Abstract
It was of interest to determine if hemispheric differences in orbitofrontal cortex (OFC) volume would be related to behavioral inhibition observed in a peer-play setting. Magnetic resonance imaging (MRI) was carried out in 23 individuals (19 males and 4 females) at an average age of 14.87±1.14 years who were either at high or low risk for alcohol dependence. All subjects had previously been evaluated in a preschool peer play paradigm (5.03±0.78 years) assessing behavioral inhibition. Region of interest measures were traced for the OFC and the amygdala, and confirmed with voxel based morphometry. Behavioral inhibition, a behavioral tendency that often occurs in a novel setting in reaction to strangers, includes the following: greater time spent next to the mother, greater time staring at another child, and longer latency to begin play with another child. A significant relationship was seen between greater right OFC volume and indicators of behavioral inhibition including greater time spent proximal to their mother and greater time staring at the other child. Also, larger amygdala volume was associated with more time spent proximal to the mother. Behavioral control, including both over- and under-control, is likely to be subserved by neural circuitry associated with emotion regulation including the right OFC and the amygdala.
Keywords: Behavioral inhibition, Behavioral undercontrol, MRI, Limbic neurocircuitry
1. Introduction
Temperament is thought to be relatively stable across infancy, childhood, adolescence and adulthood within the same individual. Chess and Thomas (1977) in a landmark study found that infants with a tendency to withdraw from new situations and unfamiliar people were at risk for developing avoidant or overanxious disorders in childhood. Children described as shy or inhibited typically withdraw when confronted with novelty, stopping their ongoing behavior, ceasing vocalization, and clinging to a familiar figure. The Harvard Child/Infant study systematically evaluated children using laboratory based observations (Kagan et al., 1984, Reznick et al., 1986). A consistent pattern of approach or withdrawal was determined using familiar or unfamiliar toys or persons as stimuli. Among the signs of inhibition typically observed was a long latency to interact, a tendency to remain proximal to the parent in new situations, and a tendency to stare at the other child without speaking or entering into play. From this effort, it was determined that approximately 10–15% of US born Caucasian toddlers and young children have a temperamental disposition to be behaviorally inhibited. Most of these children remain inhibited in early adolescence (Schwartz et al., 1999) suggesting that this trait is only partially influenced by environmental conditions.
Using behavioral observations in a laboratory setting has revealed that a history of behavioral inhibition in infancy or childhood is associated with an increased tendency for anxiety disorders in adolescence (Schwartz et al., 1999; McDermott et al., 2009; Rosenbaum et al., 1988, Rosenbaum et al., 1991) and is associated with familial risk for alcohol dependence (Hill et al., 1999). With the greater tendency for individuals with familial risk for alcohol dependence to also exhibit impulsive behavior, it may be the case that both under- and over-control are prominent features of alcohol dependence susceptibility. Because some alcohol-dependent individuals exhibit preexisting high levels of anxiety (Koob, 2003; Pandey, 2003), the increased likelihood of having a behaviorally inhibited temperament among young high risk offspring who have not become alcohol dependent (Hill et al., 2008) is especially intriguing. Also, because regions of the amygdala have been shown to be regulatory in emotional processing and in levels of anxiety (Koob, 2003; Charney and Deutch, 1996), functional and structural alteration of the amygdala may be involved in behaviorally inhibited temperament.
Schwartz et al. (2003) found an increased BOLD response in the amygdala to novel versus familiar faces among young adults who had been characterized as behaviorally inhibited as children. Guyer et al (2006), using a monetary reward to assess BOLD response to an incentive, found an increased response in the striatum, amygdala, and nucleus accumbens in adolescents who had been judged to be behaviorally inhibited during infancy. These alterations in BOLD response suggest the possibility of underlying structural differences in the amygdala and associated limbic circuitry involved in modulation of emotion and reward processing in association with behaviorally inhibited temperament.
The amygdala and the orbitofrontal cortex (OFC) have been investigated for possible structural variation in those at higher genetic risk for developing alcohol dependence (Hill et al., 2001; Benegal et al., 2007; Hill et al., 2009a). Offspring at genetic risk for alcohol dependence show reduced volume of the right OFC relative to the left (Hill et al., 2009) with reduced white matter volume of the right associated with greater impulsivity. Greater behavioral influence of the right hemisphere in impulsive behaviors has previously been shown for the ventromedial prefrontal cortex (PFC), an area that includes the medial and lateral portions of the OFC (Bechara et al., 2002, Tranel et al., 2005). Severe deficits in social/emotional and decision making processes have been shown in rare neurological patients with unilateral right ventromedial PFC ablation (Tranel et al., 2005).
Characterizing the brain/behavioral relationship between right OFC volume and the behavioral tendency to impulsively approach or, alternatively, cautiously withdraw from new situations or individuals as a continuum would predict that greater volume of the right OFC might be associated with cautious restraint or behavioral inhibition. Accordingly, volumetric differences in the OFC may provide one source of the neurological underpinnings involved in the network of structures responsible for extreme variations in temperament that include behavioral inhibition at one extreme and impulsive behavior at the other.
