Abstract
Identification of cancer cells in the lymph nodes surrounding a tumor is important in establishing prognosis. Optical detection techniques such as fluorescence and photoacoustic tomography (PAT) have been reported in preclinical studies for noninvasive sentinel lymph node (SLN) mapping. A method for validation of these techniques is needed for clinical trials. We report the use of a multimodal optical-radionuclear contrast agent as a validation tool for PAT in a preclinical model. Methylene blue (MB) was radiolabeled with 125I for multimodal SLN mapping and used in conjunction with MB to assess the feasibility of multimodal SLN mapping in a rat model by PAT and SPECT. MB provided sufficient contrast for identifying SLNs non-invasively with a PAT system adapted from a clinical ultrasound imaging system. The signal location was corroborated by SPECT using 125I labeled MB. The translation of PAT into the clinic can be facilitated by direct comparison with established imaging methods using a clinically relevant dual SPECT and photoacoustic imaging agent. The new high resolution PAT is a promising technology for sensitive and accurate SLN detection in cancer patients.
Introduction
Until recently, radical lymph node resection with ex vivo histopathology was routine for staging cancers including breast and prostate cancers. This procedure often resulted in significant morbidity from local fluid retention and consequent infection, pain and limited limb use 1 and is no longer considered the standard of care 2. Selective biopsy of lymph nodes that are first encountered by metastatic cells is a less traumatic diagnostic method and has been found to be more useful for staging without impacting treatment in most cases. In cancer staging, detection of tumors in the sentinel lymph nodes (SLNs), which are the first nodes that drain the area of interest, is an important prognostic factor that directs treatment planning. Lymphoscintigraphy for SLN mapping was introduced in 1977 using radiolabeled colloidal gold 3. Tracer agents were selected by the rate of uptake in the lymph nodes and limited diffusion outside of the lymphatic system 4. SLNs can be detected by regional subcutaneous injection of contrast agents. For example, 99mTc sulfur colloid is widely used pre-operatively and intraoperatively to detect SLNs 5.
SLN mapping is complex due to the variety of drainage pathways from the primary tumor site 6. The detection of lymph nodes and distinction of SLNs may be complicated by the choice of contrast agent and by lymphatic channels temporarily accumulating contrast agents that can be mistaken as lymph nodes 7. Thus, 3D nuclear imaging methods such as SPECT are useful for noninvasive localization of the SLN. Hybrid SPECT/CT instruments are now available and provide the ability to determine the exact anatomical location of SLNs and improve lymphatic mapping 8.
To aid intraoperative visualization of the lymph drainage into SLNs, vital dyes are commonly employed. One of the dyes used to guide SLN biopsy in humans is methylene blue (MB). SLN localization using vital dyes alone is limited to wide-field exposure of the peritumoral area for visualization of the distinct blue color with unaided human eye 9. This approach is not efficient because of the need to use large amounts of the dye, which can only be visualized invasively. This limitation of detection has led several groups to postulate that radioisotopes are superior to dye contrast agents for identifying SLNs in cancer patients 10, 11.
Recent studies, however, have shown that a new hybrid imaging method known as photoacoustic tomography (PAT) may alter the existing paradigm of SLN mapping. PAT combines the sensitivity of optical imaging with the spatial resolution of ultrasound. This imaging technology is capable of detecting light-absorbing compounds several centimeters below the skin surface 12. The sensitivity of PAT to contrast agents is much greater than ultrasound 13 and the ability of PAT to interrogate deep tissue noninvasively allows its use for 3D imaging and non-invasive lymph node identification for SLN biopsy. The success of this technology in small animal imaging has motivated current efforts to translate it into the clinical arena 14. A primary focus of these efforts is the validation of PAT as a viable method for imaging SLN in human patients. Since PAT does not use ionizing radiation, it is amenable to use in surgical suites. However, there is an urgent need to validate this new technology with an established imaging method. Considering the important role of SPECT in SLN mapping, one strategy to validate PAT is to use a common contrast agent for both PAT and a more established imaging modality such as SPECT. Here, we report our initial efforts in multimodal imaging with MB and 125I-MB for validating PAT of SLN with SPECT/CT.
