Abstract
The suprachiasmatic nuclei (SCN) are necessary and sufficient for the maintenance of circadian rhythms in primate and other mammalian species. The human dorsomedial SCN contains populations of non-species-specific vasopressin and species-specific neurotensin neurons. We made time-series recordings of core body temperature and locomotor activity in 19 elderly, male, end-stage dementia patients and 8 normal elderly controls. Following the death of the dementia patients, neuropathological diagnostic information and tissue samples from the hypothalamus were obtained. Hypothalamic tissue was also obtained from eight normal control cases that had not had activity or core temperature recordings previously. Core temperature was analysed for parametric, circadian features, and activity was analysed for non-parametric and parametric circadian features. These indices were then correlated with the degree of degeneration seen in the SCN (glia/neuron ratio) and neuronal counts from the dorsomedial SCN (vasopressin, neurotensin). Specific loss of SCN neurotensin neurons was associated with loss of activity and temperature amplitude without increase in activity fragmentation. Loss of SCN vasopressin neurons was associated with increased activity fragmentation but not loss of amplitude. Evidence for a circadian rhythm of vasopressinergic activity was seen in the dementia cases but no evidence was seen for a circadian rhythm in neurotensinergic activity. These results provide evidence that the SCN is necessary for the maintenance of the circadian rhythmin humans, information on the role of neuronal subpopulations in subserving this function and the utility of dementia in elaborating brain–behaviour relationships in the human.
Keywords: circadian rhythm, Alzheimer’s disease, vasopressin, neurotensin, neurodegeneration
Introduction
Circadian rhythms affect a ubiquitous array of homeostatic and behavioural functions in mammalian species. Several lines of evidence suggest that control of circadian rhythms is maintained by the suprachiasmatic nuclei (SCN), a small structure located adjacent to the third ventricle in the anterior hypothalamus. Ablation of the SCN results in a loss of circadian rhythmicity (Moore and Eichler, 1972; Stephan and Zucker, 1972). Transplant of SCN tissue from another animal will rescue the circadian rhythm of an animal bearing a previous SCN lesion (Lehman et al., 1987). SCN cells in culture maintain a circadian rhythm (Green and Gillette, 1982; Groos and Hendriks, 1982; Shibata et al., 1982). The SCN generates a 24 h rhythm of electrical activity via a network of individual cellular ~24 h oscillators (Welsh et al., 1995) that maintain an endogenously driven rhythm via a transcriptional feedback loop (King and Takahashi, 2000).
The SCN can be divided anatomically and functionally in mammalian species into two distinct subdivisions, the ventrolateral or core SCN and the dorsomedial or shell SCN (Van den Pol, 1980; Moore, 1983, 1995). The ventrolateral subdivision of the SCN primarily contains the synaptic field from the retinohypothalamic tract (RHT), which transmits photic information from melanopsin containing retinal ganglion cells (Berson et al., 2002) to neurons immunoreactive for vasoactive intestinal peptide (VIP). In addition, a population of calbindin positive neurons is located in a subregion, and these neurons appear necessary for the maintenance of circadian rhythms (LeSauter and Silver, 1999). The ventrolateral SCN projects primarily to local structures surrounding the SCN including the dorsomedial SCN, the paraventricular nucleus of the hypothalamus, the lateral subparaventricular zone, and the perisuprachiasmatic nucleus, which is a region surrounding the SCN that also receives innervation from the RHT (Leak and Moore, 2001).
The dorsomedial subdivision of the SCN does not receive direct photic innervation from the RHT but instead receives this innervation indirectly via the ventrolateral SCN (Albus et al., 2005). The primary neuropeptide expressed in the dorsomedial subdivision of the SCN, conserved across all mammalian species, is vasopressin. Other neuropeptides are also expressed in the dorsomedial SCN, however these vary between species. Calretinin (Leak and Moore, 2001), somatostatin (Tominaga et al., 1992) and other neuropeptides have been observed in the dorsomedial SCN in a variety of mammalian species. In the dorsomedial subdivision of the SCN, diurnal variability of arginine vasopressin and somatostatin have been observed that persist in constant darkness suggesting the presence of an intrinsic circadian rhythm to their output (Tominaga et al., 1992). In humans, there is a unique population of neurotensin containing neurons located mostly in the dorsomedial subdivision that extends into the ventrolateral SCN and anterior hypothalamus (Mai et al., 1991; Moore, 1993).
Evidence for circadian disturbance in patients with Alzheimer’s disease and other neurodegenerative dementias comes from both direct observation of the circadian rhythm of core body temperature and observation of behavioural output influenced by the circadian rhythm. The endogenous circadian rhythm of core body temperature has been shown to be phase delayed in patients with confirmed Alzheimer’s disease compared to normal elderly (Harper et al., 2005). Sleep is also markedly disturbed in patients with Alzheimer’s disease (Vitiello and Borson, 2001) and that disturbance increases as a function of severity (Bliwise et al., 1995) with evidence of weakening of the circadian rhythm by increasing nocturnal awakenings and diurnal naps (Prinz et al., 1982).
Locomotor activity in patients clinically diagnosed with Alzheimer’s disease with moderate—severe (Satlin et al., 1995; van Someren et al., 1996; Ancoli-Israel et al., 1997; Mishima et al., 1997) and mild—moderate (Volicer et al., 2001; Hatfield et al., 2004) illness exhibits disturbances in rhythmicity. These abnormalities include increased nocturnal activity (Satlin et al., 1995; Mishima et al., 1997), delayed phase (Satlin et al., 1995; Ancoli-Israel et al., 1997) and lowered coherence to measured core body temperature circadian rhythm (Mishima et al., 1997; Harper et al., 2005). These characteristics form a unique profile of disordered activity in a population of patients with autopsy-confirmed Alzheimer’s disease when compared to those with other neurodegenerative dementias (Harper et al., 2001).