An ongoing longitudinal study of offspring from families with multiple cases of alcohol dependence (AD) or from control families provided the resource for this study. With the availability of individuals who had participated in a laboratory-based observational assessment of behavioral inhibition who continued to be involved in a longitudinal follow-up study, it was possible to test our hypothesis using a sample of adolescents who had participated in the peer play observational study (Hill et al., 1999) as preschoolers. We predicted that variation in childhood temperament would be related to structural variation of the OFC and amygdala in adolescence.
2. Methods
2.1. Subjects and assessments
This protocol was approved by the University of Pittsburgh Institutional Review board. All participating parents signed informed consent forms for themselves at the time of clinical evaluation and again at the time of each follow-up evaluation for their minor children. Adolescent participants signed assent forms for the clinical interviews, personality assessment, and magnetic resonance imaging (MRI) procedure. A total of 36 children participated in a peer play evaluation modeled after procedures used in the Harvard Child/Infant study. A subset of these (N=23) were scanned as adolescents. All are members of a larger longitudinal cohort of offspring followed in a family study.
2.2. Description of the family study
The high- and low-risk (control) children/adolescents are participants in an ongoing family study in which offspring of parents with either high genetic loading for alcohol dependence (high risk) or those whose parents had minimal loading (low risk) are contrasted. The high-risk families had been identified through a proband pair of alcohol-dependent brothers. Selection through the parental generation was achieved by identifying one member of the proband pair while in a substance abuse treatment facility in the Pittsburgh area at the time of recruitment and determining that this individual had a sibling similarly affected with alcohol dependence. Because the high-risk offspring were from multiplex alcohol dependence families selected through the presence of a pair of adult alcohol-dependent brothers, each child had an average of four first and second degree relatives who were alcohol dependent. Low-risk offspring were selected on the basis of parental low density of alcohol dependence and minimal other Axis I psychopathology.
2.3. Inclusion and exclusion criteria for high-risk multiplex families
Identification of male proband pairs with AD and their families required the screening of approximately 100 families to meet the present goals, and for the broader goals of the family studies that included a search for developmental neurobiological markers (Hill et al., 2009b) and gene finding efforts (Hill et al., 2004).
The Diagnostic Interview Schedule (DIS) (Robins et al 1981) was administered to all available relatives (adult probands, their siblings and parents [>90% of first degree relatives]). Unavailable or deceased relatives were diagnosed using a minimum of two family-history reports. Targeted families were excluded if the proband or his or her first-degree relatives showed evidence of primary recurrent Major Depressive Disorder (MDD), Bipolar Disorder (BD), Primary Drug Dependence (PDD) (i.e., drug dependence preceded alcohol dependence by 1 or more years) or Schizophrenia by DSM-III criteria, the diagnostic system in place at the time the studies were initiated. Presence of Axis II disorders was not used as either an exclusionary or inclusionary condition. No attempt was made to limit the psychiatric disorders in “marrying in” spouses who represent the parents of the children/adolescents reported here. However, available spouses were diagnosed using the same methods (DIS) as members of the “target” families.
2.4. Selection of control families
Selection of control families was based on availability of a pair of male adult siblings. Volunteers were screened for Axis I psychopathology including alcohol and drug dependence using the DIS. Control families were selected if the volunteer's first-degree relatives (parents and siblings) were similarly free of psychopathology.
2.5. Child/adolescent/young adult assessment
Each child/adolescent and his/her parent were separately administered the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al., 1985) by trained, Masters' level, clinical interviewers and an advanced resident in child psychiatry at each annual evaluation. Using DSM-III criteria that have been used throughout the follow-up, K-SADS interviewers and the resident independently provided scores for each diagnosis. A best-estimate diagnosis based on these four blinded interviews was completed in the presence of a third clinician who facilitated discussion to resolve diagnostic disagreements if needed. Nine of the participants have been followed to their 19th birthday and have entered a young adult follow-up that includes administration of the Composite International Diagnostic Interview (CIDI) (Janca et al 1992).
2.6. Adolescent temperament measures
As part of the longitudinal follow-up, subjects were administered the Multidimensional Personality Questionnaire (MPQ) (Tellegen et al., 1988). The MPQ provides 11 personality scales and 3 higher order scales. Assessment was completed within 1 year of the scans.
2.7. Peer play procedure
The peer play study had utilized multiple pairings in which each child was paired with one other child whom he/she had never met in up to three separate sessions (Hill et al., 1999). Both children had mothers present within the test room who were asked to quietly observe. Observations were made during the 30-minute play session through a one-way mirrored window supplemented by cameras which provided additional views of the playroom though monitors in the observation room where coders were located. All coders met an interrater reliability criterion of r=0.90 with other coders. The sessions were scored for: (1) amount of time spent proximal to the parent (within the parent's reach); (2) the amount of time staring at the other child, neither speaking nor playing with the child at the time staring occurred. Also, latency to speak, latency to touch the playroom toys and the total amount of speech were also recorded (Hill et al., 1999).
