SUMMARY AND CONCLUSIONS
1. Extracellular and intracellular recordings in rat hippocampal slices were used to compare the synaptic responses to perforant path stimulation of granule cells of the dentate gyrus, spiny “mossy” cells of the hilus, and area CA3c pyramidal cells of hippocampus. Specifically, we asked whether aspects of the local circuitry could explain the relative vulnerability of spiny hilar neurons to various insults to the hippocampus.
2. Spiny hilar cells demonstrated a surprising lack of inhibition after perforant path activation, despite robust paired-pulse inhibition and inhibitory postsynaptic potentials (IPSPs) in adjacent granule cells and area CA3c pyramidal cells in response to the same stimulus in the same slice. However, when the slice was perfused with excitatory amino acid antagonists [6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX), or CNQX wtlh 2-amino-5-phosphonovaleric acid (APV)], IPSPs could be observed in spiny hilar cells in response to perforant path stimulation.
3. The IPSPs evoked in spiny hilar cells in the presence of CNQX were similar in their reversal potentials and bicuculline sensitivity to IPSPs recorded in dentate granule cells or hippocampal pyramidal cells in the absence of CNQX.
4. These results demonstrate that, at least in slices, perforant path stimulation of spiny hilar cells is primarily excitatory and, when excitation is blocked, underlying inhibition can be revealed. This contrasts to the situation for dentate and hippocampal principal cells, which are ordinarily dominated by inhibition, and only when inhibition is compromised can the full extent of excitation be appreciated.
5. These results suggest that the circuitry of the dentate region may be a factor in determining the vulnerability and resistance of dentate neurons. The results also provide a clue to the particular local circuit inhibitory interneurons of the dentate gyrus that inhibit spiny hilar cells.
Many of the neurons located in the hilus of the fascia dentata are known to be selectively vulnerable to certain excitotoxic insults to the hippocampus, such as intermittent perforant path stimulation (Scharfman and Schwartzkroin 1989, 1990; Sloviter 1987, 1991) and brief periods of ischemia (Benveniste and Diemer 1988; Crain et al. 1988; Johansen et al. 1987). One of the vulnerable hilar cell types that is also the most numerous is an extremely spiny hilar cell with elaborate thorny excrescences on its dendrites, the “mossy” cell (Amaral 1978; Ribak et al. 1985). Other spiny hilar cells are also vulnerable (Scharfman and Schwartzkroin 1990), as are the aspiny hilar neurons that are immunoreactive for somatostatin and/or neuropeptide Y (Johansen et al. 1987; Sloviter 1987, 1991). However, the aspiny neurons that are immunoreactive for γ-aminobutyric acid (GABA), as welt as the dentate granule cells, are relatively resistant (Sloviter 1987, 1991).
One pressing question concerns the basis for the selective vulnerability of certain hilar cells. In the past, hypotheses have focused on differences in calcium-binding protein content (Sloviter 1989) or other intrinsic properties to account for hilar cell vulnerability. We asked whether aspects of the dentate circuitry involving spiny hilar cells could explain their selective vulnerability. The results support the hypothesis that circuitry may play an important role in establishing the vulnerability of hilar cells relative to the principal cells. In addition, the results advance our understanding of the local circuitry of the dentate gyrus.
Rat hippocampal slices were prepared as previously described (Scharfman 1991; Scharfman and Schwartzkroin 1990). Briefly, adult male Sprague-Dawley rats (100–200 g) were anesthetized with ether and decapitated. The brain was dissected and immediately immersed in 4°C, oxygenated (95% O2-5% CO2) buffer (in mM: 126 NaCl, 5 KCl, 2 CaCl2, 2 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, and 10 d-glucose, pH 7.4). Slices were cut at a 45° angle from the coronal plane, as described (Scharfman and Schwartzkroin 1990), or they were cut transversely. In each case the hippocampus was immersed in 4°C, oxygenated buffer and cut in 400-μm sections with the use of a Vibroslice (Campden Instruments). Slices were immediately transferred to a recording chamber (Fine Science Tools), where they lay at an interface of oxygenated, warmed (34–35°C) buffer and humidified air. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX; Tocris Neuramin), dl-2-amino-5-phosphonovaleric acid (dl-APV, Sigma), and bicuculline methiodide (Vega Biochemicals) were dissolved in 0.9% NaCl and stored in concentrated aliquots (1 mM) at −20°C until the day of the experiment. Immediately before use they were diluted in buffer to reach a final concentration of 10 μM CNQX, 50 μM APV, or 50 μM bicuculline.