Degeneration in the SCN has been observed in post-mortem human tissue derived from patients with Alzheimer’s disease (Stopa et al., 1999). Reduced cell counts of each constituent cellular component of the SCN have also been observed using immunohistochemistry including vasopressin (Swaab et al., 1985; Stopa et al., 1999), neurotensin (Stopa et al., 1999) and VIP in presenile dementia (Zhou et al., 1995). Loss of vasopressin and VIP neurons has been associated with the severity of the neuropathologically staged illness (Wu et al., 2007), matching the impression from studies of locomotor activity and core body temperature of deterioration of circadian rhythmicity with increasing neuropathologically determined disease state (Harper et al., 2004).
Therefore, a variety of data exist demonstrating that degeneration of the central circadian clock located in the SCN occurs in dementia, and that patients with dementia experience circadian changes. However, to date no data directly compare neurodegenerative changes in the SCN in post-mortem cases directly to the circadian changes observed in the human subjects with dementia from which the cases were derived. In this study, we examined post-mortem dorsomedial SCN tissue obtained from patients who had earlier been studied in a 72 h protocol where time-series recordings had been made of motor activity and core body temperature in order to address the following three hypotheses. (i) Evidence of neurodegeneration in the SCN, as expressed by the glia/neuron ratio, will be associated with loss of circadian function, particularly loss of amplitude or abnormal phase relationship to the environment, in patients studied antemortem. (ii) Loss of vasopressin or neurotensin neurons in the dorsomedial SCN will be associated with specific circadian, functional deficits. (iii) Vasopressin and neurotensin will show evidence of a circadian rhythm in their output.
Methods
Subjects
Alzheimer’s disease group
The subjects were elderly, male patients (n = 19) residing at the E. N. Rogers Memorial (ENRM) Veterans Administration (VA) Hospital Geriatric Research, Education and Clinical Center (GRECC) in Bedford, MA, admitted with a diagnosis of possible or probable Alzheimer’s disease. The Institutional Review Boards of the McLean and ENRM VA Hospitals approved the protocol and informed consent for this study. The subjects were all severely impaired and bed-ridden and required 24 h nursing care for performance of routine activities (ambulation, feeding, etc.). Informed consent was obtained from the subject’s next-of-kin in accordance with the Veterans Administration’s human subjects’ research policies on surrogate consent. Physiological recordings were obtained every 6 months for the duration of the 3-year data collection portion of the study. The age of patients at time of last study was (mean ± SD) 69.5 ± 6.5 years. Upon death of the subjects, consent was obtained from the subjects’ next-of-kin for autopsy and subsequent donation of their brains to the Alzheimer’s Disease Research Center Brain Bank at the ENRM VA Hospital. Consent for this portion of the study was obtained independently without any linkage to the prior, physiological data collection. At time of death subjects’ mean age was 70.1 ± 6.2 years with a mean latency from last physiological recording to death of 0.52 ± 0.58 years. All subjects were free from significant intercurrent illness and were taking no antipyretic medication for at least 24 h prior to the time of physiological recording.
Comparison group—physiological recordings
Elderly, male comparison subjects (n = 8) were recruited from the Harvard Cooperative Project on Aging and the Massachusetts Institute of Technology’s (MIT) Clinical Research Center (CRC). The mean age of comparison subjects was 72.8 ± 6.0 years. All comparison subjects were free from dementia as verified by Mini-Mental State Examination and an evaluation by a board-certified psychiatrist. Except for the absence of dementia, they met all other inclusion and exclusion criteria as the Alzheimer subjects. No tissue was recovered from this group for neuropathological analysis.
Comparison neuropathological cases
An independent group was established to provide neuropathological comparisons to the Alzheimer’s disease patients. These cases were gathered from aged, male hospital patients following donation to the McLean Hospital Brain Tissue Resource Center. Control cases (n = 9; 5 males; 4 females) were chosen with no observed clinical history of dementia or affective illness, and they had no pathological evidence of AD or other degenerative dementia. Mean age was 64.3 ± 10.8 years. Causes of death included myocardial infarction, congestive heart failure, colon cancer, lung cancer, renal failure and pneumonia.
Procedures
Physiological data
Following informed consent, Alzheimer patients and normal comparison subjects participated in a 72 h, time-series, simultaneous, core body temperature and locomotor activity data collection protocol. Alzheimer patients were studied in their accustomed environment on the GRECC unit following standard ward routines. No effort was made to control light, food, nursing care, the patient’s sleep schedule or any other masking influences during data collection. Normal comparison subjects were studied at the MIT CRC in a setting designed to replicate a hospital’s environment, procedures and schedules. Core body temperature was recorded by use of a rectal thermistor (YSI, series 400, Yellow Springs, OH) inserted to 10 cm. The thermistor was connected to an ambulatory monitor that sampled the temperature data in 6 min intervals over the 72 h data-collection period. Activity was collected by an ambulatory activity monitor (AM-16, Ambulatory Monitoring Inc., Ardsley, NY) featuring a piezoelectric bender sensitive to accelerations of 0.01 g. Activity counts were accumulated over 5 min epochs and written to memory. Patients were assessed every 6 months following this data-collection protocol for the duration of their hospitalization. The last recording taken prior to the death of the subject was included for analysis in this study.
Neuropathological examination
All brains were routinely fixed in neutral buffered formalin for more than 4 weeks to standardize shrinkage and subjected to a standardized neuropathological examination. Brains were coronally sectioned and the hypothalamus was dissected away for detailed examination of the suprachiasmatic nucleus. Fourteen additional brain areas were sampled based on their suitability for the differential diagnosis of Alzheimer’s disease, dementia with Lewy bodies and other neurodegenerative dementias. Differential neuropathological diagnosis was made in accordance with standardized criteria (McKee, 1999; Stopa et al., 1999) and involved consensus of two board-certified neuropathologists. All subjects with available data were included in the results regardless of diagnosis.
