Abstract
The prevalence of overweight and obese children is increasing, a tendency that can be expected to increase the risk of adverse outcomes in adulthood. The aim of this study was to determine if prenatal exposure to alcohol, cigarettes, and street drugs would be associated with differences in body mass index (BMI) in childhood and adolescence in offspring from families at high and low genetic risk for developing alcohol dependence. Annual follow-up of offspring (N =288) provided 1200 height and weight assessments for analysis. Maternal substance use data were available for 235 offspring from families stratified for familial/genetic risk for alcohol dependence (high or low risk), providing the opportunity to assess prenatal exposure and familial/genetic risk in relation to BMI in the offspring. When data were grouped by the presence or absence of any prenatal cigarette exposure, a significant difference in offspring BMI was seen for 8- to 11-year-olds. Significant group differences were also seen at ages 12–15 and 16–18 years. A dose–response relationship between cigarette use by the mother and offspring BMI was also seen. With the strong tendency for individuals who are overweight in childhood and adolescence to become overweight adults, prenatal exposure to nicotine may be a harbinger of increased risk for numerous adult-onset, weight-related health problems.
Keywords: Risk for obesity, Childhood obesity, Prenatal smoking, Prenatal use of substances, Body mass index, Genetic susceptibility, Familial risk for alcohol dependence
1. Introduction
It is now well recognized that prenatal exposure to alcohol and cigarettes plays a critical role in determining newborn body size and head circumference (Jacobson et al., 1994). An association between maternal smoking during pregnancy and low birth weight has been recognized for some time now (Butler et al., 1972). The long-term effects of exposure to alcohol and cigarettes are not so well understood. A few longitudinal studies of prenatal exposure to alcohol and other substances find shorter height and lower body weight during childhood and adolescence in exposed offspring (Day et al., 1999, 2002; Streissguth et al., 1994). Recently, attention has also focused on increased weight or body mass index (BMI) during childhood in association with prenatal exposure to cigarettes.
Cross-sectional studies have demonstrated significant elevations in the odds that young offspring (5–7 years) of mothers who smoked during pregnancy would meet criteria for being overweight or obese (von Kries et al., 2002; Toschke et al., 2002; Wideroe et al., 2003). Two of the studies were based on data from German children living in both urban and rural communities (von Kries et al., 2002; Toschke et al., 2002). These studies reported increased odds that children would be overweight (OR: 1.43, CI: 1.07–1.90; and OR: 1.58, CI: 1.23–2.04, respectively) or obese (OR: 2.06, CI: 1.31–3.23; and OR: 1.92, CI: 1.29–2.86, respectively) if their mothers had smoked during pregnancy. Similar findings were reported by Wideroe et al. (2003) using a random sample of mothers and their 5-year-old children studied in Trondheim, Bergen (Norway), and Uppsala (Sweden). This study adjusted for maternal diet, breast-feeding, maternal obesity, and socioeconomic status, finding an increased risk for being overweight (OR: 2.9, CI: 1.3–6.6) in association with maternal smoking. Another study utilized a longitudinal birth cohort initiated in 1958, which included all individuals born in England, Wales, and Scotland (Power and Jefferis, 2002). This study also found increased odds that offspring (1.55, 95% CI: 1.19–2.00 for men; and 1.44, 95% CI: 1.13–1.84 for women) would be obese by age 33 years if their mothers had smoked during pregnancy.
With a steady increase in the prevalence of overweight children in the past two decades, obesity has recently been considered an epidemic (Strauss and Pollack, 2001; Troiano et al., 1995). Moreover, obesity in childhood/adolescence often leads to obesity in adulthood along with its attendant health problems (Whitaker et al., 1997). Because obesity is a major public health problem, understanding the possible relationship between prenatal exposure to substances and BMI is of considerable importance. However, there are obstacles to finding an answer to this question. Considerable overlap can be expected among mothers who use substances during pregnancy and those having a genetic susceptibility for one or more psychiatric disorders, particularly those with susceptibility for a substance use disorder (Hill et al., 2000). It has now been well established that individuals with substance use disorders differ from controls on several personality dimensions including behavioral disinhibition, a trait characterized by greater impulsivity and sensation seeking. It is well known that individuals with alcohol dependence exhibit higher scale scores on this trait than do nonalcoholics (Hill et al., 1990; McGue et al., 1997; Conway et al., 2002, 2003). Genetic transmission of disinhibition across generations might result in a greater likelihood that the offspring would be disinhibited with respect to substance use and food consumption. Greater likelihood of having an eating disorder appears to be associated with increased familial/genetic risk for substance use disorders (Schuckit et al., 1996; Holderness et al., 1994; Molgaard et al., 1989; Bulik, 1987; Carlat et al., 1997).
The present report is based on analyses of data obtained from two longitudinal family studies that contrast families of individuals with alcohol dependence and controls. These studies have made it possible to track the growth and development of children, adolescents, and young adults with familial backgrounds predisposing them to alcohol dependence (high-risk families) or those without such background (low-risk controls) and to evaluate the effects of maternal use of alcohol, cigarettes, and street drugs on offspring BMI. The specific goals of the analyses were to determine if significant differences in BMI would be seen in association with familial/genetic risk for alcohol dependence; to determine if prenatal exposure to alcohol, cigarettes, or street drugs would be associated with significant differences in BMI; and to test the interactive effects of prenatal exposure and familial risk on body mass index.
2. Methods
2.1. Subjects
A total of 288 children/adolescents (ages 8–18 years), who were either at high risk (n =201) or low risk (n =87) for developing alcohol dependence, were assessed multiple times (usually annually) as part of two longitudinal family studies that focus on alcoholism susceptibility (see Section 2.2). The present analyses are based on a total of 807 high-risk and 393 low-risk evaluations performed over a 10-year period.
