Abstract
A comprehensive literature search was performed to collate evidence of mitochondrial dysfunction in autism spectrum disorders (ASDs) with two primary objectives. First, features of mitochondrial dysfunction in the general population of children with ASD were identified. Second, characteristics of mitochondrial dysfunction in children with ASD and concomitant mitochondrial disease (MD) were compared with published literature of two general populations: ASD children without MD, and non-ASD children with MD. The prevalence of MD in the general population of ASD was 5.0% (95% confidence interval 3.2, 6.9%), much higher than found in the general population (∼0.01%). The prevalence of abnormal biomarker values of mitochondrial dysfunction was high in ASD, much higher than the prevalence of MD. Variances and mean values of many mitochondrial biomarkers (lactate, pyruvate, carnitine and ubiquinone) were significantly different between ASD and controls. Some markers correlated with ASD severity. Neuroimaging, in vitro and post-mortem brain studies were consistent with an elevated prevalence of mitochondrial dysfunction in ASD. Taken together, these findings suggest children with ASD have a spectrum of mitochondrial dysfunction of differing severity. Eighteen publications representing a total of 112 children with ASD and MD (ASD/MD) were identified. The prevalence of developmental regression (52%), seizures (41%), motor delay (51%), gastrointestinal abnormalities (74%), female gender (39%), and elevated lactate (78%) and pyruvate (45%) was significantly higher in ASD/MD compared with the general ASD population. The prevalence of many of these abnormalities was similar to the general population of children with MD, suggesting that ASD/MD represents a distinct subgroup of children with MD. Most ASD/MD cases (79%) were not associated with genetic abnormalities, raising the possibility of secondary mitochondrial dysfunction. Treatment studies for ASD/MD were limited, although improvements were noted in some studies with carnitine, co-enzyme Q10 and B-vitamins. Many studies suffered from limitations, including small sample sizes, referral or publication biases, and variability in protocols for selecting children for MD workup, collecting mitochondrial biomarkers and defining MD. Overall, this evidence supports the notion that mitochondrial dysfunction is associated with ASD. Additional studies are needed to further define the role of mitochondrial dysfunction in ASD.
Keywords: autism, electron transport chain, meta-analysis, mitochondrial dysfunction, regression, systematic review
Introduction
Autistic disorder, Asperger syndrome and pervasive developmental disorder-not otherwise specified comprise a heterogeneous group of neurodevelopmental disorders known as autism spectrum disorders (ASDs). ASDs are behaviorally defined by impairments in communication and social interaction along with restrictive and repetitive behaviors.1 An estimated 1 out of 110 individuals in the United States is currently affected with ASD, with a male-to-female ratio of 4.5:1.2 The etiology of ASD is not known in most cases, but a genetic component, possibly involving 15 or more loci, is widely accepted to contribute to the development of ASD.3 However, the robust phenotypic and genotypic heterogeneity among individuals with ASD4, 5 has limited the case for a purely genetic etiology.6, 7 Indeed, it is becoming apparent that many children with ASD have associated underlying medical comorbidities, such as epilepsy, sleep disruption, mitochondrial dysfunction and gastrointestinal (GI) abnormalities.8, 9, 10, 11, 12, 13
Mitochondrial dysfunction has been implicated in several psychiatric14, 15 and neurological16, 17, 18, 19, 20, 21, 22 disorders. Over 20 years ago, Coleman and Blass23 hypothesized that individuals with ASD may have an abnormality in carbohydrate metabolism, and in 1998 Lombard24 proposed that ASD may be a disorder of impaired mitochondrial function. Over the past decade, evidence has accumulated that some individuals with ASD have concomitant mitochondrial dysfunction, and some have proposed a ‘mitochondrial autism' subgroup.25 Several review articles have been recently published concerning mitochondrial dysfunction in ASD.9, 26, 27, 28 However, to date, neither a systematic comprehensive review nor a meta-analysis of this recently evolving literature has been published. In this paper, we systematically review the evidence for mitochondrial dysfunction in ASD with the following specific objectives:
To calculate the prevalence of mitochondrial dysfunction and clinical, biochemical, histological, and genetic markers of mitochondrial dysfunction in the general population of children with ASD.
To calculate the effect size of the differences in the values of biochemical markers of mitochondrial dysfunction between the general population of children with ASD and control groups.
To examine the clinical, biochemical, histological and genetic characteristics of children diagnosed with mitochondrial disease (MD) or mitochondrial dysfunction and concomitant ASD (referred to hereafter as ASD/MD) and compare these characteristics with the general ASD population and to the general childhood MD population.
To review studies examining the prevalence of ASD or autistic features in children with MD.
To review studies correlating biomarker values of mitochondrial dysfunction with the severity of ASD symptoms.
To review animal models of ASD with abnormal energy pathways.
To examine treatments of mitochondrial dysfunction in children with ASD.
Before presenting this analysis, we provide an overview of the importance of mitochondrial function in health and disease, with reference to mechanisms that overlap biochemical abnormalities associated with ASD. We also briefly discuss the standardized methods for diagnosing MD.
Mitochondrial function in health and disease
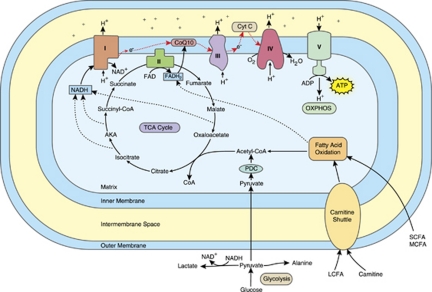
Mitochondria are distinct cellular organelles that generate adenosine triphosphate (ATP), the energy carrier in most mammalian cells, from adenosine diphosphate by oxidizing glucose and fatty acids (for a review, see Haas et al.29). Acetyl-CoA is a key intermediate generated from the oxidation of glucose and fatty acids that is further metabolized by the tricarboxylic acid (TCA) cycle. The TCA cycle produces reduced flavin adenine dinucleotide and reduced nicotinamide adenine dinucleotide. Reduced nicotinamide adenine dinucleotide and reduced flavin adenine dinucleotide transport energy to the mitochondrial electron transport chain (ETC), a series of reactions known as oxidative phosphorylation. Mitochondria contain two plasma membranes, an inner and an outer membrane. The ETC is located in the inner mitochondrial membrane and consists of five multi-subunit enzyme complexes (complexes I through V) and two electron carriers (ubiquinone, also known as co-enzyme Q10, and cytochrome c).30 See Figure 1 for an overview of mitochondrial function.
Figure 1.
Mitochondrial function and the electron transport chain (ETC). Abbreviations: ADP, adenosine diphosphate; AKA, α-ketoglutarate; ATP, adenosine triphosphate; CoQ, co-enzyme Q; Cyt C, cytochrome c; e, electron; FAD, flavin adenine dinucleotide; FADH2, reduced FAD; H, hydrogen; LCFA, long chain fatty acid; MCFA, medium chain fatty acid; NAD, nicotinamide adenine dinucleotide; NADH, reduced NAD; OXPHOS, oxidative phosphorylation; PDC, pyruvate dehydrogenase complex; SCFA, short chain fatty acid; TCA, tricarboxylic acid; I, complex I; II, complex II; III, complex III; IV, complex IV; V, complex V. The red arrows denote the flow of electrons in the ETC. The color reproduction of this figure is available on the html full text version of the article.
Mitochondria are the only organelle in mammalian cells with their own genome. The ETC is coded by both mitochondrial DNA (mtDNA) and nuclear DNA (nDNA).30 mtDNA contains 37 genes that code for 13 subunits of complexes I, III, IV and V, as well as the machinery required to translate and transcribe the mtDNA genes into ETC complex subunits. The remainder of the ETC complex subunits are coded by over 850 nDNA genes.31 nDNA also codes for mitochondrial enzymes that participate in carbohydrate and fatty acid oxidation. Thus, mutations in either genome can impair mitochondrial function and cause ETC complex deficiencies.32
The ETC is the predominant source and the major target of reactive oxygen species (ROS)20, 33 and is protected from damage caused by ROS by a mitochondrial-specific superoxide dismutase and antioxidants such as glutathione (GSH).33 Mitochondria lack the enzymes to synthesize GSH and therefore are dependent on cytosolic GSH production.34, 35 The depletion of GSH in mitochondria makes cells more vulnerable to oxidative stress and damage from ROS originating from the mitochondria.36 Additionally, factors that increase ROS production (such as, environmental toxicants, infections and autoimmune disease) can directly and indirectly lead to impairments in ETC activity,27, 37, 38 deplete GSH,37 and activate mitochondrial and non-mitochondrial-dependent biochemical cascades that result in programmed cell death (apoptosis).39
Certain mammalian cells, such as neuronal and non-neuronal brain cells, are very vulnerable to oxidative stress (for example, damage caused by ROS). The high rate of oxygen delivery and consumption in the brain provides the oxygen molecules necessary to generate ROS. The brain's ability to withstand oxidative stress is limited because of: (a) a high content of substrates that are easily oxidized, such as polyunsaturated fatty acids; (b) relatively low levels of antioxidants, such as GSH and antioxidant enzymes; (c) the endogenous generation of ROS via several specific reactions; and (d) the endogenous generation of nitric oxide (NO), a compound that readily transforms into reactive nitrogen species. Furthermore, the brain is very vulnerable to oxidative damage because it contains non-replicating cells which, once damaged, may be permanently dysfunctional or committed to apoptosis.37, 39
The number of mitochondria in each cell depends on the cellular energy demands. For example, low energy cells, such as skin cells, have fewer mitochondria, while cells that require high energy demands, such as muscle, liver, brain, cerebrovascular endothelium and GI cells, have many mitochondria. Neural synapses are areas of high energy consumption40 and are therefore especially dependent on mitochondrial function.41 Mitochondria are concentrated in the dendritic and axonal termini where they have an important role in ATP production, calcium homeostasis and synaptic plasticity.42, 43 Mitochondrial dysfunction can lead to reduced synaptic neurotransmitter release, and neurons that have high firing rates, such as GABAergic interneurons, may be the most adversely affected.27 Mitochondria also have an important role in cellular lipid metabolism, signaling and repair.44, 45
MD was once thought to be uncommon but is now considered the most recognized cause of metabolic disease.30 Despite increased recognition, the prevalence of MD is probably underestimated.46 The minimum birth prevalence of an ETC defect with onset at any age has been estimated at 1 in 7634 individuals (∼0.01%).47 More than 100 mtDNA deletions and over 150 mtDNA point mutations have been described in individuals with MD.29 MD has a broad phenotypic presentation: children with MD can have normal intelligence, mental retardation or developmental delay.48 Stressors, such as dehydration, fever and infection can lead to a functional decline and neurodegenerative regression in individuals with MD.49, 50
The diagnosis of MD can be challenging, and is based on several objective clinical, histological, biochemical, molecular, neuroimaging and enzymatic findings. Several major diagnostic criteria are used51, 52, 53, 54, 55 to classify the probability of MD into: not likely, possible, probable or definite; individuals reaching the criteria for probable or definite are typically considered to have MD. The diagnostic criteria recognize several types of clinical presentations. These include primarily muscular or central nervous system presentations or multisystem presentations.54, 55 In addition, patients can present clinically with one of the well-characterized mitochondrial syndromes.55 Other important diagnostic features include abnormal histology (such as, ragged-red or blue fibers in skeletal muscle, or skeletal muscle with reduced cytochrome c oxidase or succinate dehydrogenase staining, or electron microscopy demonstrating abnormal mitochondria or subsarcolemmal mitochondrial accumulations), abnormal enzymology (significantly impaired ETC activity), identification of an mtDNA or nDNA mutation, abnormal neuroimaging and abnormal biochemical markers.54, 55
No reliable biomarker exists to identify all cases of MD.29 Biochemical markers of mitochondrial dysfunction described in the literature include direct (lactate, pyruvate, lactate-to-pyruvate ratio, ubiquinone, alanine, alanine-to-lysine ratio and acyl-carnitine) and indirect markers (creatine kinase (CK), carnitine, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and ammonia).25, 29, 54, 56, 57 These markers can be abnormal for several reasons. For example, mitochondrial dysfunction impairs aerobic respiration, leading to a reduction in TCA cycle function resulting in an elevation in pyruvate (see Figure 1). Pyruvate is metabolized to lactate and alanine, leading to elevations in these metabolites when pyruvate metabolism is impaired.30, 56 Inhibition of the TCA cycle may result in an elevation of TCA cycle intermediates. Inhibition of aerobic respiration also impairs fatty acid β-oxidation, leading to elevations in the concentrations of acyl-carnitines. Furthermore, anaerobic respiration increases when aerobic respiration is insufficient to meet cellular energy demands. As lactate is one of the end-products of anaerobic respiration, deactivation of aerobic respiration further elevates lactate. As a result of this, the measurement of plasma lactate can be helpful in the initial workup of mitochondrial dysfunction.30, 54 However, lactate may be elevated only during illness, or not at all, in children with MD.48, 57 In fact, normal cerebrospinal fluid or serum lactate levels do not rule out MD.48, 58
Indirect markers of mitochondrial function can also be abnormal in MD. For example, depletion in total and free carnitine can occur as a consequence of excessive unprocessed fatty acids.56 Ammonia may be elevated for at least two reasons. First, under anaerobic conditions, ammonia is produced when adenosine monophosphate is broken down into inosine monophosphate in order to replenish ATP. Second, as the urea cycle is partially located in the mitochondria, mitochondrial dysfunction can result in secondary urea cycle dysfunction and an elevation in ammonia. In addition, the integrity of certain high-energy tissues, such as muscle and liver, can be compromised from mitochondrial dysfunction, resulting in elevations in indicators of tissue damage such as CK, AST and/or ALT.
Mitochondrial dysfunction can be classified as either primary or secondary.29 Primary mitochondrial dysfunction generally refers to mitochondrial dysfunction caused by a defect in a gene directly involved in the function of mitochondrial systems responsible for producing ATP, whereas secondary mitochondrial dysfunction refers to other metabolic or genetic abnormalities that impair the ability of mitochondria to produce ATP. For example, metabolites produced by toxic substances (for example, environmental toxicants) or by the dysfunction of other metabolic systems that are not specifically involved in producing ATP (for example, increased oxidative stress because of dysfunctional antioxidant pathways) can interfere with the ability of mitochondria to make ATP and lead to secondary mitochondrial dysfunction. Other reported causes of secondary mitochondrial dysfunction include: certain medications;29, 59, 60 enteric short chain fatty acids, such as propionic acid;61, 62, 63, 64, 65 elevated concentrations of tumor necrosis factor-α66, 67, 68 cerebral folate deficiency;69, 70 malnutrition;71 heme, vitamin B6, or iron deficiencies;72 elevated NO;73, 74, 75 GSH deficiency;73 oxidative stress;36 or exposure to environmental toxicants, such as heavy metals,76, 77, 78, 79 chemicals,80 polychlorinated biphenyls81 or pesticides.82, 83 Some individuals have findings consistent with MD but do not have an identifiable genetic defect and/or do not meet full criteria for definite or probable MD. It is possible that these individuals have secondary mitochondrial dysfunction9, 25, 84, 85 or may have an as yet unidentified genetic abnormality. In this review article, we collate evidence of both primary and secondary mitochondrial dysfunction in ASD.