SCN immunohistochemistry
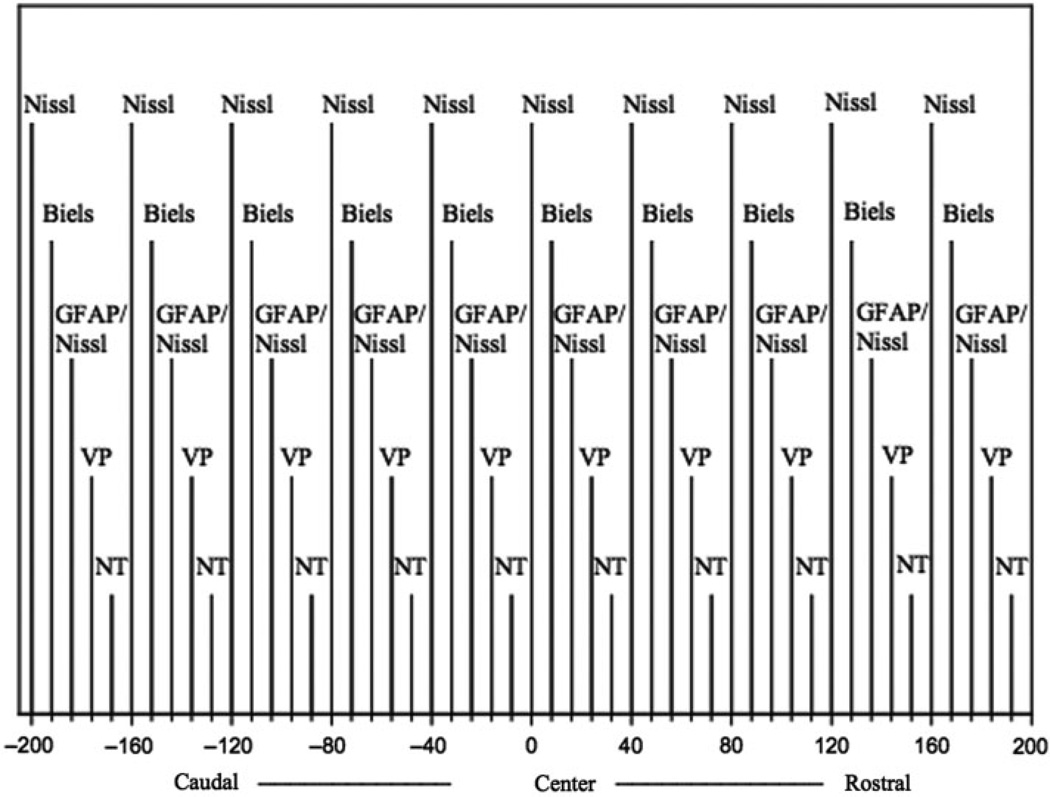
Hypothalami were blocked, processed with paraffin and serially sectioned at 8 microns in the coronal plane. The stereological sampling methods utilized in this study were designed in accordance with the principles described by Weibel for random tissue sampling of a homogeneous, three-dimensional structure (Weibel, 1989). The dorsal subdivision of the SCN was identified by examining the Nissl-stain on every fifth slide of the sectioned anterior hypothalamus using an Olympus microscope. The centre of the nucleus was then localized based on nuclear diameter and cell density. Sections from 200 microns on either side of this determined centre of the dorsal SCN were stained sequentially (Fig. 1) with Nissl, Bielschowsky silver, Nissl and glial fibrillary acidic protein (GFAP), vasopressin (Fig. 2) and neurotensin (Fig. 3). The area of greatest cell density on each slide was selected for analysis. These areas were standardized and digitized by a Scion frame grabber and subsequently analysed using NIH image on a Macintosh computer. A random, 0.1 mm2 field in the area of greatest cell density was selected from each of the 10 slides for analysis by a technician blind to the diagnosis of the case. For all cell-counting protocols (Glia/Neuron ratio, neurotensin and vasopressin) only cells with complete cytoplasmic borders and a clearly visible nucleus were recorded, and the counts obtained from 10, 0.1 mm2 fields were averaged. Glia to neuron ratio was determined by examination of GFAP/Nissl-stained sections. Cells were identified by their respective astrocytic or neuronal staining patterns.
Fig. 1.
Five series of five sections were sampled on either side of the center of the dorsolateral subdivision of the SCN. Slides were alternately stained with Nissl, Bielchowsky silver (Biels), glial fibrillary acidic protein (GFAP)—Nissl, vasopressin (VP) and neurotensin (NT). A single 0.1 mm square microscopic field was counted on each slide within the SCN in the area of maximum cell density.
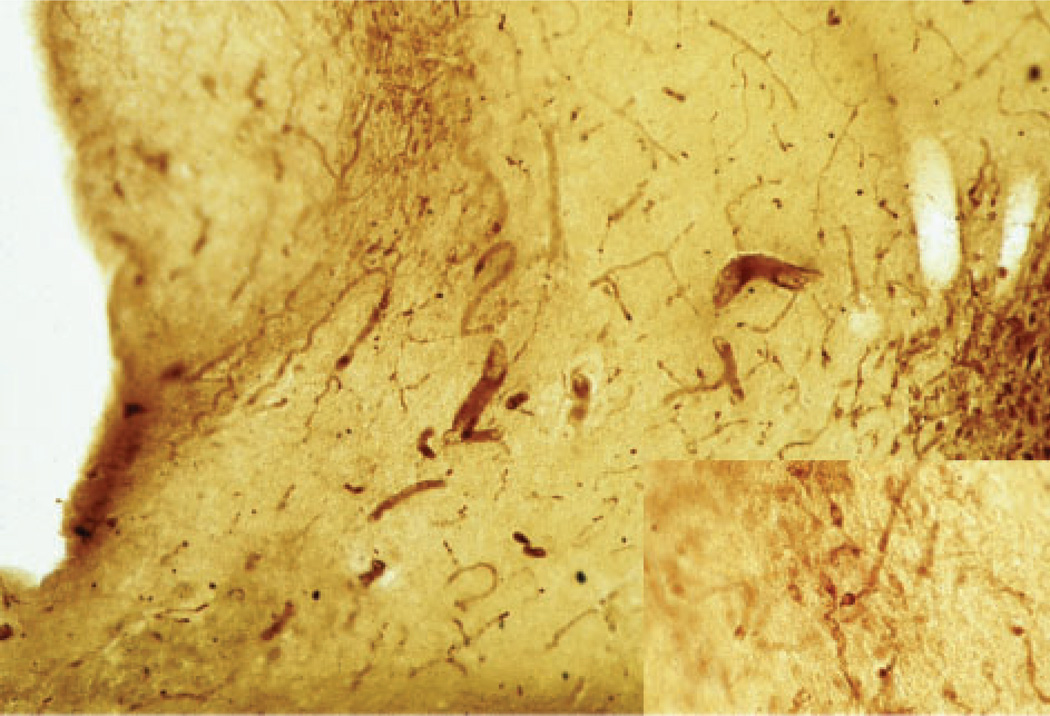
Fig. 2.
