A stop or nonsense codon is an in-frame triplet within a messenger RNA that signals the termination of translation. One common feature shared among all three nonsense codons (UAA, UAG, and UGA) is a uridine present at the first codon position. It has been recently shown that the conversion of this uridine into pseudouridine (Ψ) suppresses translation termination, both in vitro and in vivo. Furthermore, decoding of the pseudouridylated nonsense codons is accompanied by the incorporation of two specific amino acids in a nonsense codon-dependent fashion. How Ψ suppresses termination and, more importantly, enables selective decoding is poorly understood. Here, the authors provide molecular rationales for how pseudouridylated stop codons are selectively decoded. Their analysis enables prediction of potential decoding properties for Ψ-modified sense codons.
Keywords: pseudouridine, nonsense codon, molecular modeling, ribosome, tRNA
Abstract
A stop or nonsense codon is an in-frame triplet within a messenger RNA that signals the termination of translation. One common feature shared among all three nonsense codons (UAA, UAG, and UGA) is a uridine present at the first codon position. It has been recently shown that the conversion of this uridine into pseudouridine (Ψ) suppresses translation termination, both in vitro and in vivo. Furthermore, decoding of the pseudouridylated nonsense codons is accompanied by the incorporation of two specific amino acids in a nonsense codon-dependent fashion. Ψ differs from uridine by a single N1H group at the C5 position; how Ψ suppresses termination and, more importantly, enables selective decoding is poorly understood. Here, we provide molecular rationales for how pseudouridylated stop codons are selectively decoded. Our analysis applies crystal structures of ribosomes in varying states of translation to consider weakened interaction of Ψ with release factor; thermodynamic and geometric considerations of the codon-anticodon base pairs to rank and to eliminate mRNA-tRNA pairs; the mechanism of fidelity check of the codon-anticodon pairing by the ribosome to evaluate noncanonical codon-anticodon base pairs and the role of water. We also consider certain tRNA modifications that interfere with the Ψ-coordinated water in the major groove of the codon-anticodon mini-helix. Our analysis of nonsense codons enables prediction of potential decoding properties for Ψ-modified sense codons, such as decoding ΨUU potentially as Cys and Tyr. Our results provide molecular rationale for the remarkable dynamics of ribosome decoding and insights on possible reprogramming of the genetic code using mRNA modifications.
INTRODUCTION
Pseudouridine (Ψ) is a C5-glycoside rotation isomer of uridine (Fig. 1A). It is found in transfer and ribosomal RNA (tRNA, rRNA) throughout the three kingdoms of life and in spliceosomal small nuclear RNA (snRNA) in eukaryotes. Although Ψ is the most abundant RNA modification (Hamma and Ferre-D'Amare 2006), it remains to be determined whether Ψ is also present in messenger RNA (mRNA). The primary chemical change made by the U-to-Ψ conversion is the addition of a hydrogen bond donor through the N1H group. In an RNA helix, this hydrogen bond donor is in the major groove and can anchor a water molecule to bridge the interactions of this N1H group to its own and the preceding phosphate groups (Arnez and Steitz 1994).
FIGURE 1.
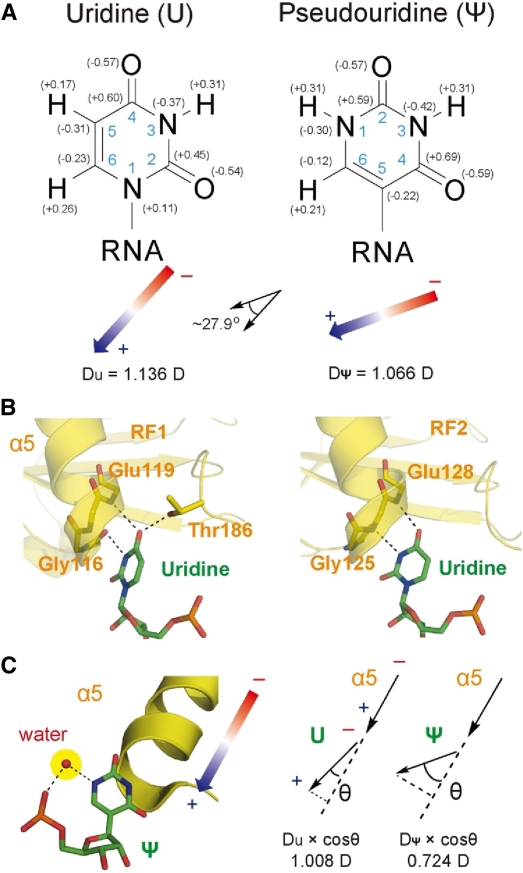
Structural comparison of U and Ψ and proposed role of decreased recognition by release factors. (A) Chemical structure of U and Ψ. Partial charge is labeled for each atom and calculated dipole moments of the two bases (debye, or D) are shown with arrays. (B) Recognition of U in a nonsense codon by RF1 (PDB code: 3D5A) and RF2 (2WH3). (C) Comparison of attractive dipole moment between the release factor protein and the nucleobase, when either a U or Ψ (assuming Ψ occupies the same position as U) is present in the active site of RF1. The extra water molecule present only with Ψ-modified nonsense codons is also shown.
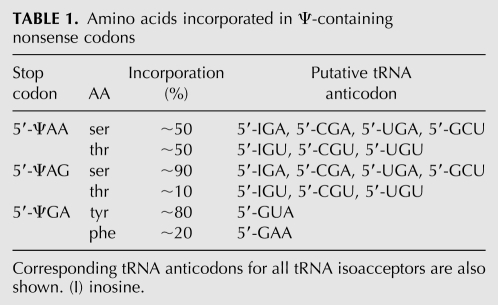
In a recent study, Yu and colleagues demonstrated that the substitution of uridine in all three nonsense codons with Ψ suppresses translational termination, both in vitro and in Saccharomyces cerevisiae (Karijolich and Yu 2011). Furthermore, they showed that Ψ-modified nonsense codons are selectively decoded as specific amino acids (Table 1). In particular, ΨAA and ΨAG are read as serine and threonine, whereas ΨGA is read as phenylalanine and tyrosine.
TABLE 1.
Amino acids incorporated in Ψ-containing nonsense codons
Decoding for unmodified mRNA codons is accomplished in multiple steps and is under strict surveillance by the ribosome. mRNA codons are usually recognized by tRNAs featuring complementary Watson-Crick sequences and sometimes wobble base pairs at the third codon position. Watson-Crick and certain wobble base pairs enable cognate tRNAs to thermodynamically outcompete other tRNA species whose sequences would introduce mismatched base pairs with the mRNA codon. Further, the mRNA-tRNA base pairs in the A-site of a ribosome are probed in a process termed “the A-site test” to ensure the fidelity of the decoded codon (Ogle et al. 2001; Schuwirth et al. 2005; Selmer et al. 2006). Specifically, the A-site test is performed by a small ribosomal subunit via A1493 and A1492 of helix 44 in the 16S rRNA (Thermus thermophilus numbering is used hereafter), which form an extensive network of hydrogen bonds in the minor groove with the mRNA-tRNA base pairs at the first and second codon position, and by G530 of loop 530 at the third codon position (Ogle et al. 2001). Such minor-groove interactions are universally conserved and serve as a key step in the fidelity control of the decoding process (Lescoute and Westhof 2006). Many tRNAs are extensively modified at nucleotide 37, the immediate 3′ nucleotide to the third anticodon nucleotide which reads the first position of the mRNA codon. Modification at nucleotide 37 could also influence the accuracy to decode the first codon nucleotide.
When the first position of the nonsense codons is modified from U to Ψ, the modified codons are not only efficiently suppressed as translation stops, they are also selectively decoded as just two amino acids (Karijolich and Yu 2011). Although the decoding pattern of the modified nonsense codons includes Watson-Crick type Ψ/A base pairs, e.g., those in tRNASer, the presence of Ψ in the first codon position also permits decoding by tRNAs with U36 at their third anticodon position, e.g., those in tRNAThr. This opens up the possibility for a mRNA-tRNA mismatched Ψ/U base pair. Furthermore, the presence of Ψ leads to additional changes in base-pairing rules in the second or even in the third codon-anticodon pairs, e.g., A/G mismatches in the case of ΨAA/ ΨAG decoding at the second position and in the case of ΨGA decoding at the third position (Table 1). No rational explanation was provided for these experimental observations.
