Abstract
A recent study has described synchronous burst discharges of dentate hilar neurons and area CA3 pyramidal cells in the presence of the convulsants 4-aminopyridine and picrotoxin in guinea-pig hippocampal slices [Müller W. and Misgeld U. (1991) J. Neurophysiol. 65, 141–147]. To examine the synchronous activity of dentate cells and area CA3 pyramidal cells further, epileptiform burst discharges were examined in morphologically and/or electrophysiologically identified non-granule cells in the hilus and granule cell layer of the rat dentate gyrus and compared to simultaneously-recorded pyramidal cells of area CA3a, b, and c. Specifically, the types of dentate cells and the types of discharge were examined, as well as the timing of burst discharge of dentate cells relative to different cells of area CA3.
In the presence of the GABAA receptor antagonist bicuculline (30 µM), all dentate cell types discharged in rhythmic, spontaneous bursts that were synchronized with area CA3 pyramidal cell epileptiform bursts. The sampled cells included hilar “mossy” cells, hilar fast-spiking cells (putative interneurons) as well as interneurons located in the granule cell layer, such as the pyramidal “basket” cells.
Simultaneous recording from dentate non-granule cells and area CA3 pyramidal cells during exposure to bicuculline demonstrated that stimulus-evoked and spontaneous epileptiform bursts occurred almost exactly at the same time; there were only a few milliseconds between the onsets of pyramidal cell bursts and dentate cell bursts, with the pyramidal cell preceding the dentate cell in almost every case. There were no systematic differences among dentate cell types in the extent they lagged behind pyramidal cells, and there were no detectable differences among area CA3 pyramidal cells. In slices that were cut between area CA3 and the dentate gyrus, epileptiform bursts occurred in area CA3 but not in the dentate.
These findings suggest that, in the absence of GABAA receptor-mediated inhibition, excitatory pathways from area CA3 to the dentate gyrus are strong and widespread. These pathways, and possibly other mechanisms, can lead to tightly synchronized action potential discharge of pyramidal cells and dentate non-granule cells. The results also suggest that disinhibition alone is insufficient to cause synchronous bursts in the dentate gyrus, in contrast to area CA3.
A recent study reported that dentate hilar cells become synchronized with area CA3 pyramidal cells in hippocampal slices that were perfused with picrotoxin and 4-aminopyridine.15 Another study showed that perfusion of slices with 4-aminopyridine and excitatory amino acid antagonists does not result in synchronous discharge of hilar cells and pyramidal cells.13 One possibility raised by these two studies is that there are excitatory synaptic connections among area CA3 pyramidal cells and hilar cells. This idea has also been suggested from previous, mainly anatomical studies, but the issue has been controversial.5,10,21,24
In the present study, synchronization of area CA3 pyramidal cells and dentate non-granule cells was examined to answer several related questions. First, what is the extent of the synchronization—does it include all hilar cells? Are interneurons located nearby, such as those in the granule cell layer, involved? Second, how closely are the dentate cells synchronized with pyramidal cells? Third, are there differences among the three subfields of area CA3? Finally, does the synchrony require 4-aminopyridine, or can it also occur merely by blocking GABAA receptors?
To answer these questions, dentate non-granule cells and pyramidal cells were recorded simultaneously in the presence of a GABAA receptor antagonist, which is known to cause synchronized burst discharge in area CA3.2,4,23,25 To address which cell type(s) burst first, two approaches were used. First, we examined randomly chosen dentate cells and pyramidal cells simultaneously during the transition from the control to hyperexcitable state, noting which cell burst first. Second, we examined the relative onset of individual burst discharges, again using simultaneous recording from dentate cells and pyramidal cells.
Non-granule cells were identified using a combination of morphological and physiological criteria that in previous studies were used to distinguish two major cell types: (i) the spiny neurons (the most common example being the mossy cell), and (ii) the relatively aspiny neurons with physiological characteristics of “fast spiking” interneurons.6,9,18,20 Although anatomical analyses have been used to describe over 20 types of hilar neurons1 and many types of interneurons in the granule cell layer,16,22 these classification schemes were not employed in the present study because in some cases only physiological information was obtained to identify non-granule cells, and using physiological characteristics alone, only the groups mentioned above can be differentiated with absolute certainty at the present time.
EXPERIMENTAL PROCEDURES
Preparation and maintenance of slices
Slices were prepared from adult female Sprague–Dawley rats that were anesthetized with ether and decapitated. Animals were treated in accordance with guidelines set by the New York State Department of Health and the National Institutes of Health for care and treatment of laboratory animals. The dissected brain was cooled in 4°C, oxygenated (95% O2, 5% CO2) buffer (in mM: 126.0 NaCl, 5.0 KCl, 2.0 CaCl2, 2.0 MgSO4, 1.25 NaH2 PO4, 26.0 NaHCO3, and 10.0 d-glucose; pH 7.4) and 400-µm slices were cut transversely using a Vibroslice (Campden Instruments). Slices were placed in a modified interface recording chamber (Fine Science Tools) and were warmed (33–35°C) and oxygenated (95% O2, 5% CO2). For experiments where slices were severed between area CA3 and the dentate, the slice was cut between the lateral tips of the upper and lower blades (Fig. 1).
Fig. 1.
Diagram of the experimental arrangement. A diagram of the hippocampal slice is shown, illustrating where electrodes were placed. A bipolar stimulating electrode (stim) was placed on the ventral portion of the fimbria, or the outer molecular layer of the upper blade. Extracellular or intracellular recordings were made near the × in the pyramidal cell layer (PCL) of areas CA3a, CA3b, or CA3c. Simultaneous intracellular recordings were made in the hilus (H) or granule cell layer (GCL). The boundaries of the hilus are the granule cell layer and the dotted line. For slices that were cut between area CA3 and the dentate gyrus, a straight cut was made between the arrows.