Photomicrograph of the anterior hypothalamus from a normal, control case stained for arginine vasopressin showing the parvocellular, vasopressin neurons of the dorsomedial SCN (upper left) and magnocellular, vasopressin neurons of the supraoptic nucleus (right) at 40× and 100× (insert).
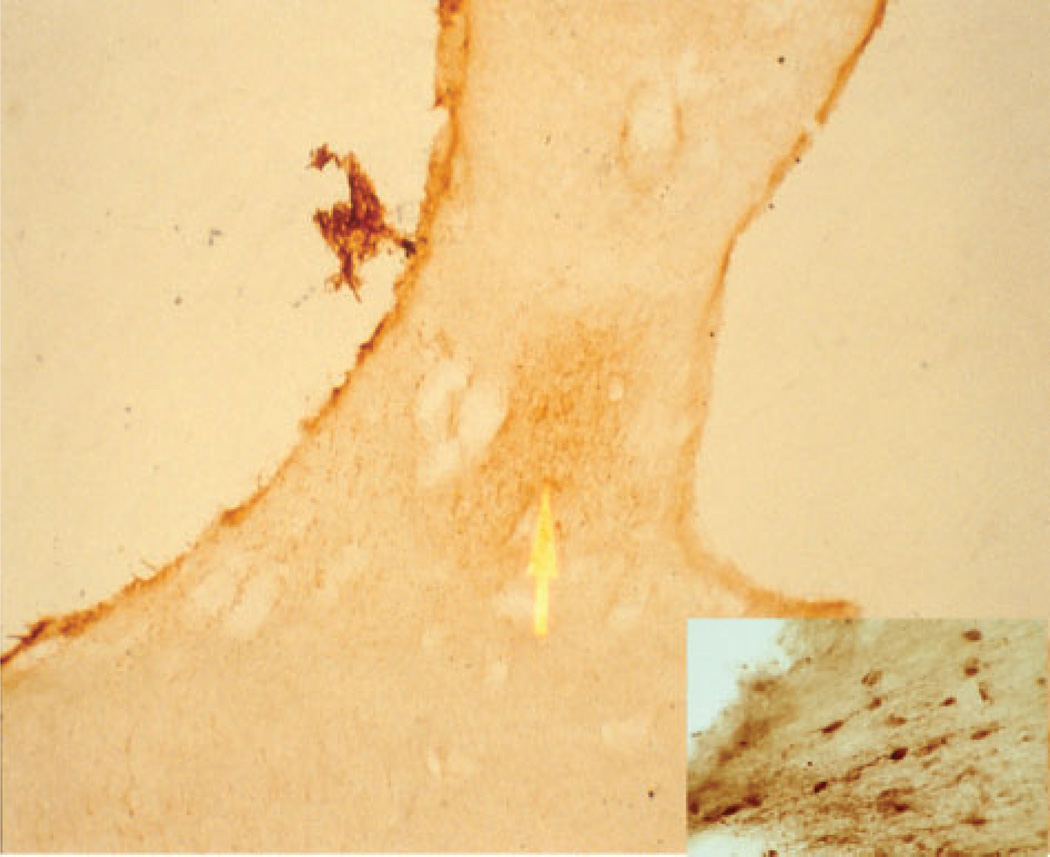
Fig. 3.
Photomicrograph of the anterior hypothalamus from a normal, control case stained for neurotensin showing the dorsomedial suprachiasmatic nucleus at 40× and 100× (insert).
Data analysis
Gross locomotor activity was assessed for maximal and minimal activity by use of the M10 and L5 statistics. (Witting et al., 1990) M10 measures the hourly activity in the 10 most active hours of the day and the L5 measures the mean hourly activity in the 5 least active hours of the day. One feature of advanced Alzheimer’s disease is a phase shift of activity and temperature away from the normal day–night schedule (Satlin et al., 1995). These phase changes are also dependent on dementia diagnosis (Harper et al., 2001). Therefore, M10 and L5 were chosen due to their ability to quantify ‘diurnal type’ (M10) and ‘nocturnal type’ (L5) activity without reference to time of day allowing for a homogeneous standard of maximal and minimal activity that would not be biased by diagnosis.
Time-series recordings of locomotor activity were assessed by both parametric and non-parametric methods. The strength of the circadian characteristics of the activity time-series data was first assessed non-parametrically by calculating the interdaily stability (IS). IS quantifies the day-to-day consistency of hourly activity by comparing the similarity of activity recorded at the same time each day in reference to the total activity (Witting et al., 1990). The final quantity is expressed from 0 to 1 with 1 indicating a perfectly consistent rhythm. In contrast, intradaily variability (IV) is a quantification of the fragmentation of the activity data with higher values reflecting greater fragmentation and suggesting frequent diurnal napping and nocturnal awakenings. The IV is quantified by measuring the hour-to-hour variance in the data and yields results between 0 and 2.
The circadian characteristics of core body temperature, locomotor activity, neurotensin and vasopressin neuron counts were also analysed by cosinor analysis. A single harmonic cosine function was modeled to the data using cosifit, a non-linear, least squares cosinor algorithm (Teicher and Barber, 1990). From this model we derived the mesor (centre of the circular function), amplitude (maximum mesor to peak difference), acrophase (time of peak of the function) in clock time and goodness-of-fit (measured variance of the data to the model). Neurotensin and vasopressin neurons counts were plotted by time of death to attempt to detect evidence of a circadian rhythm in these neurotransmitters. A single harmonic cosinor function was fitted to the neuronal counts in a time-series by time of death.