Here, we provide a molecular explanation of how the three Ψ-modified nonsense codons are suppressed as termination codons and, more importantly, are selectively decoded as specific amino acids. Taking advantage of the large number of available crystal structures of ribosomes in varying states of translation, we provide molecular models on selective decoding, A-site probing, thermodynamic and geometric considerations, and influence of certain tRNA modifications on selective decoding of Ψ-modified nonsense codons. Because no structures of eukaryotic ribosomes containing both mRNA and tRNA are available, our analysis had to rely on the structures of bacterial ribosomes. Due to the involvement of many interaction partners, it was not possible to predict the precise fraction of the two amino acids that selectively decode Ψ-containing nonsense codons. Despite these caveats, our results provide insights for the remarkable dynamics of ribosome decoding and lead to predictions of potential decoding rules for Ψ-modified sense codons which should be useful in rational reprogramming of the genetic codes using RNA modifications.
RESULTS AND DISCUSSION
Ψ has to escape recognition by release factors
Nonsense codons in the A-site of ribosome are normally recognized by release factors, thereby triggering dissociation of ribosomal subunits and releasing the newly synthesized peptide chain. In order to allow base-pairing to anticodons and subsequent incorporation of amino acids, Ψ-modified nonsense codons must first escape recognition by the release factors. In yeast, all three nonsense codons are recognized by eRF1 which forms a functional complex with eRF3, while in prokaryotes, two proteins (RF1 and RF2) recognize the nonsense codons. Due to the lack of structural information of eRF1 in the context of nonsense codon recognition in the ribosome, our analysis was performed with prokaryotic release factors (RF1 and RF2) where rich structural information is available (Korostelev et al. 2008; Laurberg et al. 2008; Weixlbaumer et al. 2008; Korostelev et al. 2010), despite the fact that the nonsense codon suppression experiments were done in yeast (Karijolich and Yu 2011).
Nonsense-codon recognition involves numerous interactions of various types (polar, hydrophobic, ionic, stacking, etc.) for E. coli RF1 and RF2 (Sund et al. 2010). Since interactions to the Watson-Crick face of uridine (or Ψ) seem inadequate to explain the decreased binding affinity of Ψ to release factors, we hypothesize that certain differences between uridine and Ψ must have weakened the recognition of the Ψ-modified nonsense codons by the release factor.
The first difference is the dipole moment: while the magnitude difference of dipole moments of U and Ψ base is small, the angular difference is obvious (∼27.9°) (Fig. 1A). The N-terminus of helix α5 from domain 2 of RF1 is in close proximity to the uridine base (Fig. 1A), and the projection for the dipole of U on the dipole of α5 is ∼30% greater than that for Ψ (Fig. 1C). Since the most favorable interaction is for colinear dipole moments, i.e., the angle θ = 0, a greater angle to helix α5's dipole moment for Ψ compared to U suggests that its interaction with helix α5 is weakened. Furthermore, the greater angle of the Ψ dipole should experience ∼1.4 times greater torque than U. This torque tends to align Ψ's dipole with that of the termination factor's α-helix, therefore coaxing Ψ in a nonideal position for hydrogen bonding to release factors (Fig. 1C). An all-atoms energy computation provides for a −3.2 kcal/mol estimate in favor of U with respect to Ψ at binding RF1; hence, the binding of Ψ-containing stop codons to release factors is expected to be significantly decreased. Although the structures of prokaryotic release factors vary significantly from those of eukaryotes (Song et al. 2000; Cheng et al. 2009), and atomic details of base recognition by eRF1 are still unclear, the rotational isomerization from U to Ψ is expected to always change the dipole-dipole interaction between a bound base and protein residues in eRF1.
Second, a water molecule is present only in the Ψ-modified codons through hydrogen bonds to the N1H and the 5′ phosphate oxygen of Ψ (Fig. 1C; Arnez and Steitz 1994; Yarian et al. 1999). This water molecule could interfere with release factor interaction through steric hindrance or reducing the rotational freedom of the Ψ base.
The weaker affinity derived from altered dipoles and water-mediated base geometry could enable some tRNA species to compete for the binding of the modified nonsense codon.
tRNA abundance cannot explain the specificity of amino acid incorporation
One trivial explanation for the specific incorporation of certain amino acids for the Ψ-modified nonsense codon (Table 1) is that decoding might be governed by an in vivo abundance of tRNA molecules. We have previously developed a tRNA microarray method to measure the relative abundance of tRNA molecules within any biological sample (Dittmar et al. 2006; Zaborske et al. 2009). Our previous results for yeast suggest that the charging level is generally high for all tRNAs under optimal growth conditions (Zaborske et al. 2009). Therefore, only the abundance of cellular tRNA should be taken into account for decoding Ψ-modified nonsense codons. However, a correlation between the tRNA abundance and selective decoding of Ψ-modified nonsense codon is not observed in yeast (Fig. 2; data from Tuller et al. 2010). For example, the abundance of all tRNAThr isoacceptors is below average, but threonine is readily incorporated at ΨAA and ΨAG codons. Further, none of the five most abundant tRNAs matches the actual amino acids incorporated at Ψ-modified nonsense codons. These results indicate that the observed specificity of amino acid incorporation is not determined by the abundance of tRNA species in cells.
FIGURE 2.
Relative tRNA abundance in yeast measured by microarray. tRNAs that could decode the Ψ-containing nonsense codons are sorted on the right. The sum of the percentage of tRNASer and tRNAThr species is shown. The anticodon sequence for each tRNA is included in the parentheses.
Selecting tRNA candidates for decoding Ψ-modified nonsense codons
The competition of various tRNA species for binding to a sense codon is determined in part by the thermodynamic preference of the Watson-Crick and certain wobble base pairs in the codon-anticodon mini-helix at the ribosomal A-site. Unlike sense codons, no complementary Watson-Crick tRNA partners exist for the nonsense codons. Therefore, decoding Ψ-modified nonsense codons has to involve mismatched base pairs. In addition, the mismatched mRNA-tRNA mini-helix has to survive the ribosomal A-site test to enable elongation of the nascent peptide chain.
The closest tRNA candidates involves two Watson-Crick base pairs in the first and second position but a mismatch in the third position. Using such tRNA candidates would predict decoding ΨAA/ΨAG as tyrosine and ΨGA as cysteine or tryptophan, but none of these predictions matches the experimental results (Karijolich and Yu 2011).
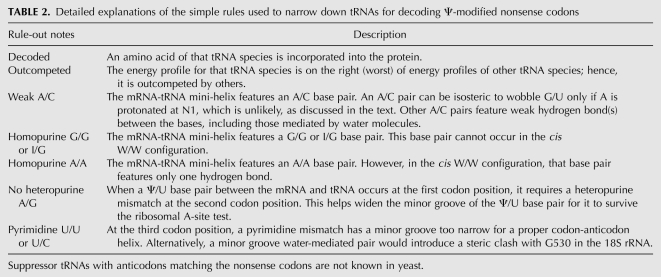
Many of the competing tRNAs can be readily ruled out by applying simple rules to the geometry of base pairs formed between the mRNA codon and tRNA anticodon. The first and second codon-anticodon base pairs are proofread extensively by the ribosome in the A-site. The base-pair geometry of the first codon-anticodon pair is particularly important because of its most extensive interactions with the A1493 residue of the 16S rRNA (Ogle et al. 2001; Lescoute and Westhof 2006). Since the canonical Watson-Crick base pairs are of the cis W/W type (Leontis-Westhof nomenclature) (Leontis and Westhof 2001), viable mismatches for the first codon-anticodon pairs should also be of the cis W/W geometry. For the first codon position, tRNA molecules having either an A or a U at the corresponding position effectively decode the Ψ-modified nonsense codons (Table 1). Thus, Ψ/A and Ψ/U pairs (codon nucleotide precedes) must be allowed. Indeed, both U/A and U/U pairs can be present in the cis W/W conformation (Leontis and Westhof 2001). This readily rules out tRNAs having a G or C at the third position of the anticodon, leaving only tRNAs with A or U at the third anticodon position for further consideration. For the second and third positions, the experimentally decoded nonsense codons indicate that hetero-purine mismatches (A/G or G/A) are allowed, which can also be found in the cis W/W state. Homo-purine mismatches can be discarded because a G/G base pair cannot occur in the cis W/W geometry, and A/A cis W/W conformation features only one hydrogen bond between the two bases and, hence, is unstable (Leontis et al. 2002).
