Abstract
Nowadays, biologists can explore the cell at the nanometre level. They discover an unsuspected world, amazingly overcrowded, complex and heterogeneous, in which water, also, is complex and heterogeneous. In the cell, statistical phenomena, such as diffusion, long considered as the main transport for water soluble substances, must be henceforth considered as inoperative to orchestrate cell activity. Results at this level are not yet numerous enough to give an exact representation of the cell machinery; however, they are sufficient to cease reasoning in terms of statistics (diffusion, law of mass action, pH, etc.) and encourage cytologists and biochemists to prospect thoroughly the huge panoply of the biophysical properties of macromolecule-water associations at the nanometre level. Our main purpose, here, is to discuss some of the more common misinterpretations due to the ignorance of these properties, and expose briefly the bases for a better approach of the cell machinery. Giorgio Careri, who demonstrated the correlation between proton currents at the surface of lysozyme and activity of this enzyme was one of the pioneers of this approach.
Keywords: Interfacial water, Intracellular diffusion, Signal transduction, Proton currents, Giorgio Careri
Up to the 1980s, most biologists had never even suspected that the physical properties of water could play a key role in the orchestration of the cell machinery. In fact, the only topic in which these properties were taken into consideration was protein folding.
Until nowadays, water, which composes 70–80% of the cell mass, was generally considered as abundant enough to allow efficient diffusion the water-soluble substances down their concentration gradients without expense of energy. Very few people imagined that cell water could participate actively, in a sophisticated manner, to the transport of metabolites and biomolecule activity.
Misinterpretation of the electron microscopy images
Most biologists were unaware that cell interior was occupied by a huge macromolecular overcrowding until this was magisterially demonstrated by Goodsell [1, 2]. This long-lived ignorance was in part due to the misinterpretation of the electron microscopy images which show more or less dark organelles (mitochondria, ribosomes, etc.) seemingly floating in a very electron-transparent medium, the hyaloplasm (from Greek : hualos = glass), often improperly named cytoplasm, the cytoplasm being sensu stricto the whole cell except the nucleus. The darkness of the objects in electron microscopy is a direct function of their mass density. It has thus been generally concluded, ill-consideredly, that hyaloplasm was quasi-totally composed of water (replaced by a resin after fixation and embedding of the material). This conception has long persisted and is probably still persisting in many minds, in spite of the warnings of eminent cytologists. Keith Porter, particularly, as early as the 1980s, suggested that the fuzzy structures observed in hyaloplasm were not artefacts but should be proteic structures playing a major role in the coordination of cell functions. He named these structures microtrabecules [3, 4]. His observations have been supported by Zierold who showed that regions apparently devoid of organelles contain not less than 20% macromolecules [5]. It is less than mitochondria or ribosomes (which each has 50% macromolecule content), which explain the low contrast of these regions in electron microscopy but is sufficient to structure the present water (80%) into a strongly constrained water, called interfacial water.
The widely ignored interfacial water
In the 1980s, very few biologists thought that cell water could differ deeply from common bulk water. Bulk water is a good solvent, therefore an appropriate medium for diffusion. The collective opinion was, and often remains, that ions and small molecules—signals and metabolites—reach their target by diffusion through hyaloplasm, since this is supposedly quasi-totally occupied by bulk water. It was still less imagined that information could be transferred along proteic structures such as suggested by Porter.
Goodsell’s illustration shows that there is little space left between macromolecules. It is clear that macromolecules constitute mechanical obstacles for diffusion. Moreover, their surfaces are patchworks of polar and apolar domains which can bind ions, polar and apolar molecules. It is then puzzling to understand how the complex and ultra-fast cell processes can depend on diffusion. Indeed, at the nanometre level, diffusion is a random phenomenon. It is only in the presence of a large number of identical molecules that emerges what is called diffusion (or concentration) gradient. But this situation is rare in cell.
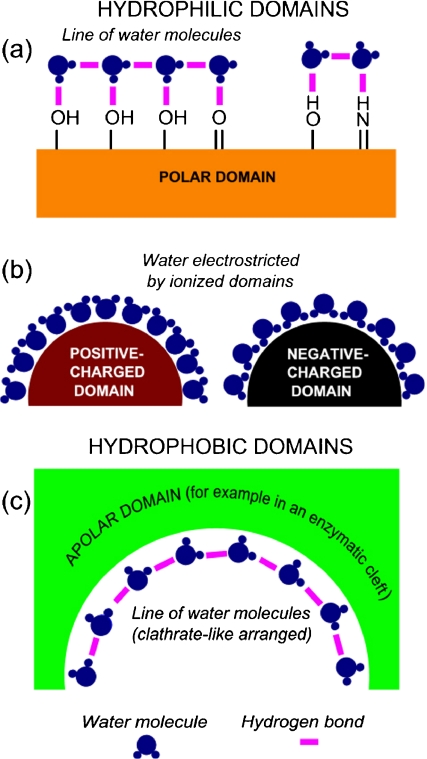
Macromolecular overcrowding slows down metabolite migration for another reason: it modifies deeply the structure of water at its contact. Indeed water molecules are electrical dipoles. At the contact of macromolecules, these dipoles arrange themselves into a non-random, very constrained manner, reflecting faithfully the patchwork of the macromolecule-surface domains, hydrophobic or hydrophilic (Mentré [6]; Figs. 1 and 2). Water molecules are strongly attracted by ionized domains (such as COO − , NH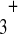 ) and form electrostricted water with a density higher than 1 [7]. They form hydrogen bonds with the polar radicals (alcohols, ketone, secondary and tertiary amines...). At the contact of apolar domains they can arrange themselves into “clathrate-like” structures [8].