All values are reported as mean ± SE. Diagnostic differences in neuropathological indices were analysed by Analysis of Variance with Tukey HSD post hoc analysis for between group differences. Equal variance assumptions were tested with the Bartlett test for homogeneity of variance (Bartlett and Kendall, 1946). Unequal variances were stabilized by log transformation. The relationships between antemortem measures of rest-activity and core body temperature and post-mortem measurements of global SCN degeneration (glia/neuron ratio) and neuronal counts (vasopressin and neurotensin) were assessed by Pearson correlation. Potential bias of the sampling based on time of death or season of death was tested by Rayleigh’s Test (Z) and Watson’s U2 test (Batschelet, 1981).
Results
SCN tissue was recovered from 19 cases where previous activity and core body temperature recordings had been obtained. Neuropathological diagnosis of these dementia subjects revealed 11 subjects with a primary diagnosis of Alzheimer’s disease (three subjects Braak stage 3–4; eight subjects Braak stage 5–6). Three subjects were diagnosed with dementia with Lewy bodies yet also showed significant Alzheimer pathology (Braak stage 5–6). Four subjects were diagnosed with varying diagnoses in the spectrum of frontotemporal dementias (two subjects Pick’s disease; two subjects corticobasal degeneration). Complete glia/neuron ratio data were available for 18 subjects and complete vasopressin and neurotensin data for 17 subjects.
Rayleigh’s test for significance in mean direction was applied to both date of death and time of death to test for seasonal and circadian bias that could influence the results. Control cases showed no tendency towards seasonal directionality (Z = 0.795; P = 0.4), but dementia cases did have significant seasonal directionality towards the spring season (Z = 3.644; P = 0.02). However, there was not a detectable mean difference between the groups (Watson’s U2 = 0.043; P = 0.5). There was no tendency towards time of death directionality that could indicate circadian bias in either the dementia or control cases (dementia: Z = 1.303; P = 0.3, control: Z = 0.841; P = 0.4).
Mean latency between the recording of core body temperature/locomotor activity and death in the dementia subjects was 0.52 ± 0.14 years. Post-mortem interval was 13.5 ± 2.6 h in the control cases, 10.81 ± 1.9 h in the AD cases and 12 ± 3.7 h in the FTD cases [F(2,26) = 0.32; P = 0.7]. Age of subjects at death was not significantly different between the groups [F(2,26) = 1.70; P = 0.2] with mean age of death being 67.9 ± 1.94 years for controls, 71.1 ± 1.56 years for AD cases and 66.1 years for FTD cases.
Dementia patients showed significant degeneration in the dorsomedial SCN (Table 1) as measured by the glia/neuron ratio [F(2,24) = 11.8; P < 0.001]. This was true for both AD (Tukey HSD; P < 0.001) and FTD (Tukey HSD; P < 0.05) when compared individually to controls. Reductions were seen in constituent neuronal subpopulations of the SCN as well with reductions in the number of vasopressin neurons [F(2,23) = 6.6; P = 0.005] and neurotensin neurons [F(2,23) = 7.6; P = 0.003]. Individual comparisons demonstrate that both diagnoses showed significant reductions in vasopressin neuronal counts whereas for neurotensin, AD cases were significantly lower than controls but FTD cases, while also reduced from control counts, did not reach significance.
Table 1.
Comparison of mean ± standard error measurements of glia/neuron ratio, vasopressin and neurotensin neuronal densities in control, Alzheimer disease (AD) and frontotemporal dementia (FTD) cases.
| Marker | Control | AD | FTD | F ratio | p value |
|---|---|---|---|---|---|
| Glia/Neuron | (9) 0.10 ± 0.10 |
(14) 0.54 ± 0.25*** |
(4) 0.44 ± 0.22* |
F(2, 24) = 12.2 | 0.0002 |
| Vasopressin | (9) 17.7 ± 7.8 |
(14) 10.2 ± 2.0** |
(3) 8.9 ±3.6** |
F(2, 24) = 7.0 | 0.004 |
| Neurotensin | (9) 10.1 ±1.6 |
(14) 6.7 ±2.1*** |
(4) 8.3 ±2.0 |
F(2, 23) = 8.6 | 0.001 |
P < 0.05;
P < 0.01;
P < 0.001 by Tukey HSD test
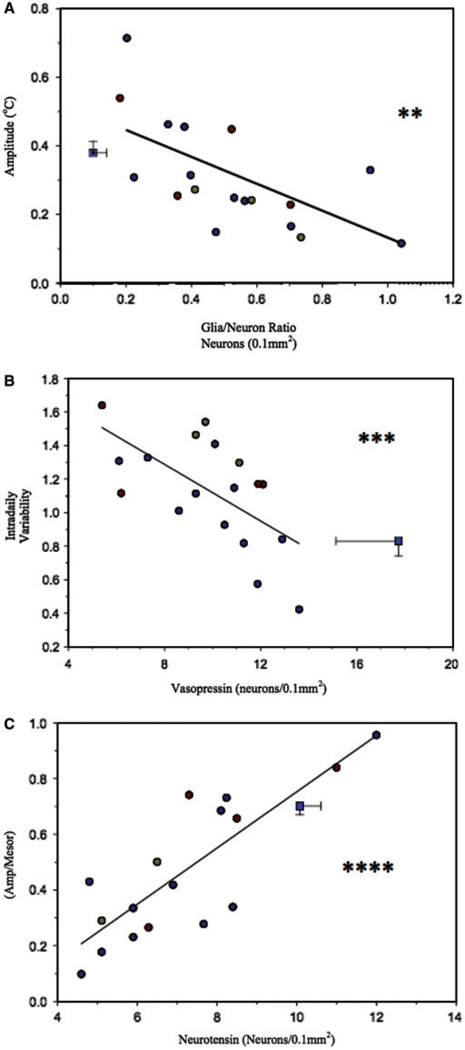
SCN degeneration as assessed by measurement of the glia/neuron ratio was associated with significant changes in the circadian rhythm of core body temperature, but not activity, in patients with dementia. Measurement of circadian core body temperature amplitude was significantly lower in patients with greater SCN degeneration (Fig. 4A) as measured by the glia/neuron ratio (r = −0.65; P = 0.004). No significant correlation with gross motor activity was observed. Circadian measures of activity (IS, relative amplitude and phase) and fragmentation of the activity pattern (IV; R = −0.07; NS) were not found to be associated with dorsomedial SCN degeneration although a trend was observed for loss of relative amplitude (r = −0.39; P = 0.1) of the activity rhythm (Table 2).