2.8. Magnetic resonance imaging and image analysis
The MRI scans were performed at the University of Pittsburgh Medical Center Magnetic Resonance Research Center with a Signa 1.5-T system (GE Medical Systems, Milwaukee, Wisconsin). A sagittal scout series verified subject position, cooperation, and image quality. A three-dimensional, spoiled gradient-recalled acquisition in the steady state pulse sequence was used to obtain 124 contiguous images with slice thickness of 1.5 mm in the coronal plane (TE=5 ms, TR=25 ms, flip angle=40°, acquisition matrix=256×192, number of excitations=1, field of view=24 cm). Coronal sections were obtained perpendicular to the anterior/posterior commissure line to provide a more reproducible guide for image orientation. Axial proton density and T2 weighted images were obtained to enable exclusion of structural abnormalities on the MRI scan. All subjects tolerated the procedure well. No sedation was used.
Regions of interest (ROIs) were drawn using BRAINS2 (Magnotta et al., 2002), a software that enables trained raters to obtain reliable and valid volumetric measures for specific structures of interest. Automated segmentation of grey, white, and cerebrospinal fluid (CSF) volumes can also be obtained. Two raters (SW and HC) blind to risk group membership with inter-rater reliability >0.95 manually traced the volumes of the OFC and amygdala in the coronal plane after first aligning the T1, T2, and proton density (PD) images. A detailed description of the ROI methodology has been presented previously and is only briefly presented here (Hill et al., 2001; Hill et al., 2009). The most anterior slice in which the temporal stem is first visible marks the initial slice to be summed to calculate amygdala volume. The most anterior slice showing the mamillary bodies is used as the posterior amygdala boundary. This landmark method is commonly used in psychiatric studies of children and adolescents using MRI (Castellanos et al 1996). The boundaries and landmarks for the OFC, right, left, and total, were obtained using the guidelines established by Lacerda et al. (2003) and are depicted in Fig. 1. Intracranial volume (ICV) was measured, including cerebral hemispheres, brainstem, and the CSF surrounding these structures (Prasad et al., 2005). Intracranial volumes were calculated by summing areas of successive coronal slices, including grey and white matter and CSF volumes, and multiplying by slice thickness.
Fig. 1.
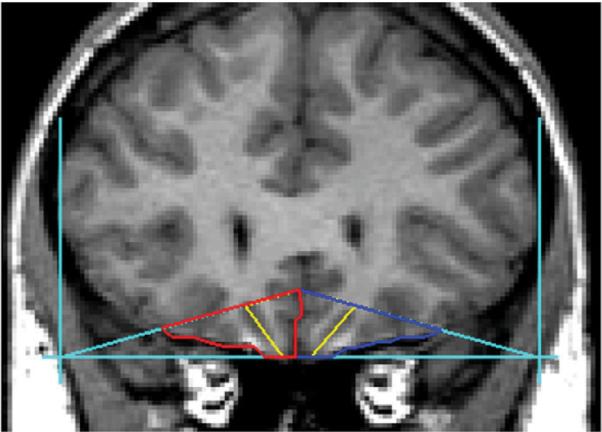
The boundaries and landmarks for the orbitofrontal cortex, right, left and total, followed the guidelines established by Lacerda et al (2003). Outlines for the left OFC are seen in blue, with the right OFC in red. The yellow line depicts the lateral and medial portions of the OFC in each hemisphere.
Our primary voxel based morphometry analysis focused on specific hypotheses concerning the OFC in the right hemisphere and the amygdala using the ROI methods outlined above. The ROI method, though labor-intensive, has long been the gold standard of choice because of its high reliability with experienced raters. However, we also conducted a whole brain voxel based analysis of the collected scans to determine if prominent differences would be seen in other regions of the brain. Also, to confirm our right hemisphere results for the OFC and total amygdala volume differences, we used masks corresponding to our ROIs from the Pick Atlas small volume correction tool (Maldjian et al., 2003). Each image was stripped of the skull using the FSL brain extraction tool (BET) to remove dura, skull, and scalp (Smith, 2002). The extracted brains were then normalized to ICBM space, tissue segmented, and smoothed with an 8-mm kernal using SPM 5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London UK). A voxel based morphometric analysis (Ashburner and Friston, 2000) was conducted using a two-sample t-test design with separate contrasts to assess between-group differences. Group placement was based on behavioral differences observed in the preschool behavioral inhbition paradigm.
2.9. Data analysis
Using a General Linear Model (GLM) (SPSS version 17; SPSS, Chicago, Illinois) approach, a series of mixed model analyses were performed with ICV as a covariate, and risk status and measures of behavioral inhibition as fixed factors for total OFC and right OFC (covarying left). Because previous results also suggested differences between high- and low-risk adolescents for right amygdala volume (Hill et al., 2001), total amygdala volume and amygdala volume in the right hemisphere were tested for a possible relationship to measures of behavioral inhibition. Mixed effects models were used to obtain unbiased estimates of brain volume by using random effects to control for the nonindependence of data collected from families with multiple siblings.
