SUMMARY AND CONCLUSIONS
Excitation of the spiny subtype of hilar neurons in the fascia dentata was characterized by intracellular recording from hilar cells in hippocampal slices. Stimulation of the outer molecular layer was used to activate the perforant path. Evoked responses were examined, as well as the large spontaneous excitatory potentials that are a distinctive characteristic of spiny hilar cells.
Excitatory potentials that occurred spontaneously, as well as those that occurred in response to outer molecular layer stimulation, were similar among the cells that were sampled, regardless of morphological variations such as the presence or absence of thorny excrescences. Spontaneous and evoked excitatory postsynaptic potentials (EPSPs) were complex depolarizations that often had several discrete peaks. Spontaneous EPSPs increased in amplitude slightly with hyperpolarization, and evoked EPSPs clearly increased with hyperpolarization.
Applications of selective antagonists of excitatory amino acid receptors were used to determine which excitatory amino acid receptor mediates EPSPs of these cells. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was used to block the receptor sub-type selective for the agonists α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid (the “AMPA/kainate” receptor). 2-amino-5-phosphonovaleric acid (APV) was used to block receptors specific for the agonist N-methyl-d-aspartate (NMDA; the “NMDA” receptor). Perfusion with CNQX (5-25 μM) completely blocked all spontaneous and evoked excitation, even when activity was examined at relatively depolarized membrane potentials and a low concentration of extracellular magnesium (0.5 mM) was used. Under these conditions, APV (25–50 μM) had no detectable effect on spontaneous activity but did increase the stimulus strength required to elicit responses to outer molecular layer stimulation.
When extracellular magnesium was lowered to 0 mM (nominally), there was strong evidence for a contribution of NMDA receptors to spontaneous and evoked EPSPs. Thus, when cells were perfused with 0 mM extracellular magnesium and 5 μM CNQX, spontaneous depolarizations were present and EPSPs could be triggered by stimulation of the outer molecular layer. Both the spontaneous and evoked EPSPs were blocked by 25 μM APV.
Because γ-aminobutyric acid (GABA)A receptors can cause depolarizations in hippocampal neurons, the GABAA receptor antagonist bicuculline was used to determine whether some of the EPSPs were mediated by GABAergic neurons that are normally activated by spontaneous release of excitatory amino acids. Bicuculline (5–25 μM) had no effect on spontaneous depolarizations, and led to an enhancement of evoked depolarizations. Therefore it does not appear that GABAA receptor-mediated depolarizations contribute to hilar cell depolarizations. However, these experiments do suggest that GABAergic inhibition is involved in limiting excitation of hilar neurons to outer molecular layer stimulation.
These results suggest that excitatory activity of dentate spiny hilar neurons is similar among spiny cells, consistent with the observed physiological homogeneity of spiny hilar cells described previously with respect to their membrane properties. In addition, the results show that AMPA/kainate receptors contribute substantially to excitation that occurs spontaneously and by afferent stimulation. NMDA receptors appear to contribute to evoked and spontaneous depolarizations when extracellular magnesium levels are extremely low. Finally, although the effects of bicuculline suggest a role of GABA and GABAA receptors in evoked responses, GABA does not appear to be involved in spontaneous or evoked depolarizations.
INTRODUCTION
The neurotransmitters and receptors mediating excitatory transmission in the hippocampus have been studied thoroughly for principal cells such as the granule cells and pyramidal cells (Lopes da Silva et al. 1990; Nicoll 1988; Schwartzkroin and Mueller 1987), but are less clear for the interneurons and other nonprincipal cells. One important population of cells for which such information is lacking is the cells found in the hilus of the fascia dentata.
The majority of hilar cells are spiny, and many of the spiny neurons bear complex postsynaptic specializations on their proximal dendrites called “thorny excrescences” (Amaral 1978). Some of the spiny hilar cells that are particularly encrusted have been referred to as “mossy” cells (Amaral 1978; Frotscher et al. 1991; Ribak et al. 1985). Spiny hilar cells vary with respect to the extent of their encrustation, their somatic size and shape, and the orientation of their dendritic trees (Amaral 1978), but they have homogeneous electrophysiological properties that are similar to those of area CA3c pyramidal cells (Scharfman 1992; Scharfman and Schwartzkroin 1988). The neurotransmitter of the spiny hilar cells appears to be glutamate (Ottersen and Storm-Mathisen 1989; Storm-Mathisen and Wold 1981). The other cells located in the hilus are relatively aspiny (Amaral 1978) and resemble “fast-spiking” cells electrophysiologically (Kawaguchi and Hama 1987; Scharfman 1992).
Hilar cells are of interest for several reasons. First, they are a substantial group of cells with an expansive axonal plexus that suggests a potentially powerful role in dentate and possibly hippocampal circuitry. For example, most (if not all) spiny hilar cells have an axon that projects throughout both the ipsilateral and contralateral dentate gyrus, terminating in the inner molecular layer (Blackstad 1956; Laurberg and Sørensen 1981; Swanson et al. 1978, 1981; Zimmer 1971). Hilar cells are also of interest because they are among the most vulnerable cells in the hippocampal formation. Anatomic as well as physiological studies have both shown that spiny hilar cells, such as the mossy cell, deteriorate dramatically when the perforant path is stimulated in an intermittent fashion for long periods of time (hours in vivo, Sloviter 1983, 1987, 1991; minutes in vitro, Scharfman and Schwartzkroin 1990a). Aspiny hilar neurons that are immunoreactive for somatostatin and neuropeptide Y are also vulnerable (Sloviter 1987, 1991). However, the granule cells and γ-aminobutyric acid (GABA)ergic cells in the same preparations are relatively resistant (Scharfman and Schwartzkroin 1990a; Sloviter 1987). Hilar cells are also selectively vulnerable to ischemia (Crain et al. 1988; Johansen et al. 1987) and traumatic brain injury (Lowenstein et al. 1992). Perhaps the most important aspect of the selective vulnerability of hilar cells is the correlation that appears to exist between death or damage to hilar cells and the development of hyperexcitability among the remaining dentate and hippocampal cells (Scharfman and Schwartzkroin 1990a,b; Sloviter 1983,1987). The relevance of this hypothesis to epilepsy is apparent from studies of human temporal lobe epilepsy, where one of the most consistent pathological features on autopsy is hilar cell loss (de Lanerolle et al. 1989; Margerison and Corsellis 1966).
In light of the intriguing association between hilar cell death and the development of hyperexcitability in the hippocampus, the need for a more complete portrait of spiny hilar neurons and their circuitry is timely. Therefore excitation of hilar cells within the normal dentate circuitry was examined, with particular emphasis on hilar cell excitation by perforant path stimulation. This stimulation site was chosen for two reasons. First, the major afferent innervation of the hilar cells derives directly and indirectly from the perforant path. The perforant path excites hilar neurons indirectly by activating granule cells (Hjorth-Simonsen and Jeune 1972; Steward and Scoville 1976) that synapse on hilar cells (Amaral 1979; Claiborne et al. 1986; Laatsch and Cowan 1966), and the perforant path also excites hilar cells directly through their synaptic contacts on hilar cells in the molecular layer (Léránth et al. 1990; Scharfman 1991b). Second, as mentioned above, because studies of selective vulnerability of hilar cells have often employed stimulation of the perforant path, analyzing excitation by perforant path was particularly appropriate to the long-term goal of understanding hilar cell selective vulnerability.
In addition to excitation by perforant path, the high level of spontaneous excitatory potentials of hilar cells was examined (Scharfman and Schwartzkroin 1988; Strowbridge et al. 1992). This spontaneous activity is one of the remarkable characteristics that distinguishes spiny hilar cells from other hippocampal neurons. The spontaneous excitatory potentials of spiny hilar cells are important because they provide a constant background level of excitation on which other activity is superimposed. The virtually incessant barrage of spontaneous excitatory potentials is evident throughout a recording session (Scharfman and Schwartzkroin 1988).
Given that excitatory input to dentate spiny hilar cells is due largely to innervation by granule cells and the perforant path, and these sources both use an excitatory amino acid as their neurotransmitter (Ottersen and Storm-Mathisen 1989; White et al. 1977), the contributions of the two best characterized excitatory amino acid receptors to hilar cell excitatory postsynaptic potentials (EPSPs), the N-methyl-d-aspartate (NMDA) receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor (also referred to as the quisqualate/kainate, or non-NMDA receptor), were examined. The synthetic agonist NMDA is selective for the NMDA receptor, whereas the agonists AMPA and kainic acid are selective for the AMPA/kainate receptor (Collingridge and Lester 1989). In addition, EPSPs were studied at different membrane potentials and extracellular magnesium concentrations, because NMDA and AMPA/kainate receptors have different profiles in these respects. The NMDA receptor is blocked by magnesium at hyperpolarized membrane potentials (Mayer et al. 1984; Nowak et al. 1984), so that lowering external magnesium or depolarization of the cell facilitates NMDA receptor-mediated events. However, the opposite situation occurs for AMPA/kainate receptor-mediated processes, because the AMPA/kainate receptor is not blocked by magnesium at hyperpolarized potentials (Collingridge and Lester 1989). Therefore events occurring as a result of AMPA/kainate receptor activation increase in amplitude with hyperpolarization, and are relatively unaffected by lowering extracellular magnesium.
A preliminary report of this study has been presented elsewhere (Scharfman 1991a).