Because available offspring from appropriate pedigrees were entered into the study at varying ages, the offspring included in the analyses completed a varying number of assessments (some children have completed their 10th annual evaluation). Analyses were based on the maximum number of repeated data assessments for each child. On average, 4.9 assessments per child have been conducted. Data for an approximately equal number of males and females within each risk group were available (high risk: 97 males and 104 females; low risk: 45 males and 42 females). Analyses were planned around three developmental periods approximately corresponding to prepubertal, pubertal, and late adolescent stages (ages 8–11, 12–15, and 16–18 years). (Tanner staging was not performed to determine pubertal status, but the 8- to 11-year-old age range was judged to be approximately equal to prepubertal status for the children included in the analysis.)
2.2. Description of family studies
These high- and low-risk (control) children/adolescents were participants in one of two family studies (Cognitive and Personality Factors in Relatives of Alcoholics and the Biological Risk Factors in Relatives of Alcoholic Women), which are currently on-going. In both studies, probands in the high-risk families were recruited from substance abuse treatment facilities in the Pittsburgh area. Probands were enrolled in treatment programs at the time of ascertainment. More than 5000 high-risk families have been screened through an available proband to net the cooperative families upon which the present report is based. The large number of families screened in treatment centers was the result of the need to find “double proband” families for the broader goals of the family study, which includes gene-finding efforts (Hill et al., 2004).
Families who lived in the Pittsburgh area were given preference for entry into the study so that it would be possible to conduct in-person interviews. All participants received detailed explanations of the study and signed a consent form before data were collected. The overall minority rate in our series of families is 13%.
2.3. Inclusion and exclusion criteria for high-risk families
Probands were selected if a pair of same-sexed adult siblings with a diagnosis of alcohol dependence (DSM-III and Feighner Criteria) was present in the family (a pair of adult alcohol-dependent sisters for the Biological Risk Factors in Relatives of Alcoholic Women study, or a pair of alcohol-dependent brothers in the Cognitive and Personality Factors in Relatives of Alcoholics study). The rationale for having initiated the studies through a double proband sampling scheme was based on the observation that restricting family ascertainment to multiplex families increases the likelihood of studying a severe form of a disorder, and increases the likelihood of finding biological markers (including genetic polymorphisms) for disease susceptibility (Morton and Mi, 1968; Anderson et al., 1986; Seidman et al., 2002; Smalley et al., 2000). Because of the requirement for multiplex sibships for the parental generation reported here, only approximately 1–2% of individuals approached in treatment centers qualified for participation. The infrequent eligibility of alcohol-dependent families was due to the multiplex sampling strategy and the requirement for minimal comorbidity. The minor offspring of the proband pair or their adult siblings from these two family studies are currently being followed in longitudinal initiatives and are the subject of this report. Considerable pedigree information is available for these offspring, who represent the third generation studied in our laboratory. Pedigree information for the offspring reveals an average of four first- and second-degree relatives with alcohol dependence, greatly increasing the risk for alcohol dependence and other substance use disorders.
2.4. Psychiatric assessment methods for adult pedigree members
Both studies used a structured psychiatric interview [Diagnostic Interview Schedule (DIS)] (Robins et al., 1981) to provide DSM-III, Axis I (American Psychiatric Association, 1980) diagnoses for adult members of the pedigree so that exclusion criteria for potential families could be applied. (The family studies were initiated at a time when DSM-III criteria were in use.) Exclusion criteria included the presence of recurrent major depressive disorder (MDD), bipolar disorder (BD), primary drug dependence (PDD) (i.e., drug dependence preceded alcohol dependence by one or more years), or schizophrenia in any first-degree relative.
The Cognitive and Personality Factors in Relatives of Alcoholics study used in-person interviews with all proband pairs and the majority of first-degree relatives of probands, while unavailable relatives were diagnosed using a minimum of two family history reports. The Biological Risk Factors in Relatives of Alcoholic Women family study also obtained diagnostic information for the proband pair using in-person interviews. First-degree relatives of the proband pair of sisters were assessed using family history methods. Similar to the Cognitive and Personality Factors family study, this study of alcohol-dependent women and their family members also attempted to limit comorbidity within families by requiring that the pair of alcohol-dependent probands have alcohol dependence as their primary disorder. If either of the pair met criteria for major depressive disorder or drug dependence, a 1-year interval of meeting criteria for alcohol dependence before developing the comorbid disorder was required.
2.5. Selection of control families
Two methods for selecting control families have been used in these family studies. In the first case, control families were selected through census tract data. Specifically, for each high-risk family studied, the place of residence was checked for census tract information to draw a control family from the same census track. Participation was solicited by phone to determine if the resident had a child between the ages of 8 and 18 years, and if there was sufficient interest in the study to warrant sending an introductory letter describing the study. Positive responses were followed with a letter of introduction and invitation for participation. These controls were not screened for the presence of psychiatric diagnoses and were designated as “unscreened” census-matched controls. In the second method, persons expressing an interest in the study were screened for the absence of all of the comorbid conditions screened from the high-risk families as well as alcohol and drug dependence among the proband’s first-degree relatives (parents and siblings). The present report is based on the third-generation offspring from those control families where the highest level of familial/genetic screening occurred (parents, aunts, uncles, and grandparents of third-generation offspring did not meet DSM, Axis I criteria for any psychiatric disorder including alcohol dependence).