Fig. 4.
(A) Evidence of global neuronal degeneration (increase in glia/neuron ratio) in the SCN was associated with loss of amplitude in the rhythm of core body temperature. (B) While reductions in neuronal density of vasopressin neurons in the dorsomedial SCN reveal no association with loss of the amplitude of core body temperature or activity. Loss of vasopressin neurons was associated with increased fragmentation of the activity rhythm. (C) Reductions in neuronal density of neurotensin neurons in the dorsomedial SCN were associated with loss of relative amplitude (amplitude/mesor) of activity. There was no evidence of association between loss of neurotensin neurons and activity fragmentation. Blue square = combined control values for neuropathological (horizontal error) and circadian (vertical error) scores. Blue circles = Alzheimer’s disease; gold circles = dementia with Lewy bodies; red circles = frontotemporal dementia; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Table 2.
Pearson correlation coefficients relating degeneration in the dorsomedial suprachiasmatic nucleus to circadian measurements of activity and temperature.
| Glia/Neuron (18) r |
Neurotensin (17) r |
Vasopressin (17) r |
|
|---|---|---|---|
| M10 | 0.03 | −0.23 | −0.14 |
| L5 | 0.19 | −0.57* | 0.10 |
| IS | 0.07 | 0.30 | 0.05 |
| IV | −0.07 | 0.20 | −0.63*** |
| Activity mesor | 0.07 | −0.36 | −0.05 |
| Activity amplitude | −0.16 | 0.16 | −0.02 |
| Relative amplitude | −0.39† | 0.83**** | −0.02 |
| Activity phase | 0.04 | 0.21 | 0.02 |
| Temperature amplitude | −0.65** | 0.70** | −0.20 |
| Temperature phase | 0.16 | −0.30 | −0.21 |
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001 by Pearson correlation;
P ≤ 0.1.
Density measurements of vasopressin neurons in the dorsomedial SCN were significantly associated with increased fragmentation of the activity rhythm. Intradaily variability (Fig. 4B) or fragmentation of the activity rhythm significantly increased with decreasing vasopressin immunoreactivity in the dorsomedial SCN (r = −0.63; P = 0.005). However, no direct circadian measure of activity such as IS (Table 2), relative amplitude (r = −0.02; NS), temperature amplitude (r = −0.20; NS) showed significant association with vasopressin neuronal density. In addition, no gross measures of activity were associated with vasopressin neuronal density (Table 2).
Density of neurotensin neurons in the SCN showed a similarly specific association with circadian amplitude measurements of core body temperature and even more robustly with activity. Nocturnal activity was higher in patients with lower density of neurotensin neurons (L5: r = −0.57; P = 0.02), but there was no association between diurnal-type activity (M10) and neurotensin (Table 2). Core body temperature amplitude was significantly lower with decreasing neurotensin neuronal density (r = 0.70; P = 0.002), and relative amplitude of activity showed a highly significant correlation to neurotensin neuronal density in the SCN (r = 0.83; P < 0.0001; Fig. 4C). There was no detectable association between activity fragmentation (IV) and neurotensin neuronal density (r = 0.20; NS), interdaily stability, or circadian phase of activity or temperature (Table 2).
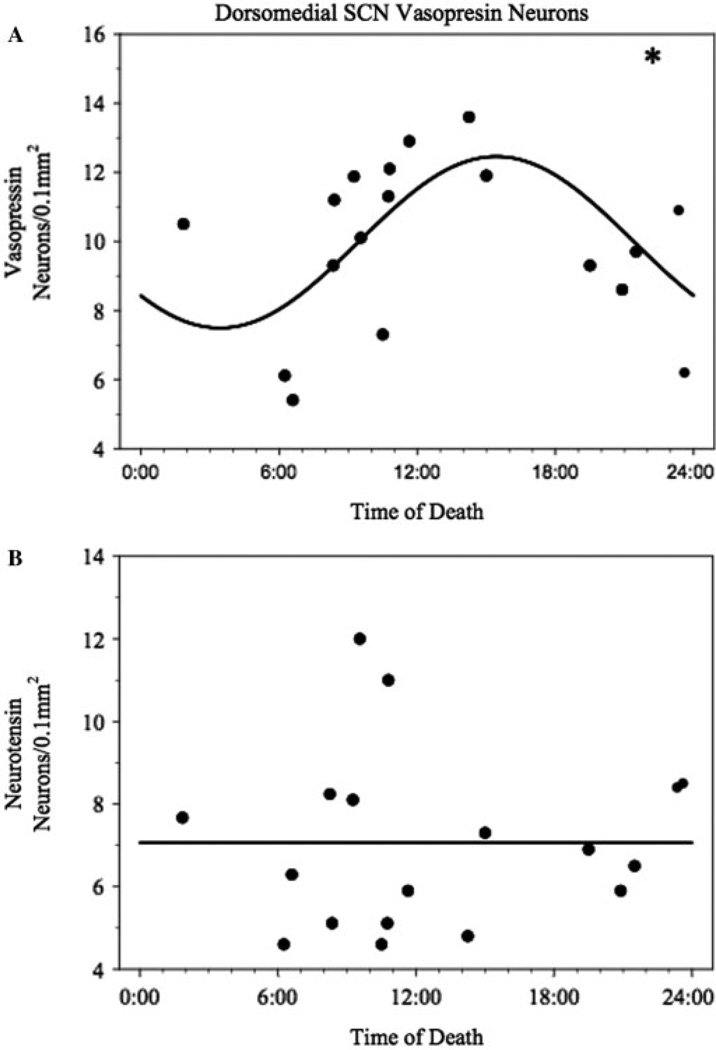
A significant circadian rhythm was detected in AD patients in the dorsomedial SCN vasopressin neuronal density when plotted by time of death (Fig. 5A). The rhythm acrophase occurred at 15:26 ± 03:54 and had a goodness-of-fit R2 = 0.35 [F(16) = 4.37; P = 0.031 compared to mesor fit]. In contrast, neurotensin neuronal counts showed no evidence of a circadian rhythm (R2 = 0.00; Fig. 5B). Control cases did not show evidence of a circadian rhythm in either vasopressin or neurotensin but that is likely due to the small sample size and resulting lack of power.