METHODS
Preparation and maintenance of slices
The use of animals was in accordance with the guidelines of the NY State Department of Health and the National Institutes of Health, Adult male Sprague-Dawley rats (100–250 g) were anesthetized with ether and decapitated. The brain was immediately removed and placed in 4°C buffer that contained (in mM) 126.0 NaCl, 5.0 KCl, 2.0 CaCl2, 2.0 MgSO4, 26.0 NaHCO3, 1.25 NaH2PO4, and 10 d-glucose; pH 7.4). Hippocampal slices (400 μm thick) were cut either in the coronal plane, parallel to the lamellae of the hippocampus (transverse slices) or parallel to the long axis of the hippocampus (longitudinal slices), using a Vibro-slice (Campden Instruments). Slices were immediately placed in a recording chamber (Fine Science Tools) that was modified so that slices lay at an interface of warmed (34–35°C), humidified air, and warmed, oxygenated (95% CO2-5% O2) buffer.
Recording and stimulation of the perforant path
Extracellular and intracellular recordings were made using borosilicate glass containing a capillary fiber in the lumen (0.6 mm ID, 1.0 mm OD, A & M Systems) pulled horizontally (Flaming Brown Model P-87) to a resistance of 1–10 MΩ (for extracellular electrodes) and 75–180 MΩ(for intracellular electrodes). Recording electrodes were filled with 1 M NaCl for extracellular recording. Either 1.5% Lucifer yellow CH (Sigma) in 1 M LiCl or 1 M KCH3COOH was used for intracellular recording. A high-input impedance amplifier with a bridge circuit was used for recording, and the bridge was balanced whenever current was passed. Data were acquired by a digital storage oscilloscope (Model 410, Nicolet) and saved on tape after digitization (Model DR-484, Neurodata).
Electrodes used to stimulate the perforant path were made of twisted metal. Teflon-coated stainless steel wire (diameter of each pole: 50 μm). The electrode was lowered so that each pole was next to hippocampal fissure. The outer molecular layer was used as an approximation of the perforant path because perforant path axons comprise the majority of axons in the outer molecular layer (Hjorth-Simonsen and Jeune 1972; Steward and Scoville 1976). Stimulation of the part of the entorhinal cortex that was not severed from the hippocampus during slicing evoked similar but smaller responses in granule cells in pilot studies, so it was not used for perforant path stimulation. Although axons of peptidergic hilar cells are also found in the outer molecular layer (Bakst et al. 1986; Kosaka et al. 1985; Roberts et al. 1984; Sloviter 1991; Sloviter and Nilaver 1987), it is unlikely that antidromic invasion of hilar cells contributed greatly to the responses to outer molecular layer stimulation, because very few hilar neurons in past studies have ever been antidromically activated by outer molecular layer stimulation.
Identification of spiny hilar cells
Cells that were injected with dye were identified as spiny hilar cells by their characteristic morphological features and electrophysiological signatures (Scharfman 1992; Scharfman and Schwartzkroin 1988). The major morphological feature of spiny hilar cells used for identification was the presence of numerous dendritic spines. The following were major physiological characteristics: 1) action potential duration > l.0 ms (measured as total duration at the base of the action potential, see below), 2) the absence of a large (>5 mV) hyperpolarizing after potential after single action potentials, 3) large (often >10 mV), frequent (>20 Hz) spontaneous subthreshold depolarizing potentials, 4) a highly variable response to depolarizing current injection that often displayed spike frequency adaptation, and 5) an excitatory response to stimulation of the outer molecular layer consisting of complex EPSPs and action potentials without an indication of inhibitory postsynaptic potentials (IPSPs). Cells that were not identified morphologically were identified solely on the basis of their physiological characteristics.
Thorny excrescences were defined on the basis of their distinctive appearance as large, complex protrusions. On hilar cells, they are found on the cell body as well as the proximal dendrites, but not distal dendrites (Amaral 1978). In contrast, relatively simple spines are thin (the stem of a simple spine may be ≤0.5 μm thick), straight, and short. Therefore “cells with excrescences” were identified by any large (>2 μm extensions from the dendrite), irregular swelling on the cell body and/or proximal dendrites. “Cells without excrescences” were defined as cells on which no large swellings were apparent using a ×40 objective. It is unlikely, given the large size difference between simple spines and excrescences, that cells with excrescences or without excrescences were mis-judged, but that possibility cannot be ruled out completely. Only those cells that were well-filled with dye were used for morphological analysis.
Data analysis
GENERAL DATA ANALYSIS
Data were analyzed off-line either by replaying data onto a chart recorder (Gould 2200S) or a digital oscilloscope equipped with software to analyze amplitude and duration of waveforms (Model 410, Nicolet). Membrane properties were defined and measured as described previously (Scharfman 199lb). Action potential amplitude was measured from an action potential evoked by a depolarizing current pulse (a 150-ms duration pulse) at threshold. Action potential duration was measured at the base, from the point where the action potential rise began to the point when the action potential repolarized. Action potential duration was measured in this way to take into account the decay phase of the action potential, because the action potentials of aspiny and spiny hilar cells differ mostly in the repolarization phase (Scharfman 1993b). Input resistance was measured from responses to a family of hyperpolarizing current pulses (−0.1 to −0.3 nA in steps of 0.05 nA) elicited near −60 mV (−57 to −63 mV). Specifically, the voltage deflection at steady state was plotted against injected current, and the slope of that function was defined as the input resistance. Larger pulses were not used because of strong rectification of spiny hilar cells (Scharfman and Schwartzkroin 1988).
QUANTITATION OF SPONTANEOUS EPSPs
When spontaneous EPSPs were sampled
Quantitation of spontaneous activity was difficult because spontaneous EPSPs could vary (Fig. 2). Therefore spontaneous activity was sampled from a period where spontaneous activity was relatively stable. Ten 1-s epochs were sampled from this stable period. Each epoch was separated from the next by 5 s. Membrane potentials were −60 to −64 mV. To sample EPSPs after drug application, spontaneous EPSPs were examined ≥ start of bath application.
FIG. 2.
Variations in spontaneous activity of the same spiny hilar cell. A: continuous record of spontaneous activity recorded from a spiny hilar cell. Spontaneous activity at the onset of the record is relatively mild but increases greatly in the 3rd, 4th, and 5th panels. The activity returns to a relatively low level in the last panel. Membrane potential = −66 mV. B: histogram of the spontaneous activity shown in A. The mean number of events (Hertz; mean ± SE) are shown for the l st and 2nd panels in A (dark bars) and 3rd and 4th panels in A (pale bars). Measurements were made to the nearest half millivolt so that the bin labeled 5–10 mV contains events between 5.0 and 9.5 mV; the bin labeled 10–15 mV contains events between 10.0 and 14.5 mV, etc.
Measurement of spontaneous EPSP amplitude
EPSP amplitude was measured from baseline to the peak of the EPSP. Baseline was the membrane potential of the cell at the lime the 1-s epochs were sampled. A single spontaneous event was defined as a depolarization >2 mV. This amplitude was chosen because 2 mV was clearly greater than the noise level, and most depolarizations were >2 mV. The peaks of large EPSPs that triggered action potentials were measured at the point where the action potential was triggered.
Measurement of spontaneous EPSP frequency
EPSP frequency was based on counting the EPSPs occurring during the 1-s epochs that were sampled for each cell. For a given cell, the total number of EPSPs was counted for each 1-s epoch and then the 10 totals were averaged. Depolarizations with multiple peaks were treated as multiple EPSPs; in such cases, the number of EPSPs was estimated as the number of discrete peaks that occurred during the depolarization.
Limitations of methods used to quantitaie spontaneous EPSPs
Clearly, these methods are limited in their ability to accurately quantitate spontaneous EPSPs. For example, even though EPSPs were sampled during periods where the spontaneous activity appeared to be stable, the activity could have been sampled during periods where changes were occurring but could not be detected. Therefore there is a chance that the calculations made for a given cell might misrepresent that cell’s actual spontaneous EPSPs. In addition, EPSP frequency was underestimated in at least three ways. First, events >2 mV were not counted. Second, events with extremely slow kinetics could have been missed because such EPSPs might not have had discrete peaks. Third, EPSP frequency was underestimated because large events that might have been composed of multiple EPSPs were treated as one event if only one peak was detected.
Dye injection and histology
Lucifer yellow was injected in the course of recording from a cell by passing hyperpolarizing DC current intermittently in brief periods (0.25–0.75 nA for 15–45 s) for a total of ~10 min of current injection. At no time did membrane properties or the response to stimulation deteriorate during current injection. Nor were there any significant differences in cells that were recorded with Lucifer yellow-filled electrodes and KCH3COOH-filled electrodes (Table 1). At the end of the recording session, the electrode was slowly withdrawn and the slice was sandwiched between filter paper soaked with fixative (3.5% paraformaldehyde). Fixed slices were refrigerated from 1 to 5 days before dehydration (0.1M NaH2P04 buffer. 5 min; 50% ethanol, 5 min; 75% ethanol, 5 min; 90% ethanol, 5 min; 95% cthanol, 10 min; 100% ethanol, 10 min). To clear the tissue, slices were placed on a slide in a drop of methyl salicylate (Sigma). Fluorescent cells were viewed either with a confocal microscope (MRC-600; Biorad) and photographed using a video printer (Sony), or sections were viewed with an epifluorescent microscope (Leitz Dialux 20) and photographed with a 35-mm camera (Tri-X black and white print film. 400 ASA Kodak).