Extracellular and intracellular recording methods were similar to those previously described (Scharfman 1991; Scharfman and Schwartzkroin 1990). A twisted, Teflon-coated stainless steel wire was placed in the outer molecular layer to stimulate the perforant path (0.05–0.2 mA, 20–300 μs, 0.2–0.075 Hz). Extracellular recordings were made with capillary-filled borosilicate glass pulled horizontally (Flaming-Brown P-87, Sutter Instruments; 1–10 MΩ) and filled with 1 M NaCl. At any given site of extracellular recording, the electrode was lowered into the slice to a depth where a maximal response was evoked. Intracellular electrodes were pulled with different settings and filled with 1 M potassium acetate (80–120 MΩ). Intracellular recordings used a high input impedance amplifier with a bridge circuit (Axoclamp 2A, Axon Instruments), and the bridge was balanced whenever current was injected intracellularly. Data were digitized (Neurodata DR-484) and stored on tape for analysis off-line. Methods to determine membrane properties and reversal potentials have been described elsewhere (Scharfman 1991; Scharfman and Schwartzkroin 1988, 1990).
In 10 slices from 10 different animals, a combination of extracellular and intracellular recording was used to examine the responses of different types of neurons before and after switching the perfusate to buffer containing CNQX. In each of these experiments, the synaptic responses of granule cells (4 cells in 4 of the 10 experiments) and/or area CA3c pyramidal cells ( 12 cells in 10 experiments) were also examined intracellularly before CNQX application. In addition, in 23 other experiments the synaptic responses of spiny hilar cells and area CA3c pyramidal cells to perforant path stimulation were examined in the same slice, in the absence of CNQX.
The first recordings of all experiments were extracellular and established that the slice was acceptable, A slice was accepted if the population spike recorded in the granule cell layer exceeded 5 mV, rode on a positive wave >5 mV (Fig. 1), and there was strong paired-pulse inhibition (Fig. 1B). The presence of paired-pulse inhibition indicated that there was strong inhibition of granule cells by paired perforant path stimulation in all experiments. In addition, several recording sites were used to show that the population spike sampled in several areas of the granule cell layer was homogeneous (Scharfman, 1991). This homogeneity indicated that many of the granule celis in the slice responded similarly to stimulation. Then pyramidal cells and/or granule cells were recorded to establish that stimulation elicited inhibitory postsynaptic potentials (IPSPs) In these cells; sometimes IPSPs appeared after brief excitatory postsynaptic potentials (EPSPs; Figs. 2 and 3). The synaptic responses of granule cells and pyramidal cells were examined at various membrane potentials so that reversal potentials could be determined. Finally, the extracellular electrode was positioned at one site in the granule cell layer, and a full range of stimulus intensities was examined for the population spike recorded at that site.
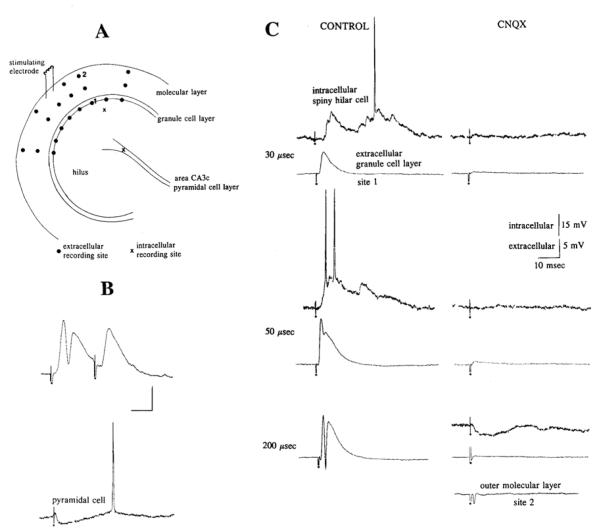
Fig. 1.