Recording and stimulation
Extracellular and intracellular recordings were made with capillary-filled borosilicate glass (A&M Systems) pulled horizontally (Sutter Instruments). Extracellular electrodes were filled with 1 M NaCl and intracellular electrodes were filled with 1 M potassium acetate, or 3% Neurobiotin (Vector Labs) in 1 M potassium acetate. A dual intracellular amplifier with a bridge circuit (Axon Instruments) was used, and bridge balance was monitored throughout recording. Recordings were saved on tape (Neurodata) and analysed off-line.
Stimulation of the fimbria or the outer molecular layer employed a twisted metal, Teflon-coated bipolar electrode. For fimbria stimulation, the electrode was placed on the ventral portion of white matter (Fig. 1). For molecular layer stimulation, the electrode was placed adjacent and parallel to the hippocampal fissure (Fig. 1). Stimulation consisted of unipolar current pulses (50–250 µA, 10–250 µs, 0.1–0.2 Hz). Stimulus strength was adjusted by changing pulse duration while maintaining constant current (i.e. 100 µA, 10 vs 20 µs).
Recordings from area CA3a were made adjacent to the upper border of the fimbria (Fig. 1). Area CA3b recordings were made at the most ventral part of the pyramidal cell layer (Fig. 1). Recordings in area CA3c were made in the portion of the cell layer lying between the upper and lower blades (Fig. 1).
Identification of cell types
Spiny hilar cells (such as mossy cells) were identified physiologically by the following characteristics: (i) broad action potential duration (over 1.0 ms total duration); (ii) responses to intracellular depolarizing current injection that varied from trial to trial, often with spike frequency adaptation; (iii) individual action potentials that were followed by depolarizing afterpotentials (at −60 mV membrane potential); and (iv) extremely large (often over 10 mV) and frequent spontaneous excitatory postsynaptic potentials (EPSPs). Fast-spiking cells (such as interneurons) were identified physiologically by the following criteria: (i) short-duration action potentials (under 1.0 ms total duration); (ii) weak spike frequency adaptation; and (iii) individual action potentials followed by afterhyperpolarizations. Morphologically, spiny hilar cells were defined by numerous dendritic spines and thorny excrescences, whereas fast-spiking cells possessed smooth and/or beaded dendrites.
Dye injection and histology
Cells were injected with Neurobiotin using 20 ms duration, +0.5–+0.8 nA, current pulses at 30 Hz for 5–10 min. Slices were fixed in 4% paraformaldehyde, embedded in 4% Agar, and 50-µm sections were cut on a Vibratome (Lancer). Sections were incubated in 0.5% Triton-X overnight, immersed in 10% methanol in 3% H2O2 for 1 h, and incubated in ABC solution (Vector Labs) for 2 h. Sections were preincubated in 0.05% diaminobenzidine (DAB) for 20 min, and immersed in DAB plus 0.003% H2O2 for 1 h. Cells were drawn with a BH-2 microscope (Olympus) and a drawing attachment (Olympus) and photographed with a 35-mm camera attachment using Techpan film (12 ASA, Kodak).
Drug application
Bicuculline methiodide (Sigma; 10 mM in 0.9% NaCl) was applied by adding an aliquot of concentrated stock solution to the perfusate so that the final concentration was 30 µM. Flow rate to the chamber was adjusted so that it required 4 min for drug solution to reach the recording chamber. This slow rate of drug perfusion was advantageous because it slowed the period of epileptogenesis, and this optimized detection of differences in the time to onset of epileptiform behavior.
Data analysis
Input resistance was defined as the maximum slope of the V–I curve constructed from measurements at steady-state of voltage responses to a family of current steps. Action potential duration was measured as total duration from an action potential elicited at threshold by a 150-ms depolarizing current pulse.
The extent to which a pyramidal cell epileptiform burst preceded a dentate cell burst was defined as the interval between the onset of a pyramidal cell burst and the onset of a dentate cell burst. The onset of an intracellularly recorded epileptiform burst was defined in two ways, as the onset of the depolarization underlying a burst, or the peak of the first action potential. Although the depolarizing event underlying the burst clearly occurs before the first action potential, and therefore is a better measure of burst onset, the start of the depolarization was sometimes difficult to measure, whereas the peak of the first action potential was always easy to discriminate. The start of the depolarization was difficult to measure in those cases where it was gradual or merged with small, spontaneous EPSPs. For calculating average values of all cell pairs, the average value of five spontaneous bursts was used for each pair.
Burst duration was defined as the duration of the paroxysmal depolarization shift (PDS), not including the afterhyperpolarization. For extracellular recording, burst duration was defined as the duration of the positivity upon which the population spikes were superimposed.
Terminology
The following definitions clarify several terms for the purposes of the present study, because they are often used with different intent in the literature. They are similar to definitions made elsewhere.23 The term “epileptiform burst” or “prolonged burst” refers to repetitive action potential discharge that occurred synchronously in dentate cells and pyramidal cells after bicuculline was applied. In spiny hilar cells and pyramidal cells, epileptiform bursts were PDSs.11,12 In contrast, the term “brief burst” refers to the shorter period of discharge that occurred immediately before prolonged bursts developed. “Afterdischarge” refers to the relatively brief cluster of action potentials that occurred upon the falling phase of the FDS, or shortly thereafter. “Synchronous” discharge refers to discharge of two or more cells that occurred in close temporal proximity (within milliseconds). “Spontaneous” discharge refers to action potentials that occurred without electrical intervention.