With support from these analyses showing differences by measures of behavioral inhibition, separate univariate analyses were planned for the OFC laterality ratio [calculated as (Right−Left)/(Right+Left)] to determine whether relationships would be seen with measures of behavioral inhibition. These ratios were of interest because previous analyses revealed laterality differences in the OFC (decreased right/left OFC ratios reflecting reductions in right OFC) in high-risk adolescents and young adults from the larger longitudinal study (Hill et al., 2009a).
The present report is based on two behavioral inhibition measures: the amount of time spent proximal to the parent, and the amount of time staring at the other child. Data were analyzed for the first session and an average measure across sessions. These variables were chosen for analysis because they had shown significant risk group differences in a previous investigation (Hill et al., 1999). To accommodate for a mixed effects model, behavioral measures were dichotomized using cut-offs that contrasted the upper tercile of scores with the lower two terciles across the three sessions. The average total time staring variable was split by those children staring at others greater than 11.33s and those staring less than 11.32s across the three sessions. The proximity to mother variable was dichotomized according to the amount of time each child spent next to the mother using 14.99s or less, contrasted with 15s or greater averaged across the three sessions. Behavioral measures were classified using the upper tercile versus the lower ones to identify those individuals who were most extreme on measures of behavioral inhibition.
3. Results
3.1. Cohort
At the time the peer play session was conducted, the children averaged 5.03±0.78 years. The subset of children who were scanned as adolescents (N=23) were studied at a mean age of 14.87±1.14 years. Included were 10 adolescents at high risk for alcohol dependence (9 males and 1 female) and 13 low-risk adolescents (10 males and 3 females). At the time of the last follow-up visit, participants were 18.09±0.85 years old. Using cases in either the upper tercile for stare or upper tercile for time spent proximal to the mother results in nine cases that may be considered the most behaviorally inhibited, versus those with neither behavior in the upper tercile (N=14). Of the nine cases who were in the upper tercile for either stare or proximity to the mother, seven were in the upper tercile for both variables. The breakdown by familial alcohol dependence risk status and behavioral inhibition is five high-risk and four low-risk subjects among the nine cases in the upper tercile, and five high-risk cases and nine low-risk cases in the lower tercile group.
Lifetime incidence of psychiatric disorders for the sample included 43.5% with no disorder, 13.0% with attention deficit hyperactivity disorder (ADHD), 8.7% with a childhood anxiety disorder (phobia, overanxious disorder), and 21.7% (5/23) with alcohol dependence, two of whom also met criteria for drug dependence. In all, 21.7% met criteria for drug dependence or abuse. Offspring from the high-risk families showed an especially high rate of substance use disorder diagnoses with 60.0% meeting criteria for alcohol or drug dependence (5 cases) and one with drug abuse. In contrast, by age 18 only one of the control offspring met criteria for alcohol and drug dependence.
3.2. Orbitofrontal cortex
Differences in OFC volume (total and right) by measures of behavioral inhibition were tested using GLM. Because the subjects had participated in multiple peer play sessions, the data are presented for the first session and for the average time across the sessions. Using a cut-off of 15 s for time spent proximal to the mother at the first session, a significant effect for proximity to mother (F=10.61, df=1, 17, p=0.005) was observed for the right OFC (covarying left OFC volume and ICV) in a model that included testing for familial risk status as a main effect. The mean right OFC volumes, corrected for covariates, for children that spent less than 15s within proximity to mother was 15.15 (±1.10) compared to 16.67 (±1.07) for those children that spent greater than 15s within proximity of mother. The main effect of familial risk status was also significant (F=6.59, df=1, 17, p=0.02). However, the interaction between time spent proximal to mother and familial risk status was not significant. The effect was specific to the right hemisphere as significant differences for total OFC volume were not seen for time spent proximal to the mother. For average time spent in close proximity to the mother, risk status was included in a model that covaried ICV. Here, the right OFC (covarying left) results were also significant (F=7.87, df=1,17, p=0.01) for proximity to mother. Risk was also significant (F=4.78, df=1, 17, p=0.04), though the interaction of familial risk and proximity was not significant.
Analyses performed to determine if right OFC volume (covarying left OFC volume) and time staring at the other child at the first peer play session would be associated (which also included familial risk status in the model) did not reveal significant effects. Analyses that were performed for right OFC volume (covarying left) relating average time staring at the other child across the sessions also did not reveal a main effect. However, a significant interaction effect in association with risk status (F=4.25, df=1, 18, p=0.05) was seen.