) and form electrostricted water with a density higher than 1 [7]. They form hydrogen bonds with the polar radicals (alcohols, ketone, secondary and tertiary amines...). At the contact of apolar domains they can arrange themselves into “clathrate-like” structures [8].
Fig. 1.
Principal types of organization of water at the contact of hydrophilic domains (a and b) and hydrophobic domains (c). Lines of H-bonded water molecules are good conductors of protons [8]. They can form along sequences of polar amino acids in polypeptide chains (a), or in clathrate-like structures (ibidem) covering apolar (hydrophobic) domains (c). They do not form at the contact of highly charged surfaces [59]
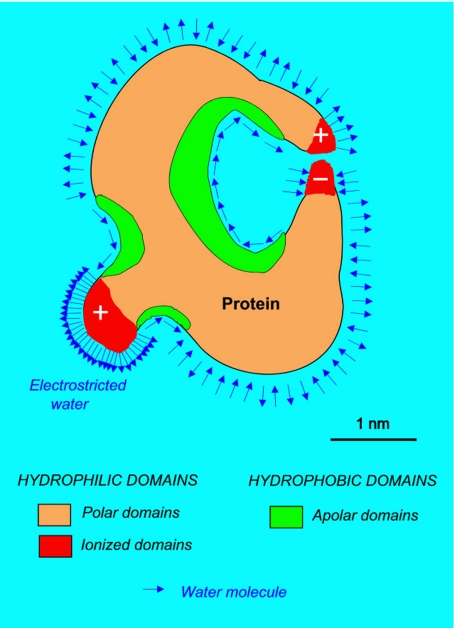
Fig. 2.
Schematic representation of the first layer of interfacial water at the surface of a protein. Its structure, highly heterogeneous, reflects the heterogeneity of the macromolecule surface. Interfacial water presents a large range of densities. Therefore, changes in protein configuration must involve changes in volume, therefore mechanical effects
When water molecules are bound to consecutive polar aminoacids (Asn, Cys, Gln, Ser, Thr, Tyr) in a polypeptide chain, they can bind together to form H-bonded lines. Hydrogen-bonded lines can also be formed along apolar surfaces in clathrates-like structures (Fig. 2). These water molecules lines are good conductors of protons ([8] and below).
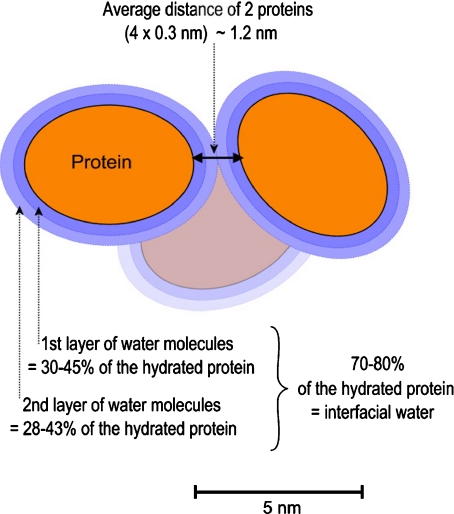
The constraints exerced on surrounding water by the surface of a macromolecule in aqueous solution extend, decreasingly, up to several layers of water molecules. These layers form interfacial water. But, in fact, there is not water enough in cell to form as many layers of interfacial water as in aqueous solutions (Fig. 3). Consequently, cell water is strongly constrained by the macromolecule surfaces. The average thickness of the space between macromolecules in a cell is small: 1.2 nm according to the estimation of Mentré [9, 10], 2 nm according to Cameron and Fullerton [11], 0.3 nm being the size of a water molecule. It is little compared to the average diameter of globular proteins (3 to 5 nm). This space is therefore occupied by water quite different from bulk water: highly constrained and also highly heterogeneous, being the faithful image of the heterogeneity of the surfaces of the macromolecules.
Fig. 3.
The whole cell water (70–80% of the total mass), statistically, is distributed into only two to three hydration layers around macromolecules. The size of a water molecule being 0.3 nm, the film of interfacial water between cell macromolecules is probably not very different from 1.2 nm (2 × 0.3 + 2 × 0.3 nm)
Misrepresentation of concentrations inside the cell
Nowadays, it is widely known that organelles can move in cells using sophisticated means of transport such as motor proteins along cytoskeleton (kinesin, dynein; Alberts et al. [12]). Interestingly, it has been shown that vesicles and secretion granules (mean diameter 100 nm) migrate much faster along cytoskeleton than isolated proteins (mean diameter 3 to 5 nm), which demonstrates the efficiency of the motor proteins [13].