Table 1. Membrane properties of spiny hilar neurons.
| All Cells | Cells With Excrescences |
Cells Without Excrescences |
Pre-CNQX | CNQX | |
|---|---|---|---|---|---|
| Sample size (n) | 58 | 9 | 13 | 24 | 24 |
| Resting membrane potential, mV | −64.7 ± 1.3 | −63.6 ± 2.3 | −64.5 ± 1.5 | −64.3 ± 1.9 | −64.9 ± 3.0 |
| Action potential amplitude, mV | 86.5 ± 1.5 | 86.1 ± 2.4 | 87.2 ± 2.3 | 86.4 ± 2.9 | 85.0 ± 2.1 |
| Action potential duration, ms | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.2 |
| Input resistance, MΩ | 82.0 ± 3.0 | 83.6 ± 2.5 | 79.6 ± 3.3 | 81.9 ± 1.7 | 82.0 ± 2.0 |
| Time constant, ms | 25 ± 2 | 25 ± 2 | 24 ± 3 | 25 ± 3 | 25 ± 4 |
All values except sample size arc means ± SE. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione. Cells with excrescences and cells without excrescences were identified morphologically after injection with Lucifer yellow (see methods). For experiments using CNQX, membrane properties were measured ≥5 min before drug application (pre-CNQX) and immediately after the maximum effect of drug application (CNQX), which was ≥ 15 min after the start of perfusion with CNQX. For measurement of membrane properties, see methods. Cells recorded in 0 or 0.5 mM magnesium, 25 μM bicuculline. or 1 μM tctrodotoxin are not included.
Drug application
The drugs applied in this study were stored as aliquots of concentrated stock solutions at 0–4°C as follows: 1 mM tetrodotoxin (in 0.9% NaCl, Sigma), 1 mM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Tocris Neuramin) in 0.05 N NaOH, 10 mM dl-2-amino-5-phosphonovalcric acid (APV, Sigma) in 0.9% NaCl and 1 mM(+) bicuculline (Sigma) in 0.5% citric acid. On the day of the experiment, aliquots were diluted in buffer to their final concentration (5 or 25 μM CNQX, 25 or 50 μM dl-APV, and 5 or 25 μM bicuculline).
Drugs were applied either by pressure application or by bath application. When a drug was pressure applied, pressure was set against the back of a low-resistance electrode filled with drug solution (Picospritzer II, General Valve Corporation). Low-resistance electrodes were pulled so that their resistance, when filled with 1 M KCI, was 1–2 MΩ. When raised in the air, a 100-ms. 20-lb/square in. pulse ejected a droplet that was 50–250 μm diam. If there were changes in membrane properties during pipette placement, that cell was discarded. If there was no detectable effect of pipette placement, a pulse of pressure (10–250 ms, 10–20 lb/square in.) was triggered. After 1 min to monitor any changes in the physiology of the cell in response to the drug, the pipette was raised in the air and placed beside the slice. At that time, an identical pulse of pressure as was used when the pipette was in the slice was triggered to judge whether the pressure pulse was effective in ejecting drug. This was important because lack of effect could be due to ineffectual drug or a clogged pipette filled with efficacious drug. The reliability of each pressure pipette was monitored by measurement of the droplet that formed at the tip of the pipette when the pipette was in air and a pressure pulse was triggered.
When drugs were added to the perfusate (“bath application”), a sufficient concentration of stock solution was added to the appropriate volume of buffer to reach the desired final concentration. When recovery from an effect of drug was examined, the source of buffer to the chamber was replaced by drug-free buffer. Both drug-free buffer and buffer containing drug were constantly oxygenated (95% CO2-5% CO2) throughout the experiment.
RESULTS
This study was based on recordings from 64 hilar cells in buffer containing 2.0 mM magnesium and 10 additional cells with buffer containing 0 or 0.5 mM magnesium and CNQX (see below). All cells had resting membrane potentials over −50 mV, overshooting action potentials, and input resistances >50 MΩ (Table 1). The cells were located in the dorsal (n = 12), middle (n = 24), or ventral (n = 38) third of the hippocampus. The planes of section were transverse (n = 43), longitudinal (n = 17), or coronal (n = 14). Cells were located close to the upper blade (n = 43), near the point where the upper and lower blades meet (n = 24), or near the lower blade (n = 7). Of the 22 morphologically identified cells, 9 possessed excrescences and 13 did not (Fig. 1). The remaining cells were identified solely by their electrophysiological properties (see methods). The results were comparable among all of the spiny hilar cells sampled, as described below.
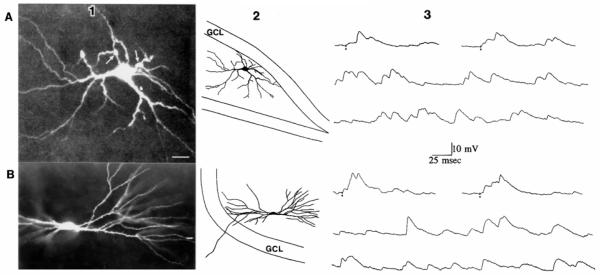
FIG. 1.
Spontaneous and evoked excitatory postsynaptic potentials (EPSPs) of different spiny hilar cells are similar. A: EPSPs of a spiny hilar “mossy” cell. A1: spiny hilar cell with excrescences on its cell body and proximal dendrites is shown. This image is a composite of 15 optical sections taken with a confocal microscope through a slice. Calibration = 15 μm. A2: drawing of the cell shown in A1 illustrates where the cell was located in the slice. Dorsal is up, area CA3 is to the left. The granule cell layer (GCL) is outlined. A3: evoked (top) and spontaneous (middle and bottom) EPSPs of the cell shown in A, 1 and 2. Small circles: stimuli to the outer molecular layer. Membrane potential = −64 mV. B: EPSPs of a spiny hilar cell without excrescences. Bl: spiny hilar cell with simple spines is shown. The cell was in a different slice from the cell in A. Calibration is the same as in A. B2: drawing of the entire cell and its location in the hilus. Dorsal is down, area CA3 is to the right. A portion of the upper blade of the dentate gyrus is outlined. B3: evoked (top) and spontaneous (middle and bottom) EPSPs of the cell shown in B.1 and 2. Stimuli were applied to the outer molecular layer. Membrane potential = −63 mV.
Spontaneous and evoked EPSPs
SPONTANEOUS EPSPs
Characteristics of spontaneous EPSPs
As noted previously (Scharfman and Schwartzkroin 1988; Strowbridge et al., 1992), the spontaneous depolarizing potentials occurring in spiny hilar cells are extremely large and frequent compared with those in other cells in the hippocampus. These depolarizing potentials are referred to below as “spontaneous” because they were shown to occur in the presence of tetrodotoxin (Livesy and Vicini 1992). Three spiny hilar cells were used to reexamine this issue, and it was found that the mean frequencies of spontaneous EPSPs were similar before and after tetrodotoxin bath application (1 μM; Table 2). The term “EPSP” is used to refer to the spontaneous depolarizations because the depolarizations were graded in amplitude and action potentials were triggered on their peaks (Figs. 2 and 3; Scharfman and Schwartzkroin 1988). No spontaneous hyperpolarizations were observed, even if a cell was depolarized to −50 mV.
Table 2. Characteristics of spontaneous depolarizing potentials in spiny hilar neurons.
| Frequency of Events, Hz | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | All events | 0–5 mV | 5–10 mV | 10–15 mV | 15–20 mV | 20–25 mV | 25–30 mV | |
| All cells | 58* | 40.2 ± 1.4 | 18.1 ± 1.1 | 10.7 ± 0.8 | 5.1 ± 0.6 | 3.1 ± 0.7 | 2.0 ± 0.6 | 0.8 ± 0.2 |
| Cells with excrescences | 9 | 38.5 ± 4.1 | 17.5 ± 2.1 | 8.9 ± 0.9 | 5.5 ± 1.0 | 3.4 ± 2.1 | 1.9 ± 0.9 | 0.9 ± 0.1 |
| Cells without excrescences | 13 | 39.4 ± 5.1 | 20.1 ± 1.5 | 10.0 ± 0.9 | 4.9 ± 0.8 | 2.8 ± 1.5 | 1.5 ± 0.5 | 1.0 ± 0.1 |
| Cells recorded with electrodes containing Lucifer yellow |
22 | 42.2 ± 4.3 | 22.2 ± 2.0 | 8.7 ± 1.0 | 5.9 ± 1.1 | 2.9 ± 0.9 | 2.0 ± 0.7 | 0.7 ± 0.1 |
| Cells recorded with electrodes containing potassium acetate |
−14* | 40.1 ± 3.9 | 17.9 ± 1.0 | 11.2 ± 0.8 | 4.8 ± 0.9 | 2.8 ± 1.9 | 2.2 ± 0.6 | 0.6 ± 0.2 |
| Cells perfused with 50 μM APV | 5 | |||||||
| Pre-APV | 41.0 ± 5.2 | 17.5 ± 4.0 | 12.4 ± 0.7 | 6.1 ± 0.9 | 3.0 ± 1.0 | 2.1 ± 0.8 | 0.9 ± 0.4 | |
| Post-APV | 39.7 ± 4.1 | 19.0 ± 3.7 | 11.1 ± 2.1 | 4.9 ± 0.7 | 3.7 ± 2.2 | 1.3 ± 0.9 | 1.2 ± 0.3 | |
| Cells perfused with low extracellular Mg2+ |
7 | |||||||
| Control (2 mM Mg2+) | 38.9 ± 3.7 | 19.2 ± 2.5 | 10.2 ± 1.5 | 5.5 ± 1.1 | 2.9 ± 0.9 | 1.0 ± 0.7 | 0.5 ± 0.1 | |
| Low Mg2+(l mM Mg2+) | 40.2 ± 5.1 | 20.0 ± 1.7 | 8.7 ± 2.1 | 5.6 ± 0.7 | 3.3 ± 1.2 | 1.6 ± 1.6 | 0.2 ± 0.2 | |
| Cells perfused with 25 μM bicuculline |
3 | |||||||
| Pre-bicuculline | 40.0 ± 3.5 | 19.6 ± 2.3 | 8.9 ± 1.1 | 6.3 ± 1.3 | 2.8 ± 0.8 | 1.9 ± 0.4 | 0.5 ± 0.2 | |
| Post-bicuculline | 41.8 ± 4.0 | 18.1 ± 1.9 | 11.2 ± 1.1 | 5.0 ± 1.0 | 3.0 ± 0.6 | 2.0 ± 0.3 | 0.7 ± 0.2 | |
| Cells perfused with 1 μM tctrodoloxin |
3 | |||||||
| Pre-tetrodotoxin | 39.2 ± 1.9 | 17.7 ± 1.1 | 9.2 ± 1.4 | 3.7 ± 0.8 | 2.0 ± 1.9 | 1.7 ± 1.2 | 1.0 ± 1.0 | |
| Post-tetrodotoxin | 38.9 ± 2.0 | 18.2 ± 2.2 | 9.6 ± 2.0 | 3.4 ± 1.4 | 1.5 ± 0.9 | 3.3 ± 1.2 | 2.0 ± 1.1 | |
All values except n values are means ± SE. APV, 2-amino-5-phosphonovaleric acid. For other abbreviations, see Table 1. Measurements of spontaneous events were made between −60 and −64 m V (see methods). Events of specific amplitudes were measured to the nearest half millivolt, so that “5-10 mV” contains events between 5.0 and 9.5 mV; 10–15 mV events include depolarizations between 5.0 and 9.5 mV, etc. Cells with excrescences were differentiated from cells without excrescences after Lucifer yellow dye injection (see methods). Pre-drug measurements were taken ≥5 min before drug application, and post-drug measurements were taken ≥15 min after switching perfusate from control buffer to buffer containing drug.