RESULTS
Development of epileptiform bursting of dentate cells and pyramidal cells
To examine whether epileptiform discharges developed first in dentate cells or whether they developed first in CA3 pyramidal cells, dentate cells and pyramidal cells were recorded simultaneously during perfusion of the slice with 30 µM bicuculline. Twenty-one of the paired recordings used simultaneous intracellular recording and the other eight involved intracellular recording from a dentate cell while recording extracellularly in the pyramidal cell layer. Dentate cells that were sampled were either spiny hilar “mossy” cells (n = 22, 17 identified by morphology and physiology; five identified by physiology alone) or fast-spiking cells (putative interneurons, n = 7, five identified by morphology and physiology, two identified by physiology alone). All areas of the CA3 region were sampled (area CA3a, n = 5; area CA3b, n = 12; area CA3c, n = 12). Fast-spiking cells were located either in the hilus (n = 3) or in the granule cell layer (n = 4). Following epilepto-genesis, additional non-granule cells were also sampled so that a total of 71 spiny hilar cells, 11 fast-spiking hilar cells, and 13 fast-spiking cells from the granule cell layer were sampled.
Changes in evoked and spontaneous activity during drug application
The development of epileptiform activity occurred in several stages that were similar from cell to cell and occurred in the same chronological order.
Disinhibition of evoked responses. Following the addition of bicuculline to the perfusate, the first detectable change was in the response to stimulation of the fimbria. For every pair of cells that was examined, the change in evoked responses occurred at similar times in the two cells. Specifically, synaptically evoked depolarizations increased in amplitude, generating more action potentials for a given depolarization, and weaker stimuli were able to evoke a response (Figs 2A, B, 4A). Analogous changes were observed in extracellular recordings; multiple population spikes developed, and weak stimulus strengths were able to evoke responses (Fig. 4A).
Fig. 2.
Epileptogenesis in a hilar mossy cell and pyramidal cell recorded simultaneously. (A, B) Recordings from a CA3b pyramidal cell (A) made simultaneous to a spiny hilar cell (B) are shown. Control: responses to stimulation of the fimbria are shown prior to addition of bicuculline to the perfusate, at three different membrane potentials (A: top = −55 mV, center = −61 mV, bottom = −73 mV, and B: top = −53 mV, center = −63 mV, bottom = −70 mV). Stimulus artifacts are marked by the dots and are clipped. Bicuculline, 9 min 10 s: following 9 min and 10 s of perfusion with 30 µM bicuculline, the response to the same stimulus as used in control produced several action potentials followed by a slow hyperpolarization in both cells. Membrane potential: −63 mV (A) and −67 mV (B). Inset: the onset of the depolarization is enlarged to illustrate that the pyramidal cell (top trace) discharged before the hilar cell (lower trace). The arrowhead marks the capacitative artifact of the pyramidal cell’s first action potential. Action potentials are clipped. Note different time calibration for inset. Bicuculline, 9 min 15 s: following 9 min and 15 s of perfusion with bicuculline, stimulation evoked prolonged action potential discharge on a longer depolarization than previous responses, despite the use of a weaker intensity stimulus. Stimulation strength in control: 0.1 mA, 100 µs; stimulation strength for responses evoked at 9 min, 15 s: 0.1 mA, 10 µs. Same membrane potentials as at 9 min 10 s. Bicuculline, 10 min: after 10 min of perfusion with bicuculline, large depolarizations with afterdischarges occurred spontaneously. The first prolonged spontaneous discharge is shown. Inset: the onset of the depolarization of the spontaneous burst is enlarged to show that the pyramidal cell discharged before the hilar cell (arrowhead marks the capacitative artifact of the pyramidal cell’s first action potential). Same time calibration as for other inset. (C, D) Morphology of the spiny hilar cell from which recordings in part B were taken. Arrowheads point to complex spines and arrows point to the axon. For both C and D, dorsal is up and area CA3 is to the right. GCL, granule cell layer; MOL, molecular layer. Scale bar in D = 25 µm for C and 75 µm for D.
Fig. 4.
Epileptogenesis of a hilar fast-spiking cell. (A–C) Simultaneous recording from the area CA3b pyramidal cell layer extracellularly (top traces) and a hilar fast-spiking cell intracellularly (lower traces). (A) Responses to fimbria stimulation (at the dots) are shown before (1) and after (2) addition of bicuculline to the perfusate. Stimulus intensity was 150 µA, 50 µs for part 1 and 150 µA, 10 µs for part 2. The first spontaneous epileptiform burst, which occurred shortly after the data in part 2 were acquired, is shown in part 3. Membrane potential = −60 mV. Stimulus artifacts are clipped. Vertical calibration for top traces (extracellular recordings), 5 mV; vertical calibration for bottom traces and all other intracellular recordings of this figure (B–D), 15 mV. Horizontal calibration for A1, 10 ms; for A2 and A3, 100 ms. (B, C) Segments of the traces that are bracketed in part A3 are expanded in B and C. (D) Some electrophysiological characteristics of the fast-spiking cell of A–C are shown. 1: Response to a +0.3-nA, 150-ms current step evoked high-frequency, non-adapting discharge, as is typical of fast-spiking cells. Horizontal calibration, shown in D2, is 50 ms for Dl and 3 ms for D2. 2: A different time-base is used to show the brief duration of an action potential and the subsequent large afterhyperpolarization, both typical characteristics of fast-spiking cells.
Development of evoked and spontaneous brief bursts. Subsequent to the disinhibition of evoked responses, stimuli evoked progressively larger responses until brief bursts of three to seven action potentials were evoked after each stimulus (Fig. 2A, B). At a similar time as the development of brief, evoked bursts, brief spontaneous bursts occurred (Fig. 3A, B), and did so simultaneously in non-granule cells and pyramidal cells (Fig. 3A, B). These brief spontaneous bursts were the first synchronized activity that was detected in the pairs that were examined. Very few (one to four) brief bursts occurred before prolonged bursts developed (see below). When there was more than one brief burst, the interval between brief bursts was variable (range: 3 to over 30 s).