Fig. 5.
Non-linear least squares fit to vasopressin (A) and neurotensin (B) neuronal densities in dementia patients. Vasopressin densities were found to show a significant circadian rhythm when compared to a mesor fit. There was no evidence of a circadian rhythm in the densities of neurotensin neurons when compared to a mesor fit.
Four of the dementia subjects were taking benzodiazepines at the time of study (lorazepam or alprazolam (0.5 mg up to 3×/day) and eight subjects were taking neuroleptics (haloperidol up to 1 mg). No systematic differences were detected in the dementia patients taking neuroleptics versus those who were not, whereas those taking benzodiazepines tended to have lower levels of locomotor activity overall and some circadian blunting of their relative activity amplitude. No effect of benzodiazepines or neuroleptics were seen in the neuronal density measurements leading us to conclude that medication was a source of experimental noise rather than exerting a systematic effect on the results (Satlin et al., 1995).
Discussion
Evidence of degeneration was seen in the dorsomedial SCN in Alzheimer and frontotemporal dementia cases as shown by an increased glia/neuron ratio in subjects with these diagnoses. Glial proliferation in response to neuronal loss is a well-recognized pathophysiological reaction that is characteristically seen in neurodegenerative diseases like Alzheimer’s disease as well as in other neurological disorders like temporal lobe epilepsy due to Ammon’s horn sclerosis. Measurements of the glia/neuron or neuron/glia ratios have been used to estimate the regional loss of neuronal or glial cell populations in a variety of disease states, as well as during normal aging (Peng and Lee, 1979; Terry et al., 1981; Hansen et al., 1988; Roy et al., 2005; Probst et al., 2007; Smyth et al., 2007).
Commensurate decrements in perceptible vasopressin and neurotensin immunoreactive neuronal density were also observed in this region. While it is possible that vasopressin and neurotensin neurons are still present and not detectable due to lower immunoreactivity in patients with dementia, it is unlikely that this is the entire explanation due to the increased glia/neuron ratio, suggesting substantial neuronal loss in the dorsomedial SCN in both patients with AD and FTD.
Previous studies have found lower counts of vasopressin immunoreactive cells in the SCN in aging and further reduced in Alzheimer’s disease (Swaab et al., 1985; Stopa et al., 1999), as well as lowered vasopressin mRNA in the SCN (Liu et al., 2000). The population of neurotensin neurons has also been found to be lower in Alzheimer cases when compared to normal elderly (Stopa et al., 1999). In this study we found decreased vasopressin and neurotensin density in dementia cases compared to elderly controls with no clinical evidence of dementia. Neuronal density of neurotensin was found to be reduced in patients with dementia diagnoses of Alzheimer’s disease and frontotemporal dementia. The reduction was commensurate with the reduction in vasopressin neuronal density further supporting the hypothesis that cell loss is occurring in the dorsomedial SCN in patients with severe Alzheimer’s disease. The density differences that we observe may be an underestimation of the total effect that might be observed in total SCN cell number (Hofman et al., 1988).
Degeneration in the SCN in AD as assessed by glia/neuron ratio was significantly associated with change in the expressed circadian rhythm of core body temperature and a trend for change in the rhythm of locomotor activity. The present results provide direct evidence of the necessity of intact SCN for the maintenance of the circadian rhythm in humans. Evidence from rats, hamsters and other species demonstrates that the SCN is necessary and sufficient for the maintenance of physiological and behavioural circadian rhythms in other mammalian species (Stephan and Zucker, 1972; Abe et al., 1979), and the current results support the hypothesis that the human SCN plays an analogous role.
Specific neuronal subpopulations in the dorsomedial SCN appear to subserve different functions in humans, as suggested by the finding that the behavioural effects resulting from loss of vasopressin neurons manifested differently from the loss of neurotensin neurons. Vasopressin neuronal loss in patients with Alzheimer’s disease was associated with increased intradaily variability, a measurement of activity fragmentation, suggesting less consolidated nighttime sleep and/or more diurnal somnolence. However, the amplitude of locomotor activity and core body temperature was not lower in response to reduced vasopressin cell density. Reduced neurotensin cell density, on the other hand, showed a strong association with reduced amplitude of the temperature rhythm and reduced relative amplitude of locomotor activity. Although the absolute activity amplitude did not show such an association, the finding of this relationship when absolute amplitude is corrected for the total activity level (mesor) suggests that differences between patients overall activity levels mask the motor activity amplitude effect. Activity fragmentation as measured by intradaily variability was not associated with neurotensin neuronal loss, further underscoring the specificity of neuronal function in the dorsomedial SCN in humans.
These findings suggest that while both major neuronal subpopulations in the dorsomedial SCN in humans affect motor activity they appear to do so via different mechanisms and therefore the consequences of the loss of these neuronal groups are expressed differently. Phenotypically, Alzheimer patients do not show significantly different intradaily variability or relative amplitude of their activity rhythm than normal comparison subjects studied under similar conditions (van Someren et al., 1996; Harper et al., 2001, 2004). Alterations in the activity rhythm that are characteristic of AD, such as phase change and loss of interdaily stability of the activity rhythm, are not likely mediated by this mechanism since we did not find changes in these measures associated with changes in neuronal density. The argument for different mechanisms is further supported by the presence of a circadian rhythm observed in vasopressin neurons in the AD autopsy cases that was not observed in neurotensin.
A major limitation in our understanding the context of the changes in the diurnal rhythm of locomotor activity and core body temperature when a comparison is made to normal controls is the fact that while the controls were studied in an environment similar to the dementia subjects, this environment was novel to the controls and subtle differences in environmental conditions may have affected the results obtained in that group. Another limitation of this study is the difference in the sexual composition of the patient group and the neuropathological control group. However, unlike VIP neuronal fields in the SCN and while the vasopressin field of the SCN has a further rostro-caudal extension in females versus males, no significant differences related to sex or age have been observed in total neuron numbers in vasopressin in the SCN (Hofman et al., 1996).