But many people persist in believing that for ions and small molecules diffusion is the principal mean of migration in the cell. This is mainly for three reasons: (1) ignorance of the physical properties of interfacial water (and even sometimes of its existence); (2) confusion between free form and bound form concentration of a substance; (3) ignorance that most of the diffusion/concentration gradients are not significant in the cell at the macromolecular level.
Ignorance of the physical properties of macromolecule-associated water
These properties have yet been investigated as early as the thirties by the Russian School of Nasonov [14]. Numerous works followed, reviewed by Troshin [15, 16] and more recently by Mentré [9, 17], Pollack [18], Pollack et al. [19], Chaplin [20]. Biologists rarely take into consideration these properties, maybe because their bases in physics are often weak, but also perhaps because they think that some biophysical results are not yet reliable enough: such as the density or the solvent power of the different forms of interfacial water, the lifetime of the different associations of water molecules, etc.
But, in any case, what is very important, and already acquired, is the knowledge that interfacial water is deeply different from bulk water. The biophysicist’s approaches, although still incomplete, open the door to a deeper understanding of the cell machinery, at the nanometre level, along the line envisioned by Erwin Schrödinger in his famous “What is Life?” [21].
Density When a macromolecule undergoes a change of form, it is clear that it does not expose the same domains to its aqueous environment. Consequently, its associated water undergoes local micro-changes in density, therefore in volume. Whatever the sign of these changes may be (increase decrease), they induce mechanical effects.
Selective solvent power One important property of interfacial water is its low and selective solvent power: for example, glucose is more excluded from the cell than urea, which circulates rapidly [22]. With constant charge; the smallest ions are the most excluded, according to the Hofmeister series [22–28]. Although recently questioned [29, 30], the classical explanation the Hofmeister series (Fig. 4) remains a good basis from which to work: the smaller an ion is, the more it retains water and therefore the more its migration is slowed down [31].
Fig. 4.
The cation Hofmeister series
Lifetime of water molecule assemblies Changes in interfacial water structure involve local variations in the lifetime of its molecular assemblies; whatever the sign of these variations may be, they induce local modifications of solvent power.
Confusion between free and bound (chelated, sequestered) forms
The classical view is that ions such as sodium and calcium, referred to as diffusible ions, can diffuse freely through cell water from concentrated to less concentrated domains. Simson and Spicer in a remarkable paper [32] explained that only free sodium and calcium can be precipitated into electron-opaque precipitates by the cytochemical pyroantimonate technique. They specified that sodium and calcium when they are complexed in insoluble salts and chelates cannot be chemically detected, except if they have a higher affinity for the pyroantimonate anion than for their complexing agents.But, in spite of this excellent analysis, many authors continued to ignore that (1) only free ions can diffuse, (2) complexed ions require the presence of still stronger complexing agents to be liberated and therefore precipitated. The consequence has been a lot of useless publications, and, unhappily, a certain discredit upon the pyroantimonate technique.It was nevertheless by using this technique (which permits to visualize the free forms and weakly bound forms of sodium and calcium) in association with electron probe microanalysis (which gives an estimation of the total concentrations), that I could deduce that water in the cell matrices must be fundamentally different from bulk water [33–35]. This result was not new for biophysicists. But it was a surprise for cytologists.Electron microscopy associated with microanalysis was accurate enough to analyze the variations of calcium at the ultrastructural level during glycogenolysis and glycogenosynthesis in the liver of mouse [36].
Ignorance that most of the diffusion/concentration gradients are not significant in cell at the macromolecular level
A concentration gradient is not a force field A given molecule does not migrate automatically downstream its concentration gradient. The motion of a given molecule is random with only a higher probability to get to less concentrated regions. Indeed in the cell, the free (“diffusible”) forms of ions and metabolites, because their concentrations are very low, are generally separated by tens—and even hundreds of nanometres. For example: the concentration of calcium in the cell is about 10 − 3 M. The quasi-totality is bound calcium. The free form oscillates between 10 − 8 and 10 − 5 M only. A simple calculation, using Avogadro’s number, shows that the mean distance between two free calcium ions is 55 nm at 10 − 5 M, 255 nm at 10 − 7 M and 550 nm at 10 − 8 M. It is very low, compared to the 12 nm separating two bound calcium ions. Because the mean size of globular proteins is 3 to 5 nm, free calcium ions are (statistically) separated by tens and even hundreds of macromolecules. Free calcium ions have to circulate inside a very narrow space, occupied by strongly constrained water. They have no chance to arriverapidly enough by diffusion where they are needed [37]: (1) because calcium ions are strongly excluded from interfacial water; (2) because having a high affinity for many macromolecules, they run a high risk of becoming trapped before reaching their target.