Fig. 3.
The onset of spontaneous burst discharge following bicuculline administration occurred simultaneously in pyramidal cells and dentate cells. (A) Simultaneous recording from a pyramidal cell (top) and spiny hilar cell (bottom) shows spontaneous activity of both cells. This record was taken 7 min after bicuculline was added to the buffer that perfused the slice. The arrow points to the first spontaneous suprathreshold discharge. (B) Expanded traces of the spontaneous event marked by an arrow in A. Arrowheads point to the capacitative artifacts of the pyramidal cell action potentials, and illustrate that pyramidal cell discharge preceded that of the hilar cell. (C) Simultaneous recording of a spontaneous epileptiform discharge of the same pyramidal cell and hilar cell as in A and B. This discharge occurred approximately 2 min after the bursts shown in A and B occurred.
Development of prolonged bursts. Within 1–2 min of the first evoked and spontaneous brief bursts, prolonged bursts followed by afterdischarges occurred, and again this change developed at the same time in dentate cells and pyramidal cells (Figs 2A, B, 4A). This change was often abrupt, such that a relatively brief evoked burst doubled or tripled in length within the 5-s interval between test stimuli (Fig. 2A, B). In other experiments brief bursts lengthened gradually (over several minutes); in these instances both the pyramidal cell and dentate cell bursts lengthened gradually. When pyramidal cells were recorded extracellularly, similar changes as those observed intracellularly occurred; brief bursts of less than five population spikes lengthened into prolonged bursts of 10 or more population spikes, and did so during the increase in the dentate cell burst.
Within seconds of the first evoked prolonged burst, the first spontaneous prolonged burst occurred, and did so simultaneously in dentate cells and pyramidal cells (Figs 2A, B, 4A). Once prolonged bursts occurred, they persisted for the remainder of the experiment (i.e. brief bursts were never observed again). Frequency of prolonged bursts varied a great deal from slice to slice (range: 0.174–0.033 Hz). Evoked and spontaneous prolonged bursts were qualitatively similar (Figs 2A, B, 4A). For the remainder of the text, prolonged bursts are often referred to as epileptiform bursts or merely “bursts.”
Comparison of prolonged bursts of dentate cells and pyramidal cells
The large depolarization shifts and associated discharge of spiny hilar cells and pyramidal cells fit the description of a PDS.11,12 Although from slice to slice there was variation in the duration, degree of discharge, and number of afterdischarges associated with PDSs, for any given pair of simultaneously recorded spiny hilar cell and pyramidal cell, the PDSs were similar. For example, the minimum and threshold stimulus strengths required to evoke PDSs were identical for any given pair of simultaneously recorded cells (threshold was defined as the stimulus strength that evoked a PDS in 50% of trials). In general, whenever a PDS occurred in the spiny hilar cell, it did in the pyramidal cell, and whenever the spiny hilar cell failed to burst, so did the pyramidal cell (Fig. 5A). The mean number of action potentials during the PDS was not different (mean 19.4 ± 1.6 for hilar cells, 21.4 ± 1.7 for pyramidal cells, n = 21 pairs, cells were assessed at approximately −65 mV membrane potential). The durations of the PDS were equivalent (mean = 237 ± 17 ms for spiny hilar cells, 236 ± 17 ms for pyramidal cells, n = 21). These values were not statistically different (Student’s t-test, P > 0.05). In some experiments, PDS duration of a given cell varied (compare the PDS of the pyramidal cell in Fig. 5B, left trace vs center trace), and in these instances the spiny hilar cell PDS duration varied in the same manner as the pyramidal cell PDS (i.e. increasing or decreasing whenever the pyramidal cell PDS increased or decreased). Finally, whenever afterdischarges occurred in a spiny hilar cell, they also did in the pyramidal cell, and there were exactly the same number of afterdischarges in both cells (mean = 1.0 ± 0.2 for hilar cells, 1.0 ± 0.2 for pyramidal cells; Figs 2A, B, 3C, 6A–C).
Fig. 5.
