [30], [50] or synergistic negative effects of co-infection on the host organism [8]. To confirm which of these hypotheses can better account for the co-infection patterns for each of the S-symbiont pairs, further experiments are needed.
Supporting Information
The locations, host plants, biotypes, and symbionts of the 61 field populations of B. tabaci collected in 2009*.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the National Science Fund for Distinguished Young Scholars (31025020), the 973 Program (2009 CB119200), the National Natural Science Foundation of China (No. 30900153), and the Key Laboratory of Vegetable Genetics and Physiology, Ministry of Agriculture. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 2.Wernegreen JJ. Endosymbiosis: Lessons in conflict resolution. PLoS Biol. 2004;2:e68. doi: 10.1371/journal.pbio.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. P Roy Soc B-Biol Sci. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandström JP, Moran NA. Amino acid budgets in three aphid species using the same host plant. Physiol Entomol. 2001;26:202–211. [Google Scholar]
- 5.Charlat S, Duplouy A, Hornett EA, Dyson EA, Davies N, et al. The joint evolutionary histories of Wolbachia and mitochondria in Hypolimnas bolina. BMC Evol Biol. 2009;9:64. doi: 10.1186/1471-2148-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science. 2004;303:1989. doi: 10.1126/science.1094611. [DOI] [PubMed] [Google Scholar]
- 7.Oliver KM, Moran NA, Hunter MS. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Pro Natl Acad Sci U S A. 2005;102:12795–12800. doi: 10.1073/pnas.0506131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver KM, Moran NA, Hunter MS. Costs and benefits of a superinfection of facultative symbionts in aphids. P Roy Soc B-Biol Sci. 2006;273:1273–1280. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Pro Natl Acad Sci U S A. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter MS, Perlman SJ, Kelly SE. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. P Roy Soc B-Biol Sci. 2003;270:2185–2190. doi: 10.1098/rspb.2003.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White JA, Kelly SE, Perlman SJ, Hunter MS. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity. 2009;102:483–489. doi: 10.1038/hdy.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montllor CB, Maxmen A, Purcell AH. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol. 2002;27:189–195. [Google Scholar]
- 13.Inbar M, Gerling D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Ann Rev of Entomol. 2008;53:431–448. doi: 10.1146/annurev.ento.53.032107.122456. [DOI] [PubMed] [Google Scholar]
- 14.Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Ann Rev Entomol. 1995;40:511–534. [Google Scholar]
- 15.Jones DR. Plant viruses transmitted by whiteflies. Eur J Plant Pathol. 2003;109:195–219. [Google Scholar]
- 16.Dinsdale A, Cook L, Riginos C, Buckley YM, De Barro PJ. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase I to identify species level genetic boundaries. Ann Entomol Soc Am. 2010;103:196–208. [Google Scholar]
- 17.Xu J, De Barro PJ, Liu SS. Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res. 2010;100:359–366. doi: 10.1017/S0007485310000015. [DOI] [PubMed] [Google Scholar]
- 18.Elbaz M, Lahav N, Morin S. Evidence for pre-zygotic reproductive barrier between the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull Entomol Res. 2010;100:581–590. doi: 10.1017/S0007485309990630. [DOI] [PubMed] [Google Scholar]
- 19.De Barro PJ, Liu SS, Boykin LM, Dinsdale A. Bemisia tabaci: a statement of species status. Ann Rev Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 20.Perring TM. The Bemisia tabaci species complex. Crop Prot. 2001;20:725–737. [Google Scholar]
- 21.Luo C, Yao Y, Wang RJ, Yan FM, Hu DX, et al. The use of mitochondrial cytochrome oxidase mtCOI gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol Sin. 2002;45:759–763. [Google Scholar]
- 22.Chu D, Zhang YJ, Brown JK, Cong B, Xu BY, et al. The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops. Fla Entomol. 2006;89:168–174. [Google Scholar]
- 23.Chu D, Wan FH, Zhang YJ, Brown JK. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ Entomol. 2010;39:1028–1036. doi: 10.1603/EN09161. [DOI] [PubMed] [Google Scholar]
- 24.Thao ML, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol. 2004;70:3401–3406. doi: 10.1128/AEM.70.6.3401-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zchori-Fein E, Brown JK. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am. 2002;95:711–718. [Google Scholar]
- 26.Thao ML, Baumann P. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr Microbiol. 2004;48:140–144. doi: 10.1007/s00284-003-4157-7. [DOI] [PubMed] [Google Scholar]