Does not include cells recorded during perfusion with 0–0.5 mM extracellular magnesium and 5 μM CNQX, cells examined with 25 μM bicuculline, or cells examined with tctrodotoxin.
FIG. 3.
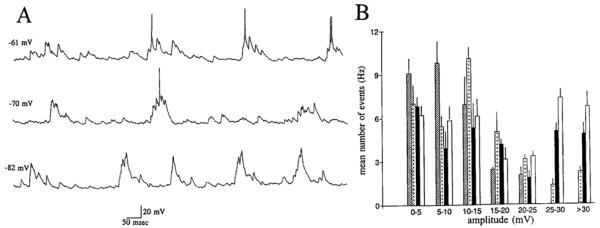
Voltage dependence of spontaneous activity of spiny hilar cells. A: 3 continuous records of spontaneous activity at 3 different membrane potentials, from the same spiny hilar cell. Membrane potentials are shown on the left. Action potentials are truncated. B: histogram shows the mean number of spontaneous events (Hertz; mean ± SE) at 4 different membrane potentials. Data were taken from the same cell as in A. Membrane potentials were −55 (bars with lines), −61 (bars with dots), −70 (dark bars), and −82 mV (clear bars).
Spontaneous EPSPs were composed of unitary-like events that occurred alone or in clusters, where they often summated (Figs. 1-3). Both single and summated spontaneous EPSPs could trigger action potentials (Figs. 2 and 3). Spontaneous EPSPs demonstrated a large range of amplitudes, even when held at a fixed membrane potential (Figs 1-3). The mean frequency of spontaneous depolarizations at a membrane potentials between −60 and −64 mV was 40.2 ± 1.4 (SE) Hz. The frequencies of spontaneous EPSPs of different amplitudes were similar among the sampled cells (Table 2).
Spontaneous EPSPs of a given cell could vary during a recording session. Large increases in the amplitude of spontaneous EPSPs could occur for seconds to minutes at any time (Fig. 2). These periods of very large spontaneous events were evident in most of the cells with excrescences (7 of 9 cells) as well as cells possessing only simple spines (10 of the 13 cells that did not have excrescences). Such periods were not necessarily evoked by any experimental manipulation, because changes could occur in the absence of stimulation, drug application, or injection of DC current. Furthermore, these periods occurred at several membrane potentials, and both early and late in the recording session. However, although these periods of larger spontaneous events could occur without an obvious stimulus, stimulation of the perforant path could evoke a period of larger events, especially if stimulation intensity was over threshold for action potential generation. After the cessation of stimulation, most cells returned to a lower level of activity within 1–5 min. In the cases where activity did not return to a lower level, cells became depolarized and the impalement was lost. During such periods of larger spontaneous depolarizations, there was no difference in the rate of spontaneous depolarizations, but the frequency of large events increased greatly (Fig. 2B). This indicates that the mechanism(s) that underly the variability in spontaneous activity could reside in the postsynaptic cells (i.e., a variable input resistance of the subsynaptic membrane of the spine or thorn could account for the changes in spontaneous activity). However, further experiments are clearly necessary to adequately explain the factors that control spiny hilar cell spontaneous activity.
Voltage dependence of spontaneous EPSPs
When membrane potential was manipulated between −45 and —95 mV, it was not clear from the raw data whether spontaneous EPSPs increased in amplitude with hyperpolarization (Fig. 3). However, histograms showed that large EPSPs (>15 mV) increased in frequency as cells were hyperpolarized, and small EPSPs (<10 mV) decreased in frequency (Fig. 3). Therefore, although unclear from the raw data, voltage dependence was demonstrated after quantitation of the spontaneous EPSPs. The mean frequency of all spontaneous events did not change with membrane potential. For the cell shown in Fig. 3, the mean number of events (Hertz) was 30.2 ± 5.1 at −55 mV, 34.1 ± 4.7 at −61 mV, 31.7 ± 6.0 at −70 mV, and 38.4 ± 4.4 at −82 mV. One simple explanation for the data is that small EPSPs increased in size with hyperpolarization. However, other possibilities cannot be excluded.
Homogeneity of spontaneous EPSPs
When compared across cells, the characteristics of spontaneous EPSPs were similar (Fig. 1; Table 2). When the spontaneous EPSPs of morphologically identified cells were compared, there were no obvious differences (Fig. 1, Table 2). Of particular interest was a comparison of spontaneous EPSPs among cells with excrescences and cells without excrescences. One might expect that cells with excrescences would exhibit different spontaneous EPSPs because the postsynaptic specialization is so different. However, there was no evidence that cells with excrescences differed from other cells with respect to their spontaneous activity. All cells demonstrated similar amplitude, frequency, and variability of spontaneous potentials (Fig. 1, Table 2).
EVOKED EPSPs
Characteristics of evoked responses
One distinct feature of evoked responses of spiny hilar cells, in comparison with the responses of granule cells, was that each response of a spiny hilar cell appeared to be composed of unitary events (Fig. 4), whereas granule cell EPSPs are smooth, graded potentials (Assaf et al. 1981; Fricke and Prince 1984; Lambert and Jones 1990). The term unitary is used because at the point where stimulus strength was raised just enough to evoke a detectable response, that response varied between one or more distinct depolarizations and no event at all (Fig. 4A). However, these apparently “unitary” EPSPs were variable in form and were often so large that they could reach threshold, characteristics that are unusual for unitary events. But these apparently unitary events were similar to the unitary EPSPs produced by single granule cells on single spiny hilar cells (Scharfman et al. 1990). This previous study supports the interpretation that the EPSPs presented here are unitary. In some cells, the unitary-like EPSPs occurred at a constant latency from the stimulus, indicating the involvement of a single axon and further supporting the supposition that they were unitary (Fig. 4A). EPSP latency of the same cell was variable when stimulus strengths near threshold were used, suggesting that more axons or synapses were recruited as stimulus strength was raised.
FIG. 4.
Characteristics of responses to outer molecular layer stimulation. A: variability of evoked responses. Five responses of a spiny hilar cell to stimulation of the outer molecular layer are shown. Stimulus strength was the lowest level that would evoke a response, yet responses were evoked in only 3 of 5 trials. Stimulus strength = 60 μA, 40 μs. Note that no response was ever elicited by a 60-nA, 30-μs stimulus in 20 consecutive trials. Stimulus frequency = 0.1 Hz. Membrane potential = −63 mV. Small circles: stimulus artifact. B: effects of stimulus strength and voltage on evoked responses. Top: responses to stimulation of the outer molecular layer of a spiny hilar cell at −61 mV. On the left is a response to a 30-μS duration stimulus (50 nA). The response to a 50-μS stimulus is shown in the next column. Longer-duration stimuli (100 μs) triggered action potentials at −61 mV and are not shown. Stimuli occurred at the dots. Center: responses of the same cell as in A to the same stimuli at −77 mV membrane potential. Bottom: responses of the same cell to the same stimuli at −93 mV membrane potential.
There was no evidence of any hyperpolarizations (IPSPs) even at membrane potentials as depolarized as −5 0 mV (Figs. 1, 4, and 6). Cells that were injected with dye dissolved in a chloride salt (which could depolarize the reversal potential of chloride-mediated IPSPs) were similar to cells impaled with KCH3COOH-filled electrodes in this respect (compare Fig. 1 with Figs. 4 and 6; Table 2). Therefore it is unlikely that use of a chloride solution made chloride-dependent IPSPs difficult to detect. Because of the large unitary size and evident lack of concomitant IPSPs, the input-output function for the spiny hilar cells was remarkably steep; the same stimulus that produced a minimal EPSP could produce one or more action potentials, and there was little difference in stimulus strength between the stimulus that evoked the minimum response and the threshold stimulus strength (Fig. 6). The frequency of stimulation was low (0.05–0.1 Hz), so that there was little possibility that one stimulus influenced the next.