2.6. Evaluation of prenatal exposure
Each mother was administered a structured interview concerning her alcohol, cigarette, and other drug use during each of her pregnancies so that the quantity and frequency of these substances could be determined. (Family history report from the father of the child was used in approximately 5% of the cases.) The interview format was developed in our laboratory and was designed to measure typical and maximal daily use by obtaining information for each of several substances, noting the quantity per occasion and the frequency of use. Daily use was multiplied by the number of days in each trimester and accumulated for all three trimesters, allowing for the total amount used throughout pregnancy to be calculated. If the mother had multiple children, she was queried concerning each child separately and asked to recall her use during each trimester. Most mothers reported that they decreased their ethanol intake by the second and third trimesters. The median consumption of ethanol during the entire pregnancy was 45 drinks (1 drink=12 oz of beer, 6 oz of wine, or 1.5 oz of liquor). As expected, drinking was more common among mothers from high-risk families, with 43.3% of these mothers reporting use of alcohol during pregnancy whereas 14.3% of the mothers of the low-risk children reported drinking at least one drink during pregnancy. Smoking during pregnancy was also more common among mothers from high-risk backgrounds with 56.6% reporting ever smoking during pregnancy, while only 17% of control mothers reported smoking while pregnant. Drug use was relatively uncommon, with the majority of mothers reporting no use during pregnancy (80.7%). Because drug use involved varying quantities taken by various routes (smoking, intravenous, and snorting), no attempt was made to analyze the data using quantity estimates. Rather, the number of days any drug was used was calculated and used in the analyses. Frequency of drug use during the entire pregnancy was used to analyze the impact of drug exposure on offspring BMI. Over the course of pregnancy, the entire group of mothers (high risk and low risk) reported drinking an average of 180 drinks (range 3–2160), smoking an average of 3535 cigarettes (range 30–16,200), and using drugs for an average of 62 days (range 1–270 days).
2.7. Determination of body mass index
At each annual evaluation, the child was weighed and height was determined by research staff. Before data analysis, body mass index (BMI) was calculated for each child using the formula: BMI=weight (lb)/height (in.)2 × 703. (The outcome measure analyzed was the log body mass index.)
2.8. Data analysis
To evaluate the effects of prenatal exposure to alcohol, cigarettes, and other drugs, level of familial loading for alcohol dependence (high risk or low risk), and the interaction of these variables on outcome (BMI) during childhood and adolescence, data were analyzed within three developmental periods corresponding to prepubertal, pubertal, and late adolescent stages (n =235). Separate analyses were performed for each stage. (A preliminary analysis using all of the data had revealed significant effects due to age.) Exposure was measured using maternal use of alcohol, cigarettes, and drugs expressed first as a dichotomous variable (any use/no use), and second, as a dose–response effect. Repeated measures analyses were performed using the acquired data. Maximum likelihood estimates were used to estimate the parameters for a general class of models where the expected values were arbitrary linear functions of a set of regression parameters and the within-subject covariates were modeled as a set of unknown covariance parameters with unstructured covariances (BMDP 5V).
In our initial model (Model 1), analyses were performed for each age group using familial risk status, prenatal exposure (sequentially using alcohol, cigarettes, and drugs), gender, family ID (a covariate that was used to control for the nonindependence of data from siblings), diagnosis of depression in the mother (MDD diagnosis by DSM-III), and the interaction of risk and gender as covariates (Table 1). A family ID variable was included to control for the fact that siblings from the same family cannot be considered to be independent samples. Also, analyses were performed to test for dose–response effects by grouping alcohol, cigarette, and drug use into three groups of approximately equal size based on the 25th, 50th, and 75th percentiles.
Table 1.
The effect of alcohol, cigarette, and drug exposure during pregnancy and child/adolescent BMI
| 8–11 years
|
12–15 years
|
16–18 years
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| df | χ2 | P | df | χ2 | P | df | χ2 | P | |
| Alcohol use | 1 | 0.55 | ns | 1 | 0.001 | ns | 1 | 0.03 | ns |
| Age | 3 | 121.39 | <0.0001 | 3 | 86.97 | <0.0001 | 2 | 19.36 | 0.001 |
| Gender | 1 | 0.24 | ns | 1 | 0.06 | ns | 1 | 1.36 | ns |
| Family type | 1 | 1.06 | ns | 1 | 1.69 | ns | 1 | 3.11 | ns |
| Sibling | 1 | 0.20 | ns | 1 | 0.05 | ns | 1 | 0.34 | ns |
| Depression in mother | 1 | 0.90 | ns | 1 | 0.17 | ns | 1 | 0.09 | ns |
| Gender × family type | 1 | 1.38 | ns | 1 | 0.98 | ns | 1 | 1.76 | ns |
| Cigarettes | 1 | 9.94 | 0.002 | 1 | 8.16 | 0.004 | 1 | 4.00 | 0.05 |
| Age | 3 | 118.44 | <0.0001 | 3 | 96.78 | <0.0001 | 2 | 17.96 | <0.0001 |
| Gender | 1 | 0.05 | ns | 1 | 0.009 | ns | 1 | 0.57 | ns |
| Family type | 1 | 0.11 | ns | 1 | 0.26 | ns | 1 | 1.08 | ns |
| Sibling | 1 | 0.55 | ns | 1 | 0.26 | ns | 1 | 0.12 | ns |
| Depression in mother | 1 | 0.01 | ns | 1 | 1.80 | ns | 1 | 0.15 | ns |
| Gender × family type | 1 | 0.47 | ns | 1 | 0.30 | ns | 1 | 0.61 | ns |
| Drugs | 1 | 0.08 | ns | 1 | 0.27 | ns | 1 | 8.29 | 0.004 |
| Age | 3 | 115.08 | <0.0001 | 3 | 97.28 | <0.0001 | 2 | 19.34 | <0.0001 |
| Gender | 1 | 0.14 | ns | 1 | 0.02 | ns | 1 | 0.91 | ns |
| Family type | 1 | 0.95 | ns | 1 | 1.31 | ns | 1 | 1.82 | ns |
| Sibling | 1 | 0.10 | ns | 1 | 0.06 | ns | 1 | 0.07 | ns |
| Depression in mother | 1 | 0.22 | ns | 1 | 0.81 | ns | 1 | 0.24 | ns |
| Gender × family type | 1 | 1.01 | ns | 1 | 0.77 | ns | 1 | 1.27 | ns |
Exposure is presented as a dichotomous variable (no exposure versus any exposure). The same model was analyzed for data obtained at each developmental period.
The women who came from high-risk families where multiple cases of alcoholism were a prominent feature were most likely to use alcohol, cigarettes, or street drugs during pregnancy. Due to this collinearity between the use of substances in pregnancy and high-risk status in the mother (Hill et al., 2000), conclusions regarding the interactive effects found could not be considered to be conclusive. However, evaluation of the main effect of familial susceptibility and prenatal exposure in the same children was expected to provide a better understanding of the association seen between specific exposures and increased BMI in offspring.