Methods
Radiolabeling
Methylene blue (MB, Sigma, St. Louis, MO) was radiolabeled with 125I sodium iodide (American Radiolabeled Chemicals, Inc. St. Louis, MO, USA) as previously described 15. 125I-NaI (37MBq) was added to freshly prepared potassium iodide/iodate solution (585 µg KI and 3.85 mg KIO3). The mixture was acidified (0.25 ml, 0.18 M HCl) and heated (60 min, 100°C.) Reaction progress was monitored by reversed phase, radio-HPLC (Altima, C18, 3 µm, 7 mm × 53 mm, 2.5 mL/min, A=0.1% TFA, water, B=0.1% TFA, acetonitrile, 30–90% B, 10 m).
In vivo Imaging
All animal studies were conducted according to guidelines on the humane care and use of laboratory animals under protocols approved by the Animal Studies Committee at Washington University School of Medicine.
A combined hand-held PAT and ultrasound (US) imaging system adapted from a clinical US array system (iU22, Philips Healthcare) was used for in vivo mapping of lymph nodes in rats 14. An optically tunable dye laser (PrecisionScan-P, Sirah), pumped by a Q-switched Nd:YAG laser (PRO-350-10, Newport), produced pulsed lasers with a duration of 6.5 ns at a repetition rate of 10 Hz. The light was coupled to bifurcated fiber bundles (CB18043, Fiberguide), which were integrated with a hand-held US array probe (L8-4, Philips Healthcare). An optical wavelength of 650 nm, close to the peak optical absorption wavelength of methylene blue (667 nm), was utilized. Light fluence on the surface was ~3 mJ/cm2, only 1/7 of the ANSI safety limit (20 mJ/cm2) 16. Generated PAT waves were detected by the linear array US probe with a nominal bandwidth of 4–8 MHz. PAT images were displayed at ~1 frame per second (fps). The imaging depth was increased by layering ~2-cm thick chicken tissue on top of the rat to demonstrate the deep-imaging capabilities of PAT at clinically relevant depths. MB (0.1 ml of 1%) was injected in the left front forepaw of 200–250 g female Sprague Dawley rats (HSD, Indianapolis, IN) (n=3), and in vivo PAT imaging was performed. Co-registered PAT and US images identify MB-enhanced SLNs (from PAT) in ratsand the surrounding anatomical structures (from US imaging).
For multimodal imaging, 125I-MB (~200 µCi) was added to 1% MB (0.1 ml, PBS). An aliquot of 0.1 ml was drawn into a 0.5 ml insulin syringe and activity measured with dose calibrator before injecting subcutaneously in the left forepaw as for PAT (n=3). The activity remaining in the syringe was measured and injected dose calculated. SPECT/CT imaging was performed immediately after injection of 125I-MB and at 1 h after injection with the NanoSPECT/CT preclinical imaging system (Bioscan, Inc., Washington, D.C.). Scanning regions were selected by side-view topogram, extending from the xyphoid to the base of the jaw. CT was performed on a 45 KVP, 177 mA and 180 400 ms projections helical CT with pitch of 1. The CT scan was followed by helical SPECT with 16 projections and 60 seconds each. Energy windows were set to 28 KeV and 30% width. CT and SPECT projections were reconstructed using InvivoScope software (Bioscan, Inc.).
After SPECT/CT imaging, planar scintigraphy was performed using the IS4000MM multimodal imaging system (Carestream Health, New Haven, CT). White light, fluorescence, X-ray and scintigraphy images were acquired. Following SPECT/CT, the rats were euthanized with pentobarbital solution (150 mg/kg, IP). The skin overlying the axillary region was removed for visualization of the axillary lymph nodes. Planar scintigraphy was performed and color images acquired with digital camera. The lymph nodes were resected and images acquired for comparison of contralateral, control lymph nodes to confirm 125I-MB uptake in lymph nodes using the optical imaging and planar scintigraphy. Activity in the lymph nodes was measured using the dose calibrator and percent injected dose per gram tissue (%ID/g) calculated.