FIG. 6.
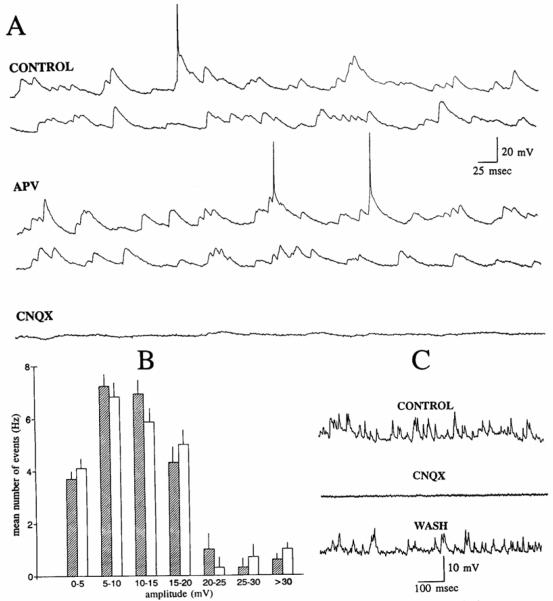
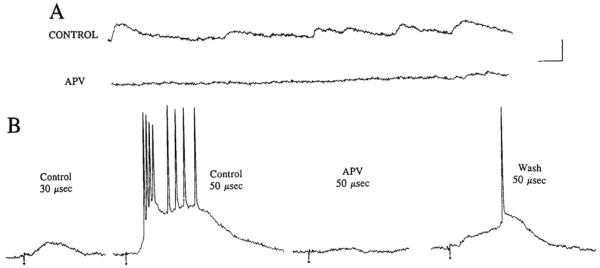
Effects of APV and CNQX on responses to outer molecular layer stimulation. CONTROL: responses to stimulation of the outer molecular layer using 4 different stimulus intensities: 40, 50, 70, and 100 μs (40 μA). Three consecutive responses (numbered 1–3) are shown in each column. Membrane potential = −60 mV. Small circles: stimulus artifacts. APV: responses of the same cell 32 minutes after 50 μM APV was added to the perfusate. There was no detectable response to a 40-μs stimulus. The responses to 2 consecutive stimuli at the other intensities are shown. Note that the responses in APV were similar qualitatively to the responses evoked in control, but higher stimulus strengths were required to evoke responses. Same membrane potential as in CONTROL. CNQX: responses of the same cell 17 min after 5 μM CNQX was added to the perfusate. Thus the perfusate contained 50 μM APV and 5 μM CNQX. There was no response to stimulation, although some spontaneous activity persisted. CNQX was added 40 min after APV perfusion began. Same membrane potential as in CONTROL.
Voltage dependence of evoked responses
When membrane potential was hyperpolarized (from approximately −50 mV to approximately −85 mV) the amplitude of evoked EPSPs increased in the majority of cells (71% of cells; Fig. 4B). EPSPs elicited by low- and high-intensity stimuli were similar in this respect (Fig. 4B). In the minority of cells that appeared to be unaffected by hyperpolarization, no change was obvious in the EPSP at all when the cell was hyperpolarized (i.e., EPSPs did not decrease in amplitude with hyperpolarization in any cell tested). Input resistance did decrease in all cells as membrane potential reached −80 to −90 mV (Scharfman and Schwartzkroin 1988), so it is likely that an increase in amplitude with hyperpolarization was underestimated.
Changes in evoked EPSPs with stimulus strength
The response to a threshold stimulus strength was similar to the response elicited at the minimum stimulus strength in that it appeared to be composed of a cluster of unitary EPSPs (Fig. 6). Threshold stimulus strength was denned as the stimulus strength that evoked action potentials in ~50% of trials. Responses tested with stimulus strengths over 1.5 times threshold stimulus strength were different from responses to lower stimulus strengths in that the underlying EPSP showed less evidence of a unitary basis and there was less variability from stimulus to stimulus (Fig. 6). This is likely to indicate more reliable activation of presynaptic neurons with higher stimulus strengths. However, another possibility is that at higher stimulus strengths, the response saturates because only a few excitatory synapses are responsible for the majority of the evoked response.
Homogeneity of evoked responses
The responses to stimulation of the outer molecular layer were similar among the cells that were sampled. Cells with and without excrescences exhibited similar evoked responses (Fig. 1; Table 2). However, in one respect the evoked responses were heterogeneous. Twenty-seven cells demonstrated a paradoxical decrease in the number of action potentials elicited by a suprathreshold stimulus (1.25–2.0 times threshold) as compared with a threshold stimulus (Fig. 8). The underlying EPSPs of cells displaying fewer action potentials were usually briefer in duration than the EPSPs of the same cells evoked in response to lower stimulus strengths (Fig. 8). This profile suggests recruitment of inhibition at higher stimulus strengths that limits the degree and duration of excitation. However, because many of the hilar cells became depolarized or unstable after long periods of stimulation using high stimulus strengths, extensive tests of the responses to high stimulus strengths were limited or avoided entirely.
FIG. 8.
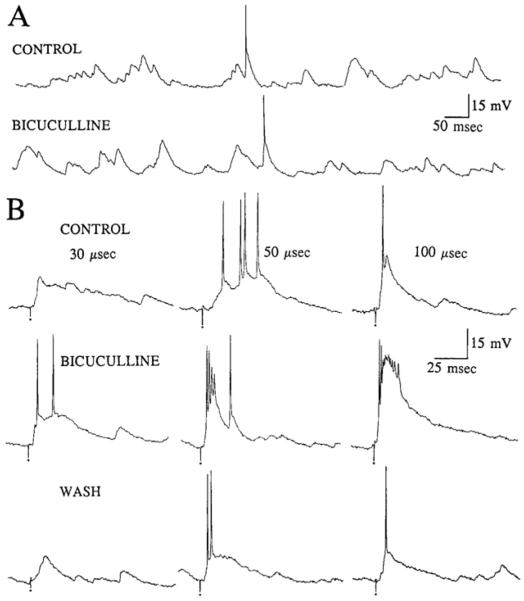
Effects of bicuculline on spontaneous and evoked activity. A: spontaneous activity of a spiny hilar cell 10 min before (CONTROL) and 25 minutes after addition of 25 μM bicuculline to the perfusate (BICUCULLINE). Membrane potential = −63 mV. B: responses to stimulation of the outer molecular layer for a different cell than that used for A. Three stimulus strengths were used: 30 μS (left). 50 μS (middle), and 100 μs (right). Small circles: stimulus artifacts. Membrane potential = −60 mV. CONTROL: representative responses to stimulation before perfusion with bicuculline. BICUCULLINE: responses of the same cell 15 min after perfusion with buffer containing 25 μM bicuculline. WASH: responses 70 min after returning to drug-free buffer.
Effects of CNQX
The effects of CNQX were evaluated on 24 cells in the presence of 2 mM extracellular magnesium. Under these conditions, CNQX was pressure applied to 9 cells and bath applied to the other 15 cells. In 10 of the bath-application experiments, CNQX was the sole drug applied to the slice; in the remaining 5, cells APV was applied to the slice before CNQX. The results of CNQX exposure did not differ between cells previously exposed to APV and cells recorded in naive slices.
SPONTANEOUS EPSPs
Initial experiments examined the effects of pressure application of CNQX (25 μM) to the proximal dendrites of spiny hilar cells to determine whether most or all of the spontaneous activity originated from thorny excrescences, which are only located on and near the cell body (Amaral 1978; Frotscher et al. 1991; Ribak et al. 1985). In these experiments, pressure pipettes were placed within 100 μM of the site where the intracellular electrode inserted into the slice. Impalements were assumed to be intrasomatic rather than intradendritic. In all nine cells, ejection of CNQX reduced the amplitude of spontaneous potentials dramatically and reversibly, but it blocked spontaneous activity completely in only two of nine cells, despite the fact thai ejections were repeated several times. These results suggest that the spontaneous activity is likely to originate from synapses located proximally as well as distally. There did not appear to be any difference between cells with and without excrescences, because of the four cells that were morphologically identified in these experiments, two were thorny and two were not.
To pursue the contribution of distal synapses to spontaneous activity, CNQX was ejected >200 μm from the cell body in the hilus and near the stimulating electrode in the outer molecular layer (n = 4 of the 9 cells). The ejection sites in the outer molecular layer were ~250 μm from the stimulating electrode. These CNQX ejections blocked most spontaneous events but did not abolish spontaneous activity. These experiments support the suggestion that spontaneous EPSPs are generated both proximal and distal to the cell body.
CNQX was bath applied to 15 other cells to determine when all spontaneous activity could be blocked if CNQX was applied to all possible receptor sites. Spontaneous activity was blocked completely in all nine cells that were held for long enough to allow the buffer containing CNQX to exchange with drug-free buffer (~15 min, given the flow rate and volume of solution to be exchanged; Fig. 6). In six other cells that were lost before CNQX-containing buffer had time to completely perfuse the slice, the majority of spontaneous EPSPs were blocked but some spontaneous activity persisted. There was no difference in the effects of the two concentrations that were used (5 μM, n = 8; 25 μM, n = 7). In two experiments where the lowest concentration of CNQX was used, it was possible to hold the cells until at least partial recovery (Fig. 5). at which point the impalements were lost. The membrane properties of the cells were unchanged at the time when CNQX had blocked spontaneous activity (Table 1).