3. Results
3.1. Main effects of familial risk and prenatal exposure to specific substances
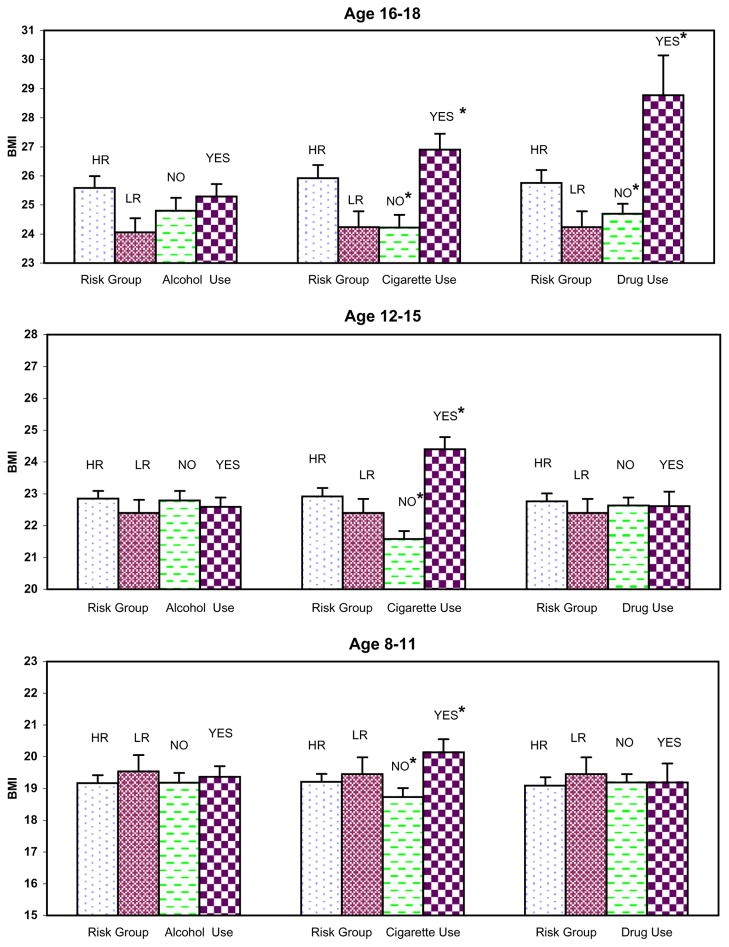
For illustrative purposes only, the main effect of prenatal exposure and the main effect of risk are presented for each substance in relation to child/adolescent body mass index for each age group (see Fig. 1). Prenatal exposure to cigarettes resulted in significant differences in child and adolescent BMI in all three developmental periods. Prenatal exposure to street drugs was associated with increased BMI for the offspring but only during the 16- to 18-year-old period, while no differences were found for maternal alcohol use. Familial/genetic risk for alcohol dependence characterized by high-risk (HR) or low-risk (LR) status was not associated with significant BMI differences for offspring.
Fig. 1.
Prenatal exposure to alcohol, cigarettes and street drugs and offspring Body Mass Index (BMI) during three developmental periods (ages 8–11, 12–15, and 16–18). Asterisks indicate significant differences at P =0.004, 0.001 and 0.01 for the age groups 8–11, 12–15, and 16–18, respectively when exposure to cigarettes versus no exposure was tested. The significant difference seen for exposure to drugs seen in the 16–18-year-old group was significant at P =0.004.
As revealed in Table 1, significant differences in BMI due to exposure to alcohol, cigarettes, or street drugs were not seen in association with gender, familial risk group, or the interaction of gender and familial risk group. Also, the presence or absence of depression did not have a significant effect on the overall results. Blocking by age ranges—prepubertal (8–11 years), pubertal (12–15), and late adolescent (16–18) periods—did not completely remove variation by age. Significant age effects could be seen within these developmental periods (Table 1). However, effects at each age within the developmental period were not analyzed due to a more limited number of cases at each age.
3.2. Familial risk for alcoholism
Contrary to expectation, no significant differences in BMI were found in association with familial/genetic loading for alcohol dependence as may be seen in Table 1.
3.3. Maternal use of street drugs
Prenatal drug use had a significant effect (see Table 1) on BMI in children over the age of 16 years, although results were not significant for the younger age groups. For the 16- to 18-year-old group, prenatal exposure to drugs (ever) was associated with greater BMI (28.77) for those exposed than those who were not exposed (24.70). Frequency of drug use during pregnancy was also significant for the 16-to 18-year-old group (χ2=8.49, df =2, P =0.01), with more frequent exposure associated with higher offspring BMI. An insufficient number of cases with reported use of particular drugs were available to detect specific exposure effects. Accordingly, drug use was analyzed as generic drug exposure. Frequency counts indicate that most of the exposures were to marihuana or cocaine, with about half of the mothers using one or both.
3.4. Maternal use of alcohol
Prenatal use of alcohol did not result in statistically significant differences in BMI values during any of the developmental periods tested.
3.5. Maternal use of cigarettes—yes/no
Table 1 summarizes findings in which cigarette use of mothers and outcome in offspring were analyzed. A significant effect of any cigarette exposure (yes/no) was seen for offspring BMI for the 8- to 11-year-olds, with exposed offspring showing higher BMI than was seen in the nonexposed group. This effect remained significant for offspring studied during the two later stages.
3.6. Maternal use of cigarettes—dose–response relationship
The data were further analyzed to determine if a dose–response relationship could be found for prenatal cigarette exposure. Three levels of cigarette use were contrasted corresponding to no use, 1/2 pack per day, and more than 1/2 pack (no use, 1–2700, and 2701–16,200 cigarettes during the pregnancy). A significant increase in offspring BMI was seen in association with the number of cigarettes smoked by the mother for 8- to 11-year-olds (χ2=13.02, df =2, P =0.002), 12- to 15-year-olds (χ2=14.04, df =2, P =0.001), and 16- to 18-year-olds (χ2=7.22, df =2, P =0.03).