Materials and methods
Search strategy
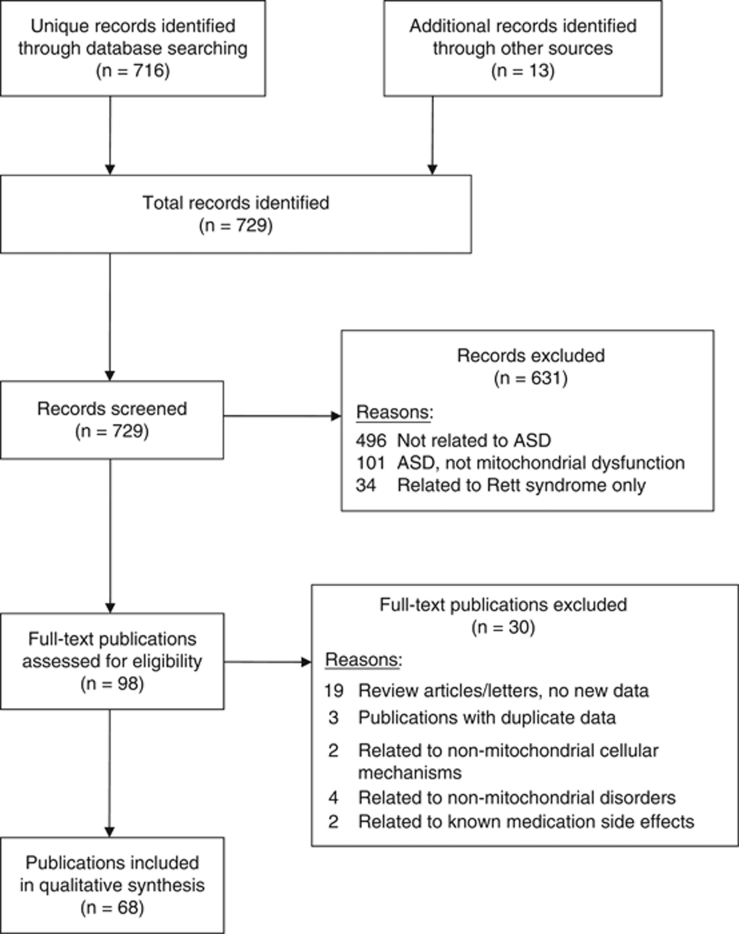
A prospective protocol for this systematic review was developed a priori, and the search terms and selection criteria were chosen in an attempt to capture all pertinent publications. A computer-aided search of PUBMED, Google Scholar, CINAHL, EmBase, Scopus and ERIC databases from inception through August 2010 was conducted to identify pertinent publications using the search terms ‘autism', ‘autistic', ‘Asperger', ‘ASD', ‘pervasive', and ‘pervasive developmental disorder' in all combinations with the terms ‘mitochondria' OR ‘mitochondrial' OR ‘lactic' OR ‘lactate' OR ‘pyruvate' OR ‘pyruvic' OR ‘ammonia' OR ‘creatine kinase' OR ‘oxidative phosphorylation' OR ‘phosphorylation' OR ‘carnitine' OR ‘acyl-carnitine' OR ‘fatty acid oxidation' OR ‘alanine' OR ‘respiratory chain' OR ‘electron transport chain' OR ‘energy' OR ‘ATP' OR ‘adenosine.' The references cited in identified publications were also searched to locate additional studies. Figure 2 depicts the publications identified during the search process.
Figure 2.
Study flow chart. Abbreviation: ASD, autism spectrum disorder.
Study selection
One reviewer screened titles and abstracts of all potentially relevant publications. Studies were initially included if they (1) involved individuals with ASD, and (2) reported at least one finding that could indicate mitochondrial dysfunction. We also included animal models of ASD with abnormal energy pathways. Abstracts or posters from conference proceedings were included if published in a peer-reviewed journal. After screening all records, 98 publications met inclusion criteria; both reviewers then independently reviewed these articles. Articles were excluded if they:
Did not involve animals or humans or human cells (for example, cellular models).
Did not present new or unique data (such as, review articles or letters to the editor).
Reported biochemical markers related to a non-mitochondrial disorder88, 89, 90, 91 or cellular mechanism.92, 93
Reported markers related to a known side effect of a medication (for example, elevated ammonia from valproic acid or rhabdomyolysis from olanzapine).94, 95
Involved only Rett syndrome or childhood disintegrative disorder.
A total of 68 publications,5, 23, 25, 28, 35, 48, 49, 58, 61, 62, 64, 65, 85, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150 describing 65 unique studies, met inclusion and exclusion criteria (see Figure 2), including three sets of publications (6 publications total) that studied the same population but provided slightly different information.28, 119, 120, 132, 133, 144
Clinical characteristics in children with ASD compared with controls
We reviewed all studies that included children with ASD and presented biochemical markers that could indicate mitochondrial dysfunction. A meta-analysis was performed based on the information derived from these studies. There are two types of measures derived from the studies reviewed: prevalence values, and comparisons of biomarker values between ASD and control groups. A mean prevalence value was computed by dividing the number of participants with abnormal values for all studies by the number of participants evaluated for all studies. A 95% confidence interval (CI) was then calculated assuming a Bernoulli distribution.151 Several statistics were computed for the comparisons of continuous biomarker values between the ASD and control groups: (a) means and variances were computed for both groups; (b) the variances of the two groups were compared using an F-ratio; (c) two different methods were used to calculate the effect size; and (d) the homogeneity statistic Q was calculated for each effect size. Calculating effect size from populations that have different variability can be problematic. Therefore, we formally compared the variability in the ASD and control groups using an F-ratio. Two approaches are used to calculate the effect size for populations with different variability. Glass et al.152 introduced the Glass's Δ, which is based only on the control s.d. Others have suggested that a statistic, such as the Hedge's g, which uses the average sample s.d., is a truer representation of the effect size.153 Thus, we calculated both Glass's Δ and Hedge's g. In addition, we calculated the homogeneity statistic Q in order to test whether or not the effect sizes are estimated from the same population means across studies.154
Clinical characteristics in children with ASD and MD compared with controls
We also reviewed all studies that reported individuals with ASD and a concomitant diagnosis of MD or mitochondrial dysfunction (ASD/MD). All of these studies described young children and adolescents, with the oldest being 20 years old. Since these studies spanned the entire childhood population, we refer to this population as children. One case series reported that the clinical characteristics of children with ASD/MD are atypical of the general ASD population.25 However, that study25 did not quantitatively compare the characteristics of these two groups. Therefore, we directly compared the prevalence of characteristics of these two groups to determine if, in fact, there are differences in the characteristics of ASD/MD as compared with the general ASD population, and, if so, which characteristics distinguish these two groups. In order to perform this analysis, we calculated the prevalence of commonly reported clinical characteristics compiled from the studies of children with ASD/MD as well as biochemical and genetic markers of mitochondrial dysfunction (see Table 3 and Supplementary Table S1). We identified a total of 11 commonly reported clinical characteristics of mitochondrial dysfunction (ataxia, cardiomyopathy, fatigue/lethargy, GI abnormalities, growth delay, hypotonia, male-to-female ratio, motor delay, myopathy, regression and seizures). We then compared the prevalence of these 11 characteristics and abnormal biomarker values for children with ASD/MD to two control groups: (a) the general population of children with ASD, and (b) the general population of children with MD. In order to determine the prevalence of these 11 clinical characteristics of mitochondrial dysfunction in the general population of children with ASD, we performed a literature search for these characteristics in ASD. The results of this search are found in the Supplementary Table S2. In order to minimize bias, we only included studies that had large sample sizes (96–987 children per study; mean 359 children; total of 2870 children), were published in high impact journals, and were either epidemiology/population-based/longitudinal studies155, 156, 157, 158, 159, 160 or large studies performed in academic settings.161, 162 The prevalence of certain clinical characteristics uncommon in the general ASD population, such as ataxia, cardiomyopathy, fatigue/lethargy, growth delay and myopathy could not be calculated for this group because adequate studies reporting these characteristics could not be identified. For the prevalence of abnormal mitochondrial biochemical markers in the general ASD population, we used the prevalence results generated from our meta-analysis (as listed in Table 1) for comparison. In order to determine the prevalence of clinical characteristics and biomarkers of mitochondrial dysfunction in the general population of children with MD, we performed a literature search for the above 11 clinical characteristics as well as the biomarkers listed in Table 3. The results of this search are found in the Supplementary Table S3. To minimize bias, we only included studies of MD that had large sample sizes (25–133 children per study; mean 61 children; total of 428 children; one study50 included 4 adults), were published in high impact journals, and were either population-based163 or from large academic/referral centers.48, 50, 51, 58, 164, 165, 166 Chi-square analysis was used to compare the prevalence rates between children with ASD/MD and the prevalence rates in the two comparison groups. We calculated the χ2 using the observed frequencies of the ASD/MD group and calculated the expected frequencies using the prevalence rates in the comparison group. Since we conducted 31 statistical tests, we controlled for inflated alpha using the Bonferroni correction. Thus, the statistical threshold was set at an alpha of 0.002 (0.05/31).
Table 1. Pooled prevalence estimates for MD in ASD and for abnormal biomarkers of mitochondrial dysfunction in ASD.
| Studies | Total N | Overall prevalence | Minimum (%) | Maximum (%) | |
|---|---|---|---|---|---|
| General ASD population | |||||
| Mitochondrial disease in ASD | 3 | 536 | 5.0% (3.2%, 6.9%) | 3.6 | 9.1 |
| Elevated lactate | 6 | 479 | 31.1% (27.0%, 35.3%) | 17 | 77 |
| Elevated pyruvate | 2 | 110 | 13.6% (7.2%, 20.1%) | 8 | 30 |
| Elevated lactate/pyruvate ratio | 1 | 192 | 27.6% (21.2%, 33.9%) | ||
| Elevated alanine | 1 | 36 | 8.3% (0.0%, 20.1%) | ||
| Low total carnitine | 1 | 30 | 90.0% (81.0%, 99.0%) | ||
| Elevated creatine kinase | 1 | 47 | 46.8% (32.4%, 61.2%) | ||
| Elevated ammonia | 1 | 80 | 35.0% (24.5%, 45.5%) | ||
| Elevated AST | 1 | 147 | 45.6% (37.5%, 53.7%)a | ||
| Elevated ALT | 1 | 87 | 7.0% (0.5%, 13.5%) | ||
Abbreviations: ALT, alanine aminotransferase; ASD, autism spectrum disorder; AST, aspartate aminotransferase; MD, mitochondrial disease.
This value was found to be significantly higher than a control group.
Results
The selected studies that reported clinical characteristics and biomarkers of mitochondrial dysfunction can be primarily separated into two groups: studies of the general ASD population, and studies of children with ASD/MD. In addition, two studies reported ASD or features of ASD in children with MD,48, 58 and two studies105, 112 examined correlations between biomarkers of mitochondrial dysfunction and the severity of ASD symptoms. Several animal models of ASD reported abnormalities in energy metabolism. Finally, some studies reported specific treatments for mitochondrial dysfunction in ASD. These studies will be discussed in subsections I through VI separately.
I. Studies examining the general ASD population
Table 1 outlines the prevalence estimates of MD and abnormal mitochondrial biomarkers in the general ASD population, while Table 2 outlines the mean differences in mitochondrial biomarker values and associated statistics in children with ASD as compared with controls. The studies reviewed and selected for individual biomarker calculations are outlined in the Supplementary Table S4.
Table 2. Pooled statistics and meta-analysis of group differences for mitochondrial biomarkers in ASD compared with controls.
|
ASD |
Control |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Number of studies | Total N | Mean (95% CI) | Total N | Mean (95% CI) | F-value | Hedge's g (CI) | Q for g | Glass's Δ (CI) | Q for Δ |
| Lactate (m l–1) | 5 | 114 | 1.73 (1.61, 1.88) | 114 | 0.91 (0.87, 0.96) | 8.72† | 1.42 (0.92, 1.92)† | 3.64 | 3.22 (2.66, 3.78)† | 45.89† |
| Pyruvate (n l–1) | 1 | 24 | 0.12 (0.11, 0.14) | 24 | 0.06 (0.06, 0.06) | 20.25† | 1.96 (0.85, 3.08)† | 6.40 (5.04, 7.76)† | ||
| Carnitine (mg ml–1) | 1 | 30 | 3.83 (3.44, 4.31) | 30 | 6.40 (6.22, 6.62) | 4.61† | 2.51 (1.61, 3.42)† | 4.21 (3.01, 5.41)† | ||
| Ubiquinone | 1 | 15 | 91.4 (81.9, 103.0) | 15 | 144.2 (130.4,161.1) | 2.13 | 1.90 (0.79, 3.01)† | 1.63 (0.62, 2.64)† | ||
| Creatine kinase | 2 | 55 | 178.8 (139.6, 226.9) | 59 | 92.2 (89.9, 121.9) | 6.93† | 0.57 (–0.15, 1.30) | 0.05 | 0.94 (0.19, 1.69) | 0.69 |
| AST | 1 | 147 | 36.3 (34.4, 38.6) | 98 | 29.7 (28.1, 31.7)a | 2.34† | 0.49 (–0.22, 1.32) | 0.67 (–0.07, 1.41) | ||
| ALT | 1 | 87 | 24.6 (19.77, 30.52) | 70 | 20.6 (18.7, 23.1) | 7.37† | 0.18 (–0.61, 0.97) | 0.38 (–0.42, 1.19) | ||
Abbreviations: ALT, alanine aminotransferase; ASD, autism spectrum disorder; AST, aspartate aminotransferase; CI, confidence interval.
Standard error of AST was misstated in the publication as 3.0 but in actuality it is 1.0.
†P<0.0001.