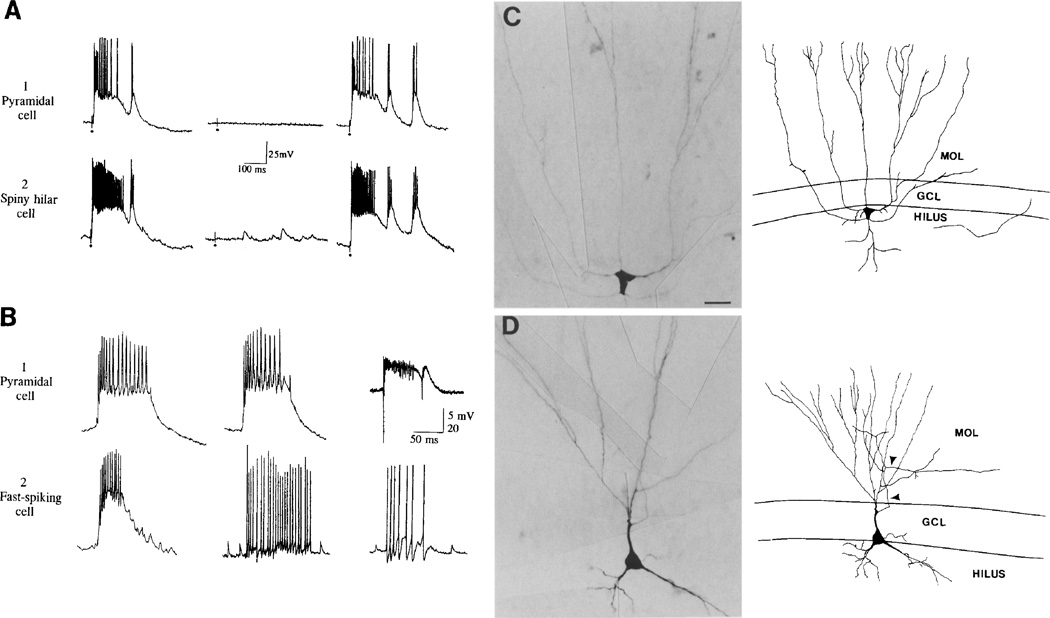
Comparison of epileptiform bursts of different cell types. (A) Simultaneous recordings from a CA3b pyramidal cell (1) and a spiny hilar cell (2) of epileptiform bursts evoked by fimbria stimulation, sampled after bicuculline application. Responses to three consecutive stimuli at 0.2 Hz are shown. Stimulus strength was very low, so that in some cases stimulation did not evoke a burst. Stimulation is at the dots and stimulus artifacts are clipped. (B) Simultaneous recordings of spontaneous bursts of CA3 pyramidal cells (1) and fast spiking cells (2). Calibration for extracellular recording, 5 mV; intracellular recording, 20 mV. Left: CA3b pyramidal cell, hilar fast-spiking cell. Center: same CA3b pyramidal cell as at left, different hilar fast spiking cell. The fast spiking cell is shown in C. Right: extracellular recording from CA3b in a different slice, intracellular recording from a fast spiking cell located in the granule cell layer. This fast-spiking cell is shown in D. (C) A photomicrograph (left) and illustration (right) of a hilar fast-spiking cell is shown. A spontaneous burst that was recorded from this cell is shown in B (center). GCL, granule cell layer; MOL, molecular layer. Scale bar = 20 µm. (D) A photomicrograph (left) and illustration (right) of a pyramidal “basket” cell is shown. A spontaneous burst that was recorded from this cell is shown in part B (right). Arrowheads point to the axon. Scale bar in C also applies to D.
Fig. 6.
Epileptiform bursts evoked by molecular layer stimulation. (A–C) While simultaneously recording from the CA3c pyramidal cell layer (1) and a spiny hilar cell (2), the molecular layer was stimulated at three different intensities: low (10 µs, A), intermediate (30 µS, B) and high (50 µs, C). Stimulation occurred at the dots and stimulus artifacts are clipped. (D, E) The parts of traces A and C in brackets are expanded in D and E, respectively. At low stimulus intensity (D), the hilar cell responds to stimulation with an action potential that occurs shortly after the stimulus, and this is followed by a burst with afterdischarges. The burst begins after the pyramidal population burst begins. At higher intensities (E), stimulation evokes a complex response in the hilar cell, and the onset of the pyramidal cell burst occurs at a short latency after the stimulus.
The bursts of fast-spiking cells were different from the PDSs of pyramidal cells that were recorded simultaneously. Specifically, all but two fast-spiking cells lacked the large underlying depolarization shift of a classical PDS (Figs 4A, 5B). The sample of fast-spiking cells included the seven fast-spiking cells examined during epileptogenesis as well as 17 other fast-spiking cells examined after epileptogenesis; the two exceptional cells were both located in the hilus. One of the fast-spiking cells that did have a large depolarization is shown in Fig. 5B (left trace). The extent of discharge during epileptiform bursts of fast-spiking cells was highly variable. Some cells discharged much more intensely than a simultaneously recorded pyramidal cell, whereas others discharged very little (Fig. 5B); in one case only one action potential occurred during a simultaneously recorded pyramidal cell burst (data not shown). Cells were evaluated at membrane potentials between −60 and −65 mV so that a paucity or excess of action potentials could not be attributed merely to a hyperpolarized or depolarized cell.
Comparison of timing of individual epileptiform bursts
The onsets of epileptiform bursts were examined to identify whether pyramidal cell bursts began precisely at the same time, before, or after dentate cell bursts. Pairs of cells that were included in this analysis included those pairs that were recorded before and during epileptogenesis (n = 21), as well as 67 other pairs of pyramidal cells and dentate cells that were obtained after epileptogenesis. The 67 pairs were composed of 45 dentate cells (spiny hilar cells, n = 31; fast-spiking hilar cells, n = 5; fast-spiking cells in the granule cell layer, n = 9) that were recorded in tandem with 67 different pyramidal cells (area CA3a, n = 3; area CA3b, n = 33; area CA3c, n = 31). In addition, 21 dentate cells were compared to pyramidal cells that were recorded extracellularly (spiny hilar cells, n = 18; hilar fast-spiking cells, n = 3; area CA3a, n = 2; area CA3b, n = 9; area CA3c, n = 10).