A: diagram of the experimental arrangement for recordings shown in B and C. Extracellular (closed circles) and intracellular (crosses) recording sites are shown in a schematic of a ventral hippocampal slice. A bipolar stimulating electrode was located at the same site in the outer molecular layer for the entire experiment. B: evidence of inhibition in granule cells and pyramidal cells, before 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) application and before the recordings in C were made. Top: initial extracellular recording in the granule cell layer demonstrated paired-pulse inhibition. Two 100-μs stimuli triggered 10 ms apart produced a population spike only after the 1st stimulus. Calibration, 5 mV, 5 ms. Stimulation occurred at the small dots. Bottom: intracellular recording from an area CA3c pyramidal cell located as shown in A. The cell was depolarized (to the level of spontaneous action potential firing, −49 mV) to illustrate that a stimulus to the molecular layer evoked an IPSP in the cell. Calibration, 20 mV, 10 ms. Extracellular responses in all figures are averages of 3 responses. Intracellular records in all figures are unaveraged responses. C: data collected from the slice diagrammed in A while simultaneously recording from site 1 extracellularly and a spiny hilar cell intracellularly. The spiny hilar cell was located at the cross symbol in the hilus of the diagram in A. Intracellular and extracellular responses to stimulation are shown before drug application (Control), and after perfusion with CNQX (CNQX). Responses were recorded with the use of 3 stimulus strengths by varying stimulus duration (30, 50, and 200 μs) while keeping current strength constant (0.1 mA). Note that the high-intensity stimulus was not tested on the impaled neuron until after CNQX application. However, the high stimulus strength was used to examine the maximal response of the granule cell population before impaling the hilar cell. That response is shown at the bottom left. At the extreme bottom right, a response to the high-intensity stimulus, recorded at site 2 in the molecular layer, is shown in the presence of CNQX. Note that, after the data in C were collected (i.e., in the presence of CNQX), responses at the sites shown by the closed circles in A were examined, and in no location were the responses any larger than those collected at sites 1 and 2. Hilar cell membrane potential, −61 mV.
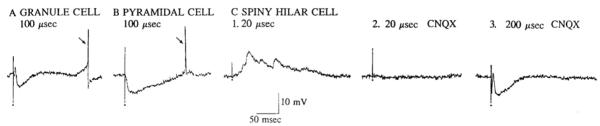
Fig. 2.
Comparison of intracellular synaptic responses of different cell types in the same slice. Responses to stimulation of the outer molecular layer are shown for 3 different cells impaled in the same slice. Responses are intracellular recordings and were obtained from a granule cell (A), an area CA3c pyramidal cell (B), and a spiny hilar neuron (C). C2 and C3: responses of the same spiny hilar cell after the slice was perfused with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). One of these responses was obtained with the use of the same stimulus intensity as that shown for the response before CNQX application (C2). The other response was elicited by using a higher stimulus strength (C3). Membrane potentials were as follows: A, −54 mV; B, −55 mV; C, −59 mV. Arrows point to spontaneous action potentials, which were truncated. Stimulation occurred at the small dots. Stimulus intensities are expressed as stimulus durations of 0.1-mA current.
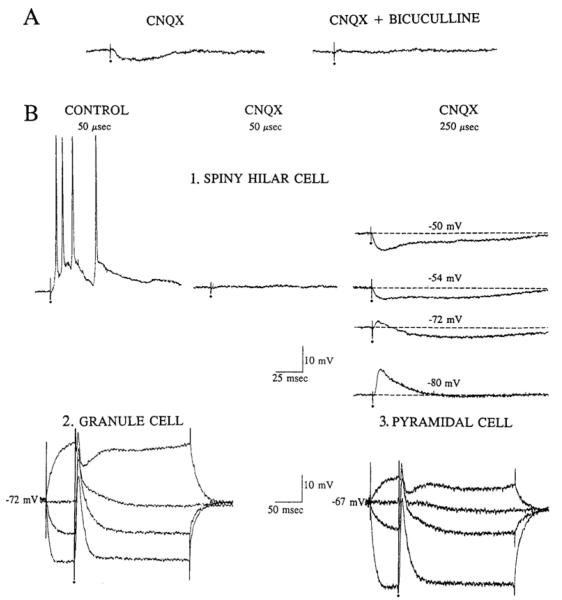
Fig. 3.