Prevalence of MD in ASD
Three studies examined the prevalence of MD in ASD. Two were large prospective studies115, 132 that examined the prevalence of MD using criteria as outlined by Bernier et al.55 and used an elevated plasma lactate to select children to undergo further workup for MD (a muscle biopsy). In these two studies, MD was diagnosed in 19%115 to 43%132 of ASD children with an elevated lactate; however, only 56%115 to 79%132 of children with an elevated lactate underwent a clinical workup for MD. The third study was a retrospective case series that examined 4194 individuals suspected of having mitochondrial dysfunction; 276 were reported to have autistic features and 14 of these had MD145 using the modified Walker criteria outlined by Morava et al.54 From these three studies, meta-analysis (Table 1) demonstrated the overall prevalence of MD in ASD was 5.0% (95% CI 3.2, 6.9%). However, it is likely that the prevalence of MD is underestimated by these three studies because: (a) individuals with a normal lactate did not undergo a thorough evaluation for MD even though normal lactate levels are found in some children with ASD/MD;25, 49, 143, 147, 150 (b) lactate alone was used in two studies115, 132 to screen for MD, whereas additional biochemical markers could have been used; and (c) children with known medical disorders, such as septo-optic dysplasia, which could be related to mitochondrial dysfunction,167 were excluded from testing for MD.132 One study was particularly robust as it was population based and examined the prevalence of MD in children with ASD identified from a population of 67 795 children.132, 133
Prevalence of abnormal biochemical markers of mitochondrial dysfunction in ASD
We reviewed studies that examined the prevalence of abnormalities in biochemical markers, which could indicate mitochondrial dysfunction (Table 1). Studies that used unusual methodology could not be included in the analysis: one study examined the prevalence of abnormalities in a combination of biomarkers rather than just one biomarker;23 another study examined the prevalence of high (alanine and ammonia) and low (free and total carnitine) biomarker values with respect to the normal mean laboratory values instead of the upper or lower limit of normal;141 and one study reported the prevalence of an elevated lactate-to-pyruvate ratio only in a subgroup of ASD children with elevated lactate.132
Six studies107, 112, 115, 116, 132, 136 examining the prevalence of elevated lactate, two studies116, 136 examining the prevalence of elevated pyruvate, one study reporting the prevalence of lactate-to-pyruvate ratio elevation,115 and another study reporting the prevalence of elevated alanine104 in the general population of ASD were included in the meta-analysis. Almost one-third of children with ASD had elevations in lactate and/or the lactate-to-pyruvate ratio, while the prevalence of pyruvate and alanine elevation was lower. The prevalence of lactate elevation varied widely from study to study. The reason for this is not clear; however, variations in blood collection technique are well known to cause differences in lactate values. Unfortunately, many of the studies that examined lactate, pyruvate or the lactate-to-pyruvate ratio did not report the technique used to collect blood (for example, fasting vs non-fasting, tourniquet vs no tourniquet). Presumably, within each study, the blood collection technique was consistent, although failure to maintain such consistency could result in an additional source of variation in these measurements.
Indirect biochemical markers of mitochondrial dysfunction also appear to be abnormal in the general ASD population with a much higher prevalence than the aforementioned direct biomarkers (see Table 1). The prevalence of low total carnitine112 and elevated ammonia116 were examined in one study each. Only one study85 looked at the prevalence of CK, AST and ALT elevation, with the prevalence of AST elevation being significantly higher as compared with a control group (children with other neurological disorders but not ASD). As these indirect markers are more nonspecific than direct markers of mitochondrial dysfunction, they could be abnormal because of other problems.
Values of abnormal biochemical markers of mitochondrial dysfunction in ASD
Table 2 outlines the mean values (with CIs) of biochemical markers of mitochondrial dysfunction, along with meta-analysis statistics, for ASD and control groups. Five studies107, 108, 112, 127, 131 reported lactate values; one study reported pyruvate values;107 one study reported carnitine values;112 and one study examined ubiquinone concentrations.110 Of note, one study examined the correlation between carnitine and lactate levels.112 Additionally, one study reported AST and ALT values,85 and two studies reported CK values.106, 127 Some studies could not be included in the analysis because of limitations. For example, one study reported significantly higher mean plasma alanine and lysine in children with ASD compared with controls,109 and another compared urinary alanine103 between ASD and control groups. However, both of these studies103, 109 only provided the information graphically. Another study reported a significantly lower mean lysine in a subgroup of children with ASD on a restricted diet, but not in those on an unrestricted diet, compared with controls; this study also reported elevated alanine prevalence but not alanine values.104 One study reported significantly increased mean alanine transport in fibroblasts from ASD children compared with controls but did not report alanine values.118 Finally, a longitudinal study reported abnormal elevations in polyunsaturated long chain fatty acids and/or saturated very long chain fatty acid-containing ethanolamine phospholipids in children with ASD compared with controls.130 Although the investigators in this latter study postulated that one mechanism that could account for these elevations was mitochondrial dysfunction,130 these abnormalities are not commonly accepted biochemical markers of mitochondrial dysfunction.
Population variability, as indexed by the F-value, was significantly greater in the ASD group as compared with the control group for all biochemical markers of mitochondrial dysfunction except ubiquinone, with the difference particularly marked for pyruvate. This is consistent with the notion that a subgroup of ASD children has abnormal mitochondrial biomarker values or that the range of biochemical marker values of mitochondrial dysfunction is much larger for the ASD population in general, suggesting that children with ASD might have a spectrum of mitochondrial dysfunction.
The effect sizes calculated using either the Hedge's g or Glass's Δ methods were large and statistically significant for lactate (1.9-fold higher in ASD), pyruvate (2.0-fold higher in ASD), carnitine (1.7-fold lower in ASD) and ubiquinone (1.6-fold lower in ASD) but not for CK, AST or ALT. Q was statistically significant for the lactate Glass's Δ effect size indicating that the effect size significantly varied across studies. Individual Glass's Δ effect sizes for lactate were statistically significant for each study and varied from a minimum of 1.56127 to a maximum of 6.41.107 Although the effect sizes for lactate varied across studies, even the smallest effect size was quite robust. Interestingly, one study reported a significant inverse correlation between carnitine and lactate levels.112
Prevalence of mtDNA abnormalities in ASD
In one study, mtDNA deletions of varying length (9.7–13.7 kb) were found in 8 of 12 (67%) children with ASD, but the specific genes affected were not reported.146 However, when specific mtDNA mutations have been investigated in the general ASD population, very few individuals with mutations have been found. For example, one study of 810 individuals with ASD that searched for mtDNA mutations (A3243A>G, T8993T>G, T8993T>C and A8344A>G) only identified two individuals (0.2%) with a mutation affecting tRNA (A3243A>G).135 Another study of 129 individuals with Asperger syndrome and 138 mothers of individuals with Asperger syndrome searched for the A3243A>G mutation, but no such mutation was found.134 Finally, in a study of 162 individuals with ASD, no significant association was observed between mtDNA haplogroup and ASD as compared with a control population.121
Prevalence of nDNA abnormalities in ASD
Three nDNA genes associated with mitochondrial function have been studied in the general ASD population. Two studies examined the inner mitochondrial membrane peptidase 2-like gene,5, 102 while 11 studies examined gene expression and/or single-nucleotide polymorphisms in the SLC25A12 gene that codes for the calcium-dependent mitochondrial aspartate/glutamate carrier (AGC) isoform 1.101, 111, 113, 114, 115, 117, 122, 124, 125, 126, 129 The evidence for an association with either of these genes was mixed and thus inconclusive, possibly due to clinical and genetic heterogeneity between studies and small sample sizes.129 Additionally, one study reported that several single-nucleotide polymorphisms in the microtubule affinity-regulating kinase 1 gene, a gene associated with mitochondrial trafficking, were associated with ASD, and microtubule affinity-regulating kinase 1 overexpression was demonstrated in postmortem frontal cortex samples in individuals with ASD as compared with healthy controls.123 However, this association has yet to be confirmed by additional studies. Thus, to date, no conclusive association between ASD and an nDNA gene associated with mitochondrial function has been found.
mRNA expression in ASD
One recent study found normal mRNA expression of the mitochondrial complex I 75-kDa subunit in the general ASD population.100
In vitro studies of mitochondrial function and mitochondrial oxidative stress in ASD
Three controlled studies compared in vitro mitochondrial function in lymphoblasts obtained from the Autism Genetic Resource Exchange between individuals with ASD and control individuals. In the first study, the ASD group demonstrated depressed complex I function, normal complex II activity and borderline elevated complex IV activity compared with controls. In addition, a higher mitochondrial maximal respiratory rate was found in the ASD group and believed to be a compensatory response to the depression in complex I function.119, 120 In another lymphoblast study, the mean baseline ATP concentration in the ASD group was equivalent to controls, but exposure to physiological concentrations of NO reduced the mitochondrial membrane potential in the ASD lymphoblasts more than in the control lymphoblasts.35 Finally, one study reported a reduced mitochondrial membrane potential in ASD mitochondria compared with controls.128 These studies support the notion that some individuals with ASD manifest mitochondrial dysfunction and compensatory mechanisms for such dysfunction.
Two studies using Autism Genetic Resource Exchange samples measured markers of mitochondrial oxidative stress in vitro between individuals with ASD and control individuals. In one study, higher concentrations of ROS and reactive nitrogen species were found in ASD mitochondria compared with controls, consistent with increased generation of mitochondrial free radicals.128 In the second study, a lower mean concentration of mitochondrial reduced GSH was found in ASD lymphoblasts compared with controls, consistent with a lower mitochondrial GSH reserve. Additionally, exposure to ethylmercury (thimerosal) led to a larger increase in the generation of free radicals and a greater reduction in the ratio of reduced GSH to the oxidized disulfide form of glutathione (GSSG) in the ASD cells compared with control cells.35 These studies suggest that some individuals with ASD manifest increased oxidative stress that may originate from mitochondrial dysfunction.
Neuroimaging in ASD
In some individuals with ASD, metabolites relating to brain bioenergetics have been measured non-invasively using magnetic resonance spectroscopy. One small magnetic resonance spectroscopy study demonstrated significantly decreased levels of phosphocreatine and esterified end products (αATP, α-adenosine diphosphate, dinucleotides and diphosphosugars) in the brains of ASD individuals compared with controls.105 Another magnetic resonance spectroscopy study measured N-acetyl-aspartate and lactate levels in the frontal lobe, temporal lobe and the cerebellum, and found significantly lower N-acetyl-aspartate levels in the cerebellum of ASD children compared with controls as well as elevated lactate in the frontal lobe of one child with ASD.108 A reduced concentration of brain N-acetyl-aspartate may be a marker of mitochondrial dysfunction,168 although this is an area of debate.169 However, changes in the other metabolites appear consistent with abnormal brain bioenergetics.
Brain pathology in ASD
In one small controlled study, post-mortem brain oxidized (carbonylated) mitochondrial protein content was nonsignificantly (P=0.12) elevated by threefold in the ASD group compared with controls with four of six (67%) ASD brains demonstrating large elevations.101
II. Studies examining children with ASD/MD
Eighteen studies reported a total of 112 children (up to 20 years of age) with mitochondrial dysfunction or MD and concomitant ASD (ASD/MD).25,28,48,49,58,85,99,115,132,133,136,139,140,142,143,144,145,147, 149,150 Many (11 of 18, 61%) of these studies did not specify the diagnostic criteria used.28, 58, 85, 99, 136, 139, 140, 142, 143, 147, 149 Only 8 of 18 (44%) studies examined family history of MD with 3 of 8 studies (37.5%) noting a positive family history. Three additional studies137, 138, 148 that did not demonstrate sufficient evidence to confirm mitochondrial dysfunction or MD were not included in the analysis: one study demonstrated elevations in several acyl-carnitines in a child148 consistent with a fatty acid oxidation defect; another reported reduced free carnitine and a mild ammonia elevation in two children;138 while the final study described elevated urinary TCA cycle metabolites in two children.137 Another study reported elevated lactate, pyruvate or alanine in 13 of 28 children with ASD/MD but did not report a prevalence for each biomarker, and therefore the prevalence of these markers could not be calculated nor included in the analysis.49 Finally, one study reported the prevalence of seven abnormal mitochondrial histological findings, but it was unclear from the study how many children had more than one abnormal finding, and therefore the prevalence of each finding could not be calculated nor included in the analysis.25
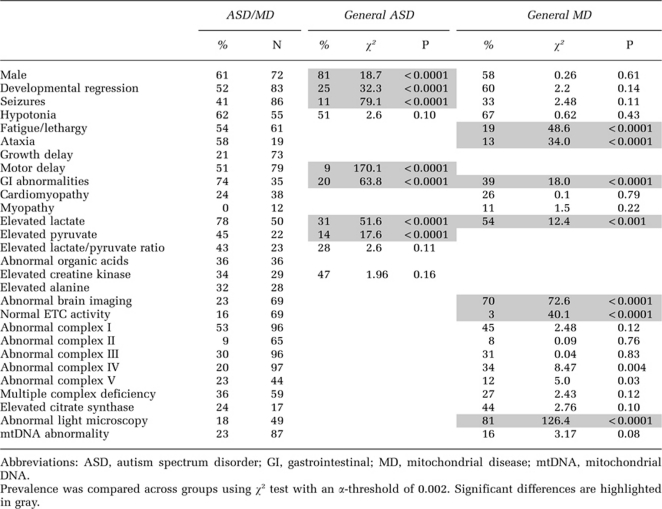
Clinical characteristics as well as the biochemical and genetic markers of mitochondrial dysfunction reported in these 112 children with ASD/MD are found in the Supplementary Table S1. Interestingly, 103 (92%) children were first diagnosed with ASD before MD, 2 (2%) were diagnosed first with MD before ASD,140, 143 while in 7 (6%) it was unclear which diagnosis was made first.48, 58, 136, 142 Several features of MD were not reported with enough frequency to calculate the prevalence of such features in children with ASD/MD. These features included sleep abnormalities,85 ammonia elevations,140 carnitine abnormalities,140 elevated alanine-to-lysine ratio85 and nDNA or chromosomal abnormalities.140, 150 The prevalence of the clinical characteristics and biomarkers of mitochondrial dysfunction in ASD/MD are provided in Table 3. Table 3 also presents prevalence estimates derived from the selected comparison papers (reviewed in Materials and Methods section) and statistically compares children with ASD/MD with the general population of ASD children and the general population of children with MD. The prevalence of abnormal organic acids and elevated alanine was relatively high in ASD/MD, but adequate studies reporting these markers could not be identified in the general population of MD in order to compare these markers.
Table 3. Prevalence of clinical characteristics and mitochondrial biomarkers in children with ASD/MD with comparison with the general ASD and MD populations.
Comparisons between children with ASD/MD and the general ASD population
Children with ASD/MD demonstrated some distinct characteristics as compared with the general population of children with ASD. Developmental regression, seizures, motor delay (such as, significantly delayed walking) and GI abnormalities (such as, reflux or constipation) were significantly more prevalent in children with ASD/MD as compared with the general ASD population. It is notable that children with ASD/MD demonstrated a relatively high prevalence of motor delay (51%), regression (52%), fatigue/lethargy (54%), ataxia (58%) and GI abnormalities (74%). Although motor delay was significantly more common in ASD/MD, the prevalence of hypotonia was not significantly different between children with ASD/MD and the general ASD population. Lactate and pyruvate, but not the lactate-to-pyruvate ratio or CK, were elevated with a significantly higher prevalence in children with ASD/MD as compared with children in the general ASD population. Interestingly, the ASD/MD population had a significantly more balanced male-to-female ratio than children from the general ASD population.
Comparisons between children with ASD/MD and the general MD population
The prevalence of male gender, developmental regression, seizures, hypotonia, cardiomyopathy and myopathy was not significantly different between MD groups with and without ASD. However, the prevalence of fatigue/lethargy, ataxia, GI abnormalities and elevated lactate was significantly higher in children with ASD/MD as compared with the general population of MD. The prevalence of an abnormal brain imaging scan was significantly lower in children with ASD/MD as compared with the general MD population.