Analyses were completed to determine if the right/left OFC ratio calculated for each subject would be related to his/her measures of behavioral inhibition (time spent proximal to the parent). The amount of time spent within close proximity to the mother (within arm's reach) at the first session was significantly associated with the ICV-adjusted right/left OFC ratios (F=7.55, df=1, 18, p=0.013) as displayed in Fig. 2. Also, a significant main effect of risk status was seen (F=5.74, df=1, 18, p=0.028) with high risk status being associated with smaller right/left OFC ratios, though a significant interaction was not seen. This tendency for lesser tissue volume in the right hemisphere has previously been reported for a sample of 107 subjects with familial multiplex alcohol dependence risk status (Hill et al., 2009a). Although OFC ratios were related to time spent proximal to the mother at the first peer play visit, this relationship was not seen when tested for the average time proximal to the mother across sessions.
Fig. 2.
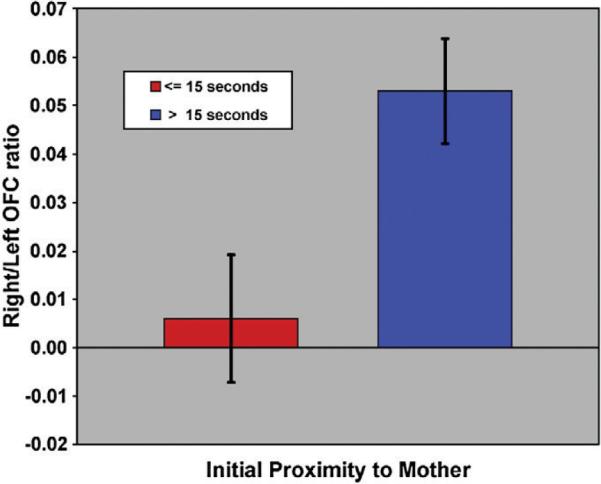
Orbitofrontal cortex ratios were determined for each participant using the formula (Right−Left)/(Right+Left). Volumes were adjusted for intracranial volume before statistical analyses were performed. Depicted here are the adjusted means (adjustment for age and risk) and the standard errors for those below 15 s for time spent proximal to the mother and those greater than 15s.
3.3. Amygdala
A linear mixed model analysis for total amygdala volume that included risk status in the model and utilized the ICV as a covariate revealed that proximity to mother at the first session was significantly related to total amygdala volume (F=12.90, df=1, 8.52, p=0.006). The interaction between familial risk and proximity to mother at this first session was not significant for total amygdala volume. A significant relationship between total amygdala volume and the average time spent proximal to the mother across sessions (covarying ICV) was also found (F=12.64, df=1, 9.67, p=0.006). The mean amygdala volume, adjusted for ICV, for children who spent more than 15 s proximal to their mother was 3.27 (±0.17) compared to 2.82 (±0.16) for those children that spent less than 15s within proximity of mother. However, the interaction between familial risk and proximity to mother across sessions was not significant. The same models were evaluated for right amygdala volume and proximity to mother for the first session and for the average time across sessions. These results did not reach statistical significance.
The amount of time staring averaged across sessions was analyzed for total and right amygdala volume. A model was evaluated that included risk as a factor and covaried ICV. The interaction between familial risk and average time staring across the three sessions was not related to right or total amygdala volume, though there was a trend for familial risk to be associated with right amygdala volume (F=3.34, df=1, 11.66, p=0.09). For the first peer play session, total time staring at other children was not related to either total or right amygdala volume.
3.4. VBM results
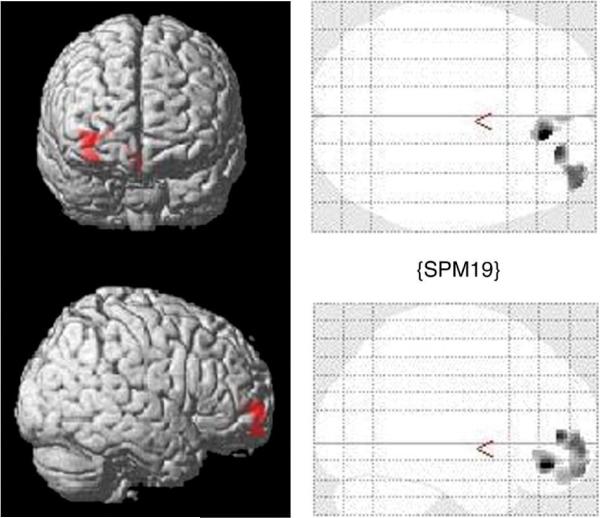
Whole brain analysis did not reveal any significant differences other than those found using the ROI measures of interest, namely, the OFC and amygdala. To confirm that ROI results obtained for the OFC were in the right hemisphere, masks corresponding to our OFC region of interest from the Pick Atlas small volume correction tool were used. This confirmed that significant differences were obtained for the right hemisphere for Brodmann areas 10, 11, and 47, regions that correspond to the OFC (Table 1). These regions were then subjected to false discovery rate (FDR) corrections. Although the VBM results did not survive the FDR correction procedure, we conclude that the uncorrected values do show convergence with the ROI manual tracings and suggest the validity of the findings. Table 1 shows the significant uncorrected increases in OFC grey matter volume for BA 11 in the inhibited children compared to the uninhibited based on the proximal to mother variable. Differences are shown in Fig. 3.