Although a purine-pyrimidine wobble should be allowed for the second and third codon-anticodon pair, A/C is not considered here because the adenosine has to be protonated to promote the formation of the base pair, even though an A/C cis W/W pair is isosteric to a G/U wobble (Stombaugh et al. 2009). The protonation state of an A/C pair is known to depend on the number of its surrounding base pairs (Siegfried et al. 2010), and the short mRNA-tRNA mini-helix in the A-site is unlikely to support the protonation of the adenosine.
Hetero-purine base pairs can exist in many conformations, two of which are most abundant: the imino (cis W/W, Saenger type VIII) and sheared (trans S/H, Saenger type XI) forms. The sequence context around the hetero-purine pair determines which one is favored over the other (Villescas-Diaz and Zacharias 2003; Yildirim and Turner 2005), The presence of either a sheared A/G (i.e., trans H/S) or G/A (i.e., trans S/H) pair would hinder the fidelity check by the A1492 residue of the rRNA which senses the sugar edge of the second codon-anticodon pair. In the sheared base-pair state, the O2′ atom of the second mRNA nucleotide is not positioned properly to make a ribose zipper with A1492. Hence, a hetero-purine base pair at the second position cannot be in the sheared state. With regard to the imino state, the cis W/W conformation offers a larger C1′-C1′ distance (∼12.5 Å compared to ∼10.3 Å for canonical Watson-Crick), but its distortion on the RNA helix is minimal. For example, sugar puckers of tandem G/A mismatches in protein database (PDB) file 1MIS (Wu and Turner 1996) are in C3′-endo anti conformation which is the same as in a canonical double-helix. A1492 may coax the imino state of the G/A or A/G base pair via an induced fit mechanism (Williamson 2000), which would then allow for a proper minor-groove base-pair recognition by A1492 of the rRNA. Inosine at position 34 in several tRNAs decodes the third codon position; since it also decodes adenosine, an adenosine-inosine mismatch should also be tolerated at the third codon position (Murphy and Ramakrishnan 2004; Murphy et al. 2004). In these studies, it has been found that the A/I mismatch is in cis W/W with both sugars in the anti conformation.
Taken altogether, these simple rules readily eliminate most of the tRNA species from considerations for decoding Ψ-modified nonsense codons (Table 2). These results, however, do not yet explain how the mRNA-tRNA mini-helix containing various mismatched base pairs is able to satisfy the ribosome A-site test. We will provide detailed explanations of this in the following sections.
TABLE 2.
Detailed explanations of the simple rules used to narrow down tRNAs for decoding Ψ-modified nonsense codons
RNA molecular modeling using MC-Sym
Although molecular dynamics (MD) simulations are routinely performed to gain insights in chemical and biological processes, in the case of RNA, however, current force fields have been shown to produce unstable trajectories or macroscopic expected values not in line with experimental results (Yildirim et al. 2009; Gong and Xiao 2010). We, therefore, choose to apply the RNA molecular modeling package, MC-Sym (Major et al. 1991; Major 2003) to generate models at the atomic level, as was done for other systems (Major et al. 1993; Lemieux et al. 1998; Wang et al. 2011). MC-Sym is not based on energetic considerations of base-pairing interactions, is capable of addressing specific base-pair types during modeling, and allows for systematic and exhaustive exploration of hypotheses on base-pair types.
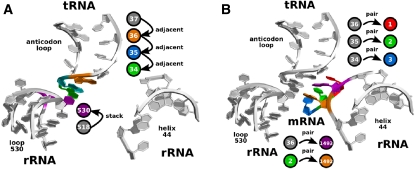
We applied MC-Sym to test and provide various mRNA-tRNA mini-helices in the context of the ribosome's helix 44 and loop 530 (Fig. 3). In our case, MC-Sym is used to sample the conformational space of the nucleotides comprising the mRNA-tRNA mini-helix and the rRNA residues directly involved in probing this mini-helix, which correspond to A1493 and A1492 from helix 44 and G530 of loop 530. Subsequently, energy minimization is applied to the MC-Sym's decoy sets, and the energy profile of each decoy set is compared to reveal which tRNA species are most likely to decode a specific mRNA codon. The energy profile derived from MC-Sym is the energy curve of the models within the given decoy set. A more negative energy profile based on our structural hypothesis of the codon-anticodon mini-helix for a given tRNA species increases the probability for using that tRNA for decoding. At this time, our energy calculations can only rank each tRNA species under consideration for their likelihood to decode Ψ-modified codons.
FIGURE 3.
Modeling steps to rebuild the A-site. (A) First, nucleotide 530 (purple) of loop 530 in the rRNA and nucleotides 34 (green), 35 (marine), and 36 (orange) of the anticodon loop are sampled using the MC-Sym computer program. An adjacency relationship (adjacent) is used to position nucleotide 36 with respect to 37 and so on down to nucleotide 34 in the tRNA. A stacking relationship (stack) positions 530 with respect to 518. (B) Second, nucleotides of the messenger RNA (1, red; 2, green; 3, marine) and the two adenosines 1492 (orange) and 1493 (purple) in the rRNA are sampled in the context of a given conformation chosen from step 1. Here, nucleotides in the mRNA are positioned using a pairing relationship (pair) with respect to their paired partner in the tRNA (anticodon 36 positions mRNA 1, 35 positions 2, and 34 positions 3). A1493 of rRNA pairs with 36 of tRNA, while A1492 of rRNA pairs with mRNA residue 2.
Stability measurements are typically reported in terms of ΔΔG. Although the computation of ΔΔG values in silico can be done via the use of thermodynamics cycles, this is computationally very expensive (Yildirim et al. 2009). Here, we report stabilities of the ribosome decoding center as internal energy or ΔH. ΔH is concerned with estimating the energy release upon hydrogen bond formation or other binding forces as the atomic groups are brought together from infinity, in contrast to ΔΔG which is concerned with the energy difference of hydrogen bonds when molecules associate compared to when these molecules are isolated in water. Although our computation does not take entropy into account, it still gives valuable information on the ability of different tRNAs for decoding particular codons. Entropic factors may be estimated, but force field issues have not yet been adequately resolved for molecular dynamics of nucleic acids, so calculations of ΔS is currently out of the scope of this work.
Because no structures of eukaryotic 80S featuring a tRNA bound to an mRNA codon in an A-site test configuration have been reported, 70S structure (1IBM) from Thermus thermophilus is used as the template for modeling, although the Ψ-modified codon experiments were performed in yeast. As such, it might be argued that a prokaryotic template is inappropriate to model translational decoding results obtained from yeast. Indeed, the eukaryotic 80S ribosome is more complex and bound by extra proteins compared to the prokaryotic 70S ribosome; however, the A-site decoding center features universally conserved and essential nucleotides: A1492, A1493, and G530 (Ben-Shem et al. 2010; Rabl et al. 2011). There are structural differences between the prokaryotic and eukaryotic ribosome, e.g., in the internal loop that anchors nucleotides A1492 and A1493. Because we model the “on,” or activated state of this internal loop, the structural template of a prokaryotic ribosome should be comparable to a eukaryotic ribosome, as both prokaryotic and eukaryotic activated A-site states should essentially be the same (Kondo and Westhof 2008).
Although ribosome P-site proofreading has also been reported recently (Zaher and Green 2009), we did not construct models for the P-site since there are no known direct readouts of the mRNA-tRNA mini-helix by any rRNA or ribosomal proteins in the P-site. We also did not take the role of A1913 of the 23S rRNA into consideration in the decoding of the third codon position (Ortiz-Meoz and Green 2011). A1913 is located on top of the first codon base and is perpendicular to the first codon base pair; it does not make a direct contact with the third codon position. A1913 may serve as a ratchet lock to help prevent slippage of the mRNA-tRNA mini-helix toward the P-site. All of our models end up with planar base pairs which should not interfere with A1913.