Evoked epileptiform bursts
CA3 pyramidal cells preceded dentate cells for all epileptiform bursts evoked by stimulation of the fimbria (tested in all experiments) or the molecular layer (in 12 experiments both the fimbria and molecular layer were tested), regardless of the definition of burst onset. (Burst onset was defined as either the start of the depolarization underlying the burst, or the peak of the first action potential.) These data differ from those of Müller and Misgeld who reported that bursts evoked by molecular layer stimulation occurred first in hilar cells and were followed by pyramidal cells (see P. 142 of Ref. 15). Some reasons for this difference may be that Müller and Misgeld sampled different cells, used picrotoxin rather than bicuculline, or that guinea-pigs were used instead of rats. In addition, there is another explanation for the discrepancy that became apparent when different stimulus intensities were tested to evaluate bursts evoked by molecular layer stimulation. When stimulus strength was weak, the response of hilar cells had two components: a short-latency, excitatory response (EPSP or one to three action potentials) that was followed after over 30 ms by a burst; the burst was synchronized with area CA3 cell bursts that were recorded simultaneously (Fig. 6). As stimulus strength was increased, there was a decrease in the delay between the short latency response and the burst that was synchronized with pyramidal cells. At high stimulus intensities, the short latency response and the synchronized burst appeared to merge, so that it appeared as if the hilar cell response to the molecular layer stimulus was one large, long-lasting burst that began shortly before the burst of pyramidal cells. Therefore the discrepany may be due to the fact that Müller and Misgeld used high stimulus intensities, whereas lower stimulus intensities were employed in the present study. The collective data, acquired using multiple stimulus intensities, indicate that responses to molecular layer stimulation occur in hilar cells before pyramidal cells, but synchronized bursts evoked by molecular layer stimulation occur first in pyramidal cells.
Spontaneous epileptiform bursts
Spontaneous pyramidal cell bursts preceded dentate cell bursts in most cases (Figs 2A, B, 3C, 4A, B, 7). Specifically, when burst onset was defined by the peak of the first action potential of a burst, there were only two pairs where the pyramidal cell did not precede the dentate cell. The afterdischarges of pyramidal cells also preceded dentate cells (Figs 4C, 6D) except for the same two pairs. The two exceptional pairs involved spiny hilar cells that preceded CA3c pyramidal cells. In these pairs, the hilar cell bursts onset preceded the pyramidal cell by 0.5–2.1 ms.
Fig. 7.
Variation in the onset of epileptiform bursts among pyramidal cells and simultaneously recorded dentate cells. (A) Recordings are shown from a CA3c pyrmidal cell (1) and a spiny hilar cell (2). The three pairs of traces are from the same cells. The first action potentials of three spontaneous epileptiform bursts are shown. Note that the onsets of the depolarizations underlying burst discharge (arrows) vary, but the intervals between action potential peaks are similar. Also note that the peak of the first action potential of the pyramidal cell always precedes the first action potential of the hilar cell. (B) Recordings from a CA3b pyramidal cell (1) and a spiny hilar cell (2, same cell as in part A). Note that the first action potential of the pyramidal cell precedes that of the hilar cell in each case, but the degree it does so varies. The peak of each action potential is marked by an arrow above the action potential.
The data were extremely variable when the onset of the burst was defined by the onset of the underlying depolarization. Even for the same pair of cells, the underlying depolarization of one cell could change relative to the other cell from burst to burst. For example, during one spontaneous burst the depolarization of the pyramidal cell could precede the dentate cell’s depolarization, but in the next spontaneous burst the depolarization of the dentate cell could precede the pyramidal cell’s depolarization (Fig. 7A). In 52 of 88 paired recordings the pyramidal cell depolarization always preceded the dentate cell, and in all other cases the dentate cell sometimes preceded the pyramidal cell.
When extracellular and intracellular bursts were compared, the timing of the first dentate cell action potential was compared to the first population spike. In 15 of 18 paired intracellular and extracellular recordings, the peak of the dentate cell’s first action potential occurred after the termination of the first population spike (Fig. 4B). In the remaining cases, the peak of the dentate cell’s action potential followed the peak of the population spike but occurred before the first population spike ended.
Comparisons of different dentate cell types and different subfields of area CA3
There were no systematic differences among the three types of dentate cells of the extent that their bursts followed CA3 pyramidal cell bursts. This was assessed by comparing all paired recordings including spiny hilar cells with pairs involving fast-spiking hilar cells and pairs involving fast-spiking cells of the granule cell layer. For each of the three groups, the interval between the onset of the pyramidal cell burst and onset of the dentate cell burst was measured. Burst onset was defined as the peak of the first action potential of the burst. The mean intervals for the three groups were 4.3 ± 0.1 ms for spiny hilar cells (n = 50), 5.0 ± 0.5 ms for fast-spiking hilar cells (n = 11), and 5.2 ± 0.5 ms for fast-spiking cells of the granule cell layer (n = 13). These means were not different (one-way ANOVA).
There also was no detectable difference in the extent that cells from different subfields of area CA3 preceded dentate cells. This analysis was performed in two ways. First, pairs were divided into three groups: pairs including CA3a pyramidal cells, pairs including area CA3b pyramidal cells, and pairs including area CA3c pyramidal cells. The mean intervals between burst onsets for each group were: 4.6 ± 0.8 ms for area CA3a cells (n = 5), 4.6 ± 0.2 ms for area CA3b cells (n = 42), and 4.2 ± 0.3 ms for area CA3c cells (n = 41). These differences were not statistically different (one-way ANOVA). The second method used to address this issue was to compare different CA3a, b, and c pyramidal cells with the same dentate cell. In such experiments one spiny hilar cell was impaled and two to nine pyramidal cells from areas CA3a, b, and c were sampled in the same slice. Five slices were examined in this manner. The mean intervals between area CA3a, b, and c cell bursts and dentate cell bursts were not different (one-way ANOVA for each slice).
Recordings in slices where the dentate gyrus was separated from area CA3
The development of epileptiform activity among area CA3 neurons and dentate neurons was examined in four slices that were cut between the lateral tips of the upper and lower blades (see Experimental Procedures). A spiny hilar cell was recorded simultaneously to an extracellular recording from area CA3b (n = 2) or CA3a (n = 2) before and during bath-application of bicuculline. Area CA3 developed spontaneous epileptiform bursts, but the dentate neurons recorded simultaneously did not. Following recording from the one dentate cell and one site in area CA3, other dentate neurons (n = 12; spiny hilar, n = 7; fast-spiking hilar, n = 3; fast-spiking granule cell layer, n = 2) and other areas of CA3 were sampled in the same slice, and the same results were obtained; area CA3 neurons burst but dentate neurons did not. Stimulation of the fimbria evoked synchronous epileptiform bursts in area CA3a and b, but the same fimbria stimulus evoked no response in dentate neurons. Conversely, stimulation of the molecular layer evoked responses (EPSPs, action potentials) in dentate cells, but not in area CA3 cells.