Table 1.
VBM results using small volume correction for regions found to differ significantly in the ROI analyses from manual tracings.
| Region | x | y | z | t value | P-valuea |
|---|---|---|---|---|---|
| BA 11 Superior Frontal | 33 | 57 | −14 | 2.70 | 0.007 |
| BA 11 Medial Frontal | 5 | 35 | −13 | 2.59 | 0.009 |
| Limbic Lobe R Amygdala | 29 | −11 | −17 | 2.51 | 0.006 |
| Limbic Lobe L Amygdala | −23 | −14 | −6 | 2.04 | 0.027 |
This is the uncorrected P value by region. None of the P-values survived FDR correction for whole brain analysis.
Fig. 3.
VBM results (SPM 5) with height threshold T=1.73, p=0.049 and extent threshold k=50 voxels for Inhibited versus Uninhibited groups based on time spent proximal to mother at <15 s versus >15s in the peer play setting at average age of 5.03 yrs±0.78 years.
3.5. Adolescent personality scale scores
We hypothesized that the offspring who were in the upper tercile for both proximity to mother and stare would show elevations in the MPQ Harm Avoidance scale. Using the upper tercile for staring at the first session, no differences in Harm Avoidance scores were seen. However, adolescents in the upper tercile for time proximal to their mother at the first session had significantly lower scores for the Well-Being scale (F=4.28, df=1, 19.59, p=0.052), a finding that was also seen when total time staring at the other child was evaluated (F=5.48, df=1, 18.57, p=0.031).
4. Discussion
The present results indicate that indices of behavioral inhibition in childhood are related to greater OFC volume in the right hemisphere and greater total amygdala volume in adolescence. The childhood behavorial indices include spending more time within close proximity to mother rather than engaging in play with the other child and spending more time staring at the other child. This relationship was studied accounting for risk status because a previous study had found differences in behavioral inhibition by familial risk status (Hill et al., 1999).
The present results add to the extant literature in suggesting that volumetric differences in the right OFC may be associated with a continuum of behavior from a disinhibited, impulsive style to an inhibited, cautiously restrained temperament. Those 5-year-olds who had shown a more extreme reaction to an unfamiliar child in a peer play setting by remaining close to their mothers and refraining from entering into play with the other child at the first peer play session exhibited greater right/left OFC ratios. Analysis of the right OFC volume, covarying left volume, was significant for both the first session and average time across sessions. Additionally, the amount of time spent staring at the other child, averaged across sessions with different children, and testing for risk status revealed an interaction suggesting that those with greater right OFC volume relative to the left tended to stare more. This is especially interesting because a previous study that examined OFC right/left ratios in 107 adolescent/ young adult participants found reduced right/left white OFC ratios were associated with increased impulsivity (Hill et al., 2009a). Other studies have demonstrated an inverse relationship between OFC grey matter volume and measures of impulsivity in healthy adults (Matsuo et al., 2009) and healthy boys ages 7–17 (Boes et al., 2009), and white matter OFC volume and impulsivity in adolescence (Hill et al., 2009a).
Utilizing offspring from families selected for multiple cases of alcohol dependence, we had previously reported that offspring from these high risk for AD families at high risk for alcohol dependence are, on the whole, more likely to be behaviorally inhibited in comparison to control children in a peer play setting where they are introduced to a child they have never seen before and measures of interaction recorded (Hill et al., 1999). The present structural MRI analyses are based on a subset of children seen in the peer play initiative approximately 10 years earlier who are currently part of a larger longitudinal follow-up effort. With an average clinical follow-up to age 18, an exceptionally high rate of alcohol and drug dependence is seen, largely due to half of the sample being from multiplex alcohol dependence families. Because multiplex family membership appears to predispose to smaller OFC volume in the right hemisphere and half of the subjects in our sample are from such background, we conclude that larger OFC volume in the right hemisphere in association with indicators of behavioral inhibition may be especially robust.
The present results also suggest that behaviorally inhibited children have larger total amygdala volume than those who are less inhibited. This is consistent with previous studies that have suggested that reduced volume of the amygdala may predispose individuals to externalizing forms of behavior including cocaine dependence (Makris et al., 2004), relapse to alcohol abuse (Wrase et al., 2008), familial risk for alcohol dependence (Hill et al., 2001), and externalizing disorders (Benegal et al., 2007). These results are also consistent with the differential BOLD response of the amygdala to novel faces in those who are behaviorally inhibited (Schwartz et al., 2003).