Ignorance of the iterative modes of propagation of ions and small molecules
The iterative propagation of granules, vesicles or organelles along the cytoskeleton by motor proteins like dynein and kinesin is well documented, as said above. Interestingly, similarly iterative modes of transport have also been described for ions, but these transports are only apparent. A given ion (phosphate, Ca2 + , H + ) seems to be transported along a chain (cascade) of macromolecules containing this ion (or another one) in a sequestered form. A signal (calcium, for example) occurring at the entry of the chain induces the liberation of the sequestered ion from the first element of the chain. And this one, in its turn, induces the liberation of the ion from the following element, etc: the ion entering the chain remains sequestered by the first element of the chain. The ion appearing at the end of the chain is liberated by the last element of the chain. This type of transport differs deeply from diffusion. It is not a transport of matter but a transfer of a level of energy (transduction).
Calcium reservoirs and phosphatase-kinase cascades It is only by the end of the eighties that authors proposed attractive models with cascades of sequestration/liberation of calcium into/out of reservoirs (Berridge and Galone [38]; Rasmussen [39]; Swann and Ozil [40]; reviewed by Mentré [37]).Rasmussen [39] proposed a model to explain that a calcium signal at the level of the cell membrane triggered a calcium burst after only few milliseconds in the nucleus (Fig. 5). For a long time, this burst had been considered as the result of a rapid diffusion of calcium into the nucleus. Rasmussen proposed that the calcium signal would be transduced, without a real transport of calcium, along a cascade of phosphatase-kinase molecules, transmitting step by step an allosteric change triggered by the liberation of phosphate. According to Philippa Wiggins (personal communication), this transfer would involve changes of structure and solvent power of water at each step of the cascade (Fig. 6a; see also: Rand [41]; Colombo et al. [42]).
Fig. 5.
a, b Transduction of calcium signal simulating a transport of calcium from plasma membrane to nucleus (inspired by Rasmussen [39])
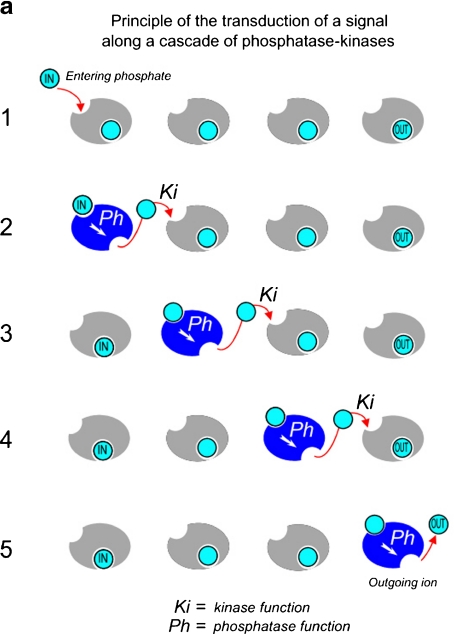
Fig. 6.
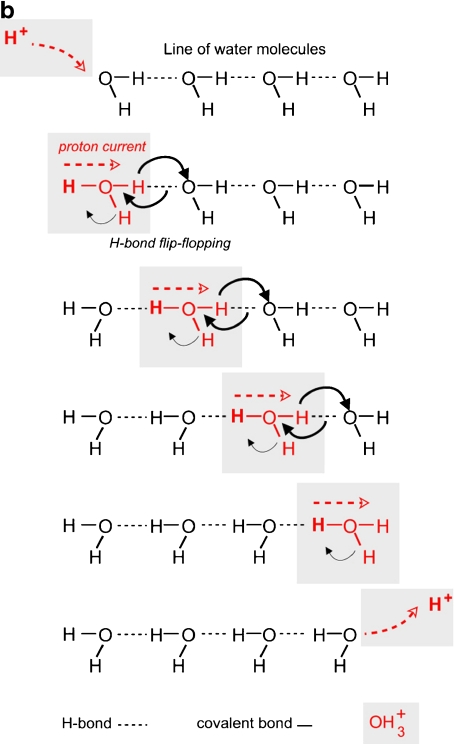
Iterative model for signal transfer. a Simplified representation of signal transduction along a cascade of phosphatase-kinase molecules. Each element of the chain has two functions: kinase (Ki) and phosphatase (Ph), the former inhibited by phosphate ion, and the latter activated, for example. A phosphate ion “IN,” liberated from one element of the chain, binds (by Ki action of this element) to the following one (1). This one undergoes an allostery transformation (in blue) which activates its Ph function (2). It exercises on itself its own phosphatase activity to liberate the phosphate ion which inhibits its Ki function (2). This process is iterative (3), (4), (5). At the end of the process, the entering ion has not left the first element of the cascade. The outgoing ion is the provided by the last element of the cascade (5). b Proton current along a linear chain of water molecules, according to the Grotthus representation. As in (a), the entering proton does not leave the first water molecule of the chain. The outgoing proton is provided by the last water molecule of the chain
Linear chains of H-bonded water molecules As said above, chains of H-bonded water molecules are good conductors of protons.1 In fact, like calcium or phosphate ions in the transduction cascades, protons do not really migrate along the water chains. The Grotthus representation illustrated by Fig. 6b gives a simplified idea of the mechanism: when a proton arrives on the first water molecule of a chain, it does not jump to the following one. The electrostatic equilibrium broken by the formation of OH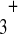 is established by the liberation of another proton of this water molecule. This proton jumps to the following water molecule. This process, involving a successive “flip-flop” of the H-bonds, is repeated, step by step, until the last water molecule of the chain. Finally, the entering proton does not go farther than the first water molecule of the chain and the outgoing proton is provided by the last water molecule of the chain. This transfer simulates a migration of matter. It is in fact a transfer of level of energy, far more rapid than diffusion [43].