The prevalence of complex I, II, III, IV, V and multiple complex deficiencies was not significantly different between children with MD with and without ASD, nor was the prevalence of elevated citrate synthase or mtDNA abnormalities. However, the prevalence of abnormal histology on light microscopy was significantly more common in the general population of MD compared with ASD/MD. The most common complex deficiency in children with ASD/MD was in complex I (53%). Children with ASD/MD were also significantly more likely to have normal ETC activity. Of note, complex V activity was only reported in five studies of ASD/MD,48, 49, 58, 132, 139 although it was abnormal in a relatively high percentage in some studies.132, 139
Mitochondrial histology and ultrastructural abnormalities in children with ASD/MD
One case series reported several mitochondrial histological abnormalities, including variations in muscle fiber size, regenerating fibers, atrophic fibers, muscle inflammation, increased myofiber lipid content and reduced cytochrome oxidase staining.25 Only three studies reported ‘ragged-red fibers' in children with ASD/MD,25, 99, 139 which is not surprising as the presence of ragged-red fibers is an unusual finding in children, even in those with MD.30
The ultrastructural abnormality most commonly reported was mitochondrial proliferation.25, 28, 49, 138, 139, 140, 142, 144 Some studies described specific ultrastructural abnormalities, such as mitochondria with abnormal cristae,139 inclusions,139 and abnormally shaped and sized mitochondria.25, 139, 149 However, these features were not reported with enough frequency to calculate accurate prevalence values or to compare them with the general population of MD.
Genetic abnormalities in children with ASD/MD
Only 24 of 112 (21%) children with ASD/MD had an mtDNA, nDNA or chromosomal abnormality. Table 4 reviews the mtDNA abnormalities reported in ASD/MD, including mtDNA depletion, mutations and deletions. mtDNA abnormalities were tested in 87 of 112 (78%) children with ASD/MD with 20 of 87 (23%) demonstrating an abnormality (see Supplementary Table S1). mtDNA depletion and deletions were reported in three49, 142, 145 and five individuals with ASD/MD,139 respectively. Pathogenic mtDNA mutations were described in 12 children with ASD/MD.25, 99, 142, 145, 147 Interestingly, no abnormalities were found in the majority (77%) of children with ASD/MD in which mtDNA was examined.
Table 4. Number of cases of mtDNA abnormalities reported in the general population of children with ASD and in children with ASD/MD.
| MD | MD unknown | Positive family history | |
|---|---|---|---|
| mtDNA depletion | 3 | 1 | |
| mtDNA deletions | 5 | 8 | |
| mtDNA mutations | |||
| tRNA | 7 | 2 | 2 |
| 3243A>G | 4 | 2 | 2 |
| Complex I | 3 | ||
| Primary LHON | 2 | ||
| POLG | 2 | ||
| Summary for mtDNA mutations in MD above |
Reference |
||
| 3397A>G (complex I) and 4295A>G (tRNA) | Weissman et al.25 | ||
| 3243A>G MELAS × 2, POLG × 2, LHON × 2 | Scaglia et al.145 | ||
| 3243A>G × 2 | Pons et al.142 | ||
| G8363A (tRNA) | Graf et al.147 | ||
| G10406A (tRNA) | Pancrudo et al.99 | ||
Abbreviations: ASD, autism spectrum disorder; LHON, Leber Hereditary Optic Neuropathy; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis and stroke syndrome; MD, mitochondrial disease; mtDNA, mitochondrial DNA; POLG, polymerase gamma; tRNA, transfer RNA.
Additionally, one study reported an nDNA mutation (SCO2) in an individual with ASD/MD.145 This mutation could adversely affect the synthesis of cytochrome c oxidase.170 Two chromosomal abnormalities were reported to be associated with ASD/MD: two children had an inverted duplication of 15q11–q13140, and one child had a deletion in 5q14.3.150 However, the relationship between these chromosomal abnormalities and ASD or mitochondrial dysfunction is unclear.
III. Studies correlating biomarkers of mitochondrial dysfunction with ASD symptoms
In one study of 11 individuals with ASD and 11 normal controls, abnormal levels of brain markers of mitochondrial function measured by magnetic resonance spectroscopy (including phosphocreatine, αATP, α-adenosine diphosphate, dinucleotides and diphosphosugars) significantly correlated with the severity of language and neuropsychological deficits in the ASD group but not in the control group.105 In another study of 30 children with ASD, those with severe ASD (as measured by the Childhood Autism Rating Scale) had both significantly lower carnitine and higher lactate concentrations than those with mild or moderate ASD.112 Additional studies are needed to evaluate these apparent correlations.
IV. Prevalence of ASD or autistic features in MD
Two retrospective case series of children with MD reported ASD or features of ASD in some of the children;48, 58 one other case series presented duplicate data and was not included in the analysis.51 The overall prevalence of ASD or ASD features in children with MD was calculated to be 4.6% (95% CI 0.3, 8.9%). However, since these studies were not population based, they may not accurately reflect the true prevalence of ASD in children with MD.
V. Animal models of ASD with abnormal function of the energy pathway
Several animal models of ASD exhibit mitochondrial dysfunction. For example, a recent rat model of ASD demonstrated that the administration of propionic acid, a fermentation end product of enteric bacteria, induced mitochondrial dysfunction and led to certain brain, behavioral and metabolic changes consistent with ASD, including features such as repetitive behaviors, social interaction problems, hyperactivity, oxidative stress, lowered GSH levels, microglial activation and altered carnitine levels.61, 62, 64, 65 In a mouse model, mutations in the SLC25A12 gene, a susceptibility gene for ASD, which codes for a mitochondrial AGC that is crucial for supporting oxidative phosphorylation and ATP production, resulted in neurofilamentous accumulations in neurons and myelination deficits. Interestingly, the myelin deficits could be reversed by the administration of pyruvate in vitro.97 The maternal ubiquitin protein ligase E3A deficiency mouse is a model of Angelman's syndrome, a syndrome that frequently includes ASD features. Recently, this mouse model was reported to have abnormal mitochondrial morphology and a partial oxidative phosphorylation defect in complex III in the hippocampal region.171 The MECP2-null mouse is a mouse model of Rett syndrome, a neurodegenerative disorder that includes ASD features. This mouse demonstrates decoupling of the respiratory complexes and overexpresses the nuclear gene for ubiquinol-cytochrome c reductase core protein 1, a gene that codes for a subunit of ETC complex III.172 Creatine helps to maintain adequate ATP levels in tissues with high energy requirements and a specific creatine transporter is required for the uptake of creatine by cells.173 A mouse model of a creatine transporter gene mutation (SLC6A8) reported seizure activity and autistic-like behavior.98 Finally, a mouse model of neuronal glucose transporter isoform 3 deficiency demonstrated certain autistic characteristics including seizure activity, abnormal social behavior and stereotypies.96 Glucose transporter isoform 3 mediates the uptake of glucose by neurons and impaired glucose transporter isoform 3 activity in vitro has been associated with subsequent ATP depletion.174
VI. Treatment of mitochondrial dysfunction in ASD
Several studies suggested that nutritional supplements and/or antioxidants may be beneficial in some children with ASD who have MD or abnormal biomarkers of mitochondrial function. Carnitine was the most commonly noted supplement to be helpful.28, 85, 130, 138, 140, 150 Along with carnitine, some investigators reported clinical improvements with co-enzyme Q1085, 143, 150 and high doses of B-vitamins, including thiamine or riboflavin.85, 140, 150 Cerebral folate deficiency was described in one child with ASD/MD,49 and specific treatment with folinic acid and a milk-free diet has been reported to result in significant improvements in ASD symptoms in children with cerebral folate deficiency.175, 176 Unfortunately, a majority of the reviewed studies did not report on any potential treatments for mitochondrial dysfunction in ASD.
Discussion
In this systematic review and meta-analysis, we examined the evidence for an overlap between ASD and mitochondrial dysfunction and/or MD. From our analysis, MD was reported in 5.0% of children with ASD. We also found that there is a rather high prevalence of abnormal values in direct (that is, lactate, pyruvate and lactate-to-pyruvate ratio) and indirect (that is, carnitine, ammonia, CK and AST) biochemical markers of mitochondrial dysfunction in the general population of children with ASD, and that children with ASD, as a group, have significantly abnormal values for direct (that is, lactate, pyruvate and ubiquinone) and indirect (that is, carnitine) biochemical markers of mitochondrial dysfunction compared with controls. The variability in the values of these biochemical markers was, for the most part, significantly larger in the ASD group as compared with the control groups, indicating that the ASD population manifests a wide variety of values of biochemical markers for mitochondrial dysfunction. Two studies reported significant correlations between markers of mitochondrial dysfunction and autism severity. Additionally, there appears to be a high prevalence of mtDNA deletions, but not pathogenic mtDNA mutations, in the general ASD population, and studies of nDNA have not found consistent abnormalities. In vitro studies with limited sample sizes have identified mitochondrial dysfunction, lower mitochondrial GSH reserve and higher mitochondrial oxidative stress in the cells of individuals with ASD compared with controls. Neuroimaging studies have also reported abnormalities in biomarkers of energy metabolism in children with ASD. One study reported a trend for higher levels of brain oxidized mitochondrial protein content in ASD compared with controls. Although many of the studies included in this review have limitations, their cumulative findings suggest that there is evidence for mitochondrial dysfunction in ASD, or at the least, in a subset of children with ASD.
Our review also demonstrates that children with ASD/MD have distinct characteristics as compared with the general population of children with ASD and have many similarities to the general population of children with MD. Abnormalities in mitochondrial histology and ultrastructure were reported in some children with ASD/MD. Surprisingly, only a relatively modest percentage (21%) of children with ASD/MD had a genetic abnormality that might account for mitochondrial dysfunction. Although two studies suggested that children with ASD or features of ASD constitute a small proportion of the general MD population,48, 58 certain limitations of these studies indicate they may not accurately reflect true prevalence. Several animal models of ASD reported mitochondrial dysfunction. A limited number of studies reported various improvements in children with ASD and mitochondrial dysfunction with certain treatments. Below we will discuss some important aspects of this review, limitations of our analyses, and important avenues for further study.
Children with ASD/MD: a distinct subgroup of children with ASD or a distinct MD syndrome?
Mitochondrial dysfunction is the most common metabolic abnormality associated with ASD.115, 177 Meta-analysis of three studies115, 132, 145 indicated that the prevalence of MD in ASD was 5.0% (95% CI 3.2, 6.9%), which is significantly higher than found in the general population (∼0.01%),47 indicating that an association between ASD and MD is likely, at least in a subgroup of individuals with ASD.25 However, this 5% prevalence is most likely an underestimate of the true prevalence of MD in individuals with ASD since these studies excluded children from testing for MD who had either normal lactate or medical disorders potentially related to mitochondrial dysfunction. Additionally, two of these three studies exclusively used lactate to identify children to be fully assessed for MD.115, 132 This latter limitation appears to be common in the reviewed studies, as only a few studies evaluated ASD children who had a normal lactate for MD.142, 143, 147, 150 Using only lactate as a screening test for MD may miss some individuals with MD. In the future, the development of sensitive and specific non-invasive or minimally invasive methods for identifying MD, such as the routine examination of mitochondrial function in lymphocytes, should make the identification of the true prevalence of MD in ASD children more realistic.
In general, MD and mitochondrial dysfunction may be underrecognized in the general ASD population,9 especially because the identification of children with ASD and concomitant mitochondrial dysfunction can be quite challenging. For example, biochemical markers may be abnormal only during illness,25, 49, 142, 143, 147, 150 tissue mitochondrial heteroplasmy can result in mitochondrial dysfunction being limited to only certain body tissues,99, 139 and known genetic defects are not commonly found in ASD/MD. Of note, routine laboratory testing to screen for mitochondrial dysfunction in children with ASD is not often performed,132 perhaps because previous investigators have suggested that mitochondrial dysfunction is rare in ASD177 and should only be considered when there is a family history of MD or neurological features suggestive of MD.134, 142 However, our review indicates that many children with ASD/MD do not have a family history of MD or neurological features that distinguish them from the general ASD population.25, 132, 133, 142 In fact, the phenotypic presentation of mitochondrial dysfunction in ASD is quite broad, ranging from significant to no neurological problems,25, 139, 142, 147 with some investigators describing children with ASD/MD as being indistinguishable from other children with ASD and others describing them as atypical for ASD.25, 132, 133, 142 Furthermore, our review suggests that some ASD children with abnormal markers of mitochondrial function do not meet definite or probable criteria for MD but rather may have a mild form of mitochondrial dysfunction, such as partial complex deficiencies, that may not be considered significant by some clinicians.9, 25, 35, 85
Interestingly, two studies reported a significant correlation between biochemical markers of mitochondrial dysfunction and the severity of ASD symptoms.105, 112 This correlation suggests that mitochondrial dysfunction exists on a continuum rather than in a discrete subgroup of ASD children. Such a notion is consistent with many of the findings in this review, including the much higher prevalence of abnormal biochemical markers of mitochondrial dysfunction as compared with the prevalence of MD in the general ASD population, and the particularly high variability in biochemical markers of mitochondrial dysfunction in the general ASD population. Clearly, additional studies are needed to better define the clinical and biochemical characteristics of ASD/MD children and to determine if a discrete ASD/MD subgroup exists or whether mitochondrial dysfunction in ASD is better represented on a continuum.
Our review of the 112 children with ASD/MD reported in the medical literature found several distinct clinical characteristics in this group, such as developmental regression, seizures, motor delay, GI abnormalities, and a higher prevalence of elevated lactate and pyruvate, as compared with the general ASD population. Additionally, children with ASD/MD had many similarities to the general population of children with MD, including the prevalence of male sex, regression, seizures, hypotonia, cardiomyopathy, myopathy and ETC abnormalities. This suggests that these clinical characteristics are more likely due to mitochondrial dysfunction than to ASD. Of course, this might also be an artifact of using diagnostic criteria for MD to define children with ASD/MD. However, other clinical characteristics which were significantly more prevalent in children with ASD/MD as compared with the general population of MD, such as fatigue/lethargy, ataxia, GI abnormalities and elevated lactate, are unlikely to be due simply to the use of diagnostic criteria. From our review, it also appears unlikely that these abnormalities reported in ASD/MD are due to factors, such as poor diet, anxiety or GI symptoms. Although three studies did attempt to identify MD from the general ASD population, these studies did not fully define the clinical or biochemical characteristics of the individuals identified.115, 132, 145
The finding of a significantly elevated prevalence (74%) of GI abnormalities (such as reflux, constipation, diarrhea and inflammation) in children with ASD/MD compared with both children with ASD and children with MD is intriguing and deserves further study. Recently, increased awareness of GI problems in ASD has been noted12, 178 and the findings of this review suggest that GI abnormalities in ASD may be related, in part, to mitochondrial dysfunction. Interestingly, mitochondrial dysfunction has been demonstrated in a mouse model of inflammatory bowel disease, and carnitine supplementation reversed both the metabolic and clinical abnormalities in this model.179 It is also possible that some metabolites originating from the GI tract, such as propionic acid produced by Clostridia, other enteric short chain fatty acids and certain cytokines might act as mitochondrial toxins. These factors might also contribute to seizure activity and our analysis found that seizures were significantly more common in ASD/MD compared with the general population of ASD. Given the high energy demands of both the GI tract and cerebrovascular endothelium, mitochondrial dysfunction may also contribute to barrier dysfunction in the brain and GI tract in ASD. Additionally, as mitochondria have an important role in lipid metabolism, previous reports of abnormalities in lipid metabolism180, 181 and lipid peroxidation182 in some individuals with ASD could be due to mitochondrial dysfunction. Further studies are needed to determine the role of mitochondrial dysfunction in barrier dysfunction, lipid abnormalities, GI dysfunction and seizure activity in ASD.