FIG. 5.
Blockade of spontaneous activity of a spiny hilar cell by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) but not the N-methyl-d-aspartate NMDA) receptor antagonist 2-amino-5-phosphonovaleric acid (APV). A: CONTROL: continuous record of spontaneous activity of a spiny hilar cell. Membrane potential = −60 mV. APV: continuous record of spontaneous activity from the same cell as in A. recorded 45 min after 50 μM APV was added to the perfusate. Same membrane potential as in control. CNQX: continuous record from the same cell, recorded 15 min after 5 μM CNQX was added to the perfusate. CNQX was added at approximately the same time that the record in APV (shown above) was sampled. Same membrane potential as in control. B: histogram shows the mean number of spontaneous EPSPs (Hertz; mean ± SE) for the cell in A, in control (shaded bars), and 45 min after perfusion with APV (clear bars). C: spontaneous activity of a spiny hilar cell before 5 μM CNQX application (CONTROL), during bath application (CNQX), and 50 min after CNQX-free buffer was reintroduced to the slice (WASH).
EVOKED EPSPs
The effects of CNQX on evoked responses were tested on all cells where CNQX was bath applied. Responses to outer molecular stimulation were blocked by perfusion with CNQX (Fig. 6). By using a slow perfusion rate, it was possible to watch the gradual blockade of the response, and this was informative. The first detectable change was not in the responses themselves, but in the stimulus strengths required to evoke them. At this time, spontaneous activity did not appear to be affected. After continued perfusion with CNQX, responses were depressed, and eventually no responses could be evoked even when stimulus strength was set to equal the maximum stimulus strength tested before drug application (i.e., 1.25–2 times threshold stimulus strength). At this time, despite complete block of evoked responses, there was a large decrease in spontaneous activity, but it was not blocked completely (Fig. 6). Subsequent to further perfusion with CNQX, spontaneous activity was blocked completely in all cells (Fig. 5).
Effects of APV
APV was applied to 10 cells by pressure application and 15 cells by bath application, in the presence of 2 mM extracellular magnesium. In 5 of the 15 experiments where APV was bath applied, APV was followed by perfusion with CNQX.
SPONTANEOUS EPSPs
Pressure application of 100 μM APV within 100 μm of the intracellular electrode, and additional application elsewhere in the hilus and near the stimulating electrode, did not produce a detectable effect. However, a subtle effect on spontaneous activity might have been missed because of the problems inherent in quantitation of spiny hilar cell spontaneous activity.
Bath application of 50 μM APV also had no detectable effect on spontaneous activity (Fig. 5; Table 2). However, there was a detectable effect on responses to outer molecular stimulation (see below).
EVOKED EPSPs
The effects of bath-applied APV were uniform. In every cell, there was an increase in stimulus strength required to evoke a response (Fig. 6). The entire input-output curve appeared to be shifted equally. For example, in Fig. 6, the stimulus strength required to evoke a detectable response rose from 30 to 50 μs, and the stimulus strength required to reach threshold for action potential generation rose from 50 to 70 μs. The stimulus strength that was just above threshold (i.e.. the lowest stimulus strength that evoked ≥1 action potential in all trials) was increased from 70 to 90 μs. However, there was little qualitative change in the evoked responses (Fig. 6). Similar results were obtained with 25 μM (n = 5) and 50 μM APV (n = 5).
In contrast, in 15 spiny hilar cells that were part of a different study, the minimum stimulus strength required to evoke a response, and the threshold stimulus strength, did not change over 30–75 min of recording from a single cell. Furthermore, during application of 5 μM bicuculline or buffer containing 1.0 mM extracellular magnesium (see below), there was no change in the stimulus strength required to evoke a minimum response and no change in the stimulus required to reach threshold. Therefore, bath application of APV, as opposed to nonspecific changes, appears to underly the changes in evoked responses.
MANIPULATION OF EXTRACELLULAR MAGNESIUM
Spontaneous and evoked hilar cell activity was recorded under two conditions that were chosen to optimize NMDA receptor activation. Cells were examined 1) at depolarized membrane potentials and 2) in the presence of a low concentration of extracellular magnesium.
When the concentration of extracellular magnesium was 2 mM and cells were depolarized positive to −50 mV, continuous action potential discharge made changes in EPSP amplitude difficult to detect. Gradual depolarization to cause inactivation of sodium channels was not able to reduce spontaneous discharge. Moreover, cells appeared to deteriorate electrophysiologically during extended periods of depolarization; after depolarizing current injection was terminated, resting potential was relatively depolarized, input resistance decreased, action potential amplitude had decreased, and action potential duration had increased.
Low levels of extracellular magnesium (0–0.5 mM) were difficult to employ without concomitant CNQX because slices became epileptiform under these conditions, as has been reported (Anderson et al. 1986; Mody et al. 1987). No hilar cells were encountered with intracellular electrodes in those slices, although granule cells could be impaled. However, slice viability was not apparently compromised when extracellular magnesium was 1.0 mM, and hilar cells were recorded successfully under such conditions without CNQX. In these experiments, when buffer was switched from one containing 2.0 rnM to one containing 1.0 mM magnesium, there were no detectable changes in spontaneous activity or the responses to stimulation of the outer molecular layer (n = 7; Table 2). In other experiments {n = 10), buffer was perfused containing an even lower concentration of magnesium (0.5 mM, n = 5; 0 mM, n = 5) if CNQX (5 μM) was perfused simultaneously. In the experiments using 0.5 mM magnesium, absolutely no spontaneous activity nor synaptic responses to outer molecular stimulation were detected at any membrane potential between −50 and −95 mV. However, in all experiments where 0 mM magnesium was employed, spontaneous depolarizations were apparent in the presence of CNQX (Fig. 7). In addition, depolarizations that triggered action potentials could be evoked by stimulation ofthe outer molecular layer (Fig. 7). The spontaneous and evoked activity was blocked by 25 μM APV in all four cells where its effects were tested (Fig. 7).
FIG. 7.
Effects of perfusing buffer containing low concentrations of magnesium. A: Top: spontaneous activity of a spiny hilar cell when the buffer used to perfuse the slice contained 0 mM magnesium and 5 μM CNQX. Bottom: spontaneous activity of the same cell 20 min later, during perfusion with buffer containing 0 mM magnesium, 5 μM CNQX. and 25 μM APV. Top and bottom traces were recorded at the same membrane potential, −65 mV. Calibration = 5 mV, 25 ms. B: 4 responses of another cell to stimulation of the outer molecular layer. Buffer contained 0 mM magnesium and 5 μM CNQX. Membrane potential for all responses was −60 mV. Calibration (in A) = 10 mV, 25 ms. Small circles: stimulus artifacts. Control 30 μs and Control 50 μs: evoked responses of a spiny hilar cell at 2 stimulus strengths. APV 50 μs: response to a 50-μs stimulus is shown after addition of 25 μM APV to the perfusate. Wash 50 μs: response to the 50-μs stimulus is shown after returning the perfusion medium to one with 0 mM magnesium and 5 μM CNQX, but not APV.
Effects of bicuculline
The GABAA receptor antagonist bicuculline was bath applied to 10 cells (5 μM, n = 7; 25 μM, n = 3). In all cells tested, there was no detectable effect ofbicuculline on spontaneous activity at any membrane potential examined (−45 to −95 mV; Fig. 8, Table 2).
Responses elicited with suprathreshold stimulus strengths (equivalent to 1.25–2 times threshold stimulus strength) were enhanced by 5–25 μM bicuculline (Fig. 8). Specifically, the suprathreshold responses, ordinarily composed of one to three action potentials, were converted to a prolonged burst discharge (Fig. 8). This burst was similar from stimulus to stimulus, and was composed of initial fast action potential(s) followed by a large, slow depolarization (Fig. 8). Bicuculline (25 μM) also enhanced the responses to subthreshold stimulation (Fig. 8), although 5 μM bicuculline were ineffective in this regard. One possible explanation for this dose-dependent effect is that two mechanisms of GABAA receptor-mediated inhibition are exerted on spiny hilar cells, one primarily exerting its effects at high stimulus strengths and the other even at low stimulus strengths.
DISCUSSION
Summary
The results documented several aspects of excitatory circuitry involving spiny hilar neurons. First, the results described the spontaneous and evoked excitation of spiny hilar cells, which was quite similar among the spiny hilar cells sampled, and quite different from other hippocampal cells. Excitatory potentials were composed of large, unitary-like events, lacked hyperpolarizations, and responded to changes in stimulus strength in an unusual manner (i.e., steep input-output curve, several action potentials at threshold, often fewer action potentials at suprathreshold stimulus strengths). The high level of spontaneous activity also contrasted with other hippocampal neurons.
The results also demonstrated the effects of excitatory amino antagonists on spiny hilar cell excitation. The experiments showed that AMPA/kainate receptor activation contributed to spontaneous and evoked EPSPs, and that NMDA receptors contribute as well, particularly when external magnesium was low. The use of the GABA antagonist bicuculline showed that GABAergic depolarizations are not involved in excitation. However, GABAergic inhibition does play a role in the circuitry.
AMPA/kainate receptors
One of the major findings of this study was that the selective antagonist of the AMPA/kainate receptor, CNQX, blocked all spontaneous events and evoked responses to outer molecular layer stimulation when perfused onto the slice. The voltage dependence of spontaneous and evoked EPSPs was consistent with events mediated by AMPA/kainatc receptors on the spiny hilar cells, as opposed to NMDA receptors, because EPSPs increased in amplitude with hyperpolarization. The selective antagonist of the NMDA receptor, APV, had very little effect unless 0 mM external magnesium was used. Bicuculline, a GABAA receptor antagonist, had no detectable effects on spontaneous activity and facilitated EPSPs. Therefore any contribution of GABA to synaptic depolarizations is unlikely.