is established by the liberation of another proton of this water molecule. This proton jumps to the following water molecule. This process, involving a successive “flip-flop” of the H-bonds, is repeated, step by step, until the last water molecule of the chain. Finally, the entering proton does not go farther than the first water molecule of the chain and the outgoing proton is provided by the last water molecule of the chain. This transfer simulates a migration of matter. It is in fact a transfer of level of energy, far more rapid than diffusion [43].
Role of proton currents Proton currents are involved in many cellular processes, which suppose the existence of chains of water molecules for their conduction, topologically well-defined at the surface of macromolecules, or inside of them [44–48].Giorgio Careri was one of the pioneers who suspected the participation of interfacial water in the activity of macromolecules. He presented a magisterial demonstration of the role of proton currents in the activity of lysozyme [49–51]. In hydrating progressively dehydrated lysozyme powder, he observed that the activity appears when there are enough water molecules to percolate the surface of the enzyme, i.e. to form proton conducting chains. To make my students in Biophysics understand this very important process, I conceived a little diagram that I showed for advice to Prof. Careri who gave me the green light (Fig. 7).
Fig. 7.
Proton currents and enzymatic activity. Dehydrated lysozyme powder is progressively rehydrated. Water coverage exhibits a percolative transition at 0.25 water/g dry weight, detectable by the sudden appearance of proton conductivity, with a parallel emergence of protein mobility and catalytic activity (diagram realized from Careri, with his approval; reproduced from Mentré [60], with the authorization of Prof. Greppin of the University of Geneva)
What about orchestration of the reactions inside of the cell?
In vitro, the reactants of a given reaction are few in number, but each one is present in a huge number of copies. The classical laws of chemistry, which are statistical laws, can be applied. There is no problem of timing and synchronization. The molecules of the reaction, generally in aqueous medium, enter into contact by diffusion, quasi-instantaneously, from the experimenter’s perspective.
In vivo, things are deeply different [10, 17]. Because of their huge variety, most of the chemical species present in cell are present in only a very low number of copies. Moreover, they cannot diffuse rapidly because they are separated by tens of macromolecules. Bulk (“free”) water is quasi-absent, appearing only transiently at the nanometre level during configuration changes undergone by macromolecules. Interfacial water, which constitutes the quasi-totality of cell water, has a low and selective solvent power which makes diffusion a poor means of transport to perform complex and ultra-fast cell processes.
Long-distance transfer of signals As said above, phosphatase-kinase cascades seem able to transduct signals in cells over long distances, probably from plasma membrane into the nucleus. But that raises new questions:
It is difficult to conceive that all the receptors located on the plasma membrane are specifically connected to their specific targets everywhere inside of the cell. Their number is so considerable that cell would be occupied by an incredible tangling of chains of transduction, leaving little space to the other organelles, looking like a town obstructed by as many telephone wires as telephone subscribers!
If the signal-transduction chains, such as telephone lines, could permit transmitter-receiver recognition, their number would be considerably low. But this raises a question: what would be the nature of “dialing”?
Intracellular pH It would be worth concluding this short review of misunderstandings with intracellular pH. It is classically measured with coloured or fluorescent indicators. Changes are visualized at the scale of light microscopy. Of course, they give only a “global” value for the whole cell. This method is not without interest since it can indicate which factors modify this “global” intracellular pH. But it gives a wrong idea of what occurs at the nanometre level. Indeed, if the whole cell was at the same pH, it is doubtful that it could function perfectly, since the cell macromolecules do not have all the same optimal pH.In fact, like diffusion and the law of mass action, pH is a statistical “macroscopic” notion. It is meaningful only in vitro where molecules and H + ions are in large numbers. Indeed, at pH 7.0, the mean distance between two protons is 250 nm. What does it mean at nanometre level? How it is perceived by macromolecules? As said above, a concentration gradient is not a force field. It is only a probability gradient. In vitro, in aqueous solution, protons and macromolecules enter randomly into collision with a high frequency. It can then be conceived that, to function optimally, macromolecules need that the frequency of these collisions corresponds to their own rhythm of activity. Protons must arrive at an optimal time in their reaction cycle, not to soon, not too late. But in the cell, protons are in countable quantity: for example, just few tens in bacteria or in a mitochondrion [52]. They are not abundant enough to enter into collision with all the macromolecules which need them at the same time.Indeed, protons can move rapidly along lines of water molecules. But these lines, very long in Ih-ice, are never long in cell. They display only few units, either along short sequences of polar amino acids or in clathrate-like structures (Fig. 1). Proton currents can just be very short stages for signal transduction (for example to bring information into the very heart of a biomolecule, such as cytochrome, as discussed by Wickström [47]), or can coordinate the activity of an enzyme [45, 49, 50].