Inhibitory postsynaptic potentials (IPSPs) evoked in spiny hilar neurons in the presence of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were similar to those elicited in granule cells and pyramidal cells in the absence of CNQX. A: the IPSP evoked in the cell from Fig. 1C in the presence of CNQX is shown on the left (CNQX), and the response to the same stimulus is shown again on the right, after perfusion was switched to a solution containing both CNQX and bicuculline (CNQX + bicuculline). Calibration is in Fig. 1C. B1: responses to outer molecular layer stimulation are shown for a different spiny hilar cell in a different slice from A. Response to a low-intensity stimulus (50 μs, 0.125 mA) is shown before (Control) and after (CNQX) CNQX application. CNQX blocked the response to the 50-μs stimulus. However, a stronger stimulus (250 μs, 0.125 mM) produced an IPSP. This IPSP is shown at several different membrane potentials on the right. B2: responses of a granule cell to stimulation of the same stimulus site in the same slice as B1, before perfusion with CNQX. Several responses to a fixed stimulus (150 μs, 0.125 mA), triggered on depolarizing or hyperpolarizing current steps (250-ms duration) from the resting potential of the cell (−72 mV), are superimposed. B3: responses of an area CA3c pyramidal cell to stimulation of the outer molecular layer in the same slice. Several responses to the same stimulus are superimposed, similar to B2. Resting potential, −67 mV. Capacitative and stimulus artifacts were clipped, and the small dots mark the stimulus artifacts.
After principal cells were sampled, a spiny hilar cell was impaled. Hilar cells were accepted if they had resting membrane potentials over −60 mV, overshooting action potentials, and electrophysiological characteristics consistent with those of morphologically identified spiny hilar cells described previously (Scharfman and Schwartzkroin 1988). The spiny hilar cells are extremely different electrophysiologically from the other hilar cells, the aspiny “fast-spiking” cells (Scharfman 1991), so they were distinguished in this study without morphological confirmation. The responses of each spiny hilar cell were examined at several stimulus intensities and several membrane potentials simultaneous to the population spike recordings. However, extremely high intensities were avoided because these types of stimulation usually led to depolarization and/or deterioration of the neuron. Low stimulus strengths were sufficient to examine subthreshold and suprathreshold responses of spiny hilar cells, because these cells have much lower thresholds than granule cells (Scharfman 1991). Hyperpolarizations were never observed, even when spiny hilar cells were depolarized to membrane potentials positive to −60 mV.
A 10- to 15-min period was used to establish that the extracellular and intracellular responses were stable and to examine both the evoked responses and membrane properties of the spiny hilar cell at a variety of membrane potentials (−45 to −90 mV). The spiny hilar cells were homogeneous in their responses to stimulation. Responses consisted of complex EPSPs with numerous peaks, often triggering action potentials; there was no evidence of IPSPs or afterhyperpolarizations even at depolarized membrane potentials (Figs. 1C, 2, and 3). This differed slightly from the results obtained from stimulation of spiny hilar cells in guinea pig slices, where EPSPs were simpler and sometimes small IPSPs were evident (Scharfman and Schwartzkroin 1988). This difference could be due to the different circuitries of the dentate gyrus in the rat and guinea pig.