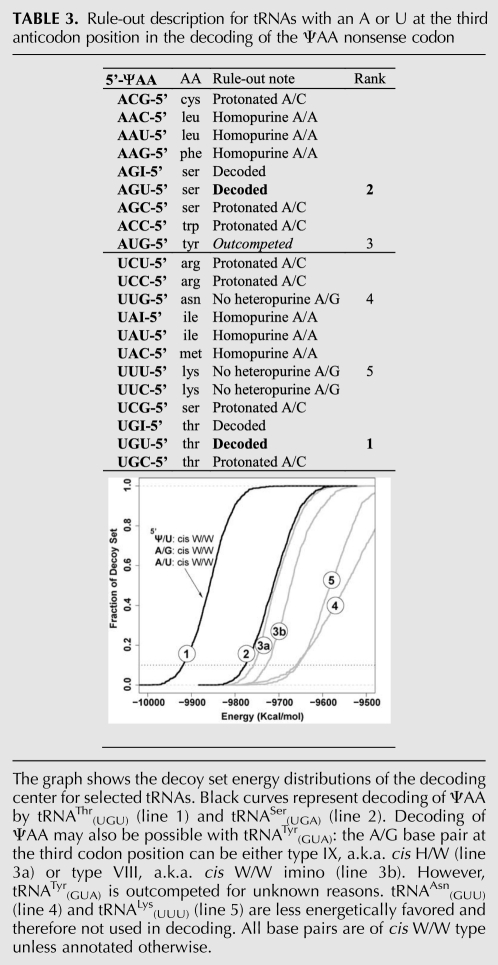
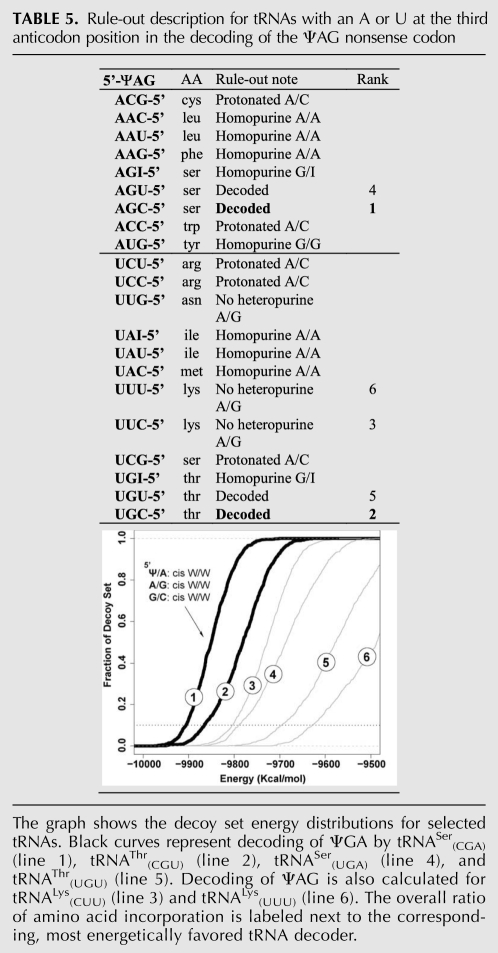
Instead of going over every codon-anticodon base-pairing in detail, we describe a few case studies to illustrate the considerations that went into our modeling. Complete rule-out descriptions for ΨAA are presented in Table 3, for ΨGA in Table 4, and for ΨAG in Table 5.
TABLE 3.
Rule-out description for tRNAs with an A or U at the third anticodon position in the decoding of the ΨAA nonsense codon
TABLE 4.
Rule-out description for tRNAs with an A or U at the third anticodon position in the decoding of the ΨGA nonsense codon
TABLE 5.
Rule-out description for tRNAs with an A or U at the third anticodon position in the decoding of the ΨAG nonsense codon
Case study I: Decoding ΨAA with tRNAThr(UGU)
The U/U cis W/W base pair is viable in the mRNA-tRNA mini-helix at the first codon position pairing with the third anticodon nucleotide of tRNAThr(UGU). However, the minor-groove width of such a U/U pair has to be properly maintained so that A1493, which interacts with both nucleotides of this base pair, can correctly recognize it. We reason that in order to widen the minor groove of the U/U base pair, two prerequisites need to be met simultaneously: water molecule(s) at the Watson-Crick edge and a hetero-purine mismatch at the second codon position pairing with the middle nucleotide of the tRNA anticodon. Hence, tRNAs bearing a uridine at position 36 can be ruled out except when there is a hetero-purine mismatch at the second codon position.
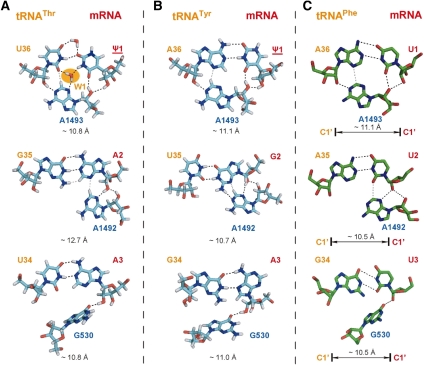
We propose that the only tRNA capable of decoding ΨAA for threonine is tRNAThr(UGU). In this case, the codon-anticodon base pairs contain a Ψ/U pair at the first position and an A/G imino pair at the second position. To provide an explanation of the decoding mode at the molecular level, extensive modeling was performed with MC-Sym, and the output models were subjected to energy minimizations (see Materials and Methods for details). One best solution from the ensemble is shown in Figure 4A. At the first position, the cis W/W type Ψ/U base pair consists of one direct hydrogen bond between the O2 atom of Ψ1 from mRNA and the N3 of U36 from tRNA and two water-mediated interactions bridging Ψ1-O2/U36-O4 and Ψ1-N3/U36-O2 (Fig. 4A, top). The physical presence of the water molecule W1 also widens the minor groove, in addition to forming a hydrogen bond to the N3 of A1493 to enable recognition. This type of U/U base pair, bridged by a water molecule, can be found in helix 44 of the ribosome in close proximity to the A1492-A1493 decoding center (Vicens and Westhof 2003). This U/U base pair with the bridging water is isosteric to canonical Watson-Crick base pairs and preserves the C1′-C1′ distance in a standard A-form helix. At the second position, an A/G imino pair is present, and this hetero purine-purine pair widens the minor groove (Fig. 4A, middle). Although the hydrogen bond to G530 is absent, the A1492 nucleoside which interacts only with the codon nucleotide from mRNA and not with the anticodon nucleotide from the tRNA can still make the ribose zipper. In terms of other base-pairing modes, a cis H/W conformation is not supported, as the H8 atom of A2 would clash with A1492-H2 in the A-minor interaction. MC-Sym is also able to build a trans H/S A/G pair, but the energy of this conformation is unfavorable. At the third position, a canonical Watson-Crick A/U pair is accommodated as anticipated.
FIGURE 4.
Roles of the A-minor motifs in the decoding recognition. (A) tRNA-mRNA base pairs between tRNAThr(UGU) and ΨAA. (B) tRNA-mRNA base pairs between tRNATyr(GUA) and ΨGA. (C) tRNA-mRNA base pairs between tRNAPhe(GAA) and UUU taken from the crystal structure of 1IBM is also shown for comparison. Distances between C1′ atoms of all base pairs are measured and annotated. Black dotted lines represent hydrogen bonds, and gray lines represent weaker interactions. Carbon atoms are colored in green (for crystal structures) or cyan (modeled structures), nitrogen in blue, oxygen in red, and hydrogen in white.
The energy profile predicts a ranking of tRNAThr(UGU) > tRNASer(UGU) ≥ tRNATyr(GUA) >> tRNAAsn(GUU) ≈ tRNALys(UUU) (Table 3). The experimental results show that ΨAA is primarily decoded by Thr and Ser, in good agreement with our rankings. tRNAAsn(GUU) and tRNALys(UUU) feature a Ψ/U pair at the first position, yet lack an A/G hetero-purine pair at the second position. The resulting energetic curves are in good agreement with our hypothesis that a Ψ/U pair is viable only when it coexists with an A/G pair. tRNATyr(GUA) is the only tRNA that shows a comparable energy profile to tRNASer(UGU), but tRNATyr(GUA) is not used in decoding. We do not know at this time why tRNATyr(GUA) is outcompeted at the A-site.