Results
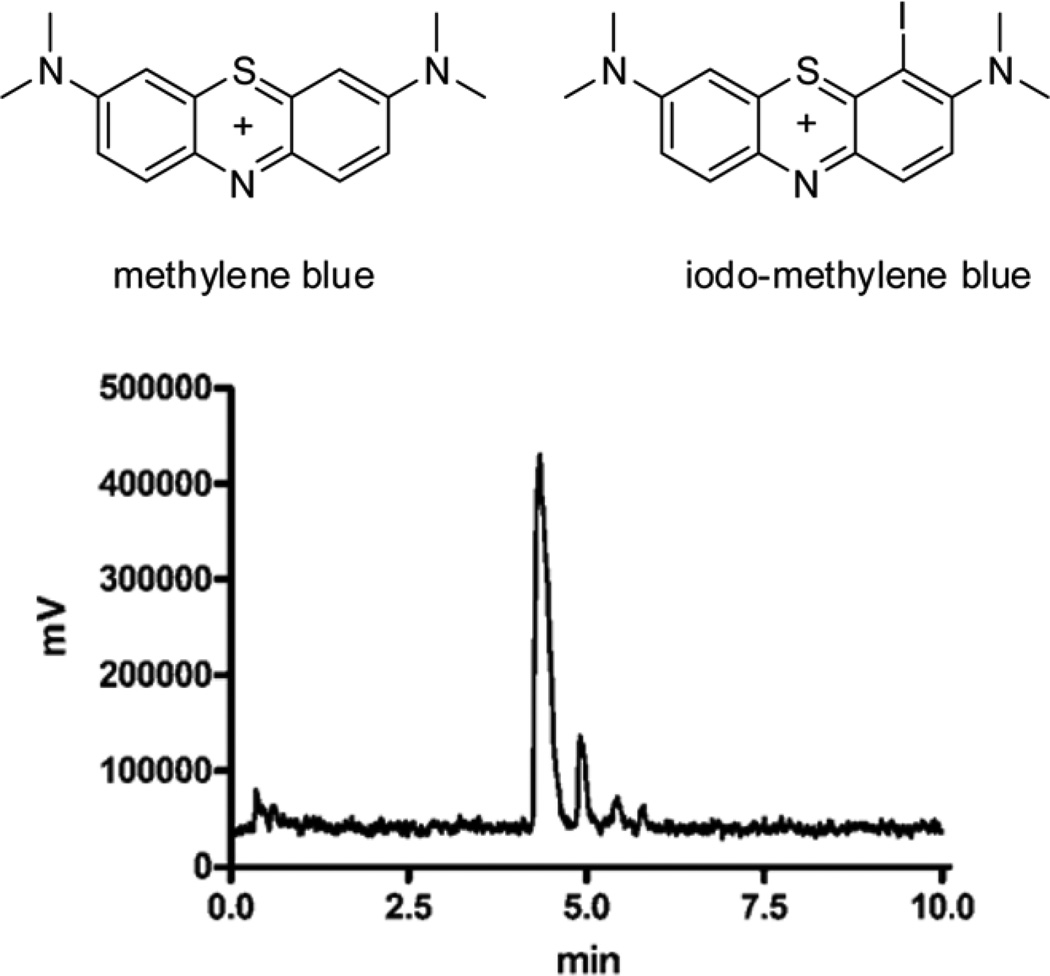
Initial attempts to prepare iodo-methylene blue using the standard iodogen method resulted in the appearance of a major impurity that was not further characterized. To overcome this problem, we utilized KI/KIO3 under acidic conditions instead of the iodogen method 15. Purification of the mixture yielded 125I –MB 17 (Figure 1), which has a retention time of 4.86 min and 97% purity based on the peak area. The unlabeled methylene blue eluted approximately 2 minutes before the iodinated species. A minor component that eluted at 4.95 min was the putative di-iodinated species that has been previously described 15. Incorporation of 125I in I-MB was quantitative and complete in 10 minutes. Because specific activity was not an issue in this study, the isolated 125I-MB was not further purified.