These results are similar to those described by Livesy and Vicini (1992), who examined spontaneous excitatory postsynaptic currents (EPSCs) in hilar neurons. They recorded from hilar cells of immature rat (11-26 days postnatal) using whole-cell recording techniques at room temperature. They also found that spontaneous EPSCs were composed of large, variable events. EPSCs evoked by glutamate ejection on or near granule cells were consistently blocked by CNQX, and effects of APV were found in only a few exceptional cases. Therefore it appears that in both the immature animal and the adult, and regardless of different recording techniques and temperature differences, AMPA/ kainate receptors are important to excitation of spiny hilar neurons.
It is likely that the relevant AMPA/kainate receptors and NMDA receptors (as discussed below) that are responsible for spontaneous EPSPs are located on the dendrites of spiny hilar cells, because spontaneous EPSPs are unaffected by tetrodotoxin and therefore presynaptic inputs (such as spontaneous firing of granule cells). However, the receptors that contribute to evoked EPSPs could be located in several areas of the local circuitry. Perhaps the most obvious possibility is that the receptors could be located on the spiny hilar cell dendrites, postsynaptic to excitatory input. However, AMPA/kainate receptors that are located presynaptic to the hilar cells, such as on the granule cells, are important to consider. Because AMPA/kainate receptors and NMDA receptors on granule cell dendrites mediate granule cell excitation to stimulation of the perforant path (Lambert and Jones 1990), blockade of those receptors could prevent excitation of granule cells as well as the targets of granule cells, i.e., the hilar neurons. Receptors that contribute to EPSPs of hilar cells may also be located on the terminals of the neurons presynaptic to the hilar cell. The above experiments do not discriminate among these possibilities.
NMDA receptors
Although there was evidence that NMDA receptors contributed to spiny hilar cell excitation, this contribution was minor in the presence of 0.5-2.0 mM extracellular magnesium. Thus, when extracellular magnesium was 1.0 mM, there was no detectable effect of APV on spontaneous potentials and only a small increase in stimulus strength required to produce responses to outer molecular layer stimulation. This weak evidence for NMDA receptors was difficult to reconcile with the efficacy of APV in blocking the deleterious effects of prolonged perforant path stimulation (Scharfman and Schwartzkroin 1989). However, experiments using nominally 0 mM magnesium revealed APV-sensitive spontaneous and evoked responses. Thus functional NMDA receptors do appear to exist on hilar cells, and the degree of magnesium block of the receptor is critical for their activation. It is possible that, during prolonged stimulation in 2 mM extracellular magnesium, depolarization mediated by AMPA/kainate receptors unblocks NMDA receptors.
GABA receptors
The effects of the GABAA receptor antagonist bicuculline on spontaneous and evoked responses were examined to determine whether some of the depolarizing postsynaptic potentials (PSPs) were mediated by GABA. Although a depolarizing potential mediated by GABA is unusual, there is precedence for it nevertheless (Alger and Nicoll 1982; Avoli and Perreault 1987). Moreover, it has been shown that GABA may be excitatory among hilar neurons (Michelson and Wong 1991). The innervation of hilar cells by GABAergic neurons is likely, given the number of GABA-immunoreactivc fibers and varicosities in the hilus (Gamrani et al. 1986; Woodson et al. 1989) and that GABA-immunoreactive terminals surround the processes of hilar cells (Misgeld and Frotscher 1986). Furthermore, bicuculline and baclofen alter the physiology of hilar cells in guinea pig hippocampal slices (Misgeld and Frotscher 1986; Misgeld et al. 1989). Even though CNQX blocked depolarizing EPSPs and EPSPs evoked by outer molecular layer stimulation, it was still possible that some of the PSPs were mediated by GABAergic neurons that were activated by excitatory amino acids. Since bicuculline blocks not only GABAA receptor-mediated IPSPs, but also depolarizing responses to exogenously applied GABA (Alger and Nicoll 1982), and synaptic depolarizations mediated by GABA (Avoli and Perreault 1987), bicuculline was a logical choice to examine hilar cell depolarizations that might be mediated by GABA.
Although these studies provided no evidence that GABAergic synaptic depolarizations were involved in spontaneous or stimulus-evoked EPSPs, they did demonstrate a role of GABA in spiny hilar circuitry. Specifically, the results provided evidence that inhibition normally limits excitation. One indication of this action was in the comparison of evoked responses using different stimulus strengths. It was often the case that fewer action potentials and briefer EPSPs were evoked by higher stimulus strengths than the stimulus strength required to reach threshold. Therefore recruitment of GABAergic inhibition may occur at high stimulus strengths. This possibility was supported by the data showing that low doses of bicuculline preferentially enhanced the excitatory response evoked with high stimulus strengths. However, the effects of synaptically released GABA are undoubtedly complex, because higher concentrations of bicuculline could enhance subthreshold responses also; the latter effect could be due to blockade of feedforward inhibition. Therefore GABA does play a role in hilar cell circuitry, but it does not appear to contribute to the spontaneous or evoked depolarizations.
Similarity of excitation among spiny hilar cells
Spontaneous activity and perforant path-evoked responses were very similar among spiny hilar cells, regardless of their different locations within the hilus and regardless of morphological variation. One important comparison was among the spiny cells with thorny excrescences on their dendrites, the cells that correspond best to mossy cells described by Amaral (1978), and the spiny hilar cells that lacked excrescences. Because the two variations of spiny hilar cell were similar in their EPSPs, it did not appear that the excrescence played a determining role in spiny hilar cell excitation. This conclusion was further supported by the inability of pressure application to the proximal dendrites to totally block spontaneous activity; thus the thorny excrescences that are situated proximally do not have a monopoly on spontaneous activity. Instead, it is likely that spontaneous activity emerges from many different sites along the dendrites of spiny hilar cells.
There were some subtle differences between spontaneous EPSPs and EPSPs evoked by stimulation of the outer molecular layer. For example, spontaneous EPSPs displayed weak voltage dependence compared with evoked EPSPs. Interestingly, unitary EPSPs evoked by action potentials in granule cells were voltage dependent (Scharfman et al. 1990), behaving similarly to EPSPs evoked by outer molecular layer stimulation. One possible reason for the weak voltage dependence of spontaneous EPSPs relative to evoked EPSPs is that spontaneous EPSPs represent the activity of a population of synapses on hilar cells that may be constantly changing. In contrast, evoked responses are more likely to represent activation of synapses that are similar from stimulus to stimulus.
Spontaneous EPSPs were also different from evoked responses in that spontaneous EPSPs were relatively resistant to CNQX. These differences indicate that there may be a subtle difference in transmitter release, site of action, or the receptor population that mediates spontaneous release and the release evoked by outer molecular layer stimulation or granule cells action potentials. It indicates that transmitter release after action potential invasion may have slightly different consequences than transmitter release occurring spontaneously. These intriguing but somewhat heretical possibilities necessitate further experimentation before they can be explained.
On the origin of spontaneous and evoked EPSPs
Because of the strong innervation of hilar cells by granule cells (Claiborne et al. 1986), and the similarity of the spontaneous and evoked EPSPs of spiny hilar cells to the unitary EPSPs documented in simultaneous paired recording between granule cells and spiny hilar cells (Scharfman et al. 1990), it is likely that the spontaneous and evoked EPSPs of hilar cells are due to granule cells. Another, albeit simplistic, argument for a role of granule cells in the large EPSPs of hilar cells is that the granule cells have the complex, large terminals that one might expect would be involved in such large, complex EPSPs. However, the perforant path input is also likely to contribute to the EPSPs, because the perforant path synapses directly onto hilar cells with dendrites in the molecular layer (Léránth et al. 1990). Other excitatory inputs that have not been identified to date may also be involved in the EPSPs occurring subsequent to molecular layer stimulation. Further experiments will be necessary to differentiate the contribution and mechanisms of hilar cell excitation by their various excitatory inputs.
Acknowledgments
I thank Drs. R. S. Sloviter. D. H. Lowenstein. and J. H. Goodman for comments on the manuscript, and R. Marshall for secretarial assistance. I also thank Dr. P. R. Adams and the Howard Hughes Medical Institute for the use of their confocal microscope.
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS-30831.