DISCUSSION
Primary conclusions
This study elucidated several aspects of the synchronization of CA3 pyramidal cells and dentate non-granule cells that is produced by disinhibition. First, synchronization of area CA3 pyramidal cells and dentate cells in the presence of bicuculline was widespread, involving all pyramidal cells, hilar cells and other dentate non-granule cells that were sampled. Pyramidal cells were sampled from areas CA3a, b, and c, and no differences were detected among the pyramidal cells. The dentate cell types that were sampled were spiny hilar cells, including mossy cells and other spiny neurons, and fast-spiking cells, including neurons located in the hilus and pyramidal-shaped “basket” cells located in the granule cell layer. Although it is possible that some cell types were not sampled, the data suggest that the majority of non-granule cells in the dentate gyrus become synchronized with the majority of pyramidal cells in the presence of bicuculline.
Second, the pyramidal cells and dentate cells were tightly synchronized, since burst discharges were only milliseconds apart. In almost every case, the onset of burst discharges began first in pyramidal cells and was followed by dentate cells. During the development of hyperexcitability the cells were also tightly synchronized, and the first burst discharges of pyramidal cells preceded those of dentate cells by milliseconds. Although it is difficult to define the exact moment when a cell or a population becomes hyper-excitable, the data indicated that epileptiform behavior developed at a similar time among the sampled cells.
Third, synchronization of pyramidal cells and dentate non-granule cells can occur merely by blocking inhibition with a GABAA receptor antagonist. This is informative because in previous studies showing synchronization following exposure to 4-aminopyridine and picrotoxin, it was unclear what mechanisms, out of the numerous processes affected by the two drugs, were responsible for synchronization. It is also important in the light of a different study which showed that dentate hilar cell burst discharges do not become synchronized with pyramidal cells bursts if 4-aminopyridine is applied with excitatory amino acid antagonists.13 Taken together, these studies suggest that the synchronization of hilar cells with pyramidal cells is dependent on inhibitory and excitatory circuitry, and the latter supports the hypothesis that there are excitatory synaptic connections among hilar cells and pyramidal cells. Collectively, the studies show that hilar cell burst discharge is not always synchronized with burst discharge in pyramidal cells. However, the converse, at least as studied to date, appears to be true; under conditions where area CA3 pyramidal cells burst. hilar cells are also excited.
Similarities and differences among epileptiform bursts of different cell types
This study also examined the different forms of excitation occurring in the presence of bicuculline. Epileptiform bursts of all spiny hilar cells and pyramidal cells sampled were extremely similar, consisting of a PDS with extensive action potential discharge and afterdischarges. However, the activity of fast-spiking cells was variable compared to pyramidal cells. The degree of action potential discharge, pre ence of a depolarization shift, and afterdischarges were variable. These results may indicate that the excitatory circuitry underlying burst discharge is different for spiny hilar cells and fast-spiking cells, with a stronger excitatory pathway to spiny hilar cells. However, the differences could also arise from differences in the repertoire of voltage-dependent and ligand-gated channels on spiny hilar cells and fast-spiking cells, irrespective of the circuitry.
The homogeneity of burst discharges among spiny hilar cells strengthens the case that, at least functionally, spiny hilar cells can be classified as a single group. This has been argued previously,18 despite anatomical differences such as the density of thorns, presence or absence of dendrites in the molecular layer, fusiform or multipolar cell body, etc.1 The data also support the separation of fast-spiking cells from spiny hilar cells, as was previously proposed.18
There were no detectable differences in the relative timing of burst discharge among the pyramidal cells from different subfields of area CA3 and different dentate cell types. These results differ from those which one would predict from other studies of synchronized burst discharge in area CA3, where area CA2–CA3 pyramidal cells initiated burst discharge.25 Based on that study, one would expect a greater interval between the bursts of area CA2–CA3 and dentate cells than the interval between bursts of area CA3b/c pyramidal cells and dentate cells. The fact that such a difference was not identified suggests that dentate non-granule cells do not function as an extension of the CA3 pyramidal cell layer, i.e. as a “CA4” region. Rather, the dentate non-granule cells appear to function as a mixed group that are not related any more to area CA3bjc than CA3a. However, further studies might reveal a difference if more restricted comparisons were made, such as examining merely the basket cells or the axo-axonic cells instead of grouping these relatively aspiny cells together.
Evidence that CA3 pyramidal cells excite dentate non-granule cells and vice versa
Burst discharges of all dentate cell types were abolished by making a cut between the lateral tips of the upper and lower blades, but this did not appear to affect burst discharges in area CA3. This is consistent with the work of Müller and Misgeld15 who placed tetrodotoxin drops in the dentate or area CA3 and reported that epileptiform discharges of unidentified dentate cells were blocked. These findings support the hypothesis that area CA3 neurons excite dentate non-granule cells. Although an important alternate hypothesis to excitatory synaptic connections is excitation induced by field effects or gap junctions, the extensive delay between some burst discharges (up to 12 ms) and the fact that gap junctions have only been noted among interneurons,7 argue against nonsynaptic excitation.