FIGURE 1.
Structures of methylene blue and iodo-methylene blue 17 (top). Reversed phase radio-chromatogram of 125I-iodo-methylene blue (bottom).
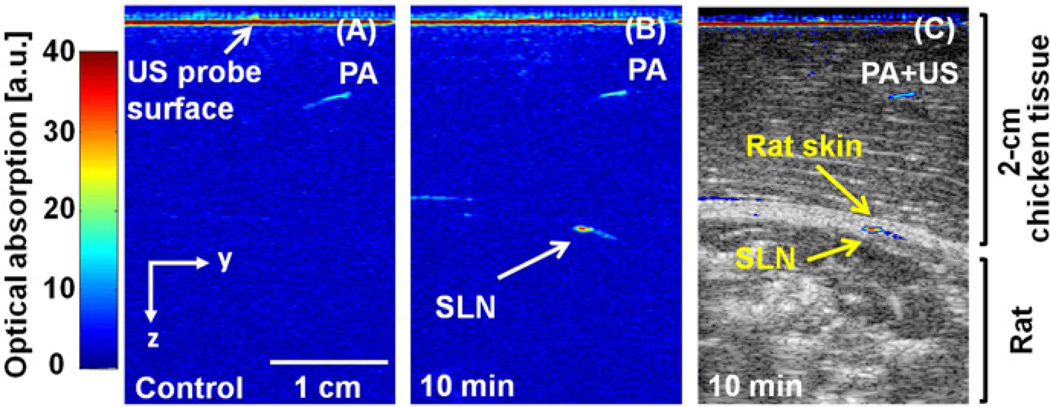
PAT images show clearly localized lymph nodes containing blue dye (Figure 2). A control PAT image was acquired before injection of MB (Figure 2A). Soon after injection, MB accumulation in the SLN was detected photoacoustically. Figure 2B shows the PAT image of the MB-dyed SLN acquired at 10 min post-injection. An overlaid PAT and US image (Figure 2C) provides both functional (MB uptake in the SLN) and structural information. Post-mortem photographs taken after PAT imaging confirmed MB uptake in the SLN (vide infra).
FIGURE 2.
PAT imaging of lymph nodes after injection of MB. A) Control PAT image obtained before MB injection. B) PAT image taken at 10 minutes post-injection. C) Overlaid PAT (pseudo color) and US (gray scale) image. PAT, photoacoustic tomography; US, ultrasound imaging; MB, methylene blue; and SLN, sentinel lymph node.
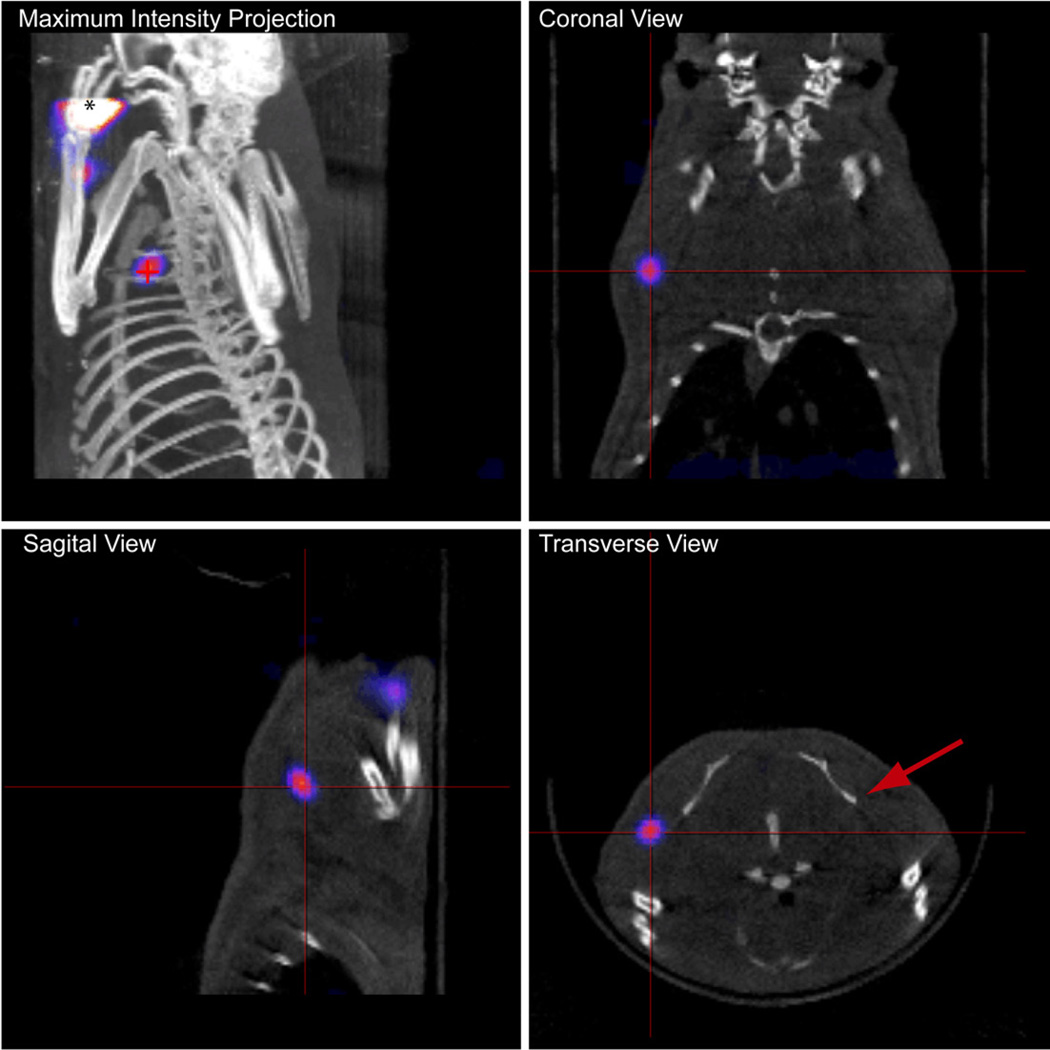
SPECT imaging showed localized signal from 125I-MB in the axillary region within 1 h after injection (Figure 3). The high intensity regions at the injection site, in the forelimb and at the location of the axillary lymph nodes were easily distinguished, allowing accurate mapping of the lymph fields. Approximately 9% (17.7+/−0.48 µCi, n=3) of the injected dose was detected in the axillary lymph node within 1 h after injection while almost no signal was detected in the region of the contralateral lymph node (Figure 3). High uptake was confirmed by planar scintigraphy and post-mortem optical imaging (vide infra).
FIGURE 3.
SPECT/CT projection images of rat acquired 1 h after subcutaneous injection of 125I-MB in the left forepaw. * indicates location of injection and crosshairs indicate location of signal from axillary lymph node. The red arrow indicates the contralateral lymph node in the transverse view. The lymph node was detected about 3 mm below the surface of the skin, correlating well with the PAT finding.
We further validated that both PAT and SPECT signals originated from the same lymph node. Since our PAT and SPECT/CT imaging systems are not co-localized in the same lab area, we explored a multimodal imaging platform. In principle, 125I-MB can provide photoacoustic, fluorescence, and gamma imaging signals. However, it was difficult to detect MB fluorescence through the skin by planar reflectance optical imaging. After removing the skin in the MB injection area, the lymph node and vessels were detected visually by dark blue stains (Figure 4A). The lymph tracts leading to the lymph node can be seen as well. Even with the exposed skin, only low fluorescence signal was detected in the forelimb and in the lymphatic vasculature, but was particularly dim from the area of the lymph node. This low nodal fluorescence could be attributed to the low fluorescence quantum yield of MB and likely quenching within the highly stained lymph nodes. The strength of the PAT signal suggests that much lower concentrations of MB can be used in future studies.