REFERENCES
- ALGER BL, NICOLL RA. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J. Physiol. Lond. 1982;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMARAL DG. A Golgi study of cell types in the hilar region of the hippocampus in rat. J. Comp. Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- AMARAL DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anal. Embryol. 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- ANDERSON WW, LEWIS DV, SWARTZWELDER HS, WILSON WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986;398:215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- ASSAF SY, CRUNELLI V, KELLY JS. Depolarizing postsynaptic actions of GABA in the rat dentate gyrus. In: DeFeudis FV, Mandel P, editors. Amino Acid Neurotransmitters. Raven; New York: 1981. pp. 239–248. [PubMed] [Google Scholar]
- AVOLI M, PERREAULT P. A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 1987;400:191–195. doi: 10.1016/0006-8993(87)90671-8. [DOI] [PubMed] [Google Scholar]
- BAKST I, AVENDANO C, MORRISON JH, AMARAL DG. An experimental analysis of the origins of somatostatin-likeimmunoreactivity in the dentate gurus of the rat. J. Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKSTAD TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J. Comp. Neurol. 1956;105:417–538. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- CLAIBORNE BJ, AMARAL DG, COWAN WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- COLLINGRIDGE GL, LESTER RAJ. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol. Rev. 1989;40:143–210. [PubMed] [Google Scholar]
- CRAIN BJ, WESTERKAM WD, HARRISON AH, NADLER JV. Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- DAHL D, BURGARD EC, SARVEY JM. NMDA receptor antagonists reduce medial but not lateral perforant path-evoked EPSPs in dentate gyrus of rat hippocampal slice. Exp. Brain Res. 1990;83:172–177. doi: 10.1007/BF00232206. [DOI] [PubMed] [Google Scholar]
- DE LANEROLLE NC, KIM JH, ROBBINS RJ, SPENCER DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- FRICKE RA, PRINCE DA. Electrophysiology of dentate gyrus granule cells. J. Neurophysiol. 1984;51:195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- FROTSCHER M, SERESS L, SCHWERDTFEGER W, BUHL E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J. Comp. Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- GAMRANI H, ONTENIENTE B, SEGUELA P, GEFFARD M, CALAS A. Gamma-aminobutyric acid-immunoreactivity in the rat hippocampus. A light and electron microscopic study with anti-GABA antibodies. Brain Res. 1986;364:30–38. doi: 10.1016/0006-8993(86)90984-4. [DOI] [PubMed] [Google Scholar]
- HJORTH-SIMONSEN A, JEUNE B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J. Comp. Neurol. 1972;144:215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- JOHANSEN FF, ZIMMER J, DIEMER NH. Early loss of somato-statin neurons in dentate hilus after cerebral ischemia in the rat precedes CA-1 pyramidal cell loss. Acta Neuropathol. 1987;73:110–114. doi: 10.1007/BF00693775. [DOI] [PubMed] [Google Scholar]
- KAWAGUCHI Y, HAMA K. Fast-spiking non-pyramidal cells in the hippocampal CA3 region, dentate gyrus and subiculum of rats. Brain Res. 1987;425:351–355. doi: 10.1016/0006-8993(87)90518-x. [DOI] [PubMed] [Google Scholar]
- KOSAKA T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983;277:347–351. doi: 10.1016/0006-8993(83)90943-5. [DOI] [PubMed] [Google Scholar]
- KOSAKA T, KOSAKA K, TATEISHI K, HAMAOKA Y, YANAIHARA N, WU J-Y, HAMA K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J. Comp. Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- KOSAKA T, WU J-Y, BENOIT R. GABAergic neurons containing somatostatin-like immunoreactivity in the rat hippocampus and dentate gyrus. Exp. Brain Res. 1988;71:388–398. doi: 10.1007/BF00247498. [DOI] [PubMed] [Google Scholar]
- LAATSCH RH, COWAN WM. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J. Comp. Neurol. 1966;128:359–396. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- LAMBERT JDC, JONES RSG. A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J. Neurophysiol. 1990;64:119–132. doi: 10.1152/jn.1990.64.1.119. [DOI] [PubMed] [Google Scholar]
- LAURBERG S, SØRENSEN KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentate and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- LERANTH C, MALCOLM AJ, FROTSCHER M. Afferent and efferent synaptic connections of somatostatin-immunoreactive neurons in the rat fascia dentata. Comp. Neurol. 1990;295:111–122. doi: 10.1002/cne.902950110. [DOI] [PubMed] [Google Scholar]
- LIVESY CT, VICINI S. Slower spontaneous excitatory postsynaptic currents in spiny versus aspiny hilar neurons. Neuron. 1992;8:745–755. doi: 10.1016/0896-6273(92)90095-u. [DOI] [PubMed] [Google Scholar]
- LOPES DA SILVA FH, WITTER MP, BOEIJINGA PH, LOHMAN AHM. Anatomic organization and physiology of the limbic cortex. Physiol. Rev. 1990;70:453–511. doi: 10.1152/physrev.1990.70.2.453. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN DH, THOMAS MJ, SMITH DH, MCINTOSH TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGERISON JH, CORSELLIS JAN. Epilepsy and the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- MAYER ML, WESTBROOK GL, GUTHRIE PB. Voltage dependent block by magnesium of NMDA responses in spinal cord neurones. Nature Lond. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- MICHELSON H, WONG RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science Wash. DC. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- MISGELD U, FROTSCHER M. Postsynaptic-GABAergic inhibition of non-pyramidal neurons in the guinea-pig hippocampus. Neuroscience. 1986;19:193–206. doi: 10.1016/0306-4522(86)90015-1. [DOI] [PubMed] [Google Scholar]
- MISGELD U, MÜLLER W, BRUNNER H. Effects of (-) baclofen on inhibitory neurons in the guinea pig hippocampal slice. Pfluegers Arch. 1989;414:139–144. doi: 10.1007/BF00580955. [DOI] [PubMed] [Google Scholar]
- MODY I, LAMBERT JDC, HEINEMANN U. LOW extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J. Neurophysiol. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- MONAGHAN DT, HOLETS VR, TOY DW, COTMAN CW. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature Lond. 1983;306:176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- NICOLL RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science Wash. DC. 1988;241:239–254. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- NOWAK L, BREGESTOVSKI P, ASCHER P, HERBERT A, PRO-CHIANTZ A. Magnesium gates glutamate activated channels in mouse central neurones. Nature Lond. 1984;307:462–464. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- OTTERSEN OP, STORM-MATHISEN J. Excitatory and inhibitory amino acids in the hippocampus. In: Chan-Palay V, Köhler C, editors. The Hippocampus: New Vistas. Liss; New York: 1989. pp. 97–117. [Google Scholar]
- RIBAK CE, SERESS L, AMARAL DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J. Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- ROBERTS GW, WOODHAMS PL, POLAK JM, CROW TJ. Distribution of neuropeptides in the limbic system of the rat: the hippocampus. Neuroscience. 1984;11:35–77. doi: 10.1016/0306-4522(84)90214-8. [DOI] [PubMed] [Google Scholar]
- SCHARFMAN HE. Excitatory transmission from dentate granule cells to hilar “mossy” cells is mediated primarily by quisqualatc/kainate receptors. Epilepsia. 1991a;32:48. [Google Scholar]
- SCHARFMAN HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J, Neurosci. 1991b;11:1660–1773. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFMAN HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny “fast-spiking” cells. In: Ribak CE, Gall C, Mody I, editors. The Role of the Dentate Gyrus in Seizures. Elsevier; Amsterdam: 1992. pp. 93–109. [PMC free article] [PubMed] [Google Scholar]
- SCHARFMAN HE. Spiny neurons of area CA3c of rat hippocampal slices have similar electrophysiological characteristics and synaptic responses despite morphological variation. Hippocampus. 1993;3:9–28. doi: 10.1002/hipo.450030103. [DOI] [PubMed] [Google Scholar]
- SCHARFMAN HE, KUNKEL DD, SCHWARTZKROIN PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- SCHARFMAN HE, SCHWARTZKROIN PA. Electrophysiology of morphologically identified mossy cells recorded in the dentate hilus of guinea pig hippocampal slices. J. Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFMAN HE, SCHWARTZKROIN PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science Wash. DC. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- SCHARFMAN HE, SCHWARTZKROIN PA. Consequences of prolonged afferent stimulation of the rat fascia dentata: epileptiform activity in area CA3 of hippocampus. Neuroscience. 1990b;35:505–517. doi: 10.1016/0306-4522(90)90325-x. [DOI] [PubMed] [Google Scholar]
- SCHARFMAN HE, SCHWARTZKROIN PA. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990a;35:491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- SCHWARTZKROIN PA, MUELLER A. Electrophysiology of hippocampal neurons. In: Jones EG, Peters A, editors. Cerebral Cortex, Further Aspects of Cortical Function, Including Hippocampus. Vol. 6. Plenum; New York: 1987. pp. 295–343. [Google Scholar]
- SLOVITER RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res. Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- SLOVITER RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science Wash. DC. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- SLOVITER RS. Permanently altered hippocampal structure, excitability and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- SLOVITER RS, NILAVER G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J. Comp. Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- STEWARD O, SCOVILLE SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Comp. Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- STORM-MATHISEN J, WOLD J,E, In vivo high-affinity uptake and axonal transport of D-[2,3-3H] aspartate in excitatory neurons. Brain Res. 1981;230:427–435. doi: 10.1016/0006-8993(81)90428-5. [DOI] [PubMed] [Google Scholar]
- STROWBRIDGE BW, BUCKMASTER PS, SCHWARTZKROIN PA. Potentiation of spontaneous synaptic activity in rat mossy cells. Neurosci.Lett. 1992;142:205–210. doi: 10.1016/0304-3940(92)90374-g. [DOI] [PubMed] [Google Scholar]
- SWANSON LW, SAWCHENKO PE, COWAN WM. Evidence for collateral projections by neurons in Ammon’s Horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J. Neurosci. 1981;5:548–559. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSON L, WYSS JM, COWAN WM, An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J. Comp. Neurol. 1978;181:681–710. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- WHITE WF, NADLER J,V, HAMBERGER A, COTMAN CW. Glutamateas transmitter of hippocampal perforant path. Nature Lond. 1977;270:356–357. doi: 10.1038/270356a0. [DOI] [PubMed] [Google Scholar]
- WOODSON W, NITECKA L, BEN-ARI Y. Organization of the GABAergic system in the rat hippocampal formation: a quantitative immunocyiochemical study. J. Comp. Neurol. 1989;280:254–271. doi: 10.1002/cne.902800207. [DOI] [PubMed] [Google Scholar]
- ZIMMER J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J. Comp. Neurol. 1971;142:393–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]