Case study II: Decoding ΨGA with tRNATyr(GUA)
According to the considerations in Table 4, tyrosine can be incorporated following the decoding of ΨGA by a GUA anticodon. This means that a consecutive G/U base pair at the first and second position and an A/G pair at the third position are tolerated. Surveys of the PDB indicate that G/U pairs are mainly found in the wobble form (cis W/W, Saenger type XXVIII), although there exist other hydrogen bonding schemes that occur much less frequently than the cis W/W type. Surveys of the PDB also shows that the A/G base pair at the third position can be in either one of four types: cis W/W (“imino,” Saenger type VIII), cis W/H (Saenger type IX), trans W/S (Saenger type X), or trans S/H (“sheared,” Saenger type XI). In all, a total of eight structural hypotheses were tested with MC-Sym (two G/U types times four A/G types). The wobble G/U pair is found to be favored over all other G/U pair types at the second position (Fig. 4B, middle). Regarding the third base pair, a trans H/S type A/G pair is ruled out since it would prevent G530 from interacting with the ribose of the third codon nucleotide. The cis H/W type A/G pair seems to be energetically preferred (Table 4): The adenosine is in syn conformation with respect to its sugar and forms two direct hydrogen bonds with the opposing guanosine (Fig. 4B, bottom). The minor groove width is ∼11.1 Å, close to 10.3 Å for a canonical base pair. The cis W/W conformation cannot be ruled out purely based on modeling, but its C1′-C1′ distance is wider than cis H/W, and it is less energetically favored (Table 4). Lastly, tRNALeu(UAA), tRNAIle(IAU), and tRNAIle(UAU), which cannot be ruled out by simple principles of base-pairing mode, seem to be energetically outcompeted. tRNACys(GCA) cannot be ruled out with energetic considerations; yet, a cysteine residue was not observed experimentally in the decoded ΨGA codon (Karijolich and Yu 2011). We will provide an explanation as to why Cys is not decoded in the “Role of Ψ in decoding” section below.
Case study III: Decoding of ΨAG
ΨAG is selectively decoded as serine and threonine, which is similar to ΨAA, yet differs in the observed ratio of amino acid incorporation. Application of the rule-out considerations in Tables 1 and 2 effectively narrows down the tRNA candidates to tRNASer(UGA), tRNASer(CGA), tRNAThr(UGU), and tRNAThr(CGU). To be extra cautious, tRNALys(CUU) and tRNALys(UUU) are also used in the decoy set energy calculation in addition to these four tRNAs (Table 5). tRNASer(CGA) (line 1) and tRNAThr(CGU) (line 2) are energetically preferred, which agrees well with the experimental results. Since the base-pairing modes observed for these two tRNAs are very similar to those shown in Figure 4 (cis W/W type Ψ/U and Ψ/A [Fig. 4A], A/G imino pair [Fig. 4B], and both tRNAs form a regular Watson-Crick G/C pair at the third position of the codon-anticodon pair), they are not illustrated in detail to avoid redundancy.
Role of Ψ in decoding
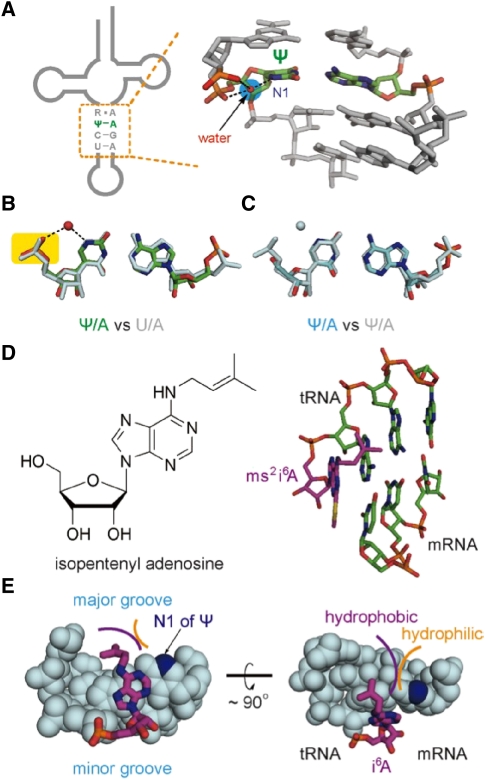
At the first position of the mini-helix between ΨGA and tRNATyr(GUA), as well as ΨAA/ΨAG with tRNASer, Ψ is in the anti conformation and forms a Watson-Crick base pair with the anticodon A36 in the tRNA. The N1 atom of Ψ is protonated at physiological pH and resides in the major groove; the N1H is frequently found to be involved in hydrogen bonding interactions with phosphate group(s) of the Ψ residue or a nearby nucleotide (Arnez and Steitz 1994; Yarian et al. 1999). For example, a Ψ/A pair is embedded within the anticodon stem of certain tRNA species (Fig. 5A; Delagoutte et al. 2000). In this helical context, previous studies have shown that a Ψ27/A43 pair is of higher stability compared to a U/A pair and should be considered as an alternative base pair for CG (Price and Gray 1998). We used the conformation of the Ψ27/A43 pair from PDB file 1F7U (Delagoutte et al. 2000) and superimposed it on the first U/A pair of the A-site codon-anticodon mini-helix from PDB file 2HGP (Yusupova et al. 2006). Because the 5′ phosphate groups of Ψ27 and U overlay very well with each other (Fig. 5B), during the decoding of ΨGA by tRNATyr(GUA), a similar water molecule is also expected to bridge the N1 atom of Ψ to its 5′ phosphate and to increase the rigidity of the base pair (Fig. 5C). The kink of the mRNA backbone between the A- and P-site is known to contribute to the discrimination of the cognate versus noncognate mRNA-tRNA mini-helix (Sanbonmatsu and Joseph 2003). Therefore, the greater rigidity of the Ψ/A pair, conferred primarily by the water molecule, may help the formation of mismatched base pairs in the second and third position of the mini-helix that eventually help the mini-helix to pass the selectivity test via the mRNA kink. Similarly, the water molecule retained in the major groove by Ψ in a Ψ/U pair (e.g., ΨAA pairing with tRNAThr(UGU)) can make favorable contributions to the formation of the mini-helix. Finally, from a kinetic point of view, increased stiffness of the phosphate backbone connecting the codon in the A-site to that in the P-site may help slow down the rate of mRNA translocation (Rodnina and Wintermeyer 2001), thereby providing extra time for the mismatched base pairs in the A-site to form.
FIGURE 5.
Proposed roles of Ψ in decoding. (A) In the high resolution structure of 1F7U, a water molecule bridges the N1 atom of Ψ and two backbone phosphate groups. (B) Overlay of Ψ27/A43 (1F7U) with U/A, first pair in the A-site of 2HGP. (C) Modeled Ψ/A pair from the mini-helix formed between ΨGA and tRNATyr(GUA) superimposed on Ψ27/A43 (1F7U). (D) Chemical structure of i6A37 on the left and a molecular view made from 3I8G showing the position of i6 group on the right. (E) Space-filled illustrations of the A-site tRNA-mRNA mini-helix show that the accommodation of the hydrophobic i6 modification is less favorable for Ψ in the first codon position compared to uridine. Color coding is the same as that used in Figure 1, except that carbon atoms in A37 are colored in magenta.
Nucleotide modifications in the tRNA anticodon loop can be another factor that influences decoding of Ψ-modified nonsense codons. For instance, hypermodified A37 and U34 are known to increase not only the stability of the mRNA-tRNA mini-helix but also the decoding capacity of tRNA (Bjork et al. 1987; Agris et al. 1997; Murphy et al. 2004; Weixlbaumer et al. 2007; Jenner et al. 2010). In yeast, both threonylcarbamoyl (t6A) and isopentenyl (i6A) groups are known modifications to the N6 amino group of A37: the former plays a role in tRNAs such as tRNAThr decoding ANN codons, while i6A37 is often present in tRNAs that read UNN codons such as tRNASer, tRNACys, and tRNATyr (Hall 1970; Nishimura 1972; Persson et al. 1994; Agris et al. 2007). Since ΨRR codons are the subjects of this study, we focused primarily on the isopentenyl modification. The lack of i6A37 modification is known to reduce the activity of the serine-inserting and tyrosine-inserting UGA suppression, and the i6 chemical group is thought to affect codon-anticodon interaction (Bjork et al. 1987). A recent ribosome crystal structure (Jenner et al. 2010) shows that the isopentenyl group of A37 in the A-site tRNA resides in the major groove of the mRNA-tRNA mini-helix right on top of the first codon-anticodon pair and also projects toward the 5′ side of the anticodon (Fig. 5D). When Ψ is present at the first codon position, its N1H group has the enhanced ability (compared to C5H group of uridine) to retain water molecule(s) in the major groove. As a result, a solvation shell around the i6 modification composed of these low-mobility water molecules can form (Fig. 5E). Because the isopentenyl group is hydrophobic, this solvation shell likely disfavors i6A37 containing tRNAs when decoding Ψ-modified nonsense codons.