Conclusion: “order from order”
Interfacial water, which constitutes the quasi-totality of cell water, undergoes numerous physical changes synchronized with macromolecular transformations. Many advances have been made demonstrating its pre-eminent role at the nanometre (macromolecule) level, in recognition, regulation and coordination (Robinson and Sligar [53]; reviewed by Mentré [6, 17, 37, 54]).
But the problem of orchestration at the micrometer (organelles) level is still in its infancy. The organization of the organelles is explored with an increased precision, but far from making the problem clearer that makes it more and more puzzling. For example, it has been shown recently that the mechanics of the kinetochore, to direct chromosome motion via microtubules and promote the arrest of cell cycle, involves not less than a hundred proteins [55].
It is tempting to consider that the cell could coordinate its activity using mechanical and electromagnetic signals. Mechanical signals could be initiated by variations of density of interfacial water accompanying variations of configuration of macromolecules. As to the electromagnetic signals, it would be interesting today to reconsider more thoroughly ideas advanced in the 1990s: transfer of signals as coherent light along interfacial water inside of microtubules [56, 57] and “vision” of infrared light by the centrosome [58].
Along the lines forseen by Erwin Schrödinger, cell orchestration proves to be far more sophisticated than biologists even envisioned, leaving less and less place for thermal dissipation, which prevails in vitro, and thus excludes chaos:
... the behaviour of living matter, whose most striking features are visibly based to a large extent on the ‘order-from order’ principle. (Erwin Schrödinger [21])
Acknowledgement
I am very grateful towards Dr. Yolène Thomas for her encouragements.
Footnotes
Organization of water into H-bonded linear chains of molecules has been often referred as “ice-like” water: indeed, this structure observed in the common Ih-ice is not present in all the types of ice.
References
- 1.Goodsell DS. Inside a living cell. Trends Biochem. Sci. 1991;16:203–206. doi: 10.1016/0968-0004(91)90083-8. [DOI] [PubMed] [Google Scholar]
- 2.Goodsell DS, editor. The Machinery of Life. 2. New York: Springer; 2009. [Google Scholar]
- 3.Porter KR. The cytoplasmic matrix and the integration of cellular function. Proc. of a Conference sponsored by Fogarty Internatl. Center, Natl. Institutes of Health. J. Cell Biol. 1984;99:1–248. doi: 10.1083/jcb.99.1.3s. [DOI] [PubMed] [Google Scholar]
- 4.Porter KR. The cytomatrix: a short history of its study. J. Cell Biol. 1984;99:3–14. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zierold K. The determination of wet weight concentrations of elements in freeze-dried cryosections from biological cells. Scanning Electron Microsc. 1986;2:713–724. [PubMed] [Google Scholar]
- 6.Mentré, P. (ed.): Coquille d’hydratation des macromolécules, eau “ liée ” et eau “ structurée ”. In: L’eau dans la cellule. Une interface hétérogène et dynamique des macromolécules, pp. 65–82. Masson-Dunod, Paris (1995)
- 7.Mentré P, Hui Bon Hoa G. The effects of high hydrostatic pressures on living cells: a consequence of the properties of macomolecules and macromolecule-associated water. Int. Rev. Cytol. 2001;201:1–84. doi: 10.1016/S0074-7696(01)01001-4. [DOI] [PubMed] [Google Scholar]
- 8.Klotz IM. Water. In: Kasha M, Pullman B, editors. Horizons in Biochemistry. Albert Szent-Györgyi dedicatory volume. New York: Academic; 1962. pp. 523–550. [Google Scholar]
- 9.Mentré, P.: An introduction to “Water in the cell”: tamed hydra? In: Water in the Cell (Mentré, P. guest ed.). Cell. Mol. Biol. 47, 709–715 (2001) [PubMed]
- 10.Leitner DM, Gruebele M, Havenith M. Solvation dynamics of biomolecules: modeling and terahertz experiments. HFSP J. 2008;2:314–323. doi: 10.2976/1.2976661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron IL, Fullerton GD. Non-bulk like water on cellular interfaces. In: Pollack G, editor. Water and the Cell. Heidelberg: Springer; 2006. pp. 315–323. [Google Scholar]
- 12.Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P. (eds): Molecular motors. In: Molecular Biology of the Cell, 4th ed., pp. 949–968. Garland Science, New York (2002)
- 13.Lasek RJ, Garner JA, Brady ST. Axonal transport of the cytoplasmic matrix. J. Cell Biol. 1984;99:212–221. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matveev, V.V.: Protoreaction of protoplasm. In: Water in the Cell (Mentré, P., ed.). Cell. Mol. Biol. 51, 715–723 (2005) [PubMed]