Interestingly, in the reviewed studies, no child with ASD/MD presented with a classic mitochondrial syndrome. Some experts have reported that autistic features are relatively common in children with MD in conjunction with a global neurological syndrome.183 However, the two studies that examined the presence of ASD characteristics in the general MD population did not support this assertion. It is possible that children with MD do not present with the typical symptoms of ASD. Alternatively, it may be that features of ASD are not specifically examined or recognized in many children who are diagnosed with MD, or that a potentially high severity of the presenting symptoms of MD does not trigger a differential diagnosis of ASD. These possibilities are supported by the finding that only 2% of children with ASD/MD were diagnosed with MD before ASD. It is possible that children with ASD/MD constitute a specific syndrome within children with MD, but further studies will be needed to better define common clinical symptoms within larger cohorts of children with ASD/MD.
Regression in children with mitochondrial dysfunction and ASD
Twelve studies reported a regression in developmental milestones in ASD children who had MD or abnormal biochemical markers of mitochondrial function.23, 25, 49, 85, 132, 137, 139, 140, 141, 142, 147, 148, 149 In these studies, regression was noted in several areas, including language, motor skills, eye contact, play skills, social interaction and receptive skills. In one case series of 25 children with ASD/MD, unusual patterns of regression, such as multiple regressions (9 children), regression with catabolic stress (7 children) and regression after age 3 (6 children) were noted.25 In some studies, factors associated with regression included illnesses25, 140, 142 and fever.49, 142 For example, in one case series of 28 children with ASD/MD, regression was reported in 17 children (61%), with a majority (12/17, 71%) experiencing regression with a fever >101°F, including 4 of 12 (33%) who developed regression with fever following routine vaccination.49 Regression after routine vaccination was also reported in another child who developed ASD/MD after a post-vaccination fever.25, 85 However, these latter three studies25, 49, 85 noted the important role of childhood vaccinations in preventing life threatening diseases. Interestingly, some children with ASD have clinical improvements during fever,184 suggesting that there are subgroups of children with ASD that respond differently to fever and that it is probably not the fever itself, but the underlying physiological process leading to fever that is related to regression in children with ASD.
It should be noted that, in each of the reviewed studies, it is not clear if mitochondrial dysfunction contributed to or caused the reported regression. Our analysis indicated that the prevalence of regression was significantly higher in children with ASD/MD as compared with the general population of children with ASD (52 vs 25%). Given that regression in individuals with MD is known to occur with stressors such as dehydration, fever and infection,50 and that the prevalence of regression in children with ASD/MD is approximately double that of the general population of ASD, it is likely that the regression reported in at least a subset of children with ASD/MD is related to mitochondrial dysfunction. This is significant because early identification of children with underlying MD who might be at risk of undergoing regression into ASD because of a metabolic stressor could lead to prophylactic measures to prevent the development of ASD, or at least minimize the severity of ASD symptoms once acquired. Although such a subset of children is likely to constitute a limited proportion of the general ASD population, given the high prevalence of ASD, the absolute number of individuals who could be protected is likely to be significant. One caveat is the assumption that these children had identifiable MD before the regression, which is empirically unproven because the MD was not identified until after the regression.
Secondary mitochondrial dysfunction in ASD
A majority (79%) of the children with ASD/MD identified in this review did not possess a genetic etiology that might account for mitochondrial dysfunction. Although a yet unidentified genetic defect may be present in some of these cases, secondary mitochondrial dysfunction is likely.9, 85 Biochemical abnormalities reported in some children with ASD could contribute to secondary mitochondrial dysfunction. For example, several studies have documented a significantly lower mean GSH concentration110, 130, 185, 186, 187, 188, 189 and a lower mitochondrial GSH reserve35 in children with ASD as compared with controls. GSH depletion is associated with impaired mitochondrial function73 and increased ROS production.36 Increased ROS can impair mitochondrial function36, 190 and may be particularly significant in individuals with ASD because they have been shown, as a group, to be under higher oxidative stress and have reduced levels of antioxidants as compared with controls.35, 110, 130, 185, 186, 187, 188, 189, 191 Furthermore, GSH protects mitochondria against the adverse effects of tumor necrosis factor-α,36 a pro-inflammatory cytokine that can inhibit mitochondrial function.66, 67 This might be particularly important since studies have reported higher tumor necrosis factor-α in lymphocytes,192 cerebrospinal fluid193 and brains194 of individuals with ASD as compared with controls.
In one reviewed study, exposure to physiological concentrations of NO reduced the mitochondrial membrane potential in ASD cells more than in control cells,35 suggesting that ASD mitochondria are more vulnerable to the adverse effects of NO. Several studies have reported a significantly higher mean concentration of NO in ASD individuals as compared with controls,110, 195, 196, 197 making it possible that individuals with ASD are not only more vulnerable to NO but also have higher baseline NO concentrations. These two factors could act synergistically to cause significant mitochondrial impairment in individuals with ASD.
Abnormalities in synaptic transmission reported in ASD could also contribute to secondary mitochondrial dysfunction. For example, an imbalance in the excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmitter systems has been implicated in the pathogenesis of ASD, with a relative increase in the glutamatergic neurotransmitter system.198 In vitro glutamate exposure has been shown to inhibit mitochondrial β-oxidation130, 199 as well as generate ROS200 and deplete GSH.130, 200 In one reviewed study, in vitro glutamate plus malate exposure adversely affected mitochondrial function in cells from individuals with ASD to a greater extent as compared with control cells.119
In another reviewed study, exposure to ethylmercury (thimerosal) led to a larger increase in free radical generation and a greater reduction in the ratio of reduced GSH to GSSG in ASD cells compared with control cells.35 These findings suggest that mitochondria from children with ASD may be more vulnerable to damage from environmental toxicants than mitochondria from typically developing children.35 In this context, exposures to environmental toxicants could contribute to secondary mitochondrial dysfunction in some children with ASD.9, 201 For example, in vitro exposure to diesel exhaust particles has been shown to inhibit mitochondrial function,80 and elevated environmental concentrations of diesel exhaust particles have been associated with ASD.202 Other environmental toxicants that inhibit mitochondrial function and have been associated with ASD include mercury,76, 77, 202, 203, 204 lead,78, 205, 206, 207 cadmium,79, 202 polychlorinated biphenyls81, 208 and pesticides.83, 209, 210, 211 Interestingly, some investigators have suggested that mtDNA deletions reported in some children with ASD may be secondary to elevated levels of ROS caused by environmental factors.146
Abnormal calcium signaling, which has been implicated in ASD,212, 213 may also contribute to secondary mitochondrial dysfunction.26, 214 For example, the mitochondrial AGC, which is coded by SLC25A12, is involved in the malate-aspartate reduced nicotinamide adenine dinucleotide shuttle and is activated, in part, through calcium signaling.215 One mouse model97 reported myelination deficits in mice with mutations in SLC25A12 and several studies111, 113, 122, 124, 125, 126 reported single-nucleotide polymorphisms in SLC25A12 were associated with ASD, although this association was not found in every study.114, 115, 117, 129, 216 However, in one study of six individuals with ASD and six controls, increased calcium levels and mitochondrial AGC transport rates were observed in all six ASD brains. The removal of calcium by a chelator led to a larger drop in mean AGC transport rate in the ASD group compared with controls.101 This finding suggests that abnormal calcium signaling contributes to AGC dysfunction in the brains of some individuals with ASD and may lead to mitochondrial dysfunction.
A recent rat model of ASD demonstrated that the administration of propionic acid induced mitochondrial dysfunction and led to certain behavioral and biochemical features of ASD, such as repetitive behaviors, social interaction problems, hyperactivity, oxidative stress, lowered GSH levels and altered carnitine levels.61, 62, 64, 65 In addition, Clostridia, an anaerobic, spore forming Gram-positive rod bacteria, is known to produce propionic acid61 and a derivative of propionic acid recovered in the urine of ASD individuals has been reported as a marker of Clostridia.217 Furthermore, significantly elevated concentrations of Clostridia in the GI tract have been reported in ASD children compared with controls218, 219, 220 with improvements noted with vancomycin treatment in some children.221, 222 Additional studies examining the roles of propionic acid and Clostridia in ASD are needed.
Currently, it is unclear if these endogenous and exogenous factors reported in some individuals with ASD contribute to secondary mitochondrial dysfunction or ASD symptoms, or if these factors are merely epiphenomena. However, these factors represent potential pathways that may impair mitochondrial function and increase the vulnerability of mitochondria to damage. Furthermore, a combination of these factors may lead to synergistic adverse effects on mitochondrial function. In this context, mitochondrial dysfunction could worsen a vulnerable system that is already under oxidative stress, resulting in an increase in the formation of ROS. As increased ROS can cause further damage to already damaged mitochondria and can directly impair mitochondrial function,190 adding mitochondrial dysfunction to a metabolic system that is already under high oxidative stress can result in the initiation of a vicious cycle that progressively impairs cellular function, leading to neurodegeneration, regression or failure of cognitive systems to properly develop.27 Clearly, further studies are needed to examine a possible link between the effects of these potentially detrimental substances and mitochondrial dysfunction in vivo. Although the effects of the environment on mitochondrial dysfunction are becoming increasingly recognized,223 and environmental pollutants, particularly environmental mercury, have been associated with an increased likelihood of ASD in epidemiological studies,202, 203, 204 the mechanism of such environmental toxicants for increasing the risk of ASD has not been well studied in vivo. Clearly, mitochondrial function is a ripe area of research when investigating the biological mechanism(s) of action of environmental toxicant exposures and indigenous abnormalities associated with ASD.
Mitochondrial dysfunction and synaptic transmission in ASD
Given the association between ASD and mitochondrial dysfunction, it is possible that mitochondrial dysfunction might have a role in the development, pathogenesis or severity of ASD. For example, mitochondrial dysfunction could be specifically detrimental to synaptic transmission because synaptic function is highly dependent on mitochondrial function.41 Furthermore, single-nucleotide polymorphisms in microtubule affinity-regulating kinase 1123 and SLC25A12122 reported in some individuals with ASD could alter synaptic plasticity and mitochondrial movement along dendrites. Such possibilities would be consistent with recent studies that have implicated synaptic dysfunction in ASD.224
Mitochondrial dysfunction can also lead to reduced synaptic neurotransmitter release, particularly in neurons with high firing rates, such as GABAergic interneurons.27 Since GABAergic neurons are inhibitory, they may be especially important between 12 and 30 months of age, because this window of development corresponds to an over-production in excitatory neurotransmitters and receptors.225, 226 Thus, without proper GABAergic neuronal function, the brain may be highly susceptible to excitotoxicity during this developmental period. Interestingly, this is the age range when regression most commonly occurs in ASD.227 Additional studies are needed to investigate these possibilities.
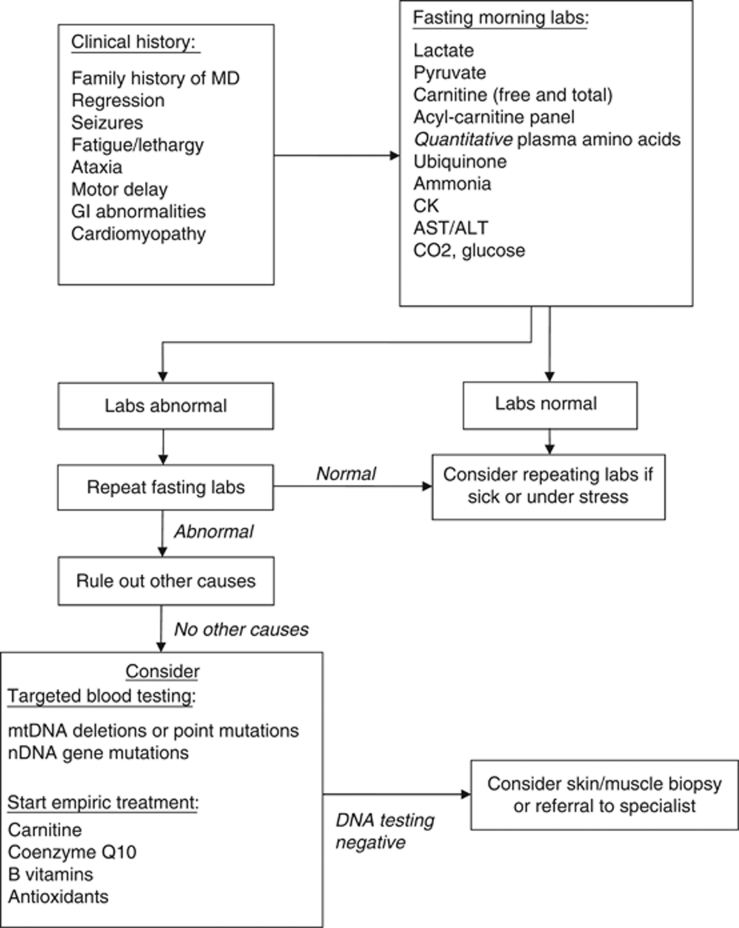
Identification of mitochondrial dysfunction in children with ASD
In the reviewed studies, the workup for MD varied widely from study to study. Lactate was by far the most commonly studied biochemical marker of mitochondrial dysfunction in ASD. Both lactate and pyruvate demonstrated a high prevalence of being abnormal in ASD, were significantly elevated in the general ASD population as compared with controls, and had a significantly higher prevalence of being abnormal in ASD/MD as compared with the general ASD population. Carnitine also had a high prevalence of being abnormal in ASD and was significantly depressed in the general ASD population as compared with controls. However, it is difficult to determine the utility of certain biomarkers (alanine, ammonia, carnitine, ubiquinone, alanine-to-lysine ratio and acyl-carnitine panel) for identifying mitochondrial dysfunction in the general population of children with ASD because the number of reports describing these biomarkers in ASD/MD was limited. Despite the lack of information that was derived from published reports, these biomarkers should be strongly considered when screening for MD in children with ASD because abnormal alanine is a recognized marker of MD54 and ammonia, ubiquinone and an acyl-carnitine panel can help identify mitochondrial dysfunction when lactate is not elevated.57, 228, 229 Further studies are needed to systematically examine the sensitivity, specificity and predictive value of these markers for diagnosing MD in the general ASD population.