Two of the top-three-ranked tRNAs under consideration for decoding ΨGA, tRNACys(GCA), and tRNATyr(GUA) contain i6A37 modification in yeast (Table 5; Sprinzl and Vassilenko 2005). The presence of i6A37 should decrease the ranking of both tRNAs. Unfortunately, we are unable to calculate the quantitative contributions of the i6A37 modification to the energy of the decoding center. For tRNACys(GCA), this modification-dependent decrease may become large enough to drop its ranking to below tRNAPhe(GAA), which would explain why Phe is used for decoding ΨGA instead of Cys.
Reprogramming the genetic code using Ψ in sense codons
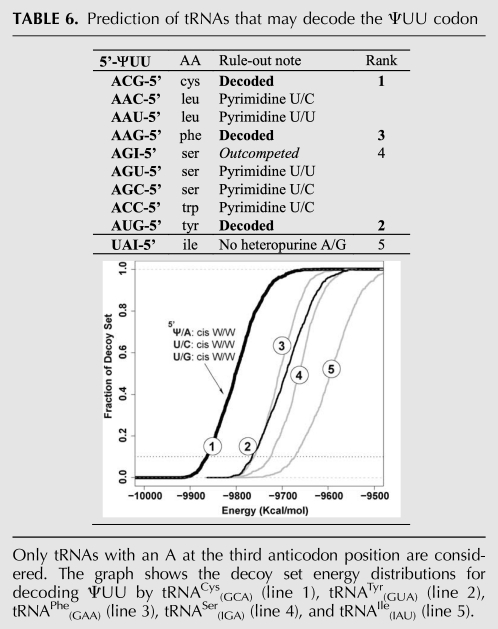
To our knowledge, no Ψ has been found in mRNA so far. Given the recent discoveries that certain Ψ modifications in U2 spliceosomal RNA can be induced under stress conditions (Wu et al. 2011), it is possible that Ψ may also be present in mRNAs under certain physiological conditions. If true, mRNA modification with Ψ in sense codons may reprogram the sense codon, allowing it to be read as amino acids distinct from the genetic code. We, therefore, performed modeling to predict how Ψ-modified sense codons might be decoded, starting with ΨUU derived from the UUU codon coding for phenylalanine (Table 6; Fig. 6).
TABLE 6.
Prediction of tRNAs that may decode the ΨUU codon
FIGURE 6.
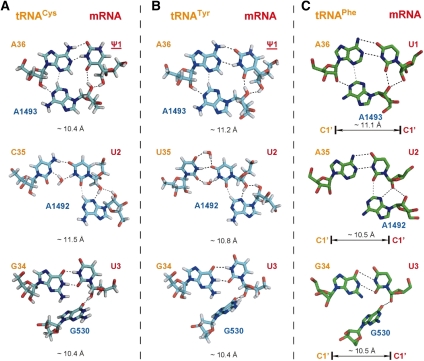
Predicting decoding of the ΨUU codon by tRNACys(GCA) and tRNATyr(GUA). (A) tRNA-mRNA base pairs between tRNACys(GCA) and ΨUU. (B) tRNA-mRNA base pairs between tRNATyr(GUA) and ΨUU. (C) tRNA-mRNA base pairs between tRNAPhe(GAA) and UUU taken from the crystal structure of 1IBM is also shown for comparison. The same color coding as from Figure 4 is used.
Based on the principles described above for selective decoding of Ψ-modified nonsense codons, tRNAs with Leu, Ser, and Trp anticodons can readily be eliminated. The remaining candidates were subjected to decoy set energy distribution analysis. The two highest ranked candidates are tRNACys(GCA) and tRNATyr(GUA) (Fig. 6). Interestingly, in the model for mRNA-tRNA mini-helix formed between ΨUU and tRNACys(GCA) or tRNATyr(GUA), a cis W/W type U/C or U/U pair is observed for the second codon-anticodon pair, and a water molecule is also found to reside in proximity to the minor groove (Fig. 6A,B, middle), similar to the situation of the Ψ/U pair for decoding ΨAA/ΨAG. It is possible that such water molecules can always help the mini-helix to survive the ribosomal A-site test through widening the minor groove of the pyrimidine-pyrimidine base pair. Our prediction of ΨUU decoding is consistent with the experimental results (Y-T Yu, pers. comm.).
CONCLUSIONS
Nonsense codon suppression is a biologically relevant event and provides unique angles to study the mechanism of translational termination (Atkins and Gesteland 2010). Yu et al. demonstrated recently that pseudouridylation of nonsense codons stimulates read-through of the Ψ-modified nonsense codons, and the modified codons are selectively decoded by just two amino acids each (Karijolich and Yu 2011). In this study, we provide a molecular rationale for the Ψ-mediated suppression of nonsense codons and how they are decoded as specific amino acids. The presence of Ψ weakens the recognition of the modified nonsense codons by release factors, allowing for the binding of competing tRNA molecules. Subsequent decoding events are not governed by the cellular abundance of tRNA, but rather by the stability of certain codon-anticodon pairs and the sterics of certain base pairs that pass the ribosome A-site test. In the minor groove, A1492, A1492, and G530 residues of the small subunit rRNA measure the width and geometry of the A-site mini-helix; we illustrate two cases in detail how mismatch-containing mini-helices can pass this A-site test. In the major groove, Ψ plays positive roles in the formation of the codon-anticodon mini-helices by stabilizing codon-anticodon base pairs and negative roles for tRNAs with hydrophobic modifications immediately 3′ to the anticodon base that pairs with Ψ.
We also extend the decoding principles derived from Ψ-modified nonsense codons to predict the decoding capacity of potential Ψ-modified sense codons, as exemplified by ΨUU. Of course, many more possible scenarios of Ψ-modified sense codons remain since uridine is present in 34 among the 61 sense codons (13 in the first codon position). Ψ in the second or third position of a codon may have different effects on decoding property compared to Ψ in the first codon position; and different rules are likely needed to understand these decoding events. Experiments designed to determine the specificity of amino acid incorporation for Ψ-modified sense codons will be highly desirable to further derive and improve the rules of decoding. The combination of computational and experimental analysis of Ψ-modified codons should be very useful in elucidating genetic code reprogramming by mRNA modifications.
MATERIALS AND METHODS
Molecular modeling
All molecular modeling attempts start with PDB file 1IBM (Ogle et al. 2001). There are more recent 70S structures, but their A-site superimposes perfectly with that of 1IBM (e.g., Selmer et al. 2006). From this template, we extracted nucleotides 30–40 from chain “Y” for the tRNA anticodon loop, nucleotides 1406–1410 and 1490–1495 of chain “A” for Helix 44, and nucleotides 516–521 and 528–533 of chain “A” for Loop 530. Nucleotides at the edges of these various stems and loops are kept fixed in 3D space, thus providing a structural context to build the decoding event of Ψ-containing nonsense codons in the ribosomal A-site.
We used the computer program MC-Sym to build the atomic-precision, 3D models of the ribosome's decoding center (Major et al. 1991; Major 2003; Parisien and Major 2008). In a typical modeling trial, a partial structure is taken as the input by MC-Sym, and nucleotides are then added to this partial structure. The addition of nucleotides is done based on relations (stacking, pairing, etc.) between nucleotides. For instance, it can load the ribosome's decoding center, and add nucleotide 530 in a stacking fashion on top of nucleotide 518 (Fig. 3A). The stacking conformations are those compiled from existing ones from solved structures in the PDB (Berman et al. 2000). MC-Sym is a constraint satisfaction problem (CSP) solver, which means that any output solution structure satisfies all the structural constraints specified in the input script. Structural constraints are explicit distance constraints or steric clashes between heavy atoms, as well as the relations between nucleotides given in the input script. The use of MC-Sym allows for explicitly controlling the types of a base pair through either the Saenger (Saenger 1984) or the Leontis-Westhof nomenclature (Leontis and Westhof 2001) and variants (Major and Lemieux 2002), hence, various structural hypothesis can be tested. The modeling of the decoding center has been broken down into two steps in order to take advantage of the divide-and-conquer principle of MC-Sym's backtracking algorithm (Major 2007; Ali et al. 2010; Wang et al. 2011), in which smaller fragments are first generated and then used as input to subsequent modeling runs.