After perfusion was switched to buffer containing CNQX, all excitatory responses to stimulation were blocked (Fig. 1C). There were no changes as determined by a t test (P value, 0.01) in the resting membrane potential (before and after CNQX, −65.5 ± 3.2 mV, mean ± SE), input resistance (before CNQX, 85.2 ± 2.5; after CNQX, 90.1 ± 4.2 MΩ), or action potential amplitude (before CNQX, 83.6 ± 4.5 mV; after CNQX, 82.9 ± 5.4 mV) in any of the hilar cells during perfusion with CNQX. As soon as excitatory transmission was blocked, the stimulus intensity was increased to the levels that were well above the threshold for a population spike and threshold for action potential generation in the hilar ceil before CNQX application (Figs. 1C, 2C, and 3B). High-intensity stimulation at this time in the experiment did not lead to deterioration of hilar neurons, probably because the deterioration is an excitotoxic effect and most, if not all, excitation was blocked pharmacologically. In all experiments an IPSP was elicited in the hilar cell by these high-intensity stimuli, whereas there was no extracellular response of granule cells recorded either in the granule cell layer or the molecular layer (Fig. 1C). This indicated that the granule cells were not involved in the circuitry underlying the newly exposed inhibition. To ensure that granule cells were not activated by stimulation at this time, the extracellular electrode was moved to many different sites in the granule cell layer (Fig. 1A), and no population spikes were detected in any experiment. Population EPSPs recorded in many sites in the molecular layer (Fig. 1A) were not detected either. However, a large volley could be recorded in the outer molecular layer (bottom right of Fig. 1C). This volley indicated that lateral perforant path axons (or, possibly, axons of aspiny hilar neurons that course in the outer molecular layer) were activated effectively by the stimulation and argues against nonspecific damage to the slice by perfusion with drug.
The IPSPs of the spiny hilar cells appeared monophasic in 7 of 10 cells (mean ± SE, Erev = −65.0 ± 1.5 mV; Figs. 1, 2, and 3A). In the three other cells the IPSPs were biphasic (Fig. 3B). Biphasic IPSPs had an early component that was similar in its reversal potential to the monophasic IPSP (Erev = −66.6 ± 2.2 mV) and in addition had a later phase that reversed at a more negative potential (extrapolated reversal potential, −82.3 ± 2.5 mV; Fig. 1B). These reversal potentials indicated that the early and late components were probably mediated by GABAA and GABAB receptors, respectively (Dutar and Nicoll 1988; Knowles et al. 1984). Consistent with this possibility, the GABAA receptor antagonist bicuculline methiodide blocked a monophasic IPSP of a spiny hilar cell in each of the three experiments in which bicuculline’s effects were tested (Fig. 3A). To add bicuculline in those experiments, perfusion with buffer containing CNQX alone was switched to buffer containing both CNQX and bicuculline.
The IPSPs of spiny hilar cells were similar in several respects to those IPSPs elicited in granule cells and pyramidal cells obtained in the same slice (Figs. 1, 2, and 3B). For example, most IPSPs were monophasic (granule cells; 2 of 4 cells; pyramidal cells: 7 of 12 cells; Figs. 1 and 2). The mean reversal potentials of these monophasic events were −65.3 mV for granule cells and −66.0 ± 2.3 for pyramidal cells. The remaining celis (2 granule cells and 5 pyramidal cells) demonstrated biphasic IPSPs. The early hyperpolarizing component of biphasic IPSPs reversed at a potential similar lo the monophasic IPSPs (for the granule cells, mean = −66.3 mV; for the pyramidal cells, −65.8 ± 2.3 mV). The late hyperpolarizing phase of the biphasic IPSPs did not always reverse polarity even when the membrane was hyperpolarized to −90 mV. The mean reversal potentials (including extrapolated reversal potentials) were −80.5 mV for granule cells and −83.4 ± 2.1 mV for pyramidal cells. These results are consistent wiih those in the literature that describes the IPSPs of granule cells and CA3 pyramidal cells (Fricke and Prince 1984; Hablitz and Thalmann 1987; Knowles et al., 1984; Muñoz et al. 1990; Thalmann 1987; Thalmann and Ayala 1982). However, the mean reversal potentials of the late IPSP are somewhat depolarized to some of the values in the literature and to Ek+ (approximately −90 mV). This is probably because of the use of extrapolation and the use of a relatively high concentration of extracellular potassium (5 mM).
In four additional experiments we attempted to control for the possibility that residual N-methyl-d-aspartate (NMDA) receptors could have activated cells in the presence of CNQX. This possibility was unlikely given the high concentration of magnesium ions in the buffer (2 mM) and the high concentration of CNQX (10 μM). Nevertheless, in four additional experiments we perfused the slice with CNQX as well as 50 μM dl-2-amino-5-phosphonovaleric acid (dl-APV), an NMDA receptor antagonist. The results were virtually identical to the experiments utilizing CNQX alone.