3.7. Comparison of mean BMI data and CDC tables
The means are plotted in Fig. 1 to illustrate that offspring whose mothers smoked during pregnancy had significantly larger BMI values than those who did not smoke (20.14 versus 18.73 at ages 8–11 years, 24.4 versus 21.58 at ages 12–15 years, and 26.9 versus 24.22 for the 16- to 18-year-olds who were exposed versus those who were not, respectively). For illustrative purposes only, data from the CDC Growth Tables (2000) were collated to provide averages for the US population of children between the ages of 8–11, 12–15, and 16–18 years (Table 2). These data provide benchmarks for the current findings and show that children in the present sample who were between the ages of 8 and 11 years, if exposed prenatally to cigarettes, are at the 90th percentile for BMI for the US population. The offspring remained in these same percentile ranks by late adolescence. The 90th percentile is above the cutoff point for being overweight by the CDC criterion, but less than the 95th percentile required to meet the CDC criterion for obesity. Being overweight has generally been defined as having a BMI greater than the 90th percentile, while being obese has been defined as the 97th percentile (von Kries et al., 2002; Toschke et al., 2002), although some studies have defined being overweight as having a BMI>85th percentile (Wideroe et al., 2003). Using the definition of BMI>85 or 90th percentile, the cigarette-exposed offspring in the present study are clearly overweight.
Table 2.
CDC norms for male and female children by age groups used in the present analysis
| P50 | P75 | P85 | P90 | P95 | P97 | |
|---|---|---|---|---|---|---|
| 16.68 | 18.26 | 19.39 | 20.33 | 22.11 | 23.64 | 8- to 11-year-old males |
| 16.87 | 18.69 | 19.96 | 21.01 | 22.93 | 24.54 | 8- to 11-year-old females |
| 16.77 | 18.47 | 19.68 | 20.67 | 22.52 | 24.09 | Mean all |
| 19.14 | 21.18 | 22.61 | 23.79 | 25.96 | 27.78 | 12- to 15-year-old males |
| 19.30 | 21.62 | 23.27 | 24.62 | 27.16 | 29.30 | 12- to 15-year-old females |
| 19.22 | 21.40 | 22.94 | 24.21 | 26.56 | 28.54 | Mean all |
| 21.56 | 23.79 | 25.30 | 26.49 | 28.62 | 30.30 | 16- to 18-year-old males |
| 21.07 | 23.59 | 25.43 | 26.98 | 29.98 | 32.64 | 16- to 18-year-old females |
| 21.31 | 23.69 | 25.36 | 26.73 | 29.30 | 31.47 | Mean all |
P50 refers to the 50th percentile.
3.8. Evaluation of the interaction between prenatal exposure and familial risk
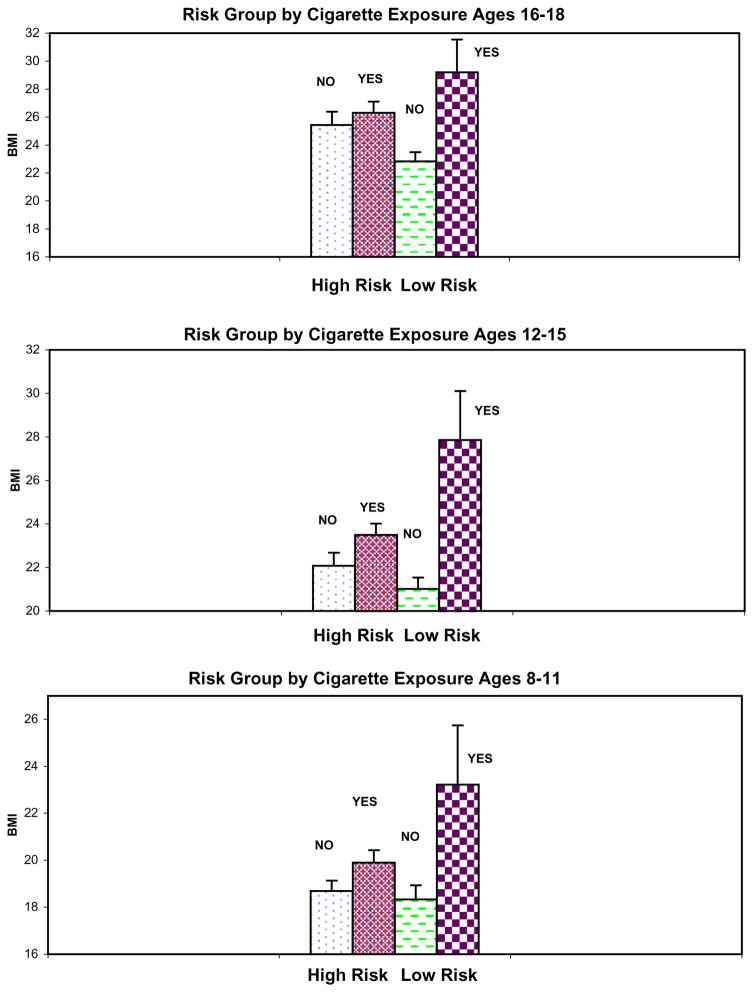
Focusing on the significant findings obtained for cigarettes, a second model was tested, which included familial risk status, prenatal exposure, gender, family ID, and the interaction terms: exposure by family type, gender by family type, gender by exposure, and the three-way interaction of exposure by family type by gender for the three age ranges (8–11, 12–15, and 16–18 years). Gender was not found to be significant in interactions with family type, exposure, or the family type by exposure comparison. Because the results of our maximum likelihood estimates are influenced by the number of variables entered, a third model was run to eliminate nonsignificant terms. Therefore, a third model was tested in which familial risk status, prenatal exposure to cigarettes, gender, family ID, age, and family risk status by exposure to cigarettes were used as predictors of the BMI outcome during each developmental period. This analysis revealed a significant difference for cigarette exposure by family type for the 12- to 15-year-olds (χ2=6.73, df =1, P =0.01) and for the 16- to 18-year-olds (χ2=5.56, df =1, P =0.02), which can be seen in Fig. 2. Further analysis of this significant two-way interaction to obtain the simple main effects within risk groups showed that while exposure to cigarettes in the high-risk group was not significant for any of the developmental periods tested, significant differences by exposure were seen in the low-risk group for each of the developmental periods. These results appear to indicate that effects of cigarette use are most clearly seen in the low-risk control children where any effects of familial/genetic loading for alcohol dependence are at a minimum.