A circadian rhythm was observed in counts of vasopressin neurons in patients with Alzheimer’s disease that was not seen in neurotensin neurons. Previous studies have observed circadian rhythms in vasopressin in the SCN in both animal models (Gillette and Reppert, 1987) and human post-mortem tissue (Hofman and Swaab, 1994). However, previous studies in human post-mortem tissue have not identified such a rhythm in patients with Alzheimer’s disease suggesting a more functional clock than had been previously supposed. Neurotensin neurons did not show a circadian rhythm when the count pattern was analysed, suggesting that neurotensin exerts its circadian effect through a different mechanism from vasopressin.
These findings support the idea of a functional although perhaps not as robust SCN in AD. The lack of a circadian rhythm in vasopressin in normal cases is probably due to a lack of statistical power rather than a rhythm not being present. No apparent rhythm was observed in neurotensin neurons in either normal or AD cases. This lack of rhythm is interesting due to the apparent dependence for expression of circadian rhythm of activity on the presence of neurotensin neurons in the dorsomedial SCN.
The present results do not elaborate on the main phenotypic finding in moderate to severe AD, that of prominent phase delay of the circadian rhythm (Harper et al., 2005). It is possible, based on studies of other species that the dysfunction leading to phase delay occurs in the core of the SCN or in the pathways innervating the SCN from the retina. Further work can be directed towards the vasoactive intestinal peptide neuronal concentration in the SCN core, the retinohypothalamic tract and the eye to understand this phenomenon.
Acknowledgements
Funded by R01-AG20654. This material is the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Administration Hospital, Bedford, MA.
Abbreviations
- GFAP
glial fibrillary acidic protein
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nuclei
- VIP
vasoactive intestinal peptide
References
- Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticoster-one, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29:119–131. doi: 10.1159/000122913. [DOI] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- Bartlett MS, Kendall DG. The statistical analysis of variances-heterogeneity and the logarithmic transformation. JRSS Suppl. 1946;8:128–138. [Google Scholar]
- Batschelet E. Circular statistics in biology. New York: Academic Press; 1981. [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Hughes M, McMahon PM, Kutner N. Observed sleep/wakefulness and severity of dementia in an Alzheimer’s disease special care unit. J Gerontol A Biol Sci Med Sci. 1995;50:M303–M306. doi: 10.1093/gerona/50a.6.m303. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Reppert SM. The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull. 1987;19:135–139. doi: 10.1016/0361-9230(87)90176-6. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Hansen LA, DeTeresa R, Davies P, Terry RD. Neocortical morphometry, lesion counts, and choline acetyltransferase levels in the age spectrum of Alzheimer’s disease. Neurology. 1988;38:48–54. doi: 10.1212/wnl.38.1.48. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, McKee AC, Satlin A, Fish D, Volicer L. Dementia severity and Lewy bodies affect circadian rhythms in Alzheimer disease. Neurobiol Aging. 2004;25:771–781. doi: 10.1016/j.neurobiolaging.2003.04.009. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, McKee A, Satlin A, Harlan PC, Goldstein RL, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. 2001;58:353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–368. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127:1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J Anat. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Zhou JN, Swaab DF. Suprachiasmatic nucleus of the human brain: an immunocytochemical and morphometric analysis. Anat Rec. 1996;244:552–562. doi: 10.1002/(SICI)1097-0185(199604)244:4<552::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn MR, Gibson M, Brittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci. 1999;19:5574–5585. doi: 10.1523/JNEUROSCI.19-13-05574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, Hoogendijk WJ, van Heerikhuize J, Kamphorst W, Unmehopa UA, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–322. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- Mai JK, Kedziora O, Teckhaus L, Sofroniew MV. Evidence for subdivisions in the human suprachiasmatic nucleus. J Comp Neurol. 1991;305:508–525. doi: 10.1002/cne.903050312. [DOI] [PubMed] [Google Scholar]
- McKee AC. Brain banking: basic science methods. Alzheimer Dis Assoc Disord. 1999;13 Suppl 1:S39–S44. [PubMed] [Google Scholar]
- Mishima K, Okawa M, Satoh K, Shimizu T, Hozumi S, Hishikawa Y. Different manifestations of circadian rhythms in senile dementia of Alzheimer’s type and multi-infarct dementia. Neurobiol Aging. 1997;18:105–109. doi: 10.1016/s0197-4580(96)00167-4. [DOI] [PubMed] [Google Scholar]
- Moore MR. Organization of the mammalian circadian system. In: Chadwick DJ, Ackrill K, editors. Circadian clocks and their adjustment. New York: John Wiley and Sons; 1995. pp. 88–99. [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- Moore RY. Organization of the primate circadian system. J Biol Rhythms. 1993;8 Suppl:S3–S9. [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Peng MT, Lee LR. Regional differences of neuron loss of rat brain in old age. Gerontology. 1979;25:205–211. doi: 10.1159/000212341. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol. 2007;114:335–345. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res Dev Brain Res. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247:154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- Smyth MD, Limbrick DD, Jr, Ojemann JG, Zempel J, Robinson S, O’Brien DF, et al. Outcome following surgery for temporal lobe epilepsy with hippocampal involvement in preadolescent children: emphasis on mesial temporal sclerosis. J Neurosurg. 2007;106:205–210. doi: 10.3171/ped.2007.106.3.205. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI. COSIFIT: an interactive program for simultaneous multioscillator cosinor analysis of time-series data. Comput Biomed Res. 1990;23:283–295. doi: 10.1016/0010-4809(90)90022-5. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Shinohara K, Otori Y, Fukuhara C, Inouye ST. Circadian rhythms of vasopressin content in the suprachiasmatic nucleus of the rat. Neuroreport. 1992;3:809–812. doi: 10.1097/00001756-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:777–796. doi: 10.2165/00023210-200115100-00004. [DOI] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Weibel E. Stereological methods. Vol. 1. New York: Academic Press; 1989. Sampling of tissue; pp. 63–100. [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]