Figure 3 outlines a suggested algorithm for screening for mitochondrial dysfunction in ASD. In order to standardize the collection and optimize the diagnosis of MD, some investigators have recommended obtaining laboratory tests in the morning while fasting38, 56 and repeating abnormal tests to verify true abnormalities.38 Obtaining laboratory values when the patient is sick or under physiological stress may also increase the sensitivity of detecting mitochondrial dysfunction.48, 57 Furthermore, a specific MD can be diagnosed by identifying a known mtDNA or nDNA gene abnormality using molecular testing that requires only a blood draw. However, in the reviewed studies, a genetic defect was not identified in the majority of children with ASD/MD. When a genetic marker cannot be identified, more invasive testing, such as a muscle or skin biopsy can be used to confirm MD. Although a muscle biopsy is more invasive than a skin biopsy, detailed pathological examination of the muscle can be particularly helpful in confirming MD. Although most reviewed studies reported ETC function, fatty acid oxidation pathways can also be examined if a skin biopsy is performed. A reduction in ETC function in two tissues can make the diagnosis of MD more definitive. Thus, if a muscle biopsy is performed, it is straightforward and advantageous to perform a skin biopsy at the same time.
Figure 3.
Suggested screening for mitochondrial dysfunction in ASD. Abbreviations: ASD, autism spectrum disorder; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; GI, gastrointestinal; MD, mitochondrial disease; mtDNA, mitochondrial DNA; nDNA, nuclear DNA.
In children with abnormal markers of mitochondrial function, it is important to consider other causes besides MD. Elevated CK in ASD can occur with muscular dystrophy89, 91 or rhabdomyolysis.95 Elevated ammonia in ASD has been associated with valproate administration94 and urea cycle disorders.90 Furthermore, false-positive elevations in biochemical markers (especially lactate) are possible, especially if the child struggles during the blood draw or if the sample is not properly processed.56 Elevations in lactate can also be found in disorders unrelated to MD.230 Finally, restrictions in diet may be important considerations in testing for MD as one study reported a significantly lower mean lysine in ASD children on a restricted diet, but not in those on an unrestricted diet, compared with controls.104
Three studies examined the prevalence of MD in ASD and the workups for MD in these studies reflect some of the difficulties that are common in the evaluation of MD. For example, in two of these studies, only 56%115 to 79%132 of ASD children with an elevated lactate were tested for MD. This likely reflects the variability in the willingness of families to allow their child with ASD to undergo invasive testing in light of the fact that the treatment for MD is limited (see below) and the yield of an invasive workup is variable. In addition, in these two studies, 19%115 to 43%132 of ASD children with an elevated lactate were found to have MD when fully assessed; this finding reflects the specificity of lactate for identifying MD in ASD and the variability in the yield of MD workups in various clinics.
It is noteworthy that older guidelines do not recommend a routine metabolic workup in children with ASD.231 However, newer reviews on the biological basis of ASD have outlined the growing number of mitochondrial and non-mitochondrial metabolic disorders associated with ASD.201, 232, 233, 234 Clearly, more studies are needed to develop sensitive and specific diagnostic techniques for MD in children with ASD. However, until such diagnostic techniques are available, we believe that all children with ASD should be screened for MD given: (a) the high prevalence of abnormal markers of mitochondrial function in ASD compared with controls; (b) the relatively high prevalence of MD in ASD; (c) some children with ASD who have MD can be phenotypically indistinguishable from typical children with ASD; and (d) the potential clinical significance of MD in children with ASD.
Treatment and management
There is no known cure for MD. However, the treatment and management of MD attempt to minimize environmentally and iatrogenically induced damage to mitochondria, treat organ dysfunction that results from mitochondrial dysfunction, and provide supplementation of important mitochondrial cofactors and ROS scavengers. It is essential that individuals with MD avoid dehydration, prolonged fasting and/or illnesses that could result in metabolic decompensation.50 Certain common medications, such as acetaminophen, and anesthetics should be avoided and an anesthesiologist familiar with MD should be consulted if anesthesia is necessary.60
Several studies suggested that treatment with mitochondrial cofactor supplementation, including antioxidants, co-enzyme Q10, carnitine and B-vitamins may improve mitochondrial function and behavior in some children with ASD. A therapeutic trial of mitochondrial cofactors and antioxidants may be reasonable in children with ASD/MD given the fact that: (a) oxidative stress is associated with impaired mitochondrial function;190 (b) antioxidant supplementation in individuals with MD increases GSH levels;235 (c) mitochondria are dependent on cytosolic GSH production;34, 35 and (d) these compounds are generally recognized as safe.9 For example, methylcobalamin and folinic acid have been reported to significantly increase GSH concentrations in children with ASD and appear to improve certain autistic behaviors.185, 187 Several other antioxidants, including vitamin C,236 carnosine237 and galantamine238, 239, 240 have also been reported to significantly improve autistic behaviors. Carnitine may be particularly helpful in children with ASD because carnitine deficiency has been implicated in ASD,112, 141 some studies have reported improvements with the use of carnitine in ASD28, 85, 130, 138, 140, 150 and carnitine may lower the toxicity241 of a potential Clostridial metabolite (a derivative of propionic acid) that has been recovered in the urine of some individuals with ASD.217 However, despite the potential therapeutic nature of these compounds, systematic studies documenting the efficacy of these treatments for mitochondrial dysfunction in children with ASD are generally lacking.
Limitations of this review and meta-analysis
Many of the reviewed studies suffered from limitations. For example, most of the studies that examined the prevalence of abnormal biochemical markers of mitochondrial function in ASD were probably susceptible to referral bias. In fact, there was only one study that was population based to prevent referral bias.132, 133 In addition, most of the studies examining the abnormal prevalence or the difference in the values of biochemical markers were based on a limited sample size (that is, <100 patients) and some studies did not contain a control group and/or were retrospective in nature.
Additionally, studies describing children with ASD/MD were based on small case series or isolated case reports and demonstrated variability in the protocols used for selecting children for MD workup, collecting biochemical markers of mitochondrial dysfunction, and defining MD. In fact, only 39% of the ASD/MD studies reviewed noted the criterion used for diagnosing MD. The lack of a standard criterion for diagnosing MD likely resulted in a heterogeneous population with varying degrees of mitochondrial dysfunction. In addition, many of the characteristics found to be more prevalent in ASD/MD are features of the criteria generally used to diagnose MD, suggesting that the clinical criteria could have influenced which children were selected to undergo evaluation for MD. Furthermore, most studies only examined a limited number of biochemical markers of mitochondrial dysfunction and contained too few participants to examine the interrelationship(s) between these markers. More studies are needed to determine how well the reviewed biochemical markers of mitochondrial dysfunction correlate with actual MD in children with ASD. For example, comparing biochemical markers (such as lactate and carnitine) with measurements of ETC activity, mtDNA abnormalities and other mitochondrial abnormalities would be helpful in examining this further.
Two of the three studies examining the prevalence of MD in children with ASD used only lactate to identify candidates for further workup.115, 132 It is likely that many of the ASD/MD case studies also used lactate as the primary biomarker to pursue a further workup for MD. Since cases have been reported in which biomarkers other than lactate are elevated in MD, it is likely that studies which identified candidates for additional workup of MD using only lactate as a criterion overlooked some patients with MD. In addition, it is unclear whether using strict criteria for diagnosing MD may eliminate ASD children who have mild mitochondrial dysfunction such as partial complex deficiencies.
In this review, we compared the prevalence of several clinical and biochemical characteristics between children with ASD/MD and two other clinical groups: children with MD and the general ASD population. The characteristics of these other clinical groups were estimated by a separate literature review. It is possible that some of the studies of the general MD population may have included a percentage of children with unrecognized ASD, thereby biasing the prevalence of certain characteristics in the childhood MD population toward the ASD/MD population. Although this percentage appears to be low from our review of the childhood MD literature, there have not been any studies specifically designed to estimate ASD prevalence in childhood MD. Similarly, some studies of the general ASD population may have included children with unrecognized MD, leading to a similar bias. The most significant limitation in defining and comparing the group of children with ASD/MD with other groups is the uncertainty regarding whether there is a well-definable subset of ASD children with MD or whether mitochondrial dysfunction in ASD is best represented on a continuum, with a subset of ASD children having mild or moderate mitochondrial dysfunction that does not fully meet the criteria for MD.
Conclusions
This systematic review and meta-analysis found that abnormal biochemical markers of mitochondrial function are relatively common in the general population of children with ASD and that a relatively high percentage of children with ASD (∼5%) have MD. However, it is unclear whether the general population of children with ASD is best characterized as having a distinct subgroup with mitochondrial dysfunction or as having a spectrum of mitochondrial dysfunction of differing severity. In this review, we examined the common characteristics of all reported cases of ASD/MD and compared these characteristics with the general ASD population and to the general population of children with MD. This analysis suggested that the ASD/MD cases, as a group, had a greater prevalence of several clinical characteristics as compared with the general population of ASD, and a similar prevalence to children with MD in many clinical and biochemical characteristics. However, an examination of the reviewed studies indicates that the methods for identifying and diagnosing MD need better characterization before a more precise group of ASD/MD can be defined.
At this point, it is not clear if mitochondrial dysfunction contributes to the development or pathogenesis of ASD or if it is merely an epiphenomenon of ASD. Several metabolic abnormalities and/or exposures to environmental toxicants could result in secondary mitochondrial dysfunction in children with ASD, or these factors could worsen mild mitochondrial dysfunction in some children, transforming mild dysfunction into more severe dysfunction. Some children demonstrated regression into ASD and MD following fever, suggesting children who develop MD may be vulnerable to external factors causing regression into ASD in children who may already have existing, but unidentified, MD. This suggests that it is important for children with MD to be identified early in life. Identification and longitudinal evaluation of the development of such individuals will allow investigators to better understand how mitochondrial dysfunction may be related to the development of ASD. In the meantime, until further studies can be performed, a reasonable approach to ASD includes a workup for MD and possible treatment with antioxidants and mitochondrial cofactors.
Acknowledgments
We thank Dr Paul Swank, PhD for his guidance on the meta-analysis and Maija Birenbaum, PhD for her editorial suggestions. This research was funded in part by the Autism Research Institute, K23NS046565, and the Jane Botsford Johnson Foundation to Dr Frye.
Daniel Rossignol has two children with ASD and is a practicing primary care physician who treats ASD children with standard and integrative treatments. He has received two grants from the International Hyperbarics Association for two studies concerning the effects of hyperbaric oxygen treatment in children with autism. Richard Frye provides expert testimony for children with mitochondrial disorders who may have been injured from vaccines. Funds from such testimony are used to support research on the biological basis of neurodevelopmental disorders.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- APA Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington, DC; 1994 [Google Scholar]
- Rice C. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, et al. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C. Subgroups in autism: are there behavioural phenotypes typical of underlying medical conditions. J Intellect Disabil Res. 1992;36 (Part 3:201–214. doi: 10.1111/j.1365-2788.1992.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Pagnamenta AT, Lamb JA, Bacchelli E, Sykes NH, Sousa I, et al. High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol Psychiatry. 2010;15:954–968. doi: 10.1038/mp.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Bradstreet JJ. Evidence of mitochondrial dysfunction in autism and implications for treatment. Am J Biochem Biotech. 2008;4:208–217. [Google Scholar]
- Canitano R. Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2007;16:61–66. doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res. 2008;17:197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, III, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125 (Suppl 1:S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Coury D. Medical treatment of autism spectrum disorders. Curr Opin Neurol. 2010;23:131–136. doi: 10.1097/WCO.0b013e32833722fa. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress Mol Psychiatry 20049684–697., 643. [DOI] [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- Coker SB, Melnyk AR. Rett syndrome and mitochondrial enzyme deficiencies. J Child Neurol. 1991;6:164–166. doi: 10.1177/088307389100600216. [DOI] [PubMed] [Google Scholar]
- Cornford ME, Philippart M, Jacobs B, Scheibel AB, Vinters HV. Neuropathology of Rett syndrome: case report with neuronal and mitochondrial abnormalities in the brain. J Child Neurol. 1994;9:424–431. doi: 10.1177/088307389400900419. [DOI] [PubMed] [Google Scholar]
- Dotti MT, Manneschi L, Malandrini A, De Stefano N, Caznerale F, Federico A. Mitochondrial dysfunction in Rett syndrome. An ultrastructural and biochemical study. Brain Dev. 1993;15:103–106. doi: 10.1016/0387-7604(93)90045-a. [DOI] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ohsawa I. Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer's disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J Alzheimers Dis. 2006;9:155–166. doi: 10.3233/jad-2006-9208. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65:1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- Coleman M, Blass JP. Autism and lactic acidosis. J Autism Dev Disord. 1985;15:1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- Lombard J. Autism: a mitochondrial disorder. Med Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L, Persico AM. Mitochondrial dysfunction in autism spectrum disorders: cause or effect. Biochim Biophys Acta. 2010;1797:1130–1137. doi: 10.1016/j.bbabio.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Hooker BS, Herbert MR. Bridging from cells to cognition in autism pathophysiology: biological pathways to defective brain function and plasticity. Am J Biochem Biotechnol. 2008;4:167–176. [Google Scholar]
- Gargus JJ, Imtiaz F. Mitochondrial energy-deficient endophenotype in autism. Am J Biochem Biotech. 2008;4:198–207. [Google Scholar]