Fig. 2.
Prenatal exposure to cigarettes and familial risk group were jointly evaluated to determine their influence on Body Mass Index (BMI) during three developmental periods (ages 8–11, 12–15, and 16–18). Asterisks indicate a significant difference. For the 8–11-year-old low-risk offspring, t =2.83, df =36, P =0.008; for the 12–15-year-olds, t =2.97, df =8.9, P =0.02; for the 16–18-year-olds, t =2.61, df =9.3, P =0.03. There were no significant differences in BMI by prenatal exposure to cigarettes that could be detected in the high-risk offspring.
4. Discussion
The goal of the present report was to determine if prenatal exposure to alcohol, cigarettes, or street drugs affected offspring BMI during childhood or adolescence. Prenatal use of drugs, which was predominantly marihuana and/or cocaine for these mothers, was associated with increased BMI, but only among offspring in the late adolescent period (ages 16–18 years). Previous studies of prenatal exposure to cocaine have reported reduced birth weight, height, and head circumference (Hurt et al., 1995; Mirochnick et al., 1995; Vance et al., 1997), but long-term differences in growth or BMI have not been found (Jacob-son et al., 1994; Richardson et al., 1996).
The present study found no effect of prenatal exposure to alcohol on offspring BMI, either increases or decreases, during childhood or adolescence. Consistent with the present results, Sampson et al. (1994) did not find any long-term differences, increases or decreases, in body weight or height in 14-year-olds who had experienced intrauterine fetal alcohol exposure (FAE). However, boys diagnosed with fetal alcohol syndrome have been shown to have significantly shorter height and lower body weight at 10 years (Spohr et al., 1993). Growth deficits seen in association with fetal alcohol syndrome (FAS) (Sokol and Clarren, 1989; Spohr et al., 1993) generally occur as a result of greater use of alcohol than was reported by the women in the present study. The present results suggest that fetal alcohol exposure does not lead to increased BMI in childhood or adolescence.
Prenatal exposure to cigarettes (ever smoked versus never smoked) was found to be strongly associated with BMI when tested in each developmental period. Using the number of cigarettes smoked during pregnancy, a linear increase in offspring BMI was seen. These findings are consistent with previous reports showing an increased prevalence of overweight or obese offspring (von Kries et al., 2002; Wideroe et al., 2003; Toschke et al., 2002; Power and Jefferis, 2002) in association with whether mothers ever or never smoked during pregnancy (dichotomous outcome). Also, this is consistent with reports finding a dose–response relationship (von Kries et al., 2002; Wideroe et al., 2003; Power and Jefferis, 2002) between BMI and quantity of cigarettes smoked prenatally. Moreover, a longitudinal study that assessed offspring at five different time points up to 33 years of age (Power and Jefferis, 2002) has confirmed this relationship between maternal smoking and offspring BMI.
Although the present findings demonstrate statistically significant differences between exposed and nonexposed offspring, the results might not be biologically meaningful if the increase in BMI associated with exposure had not resulted in the offspring moving into an overweight or obese category. Comparison of the BMI of study offspring with US norms indicated that children exposed to cigarettes prenatally were in the 90th percentile, clearly placing them in the overweight category. The nonexposed children were somewhat heavier than expected (75th percentile), possibly reflecting heavier weight trends for Pennsylvania residents (http://apps.nccd.cdc.gov/brfss).
Important sources of variation were included in the present study. Each of the exposures was evaluated alone and in combination with the presence of a familial/genetic diathesis for alcohol dependence in pedigrees screened for other major psychiatric disorders. Although the sampling frame cannot be said to be representative of all individuals with alcohol dependence, the advantages of having a set of families with an extreme form of the disorder made it possible to better estimate the contribution of familial/genetic diatheses for alcohol dependence and any impact this might have on a tendency to become overweight or obese.
While a number of confounding variables were assessed in the present study, mention should be made of the limitations. (1) All of the data were collected retrospectively. (2) This may have limited the accuracy of recall by trimester so that data could not be analyzed by trimester. (3) Parental height and weight data were not available for calculating parental BMI before and during pregnancy. (4) Offspring birth weight was not collected.
With respect to the retrospective report, this clearly is a limitation of the analysis with pregnancies occurring 8–17 years earlier. However, other studies have used retrospective report with informative results (von Kries et al., 2002; Toschke et al., 2002; 2003). Additionally, the pattern of change in alcohol consumption across trimesters seen in the present study parallels that reported by mothers who were assessed prospectively during their pregnancies (Fried et al., 1985; Robles and Day, 1990). Also, comparison of prospective and retrospective data for drinking during pregnancy has shown retrospective data to be valid (Griesler and Kandel, 1998). Moreover, follow-up of women for 4 and 5 years following their pregnancies has shown substantial reliability (r =0.53 and 0.67, respectively) between reports obtained during pregnancy and those obtained following pregnancy (Ernhart et al., 1988; Jacobson et al., 1991).
While there is some evidence that specific trimester effects occur in association with birth weight (Ohmi et al., 2002), it is important to note that one well-designed study (Toschke et al., 2003) found no significant increase in the odds ratios for childhood BMI in association with first trimester smoking versus smoking throughout pregnancy. At any rate, it appeared that women in the present study may not have been able to make distinctions by trimester accurately, requiring analyses of data for the entire pregnancy. Moreover, for those high-risk mothers who did report smoking during pregnancy, only about 15% quit smoking following the first trimester, precluding comparisons by trimester.