- 15.Troshin, A.S. (ed.): The protoplasm as a colloidal system. In: Problems of Cell Permeability, pp. 57–73 (1956). Translated from Russian by Widdas, W.F., Pergamon, Oxford (1966)
- 16.Troshin, A.S.: Do live cells have osmotic properties? In: Problems of Cell Permeability, pp. 30–56 (1956). Translated from Russian by Widdas, W.F., Pergamon, Oxford (1966)
- 17.Mentré, P. (ed.): L’eau dans la cellule. Une interface hétérogène et dynamique des macromolécules, p. 292. Masson-Dunod (1995)
- 18.Pollack GH. Cells, Gels and the Engines of Life. A New, Unifying Approach to Cell Function. Seattle: Ebner; 2001. [Google Scholar]
- 19.Pollack GH, Cameron IL, Wheatley DN, editors. Water and the Cell. New York: Springer; 2006. [Google Scholar]
- 20.Chaplin, M.: http://www.lsbu.ac.uk/water/ (2009)
- 21.Schrödinger, E. (ed.): What is Life? The Physical Aspects of the Living Cell (1944)
- 22.Ling GN. Solute exclusion by polymer and protein-dominated water: correlation with results of nuclear magnetic resonance (NMR) and calorimetric studies and their significance for the understanding of the physical state of water in living cells. Scanning Microsc. 1988;2:871–884. [PubMed] [Google Scholar]
- 23.Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888;24:247–260. doi: 10.1007/BF01918191. [DOI] [Google Scholar]
- 24.Ling, G.N.: A Physical Theory of the Living State: The Association-Induction Hypothesis, pp. 1–680. Blaisdell Waltham (1962)
- 25.Ling, G.N. (ed.): In Search of the Physical Basis of Life. Plenum, New York (1984)
- 26.Ling, G.N. (ed.): The membrane pump theory. In: A Revolution in the Physiology of the Living Cell, pp. 9–30. Krieger, Malabar (1992)
- 27.Wiggins P, MacClement BAE. Two states of water found in hydrophobic clefts: their possible contribution to mechanism of action of pumps and other enzymes. Int. Rev. Cytol. 1987;108:249–304. doi: 10.1016/S0074-7696(08)61440-0. [DOI] [PubMed] [Google Scholar]
- 28.Wiggins P, Ryn RT. Changes in ionic selectivity with changes in density in gels and cells. Biophys. J. 1987;58:585–596. doi: 10.1016/S0006-3495(90)82402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Flores SC, Lim SM, Zhang Y, Yang T, Kherb J, Cremer P. Specific anion effects on water structure adjacent to protein monolayers. Langmuir. 2010;26:16447–16454. doi: 10.1021/la1015862. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Cremer PS. Interactions between macromolecules and ions: the Hofmeister series. Curr. Opinions Chem. Biol. 2006;10:658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Glasstone, S. (ed.): Electrochemistry. In: Textbook of Physical Chemistry, pp. 884–1043. MacMillan, London (1948)
- 32.Simson JAV, Spicer SS. Selective subcellular localization of cations with variants of the potassium (pyro)antimonate technique. J. Histochem. Cytochem. 1975;23:575–598. doi: 10.1177/23.8.51037. [DOI] [PubMed] [Google Scholar]
- 33.Mentré P, Halpern S. Localization by pyroantimonate method and electron probe microanalysis of calcium and sodium in skeletal muscle of mouse. J. Histochem. Cytochem. 1988;36:55–64. doi: 10.1177/36.1.3335770. [DOI] [PubMed] [Google Scholar]
- 34.Mentré, P.: Taking into account the cell water properties for the cytochemical detection of cations: embedding into melamine after pyroantimonate fixation. In: Vasilescu, D., Jaz, J., Packer, L., Pullman, B. (eds.) Water and Ions in Biomolecular Systems. Proceed. 5th Unesco Internatl. Conf., pp. 287–294. Birkhäuser, Basel (1990)
- 35.Mentré P. Preservation of the diffusible cations for SIMS microscopy. I. A problem related to the state of water in the cell. Biol. Cell. 1992;74:19–30. doi: 10.1016/0248-4900(92)90005-L. [DOI] [PubMed] [Google Scholar]
- 36.Mentré P, Halpern S. Application of the pyroantimonate method and electron probe microanalysis to the study of glycogen metabolism in liver. Scanning Microsc. 1989;3:495–504. [PubMed] [Google Scholar]
- 37.Mentré, P. (ed.): Transports au sein de la cellule. In: L’eau dans la cellule. Une interface hétérogène et dynamique des macromolécules, pp. 207–233. Masson-Dunod, Paris (1995)
- 38.Berridge MJ, Galone A. Cytosolic oscillators. FASEB J. 1988;2:3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen H. The cycling of calcium as an intracellular messenger. Sci. Am. 1989;261:66–73. doi: 10.1038/scientificamerican1089-66. [DOI] [PubMed] [Google Scholar]