These results have been obtained with the use of spiny hilar cells in many areas of the hilus, but there were some restrictions, and this may limit the ability to generalize that the results would be predicted from any spiny hilar cell. Hilar cells were impaled in an area within 150 μm of the granule cell layer, and they were distant from the tail end of the area CA3c pyramidal cell layer that inserts between the upper and lower blades. These restrictions allowed us to be confident that we did not confuse a stray area CA3c pyramidal cell with a spiny hilar cell. In addition, extracellular recordings were obtained only from the upper blade, and stimulation was only to the upper blade.
It may also be dangerous to generalize that all hilar cells, spiny or aspiny, lack IPSPs in response to perforant path stimulation. In fact, it was shown in a previous study that some aspiny cells do demonstrate IPSPs in response to perforant path stimulation (Scharfman and Schwartzkroin 1990). However, by examining the available data for both spiny hilar cells (as discussed here) and aspiny cells (Scharfman 1991; Scharfman and Schwartzkroin 1990), an interesting and potentially important similarity between spiny and aspiny hilar cells is clear. This similarity is the association between IPSPs and vulnerability. For the spiny hilar cells, which appear to uniformly lack IPSPs, the cells also uniformly deteriorate during extended perforant path stimulation (Sloviter 1987; Scharfman and Schwartzkroin 1990). For the aspiny hilar cells, only a fraction of the cells exhibit IPSPs, and again only some of the cells are vulnerable (Scharfman and Schwartzkroin 1990; Sloviter 1987). Therefore the hypothesis that IPSP generation may be related lo vulnerability appears to be consistent not only for spiny hilar cells but also for the aspiny hilar cells. Future experiments will be required to establish that the cells that do not exhibit IPSPs are exactly the same cells that are vulnerable to prolonged perforant path stimulation; for now there exists only a possible association, albeit an intriguing one.
In summary, we have found electrophysiological evidence that, at least for many of the spiny hilar cells, there is a striking dominance of excitatory input over inhibitory input set into play by perforant path activation. Only when excitatory transmission is blocked is an existing inhibitory circuit involving spiny hilar cells revealed. This situation contrasts with the response of dentate granule cells, which respond to weak perforant path stimuli with an EPSP that is abruptly curtailed by a large IPSP. Suprathreshold stimuli evoke an action potential followed by an afterhyperpolarization that is a combination of IPSP and activation of potassium conductances by the preceding action potential. Therefore one of the factors predisposing spiny hilar cells to excitotoxic injury could be their overexcitation compared with inhibition; if there were a way to enhance inhibition or decrease excitatory drive to the spiny hilar cells, one might expect that spiny hilar cells would be less vulnerable. Furthermore, one would predict that, in the absence of inhibition, the granule cell population would be less resistant to an excitotoxic challenge.
In addition to the contribution of the results to our understanding of selective vulnerability of hilar neurons, several aspects of the unmasked inhibition were illuminating. First, the inhibition itself was extremely similar to that found for dentate granule cells and hippocampal pyramidal cells. Specifically, IPSPs were dominated by an early phase that reversed near the equilibrium potential for chloride and was therefore likely to be mediated by GABAA receptors. The second phase, when present, reversed at a more negative potential, closer to the equilibrium potential for potassium. The second phase is therefore likely to be mediated by GABAB receptors. This sequential GABAA-GABAB receptor activation has been shown to underly the IPSPs of hippocampal neurons (Dutar and Nicoll 1988; Hablitz and Thalmann 1987; Knowles et al. 1984; Thalmann and Ayala 1982). Importantly, there was no evidence for synaptic activation of a depolarizing GABA-mediated postsynaptic potential, a potential that could explain the remarkable capacity of dentate hilar cells to depolarize in synchrony in the absence of excitatory transmission (Michelson and Wong 1991; Müller and Misgeld 1990).