- 27.Weeks AR, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. P Roy Soc B-Biol Sci. 2003;270:1857–1865. doi: 10.1098/rspb.2003.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol. 2006;72:3646–3652. doi: 10.1128/AEM.72.5.3646-3652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everett KD, Thao M, Horn M, Dyszynski GE, Baumann P. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int J Syst Evol Micr. 2005;55:1581–1587. doi: 10.1099/ijs.0.63454-0. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 2008;22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- 31.Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 2010;10:142. doi: 10.1186/1471-2180-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, et al. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res. 2007;97:407–413. doi: 10.1017/S0007485307005159. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed MZ, Ren SX, Xue X, Li XX, Jin GH, et al. Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr Microbiol. 2010;61:322–328. doi: 10.1007/s00284-010-9614-5. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed MZ, Shatters RG, Ren SX, Jin GH, Mandour NS, et al. Genetic distinctions among the Mediterranean and Chinese populations of Bemisia tabaci Q biotype and their endosymbiont Wolbachia populations. J Appl Entomol. 2009;133:733–741. [Google Scholar]
- 35.Gueguen G, Vavre F, Gnankine O, Peterschmit M, Charif D, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol. 2010;19:4365–4376. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- 36.Chu D, Gao CS, De Barro P, Zhang YJ, Wan FH, et al. Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: Comparison of secondary symbionts from biotypes B and Q in China. Bull Entomol Res. 2011;101:477–486. doi: 10.1017/S0007485311000083. [DOI] [PubMed] [Google Scholar]
- 37.Shatters RG, Jr, Powell CA, Boykin LM, Liansheng H, McKenzie CL. Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: development of universal and Bemisia tabaci biotype-specific mitochondrial cytochrome c oxidase I polymerase chain reaction primers. J Econ Entomol. 2009;102:750–758. doi: 10.1603/029.102.0236. [DOI] [PubMed] [Google Scholar]
- 38.Zhang LP, Zhang YJ, Zhang WJ, Wu QJ, Xu BY, et al. Analysis of genetic diversity among different geographical populations and determination of biotypes of Bemisia tabaci in China. J Appl Entomol. 2005;129:121–128. [Google Scholar]
- 39.Arthofer W, Riegler M, Avtzis DN, Stauffer C. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environmental Microbiology. 2009;11:1923–1933. doi: 10.1111/j.1462-2920.2009.01914.x. [DOI] [PubMed] [Google Scholar]
- 40.Majerus TMO, Majerus MEN. Intergenomic arms races: detection of a nuclear rescue gene of male-killing in a ladybird. PLoS Pathog. 2010;6:e1000987. doi: 10.1371/journal.ppat.1000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson TL, Adams D, Minto LB, Douglas AE. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J Exp Biol. 2001;204:3027–3038. doi: 10.1242/jeb.204.17.3027. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol. 2002;11:2123–2135. doi: 10.1046/j.1365-294x.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 43.Simon JC, Carre S, Boutin M, Prunier-Leterme N, Sabater-Mun B, et al. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. P Roy Soc B-Biol Sci. 2003;270:1703–1712. doi: 10.1098/rspb.2003.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonardo TE, Muiru GT. Facultative symbionts are associated with host plant specialization in pea aphid populations. P Roy Soc B-Biol Sci. 2003;270:S209–S212. doi: 10.1098/rsbl.2003.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari J, Darby AC, Daniell TJ, Godfray HCJ, Douglas AE. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol. 2004;29:60–65. [Google Scholar]
- 46.Toju H, Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol. 2011;20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- 47.Osaka R, Nomura M, Watada M, Kagaeyama D. Negative effects of low temperatures on the vertical transmission and infection density of a Spiroplasma endosymbiont in Drosophila hydei. Curr Microbiol. 2008;57:335–339. doi: 10.1007/s00284-008-9199-4. [DOI] [PubMed] [Google Scholar]
- 48.Anbutsu H, Goto S, Fukatsu T. High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol. 2008;74:6053–6059. doi: 10.1128/AEM.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vautrin E, Vavre F. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 2009;17:95–99. doi: 10.1016/j.tim.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Goto S, Anbutsu H, Fukatsu T. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol. 2006;72:4805–4810. doi: 10.1128/AEM.00416-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W, Rousset F, O'Neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. P Roy Soc B-Biol Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The locations, host plants, biotypes, and symbionts of the 61 field populations of B. tabaci collected in 2009*.
(DOC)