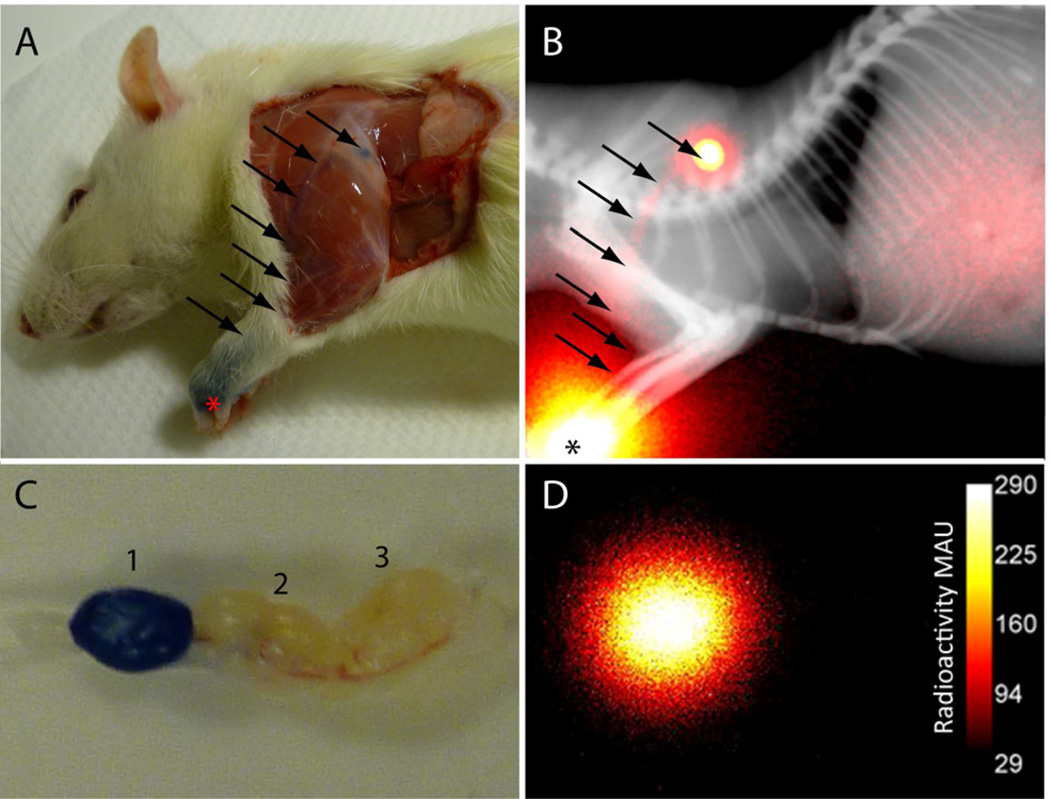
FIGURE 4.
Post-mortem optical and planar scintigraphy of rat 2 h after subcutaneous injection of 125I-MB in the left forepaw. A) The MB can be clearly seen as blue coloring in the forepaw near the site of injection (*) and at the first lymph node after removal of overlying skin and hair. The lymph tracts leading to the lymph node can be seen as well (arrows). B) Planar scintigraphy corresponds with blue color with high intensity from the left paw and lymph node. C) After dissection, the first lymph node is distinctly blue from dye accumulation while the following nodes are free of the dye. D) Ex vivo scintigraphy confirmed that the first lymph node was the source of the signal.
Unlike fluorescence imaging, planar gamma scintigraphy clearly showed high intensity from the left paw, lymphatic vessel, and lymph node (Figure 4C) corresponding with the dark blue color observed in Figure 4A. The animal was euthanized, and then the dissected lymph nodes from the left axillary region were removed. After dissection, the first lymph node is distinctly blue from dye accumulation while the following nodes are free of the dye (Figure 4B). Thus, the post-mortem photograph taken after PAT imaging confirmed MB uptake in the SLN. Similarly, the ex-vivo planar scintigraphy confirmed that the first lymph node is the source of the SPECT signal, demonstrating that 125I-MB could serve as a reliable molecular imaging probe for in vivo validation of PAT in clinical trials.