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- Zeviani M, Bertagnolio B, Uziel G. Neurological presentations of mitochondrial diseases. J Inherit Metab Dis. 1996;19:504–520. doi: 10.1007/BF01799111. [DOI] [PubMed] [Google Scholar]
- Cotter D, Guda P, Fahy E, Subramaniam S.MitoProteome: mitochondrial protein sequence database and annotation system Nucleic Acids Res 200432D463–D467.(database issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Garcia-Ruiz C, Colell A, Morales A, Mari M, Miranda M, et al. Oxidative stress: role of mitochondria and protection by glutathione. Biofactors. 1998;8:7–11. doi: 10.1002/biof.5520080102. [DOI] [PubMed] [Google Scholar]
- Enns GM. The contribution of mitochondria to common disorders. Mol Genet Metab. 2003;80:11–26. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–2383. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, et al. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273 (1 Part 1:G7–17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Lodi R, Tonon C, D′Agata V, Sapienza M, Scapagnini G, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich′s ataxia. J Neurol Sci. 2005;233:145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Munnich A, Rustin P. Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet. 2001;106:4–17. doi: 10.1002/ajmg.1391. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, et al. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., III CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18 (R2:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Goldenthal MJ, Marin-Garcia J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem. 2004;262:1–16. doi: 10.1023/b:mcbi.0000038228.85494.3b. [DOI] [PubMed] [Google Scholar]
- Marin-Garcia J, Goldenthal MJ. Heart mitochondria signaling pathways: appraisal of an emerging field. J Mol Med. 2004;82:565–578. doi: 10.1007/s00109-004-0567-7. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Crawford M, Cleary MA, Dahl HH, Dennett X, Thorburn DR. Respiratory chain complex I deficiency: an underdiagnosed energy generation disorder. Neurology. 1999;52:1255–1264. doi: 10.1212/wnl.52.6.1255. [DOI] [PubMed] [Google Scholar]
- Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126 (Part 8:1905–1912. doi: 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- Nissenkorn A, Zeharia A, Lev D, Watemberg N, Fattal-Valevski A, Barash V, et al. Neurologic presentations of mitochondrial disorders. J Child Neurol. 2000;15:44–48. doi: 10.1177/088307380001500110. [DOI] [PubMed] [Google Scholar]
- Shoffner J, Hyams L, Langley GN, Cossette S, Mylacraine L, Dale J, et al. Fever plus mitochondrial disease could be risk factors for autistic regression. J Child Neurol. 2010;25:429–434. doi: 10.1177/0883073809342128. [DOI] [PubMed] [Google Scholar]
- Edmonds JL, Kirse DJ, Kearns D, Deutsch R, Spruijt L, Naviaux RK. The otolaryngological manifestations of mitochondrial disease and the risk of neurodegeneration with infection. Arch Otolaryngol Head Neck Surg. 2002;128:355–362. doi: 10.1001/archotol.128.4.355. [DOI] [PubMed] [Google Scholar]
- Nissenkorn A, Zeharia A, Lev D, Fatal-Valevski A, Barash V, Gutman A, et al. Multiple presentation of mitochondrial disorders. Arch Dis Child. 1999;81:209–214. doi: 10.1136/adc.81.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka I. Approach for a final diagnosis of mitochondrial disease] Nippon Rinsho. 2002;60 (Suppl 4:224–228. [PubMed] [Google Scholar]
- Wolf NI, Smeitink JA. Mitochondrial disorders: a proposal for consensus diagnostic criteria in infants and children. Neurology. 2002;59:1402–1405. doi: 10.1212/01.wnl.0000031795.91814.d8. [DOI] [PubMed] [Google Scholar]
- Morava E, van den Heuvel L, Hol F, de Vries MC, Hogeveen M, Rodenburg RJ, et al. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67:1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE. 15q11.2-13 duplication, mitochondrial dysfunction, and developmental disorders. J Child Neurol. 2009;24:1316–1320. doi: 10.1177/0883073809333531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ, Filiano JJ, Sarnat HB. Skeletal muscle mitochondrial defects in nonspecific neurologic disorders. Pediatr Neurol. 1999;21:538–542. doi: 10.1016/s0887-8994(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Haas R, Stumpf DA, Parks JK, Eguren L. Inhibitory effects of sodium valproate on oxidative phosphorylation. Neurology. 1981;31:1473–1476. doi: 10.1212/wnl.31.11.1473. [DOI] [PubMed] [Google Scholar]
- Neustadt J, Pieczenik SR. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res. 2008;52:780–788. doi: 10.1002/mnfr.200700075. [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–169. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- MacFabe DF, Rodríguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Taylor AR, et al. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotech. 2008;4:146–166. [Google Scholar]
- Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, et al. Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem J. 2006;398:107–112. doi: 10.1042/BJ20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–529. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- Samavati L, Lee I, Mathes I, Lottspeich F, Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vempati UD, Diaz F, Barrientos A, Narisawa S, Mian AM, Millan JL, et al. Role of cytochrome C in apoptosis: increased sensitivity to tumor necrosis factor alpha is associated with respiratory defects but not with lack of cytochrome C release. Mol Cell Biol. 2007;27:1771–1783. doi: 10.1128/MCB.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazorla A, Quadros EV, Nascimento A, Garcia-Silva MT, Briones P, Montoya J, et al. Mitochondrial diseases associated with cerebral folate deficiency. Neurology. 2008;70:1360–1362. doi: 10.1212/01.wnl.0000309223.98616.e4. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Weis J, Sequeira JM, Quadros EV, Blau N. Mitochondrial complex I encephalomyopathy and cerebral 5-methyltetrahydrofolate deficiency. Neuropediatrics. 2007;38:184–187. doi: 10.1055/s-2007-991150. [DOI] [PubMed] [Google Scholar]
- Morava E, Rodenburg R, van Essen HZ, De Vries M, Smeitink J. Dietary intervention and oxidative phosphorylation capacity. J Inherit Metab Dis. 2006;29:589. doi: 10.1007/s10545-006-0227-x. [DOI] [PubMed] [Google Scholar]
- Atamna H, Killilea DW, Killilea AN, Ames BN. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc Natl Acad Sci USA. 2002;99:14807–14812. doi: 10.1073/pnas.192585799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vali S, Mythri RB, Jagatha B, Padiadpu J, Ramanujan KS, Andersen JK, et al. Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson′s disease: a dynamic model. Neuroscience. 2007;149:917–930. doi: 10.1016/j.neuroscience.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, Vazquez-Torres A. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem. 2008;283:7682–7689. doi: 10.1074/jbc.M708845200. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- Fowler BA, Woods JS. Ultrastructural and biochemical changes in renal mitochondria during chronic oral methyl mercury exposure: the relationship to renal function. Exp Mol Pathol. 1977;27:403–412. doi: 10.1016/0014-4800(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Guo TL, O I, Shapiro IM. Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol. 1999;157:23–35. doi: 10.1006/taap.1999.8652. [DOI] [PubMed] [Google Scholar]
- Goyer RA. Toxic and essential metal interactions. Annu Rev Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, Mihajlovic A, O′Brien PJ. Hepatocyte lysis induced by environmental metal toxins may involve apoptotic death signals initiated by mitochondrial injury. Adv Exp Med Biol. 2001;500:249–252. doi: 10.1007/978-1-4615-0667-6_38. [DOI] [PubMed] [Google Scholar]
- Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165:2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- Wong PW, Garcia EF, Pessah IN. Ortho-substituted PCB95 alters intracellular calcium signaling and causes cellular acidification in PC12 cells by an immunophilin-dependent mechanism. J Neurochem. 2001;76:450–463. doi: 10.1046/j.1471-4159.2001.00022.x. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson′s disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Morita S. Effects of pesticides on isolated rat hepatocytes, mitochondria, and microsomes II. Arch Environ Contam Toxicol. 1995;28:1–7. doi: 10.1007/BF00213961. [DOI] [PubMed] [Google Scholar]
- Hui J, Kirby DM, Thorburn DR, Boneh A. Decreased activities of mitochondrial respiratory chain complexes in non-mitochondrial respiratory chain diseases. Dev Med Child Neurol. 2006;48:132–136. doi: 10.1017/S0012162206000284. [DOI] [PubMed] [Google Scholar]
- Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. 2006;21:170–172. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, Krawiecki N, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- Sue CM, Bruno C, Andreu AL, Cargan A, Mendell JR, Tsao CY, et al. Infantile encephalopathy associated with the MELAS A3243G mutation. J Pediatr. 1999;134:696–700. doi: 10.1016/s0022-3476(99)70283-0. [DOI] [PubMed] [Google Scholar]
- Cohen BI. Use of a GABA-transaminase agonist for treatment of infantile autism. Med Hypotheses. 2002;59:115–116. doi: 10.1016/s0306-9877(02)00157-3. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Tarnopolsky M. Two children with muscular dystrophies ascertained due to referral for diagnosis of autism. J Autism Dev Disord. 2003;33:193–199. doi: 10.1023/a:1022947728569. [DOI] [PubMed] [Google Scholar]
- Gorker I, Tuzun U. Autistic-like findings associated with a urea cycle disorder in a 4-year-old girl. J Psychiatry Neurosci. 2005;30:133–135. [PMC free article] [PubMed] [Google Scholar]
- Futamura N, Kawamoto K, Takahashi K, Funakawa I, Jinnai K. Four siblings with becker muscular dystrophy (BMD) manifesting severe mental retardation. Rinsho Shinkeigaku. 2006;46:62–65. [PubMed] [Google Scholar]
- El-Ansary A, Al-Daihan S, Al-Dbass A, Al-Ayadhi L. Measurement of selected ions related to oxidative stress and energy metabolism in Saudi autistic children. Clin Biochem. 2010;43:63–70. doi: 10.1016/j.clinbiochem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Ji L, Chauhan A, Brown WT, Chauhan V. Increased activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the frontal cortex and cerebellum of autistic individuals. Life Sci. 2009;85:788–793. doi: 10.1016/j.lfs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings JA, Weckbaugh M, Nickel EJ, Cain SE, Zarcone JR, Reese RM, et al. A double-blind, placebo-controlled study of valproate for aggression in youth with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2005;15:682–692. doi: 10.1089/cap.2005.15.682. [DOI] [PubMed] [Google Scholar]
- Karakaya P, Yis U, Kurul SH, Turkmen MA. Rhabdomyolysis associated with olanzapine treatment in a child with Autism. Pediatr Emerg Care. 2010;26:41–42. doi: 10.1097/PEC.0b013e3181c39a22. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Ramoz N, Barreto M, Gazdoiu M, Takahashi N, Gertner M, et al. Slc25a12 disruption alters myelination and neurofilaments: a model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol Psychiatry. 2010;67:887–894. doi: 10.1016/j.biopsych.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Munoz C, Rosenberg EH, Jakobs C, Salomons GS. Identification, characterization and cloning of SLC6A8C, a novel splice variant of the creatine transporter gene. Gene. 2008;418:53–59. doi: 10.1016/j.gene.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pancrudo J, Shanske S, Coku J, Lu J, Mardach R, Akman O, et al. Mitochondrial myopathy associated with a novel mutation in mtDNA. Neuromuscul Disord. 2007;17:651–654. doi: 10.1016/j.nmd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurines R, Thome J, Duvigneau JC, Forbes-Robertson S, Yang L, Klampfl K, et al. Expression analyses of the mitochondrial complex I 75-kDa subunit in early onset schizophrenia and autism spectrum disorder: increased levels as a potential biomarker for early onset schizophrenia. Eur Child Adolesc Psychiatry. 2010;19:441–448. doi: 10.1007/s00787-009-0074-z. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Petek E, Schwarzbraun T, Noor A, Patel M, Nakabayashi K, Choufani S, et al. Molecular and genomic studies of IMMP2L and mutation screening in autism and Tourette syndrome. Mol Genet Genomics. 2007;277:71–81. doi: 10.1007/s00438-006-0173-1. [DOI] [PubMed] [Google Scholar]
- Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism, from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004. doi: 10.1021/pr901188e. [DOI] [PubMed] [Google Scholar]
- Arnold GL, Hyman SL, Mooney RA, Kirby RS. Plasma amino acids profiles in children with autism: potential risk of nutritional deficiencies. J Autism Dev Disord. 2003;33:449–454. doi: 10.1023/a:1025071014191. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol Psychiatry. 1993;33:762–773. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Johnson W, Caparulo BK, Young JG. Creatine phosphokinase levels in children with severe developmental disturbances. Arch Gen Psychiatry. 1976;33:683–686. doi: 10.1001/archpsyc.1976.01770060025004. [DOI] [PubMed] [Google Scholar]
- Moreno H, Borjas L, Arrieta A, Sáez L, Prassad A, Estévez J, et al. Heterogeneidad clínica del síndrome autista: un estudio en sesenta familias [Clinical heterogeneity of the autistic syndrome: a study of 60 families] Invest clin. 1992;33:13–31. [PubMed] [Google Scholar]
- Chugani DC, Sundram BS, Behen M, Lee ML, Moore GJ. Evidence of altered energy metabolism in autistic children. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:635–641. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- Kurup RK, Kurup PA. A hypothalamic digoxin-mediated model for autism. Int J Neurosci. 2003;113:1537–1559. doi: 10.1080/00207450390231482. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161:662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2:179–188. [Google Scholar]
- Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L. Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry. 2005;162:2182–2184. doi: 10.1176/appi.ajp.162.11.2182. [DOI] [PubMed] [Google Scholar]
- Blasi F, Bacchelli E, Carone S, Toma C, Monaco AP, Bailey AJ, et al. SLC25A12 and CMYA3 gene variants are not associated with autism in the IMGSAC multiplex family sample. Eur J Hum Genet. 2006;14:123–126. doi: 10.1038/sj.ejhg.5201444. [DOI] [PubMed] [Google Scholar]
- Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T, et al. Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36:1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- Germano E, Gagliano A, Magazu A, Calarese T, Calabro ME, Bonsignore M, et al. Neurobiology of autism: study of a sample of autistic children] Minerva Pediatr. 2006;58:109–120. [PubMed] [Google Scholar]
- Rabionet R, McCauley JL, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, et al. Lack of association between autism and SLC25A12. Am J Psychiatry. 2006;163:929–931. doi: 10.1176/ajp.2006.163.5.929. [DOI] [PubMed] [Google Scholar]
- Fernell E, Karagiannakis A, Edman G, Bjerkenstedt L, Wiesel FA, Venizelos N. Aberrant amino acid transport in fibroblasts from children with autism. Neurosci Lett. 2007;418:82–86. doi: 10.1016/j.neulet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Benzecry JM, Deth R, Holtzman D. Are autistic spectrum disorders an expression of mitochondrial encephalopathies. Mitochondrion. 2009;9:62. [Google Scholar]
- Holtzman D. Autistic spectrum disorders and mitochondrial encephalopathies. Acta Paediatr. 2008;97:859–860. doi: 10.1111/j.1651-2227.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- Kent L, Gallagher L, Elliott HR, Mowbray C, Chinnery PF. An investigation of mitochondrial haplogroups in autism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:987–989. doi: 10.1002/ajmg.b.30687. [DOI] [PubMed] [Google Scholar]
- Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, et al. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13:385–397. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- Maussion G, Carayol J, Lepagnol-Bestel AM, Tores F, Loe-Mie Y, Milbreta U, et al. Convergent evidence identifying MAP/microtubule affinity-regulating kinase 1 (MARK1) as a susceptibility gene for autism. Hum Mol Genet. 2008;17:2541–2551. doi: 10.1093/hmg/ddn154. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1152–1158. doi: 10.1002/ajmg.b.30739. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Buxbaum JD, Ramoz N, Schmeidler J, Reichenberg A, Hollander E, et al. Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2008;147:408–410. doi: 10.1002/ajmg.b.30614. [DOI] [PubMed] [Google Scholar]
- Turunen JA, Rehnstrom K, Kilpinen H, Kuokkanen M, Kempas E, Ylisaukko-Oja T. Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res. 2008;1:189–192. doi: 10.1002/aur.25. [DOI] [PubMed] [Google Scholar]