- 40.Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int. Rev. Cytol. 1994;152:183–222. doi: 10.1016/S0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 41.Rand RP.Raising water to new heights Science 19922566181992Sci...256..618R 10.1126/science.256.5057.618 [DOI] [PubMed] [Google Scholar]
- 42.Colombo MF, Rau DC, Parsegian VA.Protein solvation in allosteric regulation: a water effect on hemoglobin? Science 1992256655–659.1992Sci...256..655C 10.1126/science.1585178 [DOI] [PubMed] [Google Scholar]
- 43.Teissié J, Prats M, Soucaille P, Tocanne JF.Evidence for conduction of protons along the interface between water and a polar lipid monolayer Proc. Natl. Acad. Sci. U.S.A. 1985823217–3221.1985PNAS...82.3217T 10.1073/pnas.82.10.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kell DB. On the functional proton current pathway of electron transport phosphorylation. An electrodic view. Biochim. Biophys. Acta. 1979;549:55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- 45.Careri G, Giansanti A, Rupley JA.Proton percolation on hydrated lysozyme powder Proc. Natl. Acad. Sci. U.S.A. 1986836810–6814.1986PNAS...83.6810C 10.1073/pnas.83.18.6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paddock ML, McPherson PH, Feher G, Okamura MY.Pathway of proton transfer in bacterial reaction centers: replacement of serine-L223 by alanine inhibits electron and proton transfers associated with reduction of quinone to dihydroquinone Proc. Natl. Acad. Sci. U.S.A. 1990876803–6807.1990PNAS...87.6803P 10.1073/pnas.87.17.6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wikström M, Verkhovsky MI, Hummer G. Water-gated mechanism of proton translocation by cytochrome c oxidase. Biochim. Biophys. Acta. 2003;1604:61–65. doi: 10.1016/S0005-2728(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 48.Yin H, Feng G, Clore GM, Hummer G, Rasaiah JC. Water in the polar and nonpolar cavities of the protein interleukin-1β. J. Phys. Chem., B. 2010;114:16290–16297. doi: 10.1021/jp108731r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Careri G, Geraci M, Giansanti A, Rupley JA.Protonic conduction of hydrated lysozyme powders at megahertz frequencies Proc. Natl. Acad. Sci. U.S.A. 1985825342–5346.1985PNAS...82.5342C 10.1073/pnas.82.16.5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Careri G, Consolini G, Bruni F. Proton conductivity in hydrated proteins. Evidence for percolation. In: Vasilescu D, Jaz J, Packer L, Pullman B, editors. Water and Ions in Biomolecular Systems. Proceed. 5th Unesco Internatl. Conf. Basel: Birkhäuser; 1990. pp. 165–170. [Google Scholar]
- 51.Careri G, Peyrard M. Physical aspects of the weakly hydrated protein surface. Water in the Cell (Mentré, P. ed.). Cell Mol. Biol. 2001;47:745–756. [PubMed] [Google Scholar]
- 52.Albrecht-Buelher G. In defense of “nonmolecular” biology. Int. Rev. Cytol. 1990;120:191–241. doi: 10.1016/S0074-7696(08)61601-0. [DOI] [PubMed] [Google Scholar]
- 53.Mentré, P. (ed.): L’eau, une interface hétérogène et dynamique des macromolecules. In: L’eau dans la cellule. Une interface hétérogène et dynamique des macromolécules, pp. 268–275. Masson Dunod, Paris (1995)
- 54.Robinson CR, Sligar SG. Hydrostatic and osmotic pressure as tools to study macromolecular recognition. Methods Enzymol. 1995;259:395–427. doi: 10.1016/0076-6879(95)59054-4. [DOI] [PubMed] [Google Scholar]
- 55.Bloom K, Yeh E. Tension management in the kinetochore. Curr Biol. 2010;20:1040–1048. doi: 10.1016/j.cub.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jibu M, Hagan S, Hameroff SR, Pribram KH, Yasue K. Quantum optical coherence in cytoskeletal microtubules: implications for brain function. Biosystems. 1994;32:195–209. doi: 10.1016/0303-2647(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 57.Hameroff, S.R., Penrose, R. (eds.): Orchestrated reduction of quantum coherence in brain microtubules: a model for consciousness? In: Toward a Science of Consciousness—The First Tucson Discussions and Debates, pp. 507–540. Cambridge, MIT (1996)
- 58.Albrecht-Buelher G. Cellular infrared detector appears to be contained in the centrosome. Cell Motil. Cytoskelet. 1994;27:262–271. doi: 10.1002/cm.970270307. [DOI] [PubMed] [Google Scholar]
- 59.Nihonyanagi S, Yamaguchi S, Tahara T. Water hydrogen bond structure near highly charged interfaces is not like ice. J. Am. Chem. Soc. 2010;132:6867–6869. doi: 10.1021/ja910914g. [DOI] [PubMed] [Google Scholar]
- 60.Mentré P. Organization and properties of water in cell system. In: Greppin H, Penel C, Broughton WJ, Strasser R, editors. Integrated Plant Systems. Geneva: University of Geneva; 2000. pp. 3–22. [Google Scholar]