Discussion
Central to current SLN mapping is the administration of two complementary detection agents, 99mTc-labeled colloids and MB to guide SLN identification and biopsy. Although the goal of the procedure is to localize the SLN, significant differences in the size, physical and chemical properties of these two agents result in differential distributions and uptakes of the agents in the lymph nodes. These differences are not problematic when used for SLN detection and biopsy. However, validation of PAT for human use will benefit from a direct comparison of the nascent PAT method with established SPECT. Although PAT has been shown to image objects several centimeters in animal tissue, the optical properties of human tissue are different and it is important to demonstrate that PAT will not miss critical SLNs in humans.
To facilitate this validation, we explored the use of a single imaging agent for both PAT and SPECT. Previous studies have shown that MB can be radio-iodinated with different radioisotopes of iodine for imaging cancer in humans and animals 18. These studies demonstrated that the combination of radiotracer and MB increases the rate of correct SLN detection. In particular, 125I-MB has been previously reported as an alternative to the mixture of 99mTc colloid and MB for intra-operative guidance of SLN biopsy. A phase I/II clinical trial was performed in which a handheld gamma detector was used to locate hotspots followed by skin incision and excision of SLNs that were stained blue 19.
We prepared 125I-MB and used it in a different context from previously reported applications. Here, MB served as a contrast agent for PAT, which relies on light absorption by chromophores such as exogenous dyes or endogenous targets like hemoglobin. This absorption process generates local thermal expansion of tissue that is captured as an acoustic signal for PAT image reconstruction. By radiolabeling MB with 125I, it also yields SPECT signals, thus providing a direct approach to validate the application of the nascent PAT for lymph node mapping in humans. This is particularly important if PAT will be used as a standalone noninvasive imaging modality in the surgical room. We used a small animal model of lymph node mapping to explore the validity of the aforementioned strategy. Our study showed that the multimodal optical-nuclear contrast agent can be used to confirm the location of SLNs detected by noninvasive PAT in deep tissue. This strategy of validation is effective for preclinical and clinical studies using PAT.
Mapping of the lymphatic vasculature and nodes by high resolution PAT for SLN biopsy demonstrated great potential. Validation of this technique by SPECT/CT will improve whole-field identification of lymph nodes draining the tumor area. The successful demonstration of lymph node localization using the clinically compatible 125I-MB imaging agent in small animals and the ability of PAT to interrogate deep tissue suggest that this strategy will speed clinical translation of PAT for SLN biopsy.
Conclusion
SLN biopsy has become the standard of care for staging of many types of cancer. The nodal status is an important prognostic indicator after diagnosis of cancer. PAT has demonstrated great potential for non-invasive detection of SLN after injection of an optically absorbing contrast agent. Validation of PAT for SLN biopsy is needed for clinical translation. The multimodal imaging approach presented in this report is a good strategy for validation of optical imaging techniques. This multimodal technique will be useful for validating the signal location determined by non-invasive PAT using the hand-held device. SLN mapping for subsequent fine needle aspirate, core needle biopsy or minimally invasive resection can be achieved with high confidence. The next stage is to use the dual reporter imaging probe in human studies, a process that will help validate the future role of PAT in breast cancer staging.
Background
Identification of cancer cells in the lymph nodes surrounding a tumor is important in establishing prognosis. Optical detection techniques such as fluorescence and photoacoustic tomography (PAT) have been reported in preclinical studies for noninvasive sentinel lymph node (SLN) mapping. A method for validation of these techniques is needed for clinical trials.
Translational Significance
We report the use of a multimodal optical-radionuclear probe to validate PAT in a preclinical model. The approach will facilitate translation of PAT into the clinic through comparison with clinically relevant SPECT. The high resolution PAT is sensitive and accurate for SLN detection in cancer patients.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (R01 EB008458, R01 EB007276, R01 EB008111, R01 EB000712, R01 EB008085, R33 CA123537, and U54 CA136398 — the Network for Translational Research). Dr. Akers is supported an award from the National Center for Research Resources (K01RR026095). L.V.W. has a financial interest in Microphotoacoustics, Inc. and Endra, Inc., which, however, did not support this work. T.N.E. is an employee of Philips Research.