In the first step, nucleotides 34–36 of the tRNA's anticodon loop are sampled along with nucleotide 530 of the ribosome's Loop 530 (Fig. 3A). In this step, two structural features are enforced with the use of distance constraints: the anticodon loop, Loop 530, ribose zipper and the Hodgson and Fuller's rule for the anticodon loop conformation (Fuller and Hodgson 1967). Distance constraints for the ribose zipper are (530:N3)-(35:O2′) and (530:O2′)-(35:O2′), between 2.5 and 3.0 Å. Distance constraints for the Hodgson and Fuller rule: (34:PSY)-(35:PSY)-(36:PSY)-(37:PSY), between 2.5 and 6.0 Å. The stacking of nucleotides in the anticodon loop can be controlled via a distance constraint between consecutive PSY pseudoatoms, which can be found at the center of each six-member ring of each nucleotide.
In the second step, models from the first step are used as input to MC-Sym upon which the mRNA nonsense codon and the two adenosines of Helix 44 are grafted (Fig. 3B). At this point, many structural hypotheses can be tested by explicitly specifying particular base-pair types between the tRNA anticodon loop and the nonsense codon mRNA. The stringent A-site test, performed by adenosines 1492 and 1493 of Helix 44 via A-minor interactions to the tRNA-mRNA mini-helix and by G530 of Loop 530 sensing the third codon position, imposes structural constraints that are enforced using distance constraints. All MC-Sym scripts are available upon request.
While modeling, we did not enforce the G530/A1492 pairing, as MC-Sym would sometimes not yield solutions, especially in the case of the A/G mismatch at the second codon position. A-minor interactions occur by themselves and do not require A1492 to pair with G530. Furthermore, G530 is locked in the anti position through hydrogen bonds with both the sugar rings of the second anticodon nucleotide of tRNA and the third nucleotide of the codon.
Energy minimization
All models have been energy-minimized using the Tinker molecular package version 5 (Ponder and Richards 1987) and the Amber-99 force-field (Kollman et al. 2000) in the gas-phase. The minimization algorithm is the memory-limited Broyden–Fletcher–Goldfarb–Shanno (L-BFGS) method. Convergence is attained when the root-mean-squared gradient value is <0.1 kcal/mol/Å. A sodium cation is appended at the apex of each phosphate group to neutralize the total charge of the complexes. During minimization, all heavy atoms of a nucleobase are kept to their original position using a spring constant of 1001 kcal/mol/Å, while all ions, protons, and backbone and sugar atoms are free to move.
The results of the modeling and minimization are decoy sets, each featuring a different structural hypothesis, i.e., base-pair types. By plotting the energy distributions of each decoy set, we can compare and rank them by energy preferences. Thus, the decoy set that features 3D models with the best energies is believed to be preferably decoded over a decoy set whose models are less favored energetically. In addition, the whole energy curves, instead of only the best energy models, are compared since the curves may convey more robust energy information than the single best energy model alone (which could happen to fall in a deep energy well but does not represent appropriately the “ensemble”). Because the energy function is not perfect (functional form, parameters, etc.), entropy is discarded, and the energy computations have been performed in the context of an implicit solvation model, we do not claim that the energy curves correlate with the efficiency of decoding, but they should, at least, provide a qualitative ranking of the various competing tRNA species.
The use of an implicit solvation model is motivated by the following observations: first, implicit solvation models use solvent accessible surface area (SASA) to determine the average contribution of water on specific atomic groups. Here, the presence of a few explicit water molecules will decrease the SASA for the atomic groups in direct contact with these explicit water molecules, therefore preventing a double-count of water contribution. Second, soaking the modeled system in explicit water will increase the number of atoms significantly, hence offsetting the energetic differences upon mRNA:tRNA nucleotide mutations. Although the use of an explicit water model could lead to a better modeling of the dynamics of the mRNA:tRNA:rRNA interactions, it is beyond the scope of this paper.
Depicted models are those that boast a specified network of hydrogen bonds, more specifically the ribose zippers for nucleotides 530, 1492, and 1493, and the proper hydrogen bonds between the mRNA and the tRNA. Hydrogen bond strengths are computed as suggested (Boobbyer et al. 1989), which takes into account the directionality of hydrogen bonds.
Molecular graphics figures were prepared with PyMOL.
ACKNOWLEDGMENTS
This work was supported by a grant from NIH (GM088599 to T.P.) and by the Chicago Fellows program (to M.P.). We thank Drs. Yi-tao Yu and Timothy Nilsen for stimulating discussions.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.031351.111.
REFERENCES
- Agris PF, Guenther R, Ingram PC, Basti MM, Stuart JW, Sochacka E, Malkiewicz A 1997. Unconventional structure of tRNALysSUU anticodon explains tRNA's role in bacterial and mammalian ribosomal frameshifting and primer selection by HIV-1. RNA 3: 420–428 [PMC free article] [PubMed] [Google Scholar]
- Agris PF, Vendeix FA, Graham WD 2007. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 366: 1–13 [DOI] [PubMed] [Google Scholar]
- Ali M, Lipfert J, Seifert S, Herschlag D, Doniach S 2010. The ligand-free state of the TPP riboswitch: A partially folded RNA structure. J Mol Biol 396: 153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnez JG, Steitz TA 1994. Crystal structure of unmodified tRNAGln complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry 33: 7560–7567 [DOI] [PubMed] [Google Scholar]
- Atkins JF, Gesteland RF, ed. 2010. Recoding: Expansion of decoding rules enriches gene expression. In Nucleic acids and molecular biology, Vol. 24. Springer Science+Business Media, New York [Google Scholar]
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M 2010. Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE 2000. The protein data bank. Nucleic Acids Res 28: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork GR, Ericson JU, Gustafsson CE, Hagervall TG, Jonsson YH, Wikstrom PM 1987. Transfer RNA modification. Annu Rev Biochem 56: 263–287 [DOI] [PubMed] [Google Scholar]
- Boobbyer DNA, Goodford PJ, Mcwhinnie PM, Wade RC 1989. New hydrogen-bond potentials for use in determining energetically favorable binding-sites on molecules of known structure. J Med Chem 32: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Cheng Z, Saito K, Pisarev AV, Wada M, Pisareva VP, Pestova TV, Gajda M, Round A, Kong C, Lim M, et al. 2009. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev 23: 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delagoutte B, Moras D, Cavarelli J 2000. tRNA aminoacylation by arginyl-tRNA synthetase: Induced conformations during substrates binding. EMBO J 19: 5599–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KA, Goodenbour JM, Pan T 2006. Tissue-specific differences in human transfer RNA expression. PLoS Genet 2: e221 doi: 10.1371/journal.pgen.0020221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller W, Hodgson A 1967. Conformation of the anticodon loop in tRNA. Nature 215: 817–821 [DOI] [PubMed] [Google Scholar]
- Gong Z, Xiao Y 2010. RNA stability under different combinations of amber force fields and solvation models. J Biomol Struct Dyn 28: 431–441 [DOI] [PubMed] [Google Scholar]
- Hall RH 1970. N6-(delta 2-isopentenyl)adenosine: Chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol 10: 57–86 [DOI] [PubMed] [Google Scholar]
- Hamma T, Ferre-D'Amare AR 2006. Pseudouridine synthases. Chem Biol 13: 1125–1135 [DOI] [PubMed] [Google Scholar]
- Jenner LB, Demeshkina N, Yusupova G, Yusupov M 2010. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat Struct Mol Biol 17: 555–560 [DOI] [PubMed] [Google Scholar]
- Karijolich J, Yu YT 2011. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474: 395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman PA, Wang JM, Cieplak P 2000. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem 21: 1049–1074 [Google Scholar]