In addition, the nature of the experiment allowed us to infer which types of inhibitory cells might mediate the inhibition of hilar cells. Because the known excitatory pathways were blocked in our slices, the candidate inhibitory cells must have been activated either directly or antidromically by the stimulating electrode in the outer molecular layer. Therefore the inhibitory cells were either 1) local GABAergic neurons that had dendrites in the molecular layer [i.e., the GABAergic basket cells in the granule cell layer or those in the other dentate layers (Ribak and Seress 1983)] or 2) aspiny somatostatin/neuropeptide Y-immunoreactive neurons of the hilus that have a plexus in the outer molecular layer (Bakst et al. 1986; Morrison et al. 1982; Sloviter 1991; Sloviter and Nilaver 1987). Regarding the first possibility, it is unlikely that the GABAergic basket cells of the granule cell layer project to the hilus, because their axons are known to contact granule cells primarily, if not exclusively (Ribak and Seress 1983; Seress and Ribak 1990). However, GABAergic neurons in other dentate layers, either the molecular layer or the hilus, could mediate inhibition of spiny hilar cells. The second possibility, that aspiny hilar somatostatin/neuropeptide Y-immunoreactive neurons inhibit spiny hilar cells, is attractive in light of the recent evidence that collaterals of these neurons project to hilar cells (Deller and Leranth 1990; Leranth et al. 1990). Further, these peptidergic neurons form symmetrical (putative inhibitory) synapses on hilar neurons (Deller and Leranth 1990; Leranth et al. 1990). However, there is no physiological evidence that somatostatin/neuropeptide Y-immunoreactive cells are necessarily inhibitory. Therefore future experiments will be necessary to pinpoint the origin of spiny hilar cell inhibition.
It has been puzzling in past studies that spiny hilar neurons and many aspiny hilar neurons recorded in slices of diverse orientation have lacked IPSPs, because it has been shown that GABAergic boutons are frequently found to make symmetrical synapses on the dendrites and cell bodies of these cells (Ribak et al. 1985; Ribak and Seress 1988). The present study provides an explanation for this apparent paradox, that in fact IPSPs are present, but they are ordinarily overpowered by excitatory input. Although one must be cautious that a very different situation could occur in vivo, where additional commissural (Bakst et al. 1986; Buzsáki and Czéh 1981; Goodman and Sloviter 1991; Ribak et al. 1986) and extrahippocampal (Bilkey and Goddard 1985; Freund and Antal 1988; Lopes Da Silva et al. 1991) inputs are present, these results still allow some insight into the functional circuitry of the dentate hilus and its possible relevance to selective vulnerability of hilar neurons.
Acknowledgments
I thank Dr. R. S. Sloviter, Dr. D. H. Lowenstein, and Dr. J. H. Goodman for comments on the manuscript.
This work was supported by a grant from the Epilepsy Foundation of America.
REFERENCES
- Amaral DG. A Golgi study of the cell types of the hilar region of the hippocampus in the rat. J. Camp. Neurol. 1978;185:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Bakst I, Avendano C, Morrison JH, Amaral DG. An experimental analysis of the origins of somatostatin-like immunoreactivity in the dentate gyrus of the rat. J. Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Diemer NH. Early postischemic 45Ca accumulation in rat dentate hilus. J. Cereb. Blood Flow Metab. 1988;8:713–719. doi: 10.1038/jcbfm.1988.118. [DOI] [PubMed] [Google Scholar]
- Bilkey DK, Goddard GV. Medial septal facilitation of hippocampal granule cell activity is mediated by inhibition of inhibitory interneurones. Brain Res. 1985;361:99–106. doi: 10.1016/0006-8993(85)91279-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Czéh G. Commissural and perforant path interactions in the rat hippocampus. Exp. Brain Res. 1981;43:429–438. doi: 10.1007/BF00238387. [DOI] [PubMed] [Google Scholar]
- Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forrbrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- Deller T, Leranth C. Synaptic connections of neuropeptide Y (NPY) immunoreactive neurons in the hilar area of the rat hippocampus. J. Comp. Neurol. 1990;300:433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoli RA. A physiological role for GABAB receptors in the central nervous system. Nature Lond. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature Lond. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Fricke RA, Prince DA. Electrophysiology of dentate gyrus granule cells. J. Neurophysiol. 1984;51:195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Sloviter RS. Evidence for commissurally projecting parvalbumin-imunoreactive basket cells in the dentate gyrus of the rat. Hippocampus. 1991;2:13–22. doi: 10.1002/hipo.450020103. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Thalmann RH. Conductance changes underlying a large synaptic hyperpolarization in hippocampal CA3 neurons. J. Neurophysiol. 1987;58:160–179. doi: 10.1152/jn.1987.58.1.160. [DOI] [PubMed] [Google Scholar]
- Johansen FF, Zimmer J, Diemer NH. Early loss of somatostatin neurons in the dentate hilus after cerebral ischemia in the rat precedes CA-1 pyramidal cell loss. Acta Neuropathol. 1987;73:110–114. doi: 10.1007/BF00693775. [DOI] [PubMed] [Google Scholar]
- Knowles WD, Schneiderman JH, Wheal HV, Stafstrom CE, Schwartzkroin PA. Hyperpolarizing potentials in guinea pig hippocampal CA3 neurons. Cell. Mol. Neurobiol. 1984;4:207–230. doi: 10.1007/BF00733586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Malcolm AJ, Frotscher M. Afferent and efferent synaptic connections of somatostatin-immunoreactive neurons in the rat fascia dentata. J. Comp. Neurol. 1990;295:111–122. doi: 10.1002/cne.902950110. [DOI] [PubMed] [Google Scholar]
- Lopes Da Silva FH, Witter MP, Boeijinga PH, Lohman AHM. Anatomical organization and physiology of the limbic cortex. Physiol. Rev. 1991;70:453–511. doi: 10.1152/physrev.1990.70.2.453. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J. Comp. Neurol. 1978;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science Wash. DC. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Benoit R, Magistretti PJ, Ling N, Bloom FE. Immunohistochemical distribution of pro-somatostatin-related peptides in the hippocampus. Neurosci. Lett. 1982;34:137–142. doi: 10.1016/0304-3940(82)90165-3. [DOI] [PubMed] [Google Scholar]
- Müller W, Misgeld U. Inhibitory role of dentate hilus neurons in guinea pig hippocampal slice. J. Neurophysiol. 1990;64:46–56. doi: 10.1152/jn.1990.64.1.46. [DOI] [PubMed] [Google Scholar]
- Muñoz MD, Nuñez A, García-Austi E. In vivo intracellular analysis of rat dentate granule cells. Brain Res. 1990;509:91–98. doi: 10.1016/0006-8993(90)90313-z. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J. Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L. A Golgi-electron microscopic study of fusiform neuron in the hilar region of the dentate gyrus. J. Comp. Neurol. 1988;271:67–78. doi: 10.1002/cne.902710108. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Amaral DG. The development,, ultrastructure, and synaptic connections of the mossy cells of the dentate gyrus. J. Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Peterson GM, Seroogy KB, Fallon JH, Schmued LC. A GABAergic inhibitory component within the hippocampal commissural pathway. J. Neurosci. 1986;6:3492–3498. doi: 10.1523/JNEUROSCI.06-12-03492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J. Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells recorded in the dentate hilus in guinea pig hippocampal slices. J. Neurosci. 1988;8:3412–3421. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science Wash. DC. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990;35:491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of the light and electron microscopic features of basket cells in the hippocampal dentate gyrus or the rat. Anal. Embryo. 1990;181:547–565. doi: 10.1007/BF00174627. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss or interneurons in experimental epilepsy. Science Wash. DC. 1987;235:173–176. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J. Comp. Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J. Comp. Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- Thalmann RH. Pertussis toxin blocks a late inhibitory postsynaptic potential in hippocampal CA3 neurons. Neurosci. Lett. 1987;82:41–46. doi: 10.1016/0304-3940(87)90168-6. [DOI] [PubMed] [Google Scholar]
- Thalmann RH, Ayala GF. A late increase in potassium conductance follows synaptic stimulation of granule neurons of the dentate gyrus. Neurosci. Lett. 1982;29:243–248. doi: 10.1016/0304-3940(82)90324-x. [DOI] [PubMed] [Google Scholar]