Abbreviations
- PAT
photoacoustic tomography
- SLN
sentinel lymph node
- MB
methylene blue
- SPECT
single-photon emission computed tomography
- PET
positron emission tomography
- CT
computed tomography; US = ultrasound
- US
ultrasound
- IP
intraperitneal
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read this journal’s policy on disclosure of potential conflicts of interest.
References
- 1.Amersi F, Hansen NM. The benefits and limitations of sentinel lymph node biopsy. Curr Treat Options Oncol. 2006;7:141–151. doi: 10.1007/s11864-006-0049-y. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DS, Sample WF, Fee HJ, Holmes C, Morton DL. Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg Forum. 1977;28:147–148. [PubMed] [Google Scholar]
- 4.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 5.Thompson M, Korourian S, Henry-Tillman R, et al. Intraoperative radioisotope injection for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:3216–3221. doi: 10.1245/s10434-008-0010-3. [DOI] [PubMed] [Google Scholar]
- 6.Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nucl Med. 2003;44:570–582. [PubMed] [Google Scholar]
- 7.Boxen I, McCready D, Ballinger JR. Sentinel node detection and definition may depend on the imaging agent and timing. Clinical Nuclear Medicine. 1999;24:390–394. doi: 10.1097/00003072-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 8.van der Ploeg IM, Valdes Olmos RA, Kroon BB, Nieweg OE. The Hybrid SPECT/CT as an additional lymphatic mapping tool in patients with breast cancer. World J Surg. 2008;32:1930–1934. doi: 10.1007/s00268-008-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varghese P, Abdel-Rahman AT, Akberali S, Mostafa A, Gattuso JM, Carpenter R. Methylene blue dye--a safe and effective alternative for sentinel lymph node localization. Breast J. 2008;14:61–67. doi: 10.1111/j.1524-4741.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida T, Jinno H, Sakata M, et al. Superiority of Radioisotope over Blue Dye for Sentinel Lymph Node Detection in Breast Cancer. Eur Surg Res. 2010;44:111–116. doi: 10.1159/000277937. [DOI] [PubMed] [Google Scholar]
- 11.Lin KM, Patel TH, Ray A, et al. Intradermal radioisotope is superior to peritumoral blue dye or radioisotope in identifying breast cancer sentinel nodes. J Am Coll Surg. 2004;199:561–566. doi: 10.1016/j.jamcollsurg.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats-- volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology. 2010;255:442–450. doi: 10.1148/radiol.10090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110:2756–2782. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, Erpelding TN, Maslov K, et al. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. J Biomed Opt. 2010;15 doi: 10.1117/1.3469829. 046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blower PJ, Carter NJ. Rapid preparation of 123I-labelled methylene blue and toluidine blue: potential new agents for parathyroid scintigraphy. Nucl Med Commun. 1990;11:413–420. doi: 10.1097/00006231-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 16.American national standard for the safe use of lasers. New York, NY: American National Standards Institute; 2002. [Google Scholar]
- 17.Blower PJ, Clark K, Link EM. Radioiodinated methylene blue for melanoma targeting: chemical characterisation and tumour selectivity of labelled components. Nuclear medicine and biology. 1997;24:305–310. doi: 10.1016/s0969-8051(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 18.Link EM, Costa DC, Lui D, Ell PJ, Blower PJ, Spittle MF. Targeting disseminated melanoma with radiolabelled methylene blue: Comparative bio-distribution studies in man and animals. Acta Oncol. 1996;35:331–341. doi: 10.3109/02841869609101650. [DOI] [PubMed] [Google Scholar]
- 19.Cundiff JD, Wang YZ, Espenan G, et al. A phase I/II trial of 125I methylene blue for one-stage sentinel lymph node biopsy. Ann Surg. 2007;245:290–296. doi: 10.1097/01.sla.0000242712.74502.72. [DOI] [PMC free article] [PubMed] [Google Scholar]