The present study could not address the influence of parental BMI on the obtained results. This may be a problem because childhood obesity is associated with both paternal and maternal obesity (Whitaker et al., 1997; Toschke et al., 2003). However, if parental obesity and use of alcohol, cigarettes, and other substances during pregnancy were highly associated, then one would expect that offspring of mothers who drank but did not smoke during pregnancy would have elevated BMI during childhood and adolescence as well. This was not the case as alcohol use was not associated with increased BMI in offspring.
Absence of birth weight information may be less of a problem because recent evidence suggests that birth weight is only weakly related to adult weight (Parsons et al., 2001). While undernutrition might explain low birth weight in association with alcohol and cigarette smoking, it cannot explain differing long-term outcome. Only prenatal cigarette use was found to be significantly associated with increased BMI in offspring at all three developmental periods. (“Street” drug use was associated with increased BMI in one developmental period.) Alcohol and cigarette exposure may “reprogram” tissues and organs of the body during critical periods of fetal development in differing ways. Results from the present report suggest that cigarettes may be most influential in increasing BMI several years later. The present results further extend the hypothesis offered by Barker (1997) that under-nutrition may permanently change or “program” the body, leading to increased risk of adult diseases, including cardiovascular disease and diabetes. It is of interest to speculate that these end-point diseases may be mediated by increased BMI.
Although reprogramming is thought to occur, the exact nature of the biological mechanism responsible for the programming is unknown. However, animal studies have shown that prenatal administration of nicotine results in dopaminergic alterations in the neocortex and is a factor in serotonin transporter density in the rat brain (Muneoka et al., 1999, 2001). Toschke et al. (2002) have suggested that such changes might alter impulse control in offspring, leading to increased BMI. Other speculations have included possible prenatal alteration of fat cells and hypothalamic alteration (van der Meulen, 2002). Additionally, alterations in neuroendocrine metabolic pathways leading to poorer endocrine regulation have also been offered as a mechanism to explain the recently reported association between maternal smoking during pregnancy and type II diabetes in offspring (Montgomery and Ekbom, 2002).
It should be noted that the present study was not designed to be representative of the US population. Rather, recruitment procedures were designed to achieve a collection of multiplex families in which it can be expected that a greater proportion of alcohol dependence susceptibility genes are segregating (Hill et al., 2004). Use of these families to assess the impact of prenatal substance use provides a unique opportunity to hold constant the familial/genetic susceptibility for substance use disorders while examining the specific drug use behaviors of women during their pregnancies. Availability of a longitudinal follow-up of their offspring has provided an opportunity to assess the permanence of these effects during three developmental periods ending at approximately age 18 years.
Overall, prenatal exposure to alcohol, cigarettes, and street drugs has been reported to be associated with long-term changes in growth and development, neuropsychological deficits, and behavioral sequelae such as increased use of substances in young adulthood (Baer et al., 2003). However, it has not been a common feature of these studies to include other potentially salient causes of these abnormalities such as having a familial diathesis for psychiatric disorders. A few notable exceptions are Baer et al. (1998) and Hill et al. (2000), where the joint effects of family history of substance abuse problems and specific prenatal exposures were examined. Even when both types of measures are included in the same study as was done in the present report, the collinearity between having a diathesis for alcohol or drug dependence and use of these substances during pregnancy complicates the emerging picture. Nevertheless, the dose–response effect seen for cigarettes and BMI of offspring does suggest a physiological effect that may be set during the prenatal period.
In conclusion, while prenatal use of cigarettes is known to be associated with newborn growth deficits, the long-term effect of prenatal cigarette use on BMI is less well documented. The present results, along with previous studies, suggest that prenatal exposure to tobacco results in increased BMI during childhood, a tendency that remains into late adolescence and beyond. None of the previously reported studies have been able to assess the possible conjoint impact that familial risk for alcohol dependence and prenatal exposure have on BMI in offspring assessed longitudinally during childhood and adolescence. One epidemiological sample, which studied a possible familial association between disordered eating and substance use disorders, concluded that the problems are not cross-transmitted within families and that the addiction model of eating disorders may be too simplistic (von Ranson et al., 2003). However, highly convincing data have been provided by others for a common genetic diathesis (Wang et al., 2004). While no independent effect of familial risk for alcohol dependence was seen in the present study, exposure to cigarettes appeared to have a dramatic effect on BMI during childhood and adolescence. Testing for interaction effects revealed that significant differences in cigarette exposure could be seen only when comparing exposed and nonexposed control children where the familial loading for alcohol dependence and other disorders could be expected to be at a minimum. Because women with a personal or family history of alcohol dependence are more likely to smoke during pregnancy, the collinearity of these variables may preclude seeing an effect of familial/genetic risk factors for increased BMI. It is noteworthy that almost half of the present sample smoked during pregnancy. With obesity becoming one of the most common health problems in children and adolescents, a problem that appears to persist into adulthood, considerable increased morbidity and mortality may be incurred as a result. For this reason, the present findings appear to have significant public health implications.
Acknowledgments
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA05909, AA08082, and AA11304).