If area CA3 neurons do synaptically excite dentate neurons, they may do so in a number of ways. First and most simply, they could directly innervate dentate cells. Area CA3 axons have been found in the hilus,5,10 so this is theoretically possible. However, the long intervals between some bursts of CA3 cells and dentate cells indicate that a polysynaptic pathway is more likely. One possibility for a polysynaptic circuit is one where area CA3 pyramidal cells excite other pyramidal cells before finally exciting hilar neurons. This possibility is feasible given the recurrent collaterals of area CA3 pyramidal cells.14 Excitation of dentate cells by a polysynaptic circuit involving area CA3 innervation of area CA1 neurons (such as stratum lacunosum-moleculare interneurons, which can project to the dentate8) can be ruled out because cutting just the portion of the slice between the blades of the dentate gyrus was sufficient to block all excitation of dentate cells. Excitation via the CA3–CAl-entorhinal cortex-dentate gyrus circuit can be ruled out for the same reason.
The data in support of an excitatory projection from dentate cells to area CA3 are very weak. Only in two cases was there clear evidence that a dentate cell epileptiform burst preceded a CA3 cell burst. Thus, the data strongly support an excitatory pathway from area CA3 to dentate non-granule cells, but only weakly support reciprocal excitation.
Implications
The ability of area CA3 pyramidal cells to excite dentate non-granule cells may have far-reaching implications, regardless of the mechanisms by which it occurs, because granule cells are innervated by non-granule cells.3,16,17,19,22 The results suggest that an excitatory pathway from area CA3 to the dentate might normally be limited by inhibition, but under physiological or pathophysiological conditions where inhibition becomes impaired, an underlying excitatory circuitry might emerge. Under these circumstances, area CA3 pyramidal cells could have strong effects on granule cells, a direction that is precisely the opposite of the trisynaptic circuit.
Acknowledgements
I thank Dr R. S. Sloviter and Dr D. H. Lowenstein for their comments on an earlier version of the manuscript. I also thank Dr S. Neubort for assistance with statistics, MS E. Dean for photography, and Mrs A. Curcio and Mrs R. Marshall for secretarial assistance.
Abbreviations
- DAB
diaminobenzidine
- EPSP
excitatory postsynaptic potential
- PDS
paroxysmal depolarization shift
REFERENCES
- 1.Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J. comp. Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 2.Dingledine R, Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J. Physiol. 1980;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frotscher M, Seress L, Schwerdtfeger WK, Buhl EH. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J. comp. Neurol. 1990;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- 4.Hablitz JJ. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J. Neurophysiol. 1984;51:1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. comp. Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi Y, Hama K. Fast-spiking non-pyramidal cells in the hippocampal CA3 region, dentate gyrus, and subiculum of rats. Brain Res. 1987a;425:351–355. doi: 10.1016/0006-8993(87)90518-x. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983;277:347–351. doi: 10.1016/0006-8993(83)90943-5. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel DD, Lacaille J-C, Schwartzkroin PA. Ultrastructure of stratum lacunosum moleculare interneurons of hippocampal CA1 region. Synapse. 1988;2:382–394. doi: 10.1002/syn.890020405. [DOI] [PubMed] [Google Scholar]
- 9.Lacaille J-C, Kunkel DD, Schwartzkroin PA. Electrophysiological and morphological characterization of hippocampal interneurons. In: Chan-Palay V, Köhler C, editors. The Hippocampus–New Vistas. New York: Alan R. Liss; 1989. pp. 285–303. [Google Scholar]
- 10.Li X-G, Tepper JM, Jandó G, Buzsáki G. Axon arborization of CA3 pyramidal cells in vivo: an intracellular labelling study. Soc. Neurosci. Abstr. 1992;18:320. [Google Scholar]
- 11.Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Expl Neurol. 1964;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Expl Neurol. 1964;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- 13.Michelson HB, Wong RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- 14.Miles R, Wong RKS. Excitatory synaptic interactions between CA3 neurons in the guinea pig hippocampus. J. Physiol. 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller W, Misgeld U. Picrotoxin- and 4-aminopyridine-induced activity in hilar neurons in the guinea-pig hippocampal slice. J. Neurophysiol. 1991;65:141–147. doi: 10.1152/jn.1991.65.1.141. [DOI] [PubMed] [Google Scholar]
- 16.Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J. Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- 17.Ribak CE, Seress L, Amaral DG. The development, ultrastructure, and synaptic connections of the mossy cells of the dentate gyrus. J. Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 18.Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny “fast-spiking” cells. In: Ribak CE, Gall C, Mody I, editors. The Dentate Gyrus and its Role in Seizures. Amsterdam: Elsevier; 1992. pp. 93–108. [PMC free article] [PubMed] [Google Scholar]
- 19.Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–701. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzkroin PA, Mathers L. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978;157:1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- 21.Schwerdtfeger WK, Sarvey JM. Connectivity of the hilar region of the hippocampal formation in the rat. J. Hirnforsch. 1983;24:201–207. [PubMed] [Google Scholar]
- 22.Soriano E, Nitsch R, Frotscher M. Axo-axonic chandelier cells in the rat fascia dentata: Golgi-electron microscopy and immunocytochemical studies. J. comp. Neurol. 1990;293:1–25. doi: 10.1002/cne.902930102. [DOI] [PubMed] [Google Scholar]
- 23.Swann JW, Brady RJ, Friedman RJ, Smith KJ. The dendritic origins of penicillin-induced epileptogenesis in CA3 hippocampal pyramidal cells. J. Neurophysiol. 1986;56:1718–1738. doi: 10.1152/jn.1986.56.6.1718. [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J. comp. Neurol. 1978;181:681–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 25.Wong RKS, Traub RD. Synchronized burst discharge in disinhibited hippocampal slice I. Initiation in CA2–CA3 region. J. Neurophysiol. 1983;49:442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]