- Kondo J, Westhof E 2008. The bacterial and mitochondrial ribosomal A-site molecular switches possess different conformational substates. Nucleic Acids Res 36: 2654–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostelev A, Asahara H, Lancaster L, Laurberg M, Hirschi A, Zhu J, Trakhanov S, Scott WG, Noller HF 2008. Crystal structure of a translation termination complex formed with release factor RF2. Proc Natl Acad Sci 105: 19684–19689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostelev A, Zhu J, Asahara H, Noller HF 2010. Recognition of the amber UAG stop codon by release factor RF1. EMBO J 29: 2577–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurberg M, Asahara H, Korostelev A, Zhu J, Trakhanov S, Noller HF 2008. Structural basis for translation termination on the 70S ribosome. Nature 454: 852–857 [DOI] [PubMed] [Google Scholar]
- Lemieux S, Chartrand P, Cedergren R, Major F 1998. Modeling active RNA structures using the intersection of conformational space: Application to the lead-activated ribozyme. RNA 4: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis NB, Westhof E 2001. Geometric nomenclature and classification of RNA base pairs. RNA 7: 499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis NB, Stombaugh J, Westhof E 2002. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res 30: 3497–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescoute A, Westhof E 2006. The A-minor motifs in the decoding recognition process. Biochimie 88: 993–999 [DOI] [PubMed] [Google Scholar]
- Major F 2003. Building three-dimensional ribonucleic acid structures. Comput Sci Eng 5: 44–53 [Google Scholar]
- Major FPT. 2007. RNA tertiary structure prediction. In Bioinformatics: From genomes to therapies (ed. T Lengauer), pp. 491–539. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- Major F, Lemieux S 2002. RNA canonical and non-canonical base pairing types: a recognition method and complete repertoire. Nucleic Acids Res 30: 4250–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major F, Turcotte M, Gautheret D, Lapalme G, Fillion E, Cedergren R 1991. The combination of symbolic and numerical computation for three-dimensional modeling of RNA. Science 253: 1255–1260 [DOI] [PubMed] [Google Scholar]
- Major F, Gautheret D, Cedergren R 1993. Reproducing the three-dimensional structure of a tRNA molecule from structural constraints. Proc Natl Acad Sci 90: 9408–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FV IV, Ramakrishnan V 2004. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11: 1251–1252 [DOI] [PubMed] [Google Scholar]
- Murphy FV IV, Ramakrishnan V, Malkiewicz A, Agris PF 2004. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Mol Biol 11: 1186–1191 [DOI] [PubMed] [Google Scholar]
- Nishimura S 1972. Minor components in transfer RNA: Their characterization, location, and function. Prog Nucleic Acid Res Mol Biol 12: 49–85 [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM Jr, Tarry MJ, Carter AP, Ramakrishnan V 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292: 897–902 [DOI] [PubMed] [Google Scholar]
- Ortiz-Meoz RF, Green R 2011. Helix 69 is key for uniformity during substrate selection on the ribosome. J Biol Chem 286: 25604–25610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisien M, Major F 2008. The MC-Fold and MC-Sym pipeline infers RNA structure from sequence data. Nature 452: 51–55 [DOI] [PubMed] [Google Scholar]
- Persson BC, Esberg B, Olafsson O, Bjork GR 1994. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie 76: 1152–1160 [DOI] [PubMed] [Google Scholar]
- Ponder JW, Richards FM 1987. An efficient Newton-like method for molecular mechanics energy minimization of large molecules. J Comput Chem 8: 1016–1024 [Google Scholar]
- Price D, Grey M 1998. Editing of tRNA. In Modification and editing of RNA (ed. H Grosjean, R Benne). ASM Press, Washington, DC [Google Scholar]
- Rabl J, Leibundgut M, Ataide SF, Haag A, Ban N 2011. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 331: 730–736 [DOI] [PubMed] [Google Scholar]
- Rodnina MV, Wintermeyer W 2001. Fidelity of aminoacyl-tRNA selection on the ribosome: Kinetic and structural mechanisms. Annu Rev Biochem 70: 415–435 [DOI] [PubMed] [Google Scholar]
- Saenger W. 1984. Principles of nucleic acid structure. Springer-Verlag, New York. [Google Scholar]
- Sanbonmatsu KY, Joseph S 2003. Understanding discrimination by the ribosome: Stability testing and groove measurement of codon-anticodon pairs. J Mol Biol 328: 33–47 [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH 2005. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310: 827–834 [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FV IV, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V 2006. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313: 1935–1942 [DOI] [PubMed] [Google Scholar]
- Siegfried NA, O'Hare B, Bevilacqua PC 2010. Driving forces for nucleic acid pKa shifting in an A+·C wobble: Effects of helix position, temperature, and ionic strength. Biochemistry 49: 3225–3236 [DOI] [PubMed] [Google Scholar]
- Song H, Mugnier P, Das AK, Webb HM, Evans DR, Tuite MF, Hemmings BA, Barford D 2000. The crystal structure of human eukaryotic release factor eRF1–mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 100: 311–321 [DOI] [PubMed] [Google Scholar]
- Sprinzl M, Vassilenko KS 2005. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 33: D139–D140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stombaugh J, Zirbel CL, Westhof E, Leontis NB 2009. Frequency and isostericity of RNA base pairs. Nucleic Acids Res 37: 2294–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund J, Ander M, Aqvist J 2010. Principles of stop-codon reading on the ribosome. Nature 465: 947–950 [DOI] [PubMed] [Google Scholar]
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y 2010. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141: 344–354 [DOI] [PubMed] [Google Scholar]
- Vicens Q, Westhof E 2003. Molecular recognition of aminoglycoside antibiotics by ribosomal RNA and resistance enzymes: An analysis of x-ray crystal structures. Biopolymers 70: 42–57 [DOI] [PubMed] [Google Scholar]
- Villescas-Diaz G, Zacharias M 2003. Sequence context dependence of tandem guanine:adenine mismatch conformations in RNA: A continuum solvent analysis. Biophys J 85: 416–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Parisien M, Scheets K, Miller WA 2011. The cap-binding translation initiation factor, eIF4E, binds a pseudoknot in a viral cap-independent translation element. Structure 19: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Murphy FV IV, Dziergowska A, Malkiewicz A, Vendeix FA, Agris PF, Ramakrishnan V 2007. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat Struct Mol Biol 14: 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Jin H, Neubauer C, Voorhees RM, Petry S, Kelley AC, Ramakrishnan V 2008. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 322: 953–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JR 2000. Induced fit in RNA-protein recognition. Nat Struct Biol 7: 834–837 [DOI] [PubMed] [Google Scholar]
- Wu M, Turner DH 1996. Solution structure of (rGCGGACGC)2 by two-dimensional NMR and the iterative relaxation matrix approach. Biochemistry 35: 9677–9689 [DOI] [PubMed] [Google Scholar]
- Wu G, Xiao M, Yang C, Yu YT 2011. U2 snRNA is inducibly pseudouridylated at novel sites by Pus7p and snR81 RNP. EMBO J 30: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarian CS, Basti MM, Cain RJ, Ansari G, Guenther RH, Sochacka E, Czerwinska G, Malkiewicz A, Agris PF 1999. Structural and functional roles of the N1- and N3-protons of Ψ at tRNA's position 39. Nucleic Acids Res 27: 3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim I, Turner DH 2005. RNA challenges for computational chemists. Biochemistry 44: 13225–13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim I, Stern HA, Sponer J, Spackova N, Turner DH 2009. Effects of restrained sampling space and nonplanar amino groups on free-energy predictions for RNA with imino and sheared tandem GA base pairs flanked by GC, CG, iGiC or iCiG base pairs. J Chem Theory Comput 5: 2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupova G, Jenner L, Rees B, Moras D, Yusupov M 2006. Structural basis for messenger RNA movement on the ribosome. Nature 444: 391–394 [DOI] [PubMed] [Google Scholar]
- Zaborske JM, Narasimhan J, Jiang L, Wek SA, Dittmar KA, Freimoser F, Pan T, Wek RC 2009. Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J Biol Chem 284: 25254–25267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher HS, Green R 2009. Quality control by the ribosome following peptide bond formation. Nature 457: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]