- Al-Mosalem OA, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to energy metabolism in Saudi autistic children. Clin Biochem. 2009;42:949–957. doi: 10.1016/j.clinbiochem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Essa MM, Muthaiyah B, Brown WT, Chauhan V. Mitochondrial abnormalities in lymphoblasts from autism: P1-01-03. J Neurochem. 2009;109 (Suppl 1:273. [Google Scholar]
- Chien WH, Wu YY, Gau SS, Huang YS, Soong WT, Chiu YN, et al. Association study of the SLC25A12 gene and autism in Han Chinese in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:189–192. doi: 10.1016/j.pnpbp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Pastural E, Ritchie S, Lu Y, Jin W, Kavianpour A, Khine Su-Myat K, et al. Novel plasma phospholipid biomarkers of autism: mitochondrial dysfunction as a putative causative mechanism. Prostaglandins Leukot Essent Fatty Acids. 2009;81:253–264. doi: 10.1016/j.plefa.2009.06.003. [DOI] [PubMed] [Google Scholar]
- El-Ansary A, Al-Daihan S, Al-Dabas A, Al-Ayadhi L. Activities of key glycolytic enzymes in the plasma of Saudi autistic patients. Open Access J Clin Trials. 2010;2010:49–57. [Google Scholar]
- Oliveira G, Diogo L, Grazina M, Garcia P, Ataide A, Marques C, et al. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev Med Child Neurol. 2005;47:185–189. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- Oliveira G, Ataide A, Marques C, Miguel TS, Coutinho AM, Mota-Vieira L, et al. Epidemiology of autism spectrum disorder in Portugal: prevalence, clinical characterization, and medical conditions. Dev Med Child Neurol. 2007;49:726–733. doi: 10.1111/j.1469-8749.2007.00726.x. [DOI] [PubMed] [Google Scholar]
- Kent L, Lambert C, Pyle A, Elliott H, Wheelwright S, Baron-Cohen S, et al. The mitochondrial DNA A3243A>G mutation must be an infrequent cause of Asperger syndrome. J Pediatr. 2006;149:280–281. doi: 10.1016/j.jpeds.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Zhang H, Huq A. Prevalence of common mitochondrial point mutations in autism. Neuropediatrics. 2006;37 (Suppl 1:S127. [Google Scholar]
- Laszlo A, Horvath E, Eck E, Fekete M. Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin Chim Acta. 1994;229:205–207. doi: 10.1016/0009-8981(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Shaw W, Kassen E, Chaves E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin Chem. 1995;41 (8 Part 1:1094–1104. [PubMed] [Google Scholar]
- Gargus JJ, Lerner MA. Familial autism with primary carnitine deficiency, sudden death, hypotonia and hypochromic anemia. Am J Human Gen. 1997;61:A98. [Google Scholar]
- Filiano JJ, Goldenthal MJ, Rhodes CH, Marin-Garcia J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002;17:435–439. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Juranek J, Smith M, Mays LZ, Ramos ER, Bocian M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53:801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–623. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- Pons R, Andreu AL, Checcarelli N, Vila MR, Engelstad K, Sue CM, et al. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004;144:81–85. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Tsao CY, Mendell JR. Autistic disorder in 2 children with mitochondrial disorders. J Child Neurol. 2007;22:1121–1123. doi: 10.1177/0883073807306266. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V, Brown WT.(eds).Autism: Oxidative Stress, Inflammation, and Immune Abnormalities CRC Press: Boca Raton; 2010 [Google Scholar]
- Scaglia F, Zhang S, Tan Z, Fouladi N, Schmitt E, Wong L-J. Proceedings of the American Society of Human Genetics 59th Annual Meeting. Honolulu, Hawaii; 2009. Prevalence of autism spectrum disorders in subjects with definite diagnosis of mitochondrial cytopathies. [Google Scholar]
- Smith M, Spence MA, Flodman P. Nuclear and mitochondrial genome defects in autisms. Ann NY Acad Sci. 2009;1151:102–132. doi: 10.1111/j.1749-6632.2008.03571.x. [DOI] [PubMed] [Google Scholar]
- Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D, et al. Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol. 2000;15:357–361. doi: 10.1177/088307380001500601. [DOI] [PubMed] [Google Scholar]
- Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial beta-oxidation by long chain acyl-CoA dehydrogenase. Med Hypotheses. 2004;62:970–975. doi: 10.1016/j.mehy.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Castro-Gago M, Blanco-Barca O, Gomez-Lado C, Pintos-Martinez E, Campos-Gonzalez Y, Eiris-Punal J. Association between autistic spectrum and mitochondrial pathology. Rev Neurol. 2008;47:52–53. [PubMed] [Google Scholar]
- Ezugha H, Goldenthal M, Valencia I, Anderson CE, Legido A, Marks H. 5q14.3 deletion manifesting as mitochondrial disease and autism: case report. J Child Neurol. 2010;25:1232–1235. doi: 10.1177/0883073809361165. [DOI] [PubMed] [Google Scholar]
- Mood AM, Graybill FA, Boes DC. Introduction to the Theory of Statistics. McGraw-Hill: New York; 1974. [Google Scholar]
- Glass GV, McGaw B, Smith ML. Metaanalysis in Social Research. Sage Publishing: Beverly Hills; 1981. [Google Scholar]
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic Press: New York; 1985. [Google Scholar]
- Lipsey MW, Wilson D. Practical Meta-Analysis. Sage Publications: Thousand Oaks; 2001. [Google Scholar]
- Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2009;39:405–413. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health Dev. 2010;36:437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics. 2001;108:E58. doi: 10.1542/peds.108.4.e58. [DOI] [PubMed] [Google Scholar]
- Lingam R, Simmons A, Andrews N, Miller E, Stowe J, Taylor B. Prevalence of autism and parentally reported triggers in a north east London population. Arch Dis Child. 2003;88:666–670. doi: 10.1136/adc.88.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Rice CE, Baio J. Developmental regression in children with an autism spectrum disorder identified by a population-based surveillance system. Autism. 2009;13:357–374. doi: 10.1177/1362361309105662. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Tuchman RF, Rapin I. Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics. 1997;99:560–566. doi: 10.1542/peds.99.4.560. [DOI] [PubMed] [Google Scholar]
- Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann Neurol. 2001;49:377–383. [PubMed] [Google Scholar]
- Holmgren D, Wahlander H, Eriksson BO, Oldfors A, Holme E, Tulinius M. Cardiomyopathy in children with mitochondrial disease; clinical course and cardiological findings. Eur Heart J. 2003;24:280–288. doi: 10.1016/s0195-668x(02)00387-1. [DOI] [PubMed] [Google Scholar]
- Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EO, Neish SR, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114:925–931. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- Skladal D, Sudmeier C, Konstantopoulou V, Stockler-Ipsiroglu S, Plecko-Startinig B, Bernert G, et al. The clinical spectrum of mitochondrial disease in 75 pediatric patients. Clin Pediatr (Phila) 2003;42:703–710. doi: 10.1177/000992280304200806. [DOI] [PubMed] [Google Scholar]
- Schuelke M, Krude H, Finckh B, Mayatepek E, Janssen A, Schmelz M, et al. Septo-optic dysplasia associated with a new mitochondrial cytochrome b mutation. Ann Neurol. 2002;51:388–392. doi: 10.1002/ana.10151. [DOI] [PubMed] [Google Scholar]
- Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- Su H, Fan W, Coskun PE, Vesa J, Gold JA, Jiang YH, et al. Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett. 2009;487:129–133. doi: 10.1016/j.neulet.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Paterson A, Curtis J, Guy J, Macleod N, Bird A. Gene expression analysis exposes mitochondrial abnormalities in a mouse model of Rett syndrome. Mol Cell Biol. 2006;26:5033–5042. doi: 10.1128/MCB.01665-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie DL. Functional insights into the creatine transporter. Subcell Biochem. 2007;46:99–118. doi: 10.1007/978-1-4020-6486-9_6. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Sahoo T, Hyland K, Bottiglieri T, Peters S, del Gaudio D, et al. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 2005;64:1088–1090. doi: 10.1212/01.WNL.0000154641.08211.B7. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Sequeira JM, Blau N, Quadros EV. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev Med Child Neurol. 2008;50:346–352. doi: 10.1111/j.1469-8749.2008.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, Lev D. Should autistic children be evaluated for mitochondrial disorders. J Child Neurol. 2004;19:379–381. doi: 10.1177/088307380401900510. [DOI] [PubMed] [Google Scholar]
- Buie T, Fuchs GJ, III, Furuta GT, Kooros K, Levy J, Lewis JD, et al. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics. 2010;125 (Suppl 1:S19–S29. doi: 10.1542/peds.2009-1878D. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Calvani M, Casamassimi A, Petillo O, Margarucci S, Rienzo M, et al. Experimental colitis: decreased Octn2 and Atb0+ expression in rat colonocytes induces carnitine depletion that is reversible by carnitine-loaded liposomes. FASEB J. 2006;20:2544–2546. doi: 10.1096/fj.06-5950fje. [DOI] [PubMed] [Google Scholar]
- Bell JG, Sargent JR, Tocher DR, Dick JR. Red blood cell fatty acid compositions in a patient with autistic spectrum disorder: a characteristic abnormality in neurodevelopmental disorders. Prostaglandins Leukot Essent Fatty Acids. 2000;63:21–25. doi: 10.1054/plef.2000.0186. [DOI] [PubMed] [Google Scholar]
- Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71:201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids. 2005;73:379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Enns GM. Autistic features part of global neurologic syndrome in children who have mitochondrial disease. AAP News. 2008;29:20. [Google Scholar]
- Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–e1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009;89:425–430. doi: 10.3945/ajcn.2008.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Geier MR. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009;34:386–393. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Nataf R, et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–181. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Malik M, Sheikh AM, Wen G, Spivack W, Brown WT, Li X. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology. 2011;216:80–85. doi: 10.1016/j.imbio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, Shankar S, McDougle CJ. High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004;55:434–437. doi: 10.1016/j.biopsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Sogut S, Zoroglu SS, Ozyurt H, Yilmaz HR, Ozugurlu F, Sivasli E, et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin Chim Acta. 2003;331:111–117. doi: 10.1016/s0009-8981(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Zoroglu SS, Yurekli M, Meram I, Sogut S, Tutkun H, Yetkin O, et al. Pathophysiological role of nitric oxide and adrenomedullin in autism. Cell Biochem Funct. 2003;21:55–60. doi: 10.1002/cbf.989. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Bremer J, Davis EJ. Suppression of the mitochondrial oxidation of (-)-palmitylcarnitine by the malate-aspartate and alpha-glycerophosphate shuttles. J Biol Chem. 1976;251:277–284. [PubMed] [Google Scholar]
- Tirosh O, Sen CK, Roy S, Packer L. Cellular and mitochondrial changes in glutamate-induced HT4 neuronal cell death. Neuroscience. 2000;97:531–541. doi: 10.1016/s0306-4522(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:129–136. doi: 10.1007/s11910-009-0021-x. [DOI] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C. Environmental mercury release, special education rates, and autism disorder: an ecological study of Texas. Health Place. 2006;12:203–209. doi: 10.1016/j.healthplace.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Palmer RF, Blanchard S, Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place. 2009;15:18–24. doi: 10.1016/j.healthplace.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Autism and autistic symptoms associated with childhood lead poisoning. J Appl Res. 2005;5:80–87. [Google Scholar]
- Cohen DJ, Johnson WT, Caparulo BK. Pica and elevated blood lead level in autistic and atypical children. Am J Dis Child. 1976;130:47–48. doi: 10.1001/archpedi.1976.02120020049007. [DOI] [PubMed] [Google Scholar]
- Campbell M, Petti TA, Green WH, Cohen IL, Genieser NB, David R. Some physical parameters of young autistic children. J Am Acad Child Psychiatry. 1980;19:193–212. doi: 10.1016/s0002-7138(09)60697-x. [DOI] [PubMed] [Google Scholar]
- Edelson SB, Cantor DS. The neurotoxic etiology of the autistic spectrum disorders: a replication study. Toxicol Ind Health. 2000;16:239–247. [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Krey JF, Dolmetsch RE. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol. 2007;17:112–119. doi: 10.1016/j.conb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system: seizures, migraine, and autism. Ann NY Acad Sci. 2009;1151:133–156. doi: 10.1111/j.1749-6632.2008.03572.x. [DOI] [PubMed] [Google Scholar]
- Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, et al. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281:1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2008;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci. 2010;13:135–143. doi: 10.1179/147683010X12611460763968. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35 (Suppl 1:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54 (Part 10:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998;51:133–144. doi: 10.1016/s0306-9877(98)90107-4. [DOI] [PubMed] [Google Scholar]
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- Schmidt CW. Mito-conundrum: unraveling environmental effects on mitochondria. Environ Health Perspect. 2010;118:a292–a297. [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190 (Suppl 1:S8–21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stefanatos GA. Regression in autistic spectrum disorders. Neuropsychol Rev. 2008;18:305–319. doi: 10.1007/s11065-008-9073-y. [DOI] [PubMed] [Google Scholar]
- Sim KG, Hammond J, Wilcken B. Strategies for the diagnosis of mitochondrial fatty acid beta-oxidation disorders. Clin Chim Acta. 2002;323:37–58. doi: 10.1016/s0009-8981(02)00182-1. [DOI] [PubMed] [Google Scholar]
- Quinzii CM, DiMauro S, Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PB, Hunter JV, Kaye EM. Lactic acid elevation in extramitochondrial childhood neurodegenerative diseases. J Child Neurol. 2001;16:657–660. doi: 10.1177/088307380101600906. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Jr, Dawson G, et al. Practice parameter: screening and diagnosis of autism: report of the quality standards subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- Benvenuto A, Moavero R, Alessandrelli R, Manzi B, Curatolo P. Syndromic autism: causes and pathogenetic pathways. World J Pediatr. 2009;5:169–176. doi: 10.1007/s12519-009-0033-2. [DOI] [PubMed] [Google Scholar]
- Manzi B, Loizzo AL, Giana G, Curatolo P. Autism and metabolic diseases. J Child Neurol. 2008;23:307–314. doi: 10.1177/0883073807308698. [DOI] [PubMed] [Google Scholar]
- Frye RE.AutismIn: Carney PR, Geyer JR (eds).Pediatric Practice: Neurology McGraw-Hill: New York, NY; 2010 [Google Scholar]
- Atkuri KR, Cowan TM, Kwan T, Ng A, Herzenberg LA, Enns GM. Inherited disorders affecting mitochondrial function are associated with glutathione deficiency and hypocitrullinemia. Proc Natl Acad Sci USA. 2009;106:3941–3945. doi: 10.1073/pnas.0813409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolske MC, Spollen J, McKay S, Lancashire E, Tolbert L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:765–774. doi: 10.1016/0278-5846(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Chez MG, Buchanan CP, Aimonovitch MC, Becker M, Schaefer K, Black C, et al. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J Child Neurol. 2002;17:833–837. doi: 10.1177/08830738020170111501. [DOI] [PubMed] [Google Scholar]
- Hertzman M. Galantamine in the treatment of adult autism: a report of three clinical cases. Int J Psychiatry Med. 2003;33:395–398. doi: 10.2190/JE5Q-1NFT-FL40-7PMW. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Craven-Thuss B, Smith J. A prospective, open-label trial of galantamine in autistic disorder. J Child Adolesc Psychopharmacol. 2006;16:621–629. doi: 10.1089/cap.2006.16.621. [DOI] [PubMed] [Google Scholar]
- Niederhofer H, Staffen W, Mair A. Galantamine may be effective in treating autistic disorder. BMJ. 2002;325:1422. doi: 10.1136/bmj.325.7377.1422/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke T, Perez-Cerda C, Baumgartner M, Fowler B, Sander S, Sasse M, et al. Propionic acidemia: unusual course with late onset and fatal outcome. Metabolism. 2004;53:809–810. doi: 10.1016/j.metabol.2003.12.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.