The present results show that the right OFC, in contrast to total OFC volume, is associated with measures of childhood behavioral inhibition. This suggests that tissue volume in the right hemisphere relative to the left may determine the degree to which an individual is prone to either an inhibited or impulsive temperament. Because the children were not scanned at the time of the peer play observations, we do not know if this relationship might have been found at that time. Also, observational measures of temperament that may be less susceptible to social desirability factors were not available for the adolescents at the time of scanning. However, MPQ self-report measures were analyzed contrasting those who were in the upper tercile for staring and proximity to the mother versus those who were in the lower terciles with results suggesting that those most behaviorally inhibited at age 5 scored lower at age 15 on Well-Being, an MPQ trait that indicates a tendency for a positive outlook on one's life. This finding is of interest because of reported associations between Well-Being and frontal EEG by hemisphere. Specifically, greater alpha EEG is seen in the left than right hemisphere in those scoring higher on self-reported Well-Being inventories (Davidson, 2004; Urry et al., 2004; Tomarken et al., 1992). Because MPQ Well-Being appears to have moderate heritability (h2=0.40) (Finkel and McGue, 1997), it is likely that the 5-year-olds who scored in the upper tercile for indices of behavioral inhibition were already showing signs of lesser positive affect.
A comment is in order with respect to why differences should have been found by hemisphere for the OFC. Neurodevelopmental changes in decision making are accompanied by changes in brain morphology of the OFC that differ by hemisphere. Greater involvement of the right than left prefrontal cortex is seen in tasks involving response selection and inhibition, with suboptimal response inhibition in children and adolescents related to insufficient recruitment of the right ventrolateral PFC (Rubia et al., 2001). Social/emotional and decision making processes in adults are similarly impaired when unilateral right hemisphere lesions of the ventrolateral PFC are seen in neurological patients (Tranel et al., 2005).
Functional differences by hemisphere identified using EEG asymmetry suggest a relationship to approach and avoidance behaviors (Fox, 1991; Davidson, 1992). A decrease in alpha power at electrodes over the right frontal region relative to the left hemisphere in infants has been associated with greater behavioral inhibition in comparison to infants with decreased alpha power over the left frontal region (Fox, 1994). Study of 31-month-old toddlers in a peer play setting modeled after the Kagan laboratory procedures identified those who were highly extreme in remaining proximal to their mothers and found this characteristic to be related to greater EEG activity in the right hemisphere (Davidson, 1992). EEG asymmetry has also been observed in 4-year-old children that were inhibited as infants (Fox et al., 2001), an association that was also observed at ages 10–12 years (McManis et al., 2002).
Volumetric indices of the OFC and the amygdala may serve as a marker of biological susceptibility to both behavioral inhibition and disinhibition. Children and adults with ADHD who exhibit disinhibited and impulsive behaviors have smaller OFC volumes compared with healthy subjects (Hesslinger et al., 2002; Carmona et al., 2005). Because the OFC appears to be a neural substrate for mechanisms of inhibition and has been implicated in a variety of impulsive behaviors (Dom et al., 2005), variation in OFC morphology could be the basis for a common vulnerability factor for a number of adult psychopatho-logical conditions including anxiety disorders, depression, alcohol abuse, and drug dependence. Because OFC projections to the amygdala provide local inhibitory influences through GABAergic neurotransmission, it appears likely that the OFC and amygdala are components of a neural circuit modulating inhibition and impulsivity through their interaction (Rempel-Clower, 2007).
Limitations of the study include the small sample size that precluded specifically testing the interaction of behavioral inhibition and gender on brain morphology. A further limitation of our study was that three families contributed multiple siblings. Because brain volume appears to be a heritable trait (Pfefferbaum et al., 2004; Schmitt et al., 2007), presence of multiple siblings could have decreased variance among subjects leading to incorrect conclusions. However, use of the mixed model analysis permitted us to control for family membership minimizing this limitation.
Although our sample size was modest and precluded specifically addressing the possible interaction effects of gender and behavioral inhibition, nevertheless several features appear to suggest the importance of our findings. First, our analysis was hypothesis driven and conducted using carefully drawn ROIs by experienced raters. Second, our findings were confirmed by VBM techniques using small volume correction masks. Third, our sample was drawn from among individuals who participated in a peer play procedure that allowed for direct observation of temperamental traits, a method that is clearly preferable to questionnaire-based methods (Kagan, 1994), which often rely on parental reports that can be biased by parental expectations (Mangelsdorf et al., 2000). Finally, our results show for the first time that larger volumes of the amygdala and right OFC are associated with the trait-like behavior first described by Kagan and colleagues (Kagan et al., 1984) as “behavioral inhibition”. This tendency to be behaviorally inhibited includes a predisposition to cautious restraint and reduced levels of impulsivity that increases the likelihood of adolescent anxiety disorders. In contrast, trait impulsivity is a prominent feature of bipolar disorder (Swann et al., 2009b), antisocial personality disorder (Swann et al., 2009a), and alcohol dependence (Rubio et al., 2007). A previous study from this laboratory using a much larger sample of 107 subjects demonstrated that smaller OFC volume in the right hemisphere characterizes offspring from families at exceptionally high risk for alcohol dependence because of their multiplex family background in contrast to low-risk control subjects (Hill et al., 2009a). Moreover, white matter volume of the right OFC was significantly associated with impulsivity (lower scores on the MPQ Control scale). The present results indicate that individuals who show extreme scores (upper tercile) on measures of behavioral inhibition at age 5 have larger OFC volumes in the right hemisphere at age 15. The finding appears to be sufficiently robust that it is seen even in a study in which half of the sample had a high risk for alcohol dependence background, a factor that is associated with smaller OFC volume in the right hemisphere.