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. APA; Washington, DC: 1980. [Google Scholar]
- Anderson VE, Hauser WA, Rich SS. Genetic heterogeneity in the epilepsies. Advances in Neurology. 1986;44:59–75. [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. Journal of Studies on Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Archives of General Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Bulik C. Alcohol use and depression in women with bulimia. American Journal of Drug and Alcohol Abuse. 1987;13:343–355. doi: 10.3109/00952998709001518. [DOI] [PubMed] [Google Scholar]
- Butler NR, Goldstein H, Ross EM. Cigarette smoking in pregnancy: its influence on birth weight and perinatal mortality. British Medical Journal. 1972;2:127–130. doi: 10.1136/bmj.2.5806.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlat DJ, Camargo CA, Herzog DB. Eating disorders in males: a report on 135 patients. American Journal of Psychiatry. 1997;154:1127–1132. doi: 10.1176/ajp.154.8.1127. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen JD, Rounsaville BJ, Merikangas KR. Personality, drug of choice, and comorbid psychopathology among substance abusers. Drug and Alcohol Dependence. 2002;65:225–234. doi: 10.1016/s0376-8716(01)00168-5. [DOI] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug and Alcohol Dependence. 2003;71:65–75. doi: 10.1016/s0376-8716(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Day NL, Zuo Y, Richardson GA, Goldschmidt L, Larkby CA, Cornelius MD. Prenatal alcohol use and offspring size at 10 years of age. Alcoholism, Clinical and Experimental Research. 1999;23:863–869. [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol, Clinical and Experimental Research. 2002;26:1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcoholism, Clinical and Experimental Research. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Fried PA, Barnes MV, Drake ER. Soft drug use after pregnancy compared to use before and during pregnancy. American Journal of Obstetrics and Gynecology. 1985;151:787–792. doi: 10.1016/0002-9378(85)90520-4. [DOI] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB. The impact of maternal drinking during and after pregnancy on the drinking of adolescent offspring. Journal of Studies on Alcohol. 1998;59:292–304. doi: 10.15288/jsa.1998.59.292. [DOI] [PubMed] [Google Scholar]
- Hill SY, Zubin J, Steinhauer SR. Personality resemblance in relatives of male alcoholics, a comparison with families of male control cases. Biological Psychiatry. 1990;27:1305–1322. doi: 10.1016/0006-3223(90)90501-r. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke-Wellman J, Shen S. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. Journal of Studies on Alcohol. 2000;61:661–668. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderness CC, Brooks-Gunn J, Warren MP. Co-morbidity of eating disorders and substance abuse review of the literature. International Journal of Eating Disorders. 1994;16:1–34. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hurt H, Brodsky NL, Betancourt L, Braitman LE, Malmud E, Giannetta J. Cocaine exposed children: follow-up through 30 months. Journal of Developmental and Behavioral Pediatrics. 1995;16:29–35. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicology and Teratology. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcoholism, Clinical and Experimental Research. 1994;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcoholism, Clinical and Experimental Research. 1997;21:513–520. [PubMed] [Google Scholar]
- Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. Journal of Pediatrics. 1995;126:636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- Molgaard CA, Chambers CM, Golbeck AL, Elder JP, Ferguson J. Maternal alcoholism and anorexia nervosa: a possible association? International Journal of the Addictions. 1989;24:167–173. doi: 10.3109/10826088909047281. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. British Medical Journal. 2002;324:26–27. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NE, Mi MP. Multiplex families with two or more probands. American Journal of Human Genetics. 1968;20:361–367. [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Nakatsu T, Fuji J, Ogawa T, Takigawa M. Prenatal administration of nicotine results in dopaminergic alterations in the neocortex. Neurotoxicology and Teratology. 1999;21:603–609. doi: 10.1016/s0892-0362(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in rat brain. European Journal of Pharmacology. 2001;411:279–282. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- Ohmi H, Hirooka K, Mochizuki Y. Fetal growth and the timing of exposure to maternal smoking. Pediatrics International. 2002;44:55–59. doi: 10.1046/j.1442-200x.2002.01495.x. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. British Medical Journal. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. International Journal of Epidemiology. 2002;31:413–419. [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school age children. Neurotoxicology and Teratology. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Robles N, Day NL. Recall of alcohol consumption during pregnancy. Journal of Studies on Alcohol. 1990;51:403–407. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Prenatal alcohol exposure, birthweight and measures of child size from birth to age 14 years. American Journal of Public Health. 1994;84:1421–1428. doi: 10.2105/ajph.84.9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Anthenelli RM, Bucholz KK, Hesselbrock VM, Nurnberger JI. Anorexia nervosa and bulimia nervosa in alcohol-dependent men and women and their relatives. American Journal of Psychiatry. 1996;153:74–82. doi: 10.1176/ajp.153.1.74. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Archives of General Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Del’Homme M, NewDelman J, Gordon E, Kim T, Liu A, McCracken JT. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Clarren SK. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcoholism, Clinical and Experimental Research. 1989;13:597–598. doi: 10.1111/j.1530-0277.1989.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. Prenatal alcohol exposure and long-term developmental consequences. Lancet. 1993;341:907–910. doi: 10.1016/0140-6736(93)91207-3. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. Journal of the American Medical Association. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Bookstein FL. Prenatal alcohol use and offspring development: the first fourteen years. Drug and Alcohol Dependence. 1994;36:89–99. doi: 10.1016/0376-8716(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Koletzko B, Slikker W, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. European Journal of Pediatrics. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. American Journal of Epidemiology. 2003;158:1068–1074. doi: 10.1093/aje/kwg258. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Archives of Pediatrics and Adolescent Medicine. 1995;149:1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- Vance JC, Chant DC, Tudehope DI, Gray PH, Hayes AJ. Infants born to narcotic dependent mothers: physical growth patterns in the first 12 months of life. Journal of Paediatrics and Child Health. 1997;33:504–508. doi: 10.1111/j.1440-1754.1997.tb01659.x. [DOI] [PubMed] [Google Scholar]
- van der Meulen J. Commentary: maternal smoking during pregnancy and obesity in the offspring. International Journal of Epidemiology. 2002;31:420–421. doi: 10.1093/ije/31.2.420. [DOI] [PubMed] [Google Scholar]
- von Kries R, Toschke AM, Koletzko B, Slikker W. Maternal smoking during pregnancy and childhood obesity. American Journal of Epidemiology. 2002;156:954–961. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- von Ranson KM, McGue M, Iacono WG. Disordered eating and substance use in an epidemiological sample: II. Associations within families. Psychology of Addictive Behaviors. 2003;17:193–201. doi: 10.1037/0893-164X.17.3.193. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of Addictive Disease. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wideroe M, Vik T, Jacobsen G, Bakketeig L. Does maternal smoking during pregnancy cause childhood overweight? Paediatric and Perinatal Epidemiology. 2003;17:171–179. doi: 10.1046/j.1365-3016.2003.00481.x. [DOI] [PubMed] [Google Scholar]