Acknowledgements
The research reported was supported by grants from the National Institute on Alcohol Abuse and Alcoholism: AA 005909, AA 008082, and AA 015168.
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M, Tomàs J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neuroscience Letters. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vaus YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rappoport J.l. Quantitative brain magnetic resonance imaging in attention deficit hyperactivity disorder. Archives General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Chambers W, Puig-Antich J, Hirsch M, Paez P, Ambrosini P, Tabrizi MA. The assessment of affective disorders in children and adolescents by semistructured interview: test–retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Critical Reviews in Neurobiology. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Chess S, Thomas A. Temperament and the parent–child interaction. Pediatric Annals. 1977;6:574–582. [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: neural substrates and biobehavioural correlates. Phil. Trans R. Soc. Lond. B. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Finkel D, McGue M. Sex differences and nonadditivity in heritability of the Multidimensional Personality Questionnaire scales. Journal of Personality and Social Psychology. 1997;72:929–938. doi: 10.1037//0022-3514.72.4.929. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it's not left, it's right. Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotion regulation. Introduction. Monographs of the Society for Research in Child Development. 1994;59:103–107. [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Fronto-orbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke J, Snidman N, Kagan J. Behavioral inhibition in children from families at high risk for developing alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:410–417. doi: 10.1097/00004583-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome-wide search for alcoholism susceptibility genes. American Journal of Medical Genetics, Part B (Neuropsychiatric Genetics) 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke-Wellman J, Lowers L. Psychopathology in childhood and adolescence: A prospective study of offspring from multiplex alcoholism families. Psychiatry Research. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009a;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009b;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janca A, Robins LN, Cottler LB, Early TS. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI field trials B wave II at the St. Louis site. Br J Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- Kagan J. Galen's Prophecy: Temperament in Human Nature. Basic Books, New York; New York: 1994. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism Clinical Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lacerda ALT, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: A validation study. Neuroimage. 2003;19:665–673. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreason NC, O'Leary DS, Yuh WTC, Heckel D. Structural MR image processing using BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman L, Goldstein J, Gastfriend D, Albaugh DM, Hodge SM, Ziegler D, Kennedy D, Caviness VS, Tsuang M, Hyman S, Rosen BR, Breiter HC. Decreased absolute volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf SC, Schoppe SJ, Buur H. The meaning of parental reports: A contextual approach to the study of temperament and behavior problems in childhood. In: Molfese VJ, Molfese DL, editors. Temperament and Personality Development Across the Lifespan. Erlbaum Publisher; London, UK: 2000. pp. 121–140. [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping. 2009;30:1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA. EEG asymmetry, power, and temperament in children. Developmental Psychobiology. 2002;41:169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptideY gene. Trends in Pharmacological Sciences. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Prasad KMR, Sahni SD, Rohm BR, Keshavan MS. Dorsolateral prefrontal cortex morphology and short-term ourcome in first episode schizophrenia. Psychiatry Research: Neuroimaging. 2005;140:147–155. doi: 10.1016/j.pscychresns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Annals New York Academy of Science. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Kagan J, Snidman N, Gersten M, Baak K, Rosenberg A. Inhibited and uninhibited behavior: a follow-up study. Child Development. 1986;51:660–680. [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Gersten M, Hirshfeld DR, Meminger SR, Herman JB, Kagan J, Reznick JS, Snidman N. Behavioral inhibition in children of parents with panic disorder and agoraphobia. A controlled study. Archives of General Psychiatry. 1988;45:463–470. doi: 10.1001/archpsyc.1988.01800290083010. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld DR, Bolduc EA, Faraone SV, Kagan J, Snidman N, Reznick JS. Further evidence of an association between behavioral inhibition and anxiety disorders: results from a family study of children from a non-clinical sample. Journal of Psychiatric Research. 1991;25:49–65. doi: 10.1016/0022-3956(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Marinez I, Iribarren MM, Jimenez-Arriero MA, Ponce G, Avila C. Varieties of impulsivity in males with alcohol dependence: the role of Cluster-B personality disorder. Alcoholism: Clinical and Experimental Reseach. 2007;31:1826–1832. doi: 10.1111/j.1530-0277.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Schmitt EJ, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Research and Human Genetics. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. Journal of Psychiatric Research. 2009a;43:1057–1063. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disorders. 2009b;11:280–288. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A, Lykken DT, Bouchyard TJ, Jr, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. Journal of Personality and Social Psychology. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Making a life worth living: neural correlates of well-being. Psychological Science. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]