Abstract
Activating and inhibiting receptors of lymphocytes collect valuable information about their mikròs kósmos. This information is essential to initiate or to turn off complex signaling pathways. Irrespective of these advances, our knowledge on how these intracellular activation cascades are coordinated in a spatiotemporal manner is far from complete. Amongst multiple explanations, the scaffolding proteins have emerged as a critical piece of this evolutionary tangram. Amongst many, IQGAP1 is one of the essential scaffolding proteins that coordinate multiple signaling pathways. IQGAP1 possesses multiple protein interaction motifs to achieve its scaffolding functions. Using these domains, IQGAP1 has been shown to regulate a number of essential cellular events. This includes actin polymerization, tubulin multimerization, MTOC formation, calcium/calmodulin signaling, Pak/Raf/Mek1/2-mediated Erk1/2 activation, formation of maestrosome, E-cadherin and CD44-mediated signaling and GSK3/APC-mediated β-catenin activation. In this review we summarize the recent developments and exciting new findings of cellular functions of IQGAP1.
Introduction
Incessant communications of immune cells with their environment govern their development, trafficking, recognition of target antigens/cells, phenotypic conversion from effector to memory or apoptotic death. Recent studies indicate the bygone era of linear signaling pathways and a paradigm shift to complex network circuitries. Integration of ‘spacetime relativity’ is obligatory to execute intended biological functions in lymphocytes. Multiple mechanisms have been described that facilitate the spatiotemporal coordination of signaling events in lymphocytes (1). Among these, scaffolding proteins play an important role. Scaffolding proteins are defined as proteins that can recruit, coordinate and facilitate the spatiotemporal organization and the sequential activation of signaling molecules to achieve optimal functional outcomes. Cytoplasmic scaffolding proteins such as IQGAP, Carma1, KSR1, Ste5, MP1 Paxillin or PSD-95 can function as processing centers of kinases and their substrates. IQGAP1 is one of the most evolutionarily conserved (>90%) scaffolding proteins and is present in a variety of organisms. There are three isoforms of IQGAPs (1, 2 and 3) that are described in human and mouse. Among these, IQGAP1 is ubiquitously found (2) while the expressions of IQGAP2 (liver, platelets, kidney, stomach, prostate, thyroid and salivary glands) (3–6) and IQGAP3 (brain, lung, testis, small intestine and colon) (3,7) are restricted. Amongst lymphocytes, NK (8), B and T cells (Malarkannan, unpublished) predominantly express IQGAP1. This review abridges the recent exciting information on IQGAP1 and its cellular functions (8).
Protein structure and cellular partners of IQGAP1 scaffolding protein
IQGAP1 is a 190-kDa protein and its functional domains indicate that it is a critical regulator of development and functions in multiple cell types. It was identified in 1994 as a widely expressed IQ domain-containing protein (9). This 1657 amino acid long scaffolding protein has been described to associate with more than 50 different protein partners (10). Knockout mouse for IQGAP1 has been generated, where the null mutants bred at normal frequencies and no other major physiologic defects could be identified except a significant onset of gastric hyperplasia later in their life (11). A possible explanation for these observations could be the presence of IQGAP2 and IQGAP3 isoforms. Multiple cell surface receptors have been described to directly recruit IQGAP1 through their cytoplasmic tails, including Cadherins. The E- and N-cadherins recruit IQGAP1 in order to maintain their trans interactions in the tight-and adherens junctions (12). E-cadherins are primarily expressed on epithelial cells but their ability to interact with receptors such as KLRG1 (13–18) that has been shown to play a critical role in the homeostasis of NK cells (19) further emphasize the potential roles of IQGAP1 in the immune system. Recent studies show that E-cadherin also interacts with integrin CD103 (20–22) that is expressed in specific T cell subsets (20,23,24) and CD11c high dendritic cells (25). These studies provide a functional framework of how E-Cadherin on mucosal epithelia or thymus can influence the maturation and migration of CD103+ T cells and dendritic cells. Additional future works are required to precisely determine the role of IQGAP1 in these cell-cell interactions. In a recent study, IQGAP1 has been shown to be part of a large cytoplasmic complex that contained phosphorylated NFAT1, long intergenic non-coding RNA (lincRNA), non-coding (RNA) repressor of NFAT (NRON) and three of the kinases that are responsible for NFAT1 phosphorylation (26). This study further showed that the IQGAP1 preferentially interacted with phosphorylated NFAT1. Lack of IQGAP1 increased the dephosphorylation of NFAT1, its entry into the nucleus resulting in an augmented production of IL-2 or IFN-γ from T cells after mitogenic activation (26). These results while defining the potential scaffold functions of lincRNA also emphasize the novel functions mediated by IQGAP1 in immune cells.
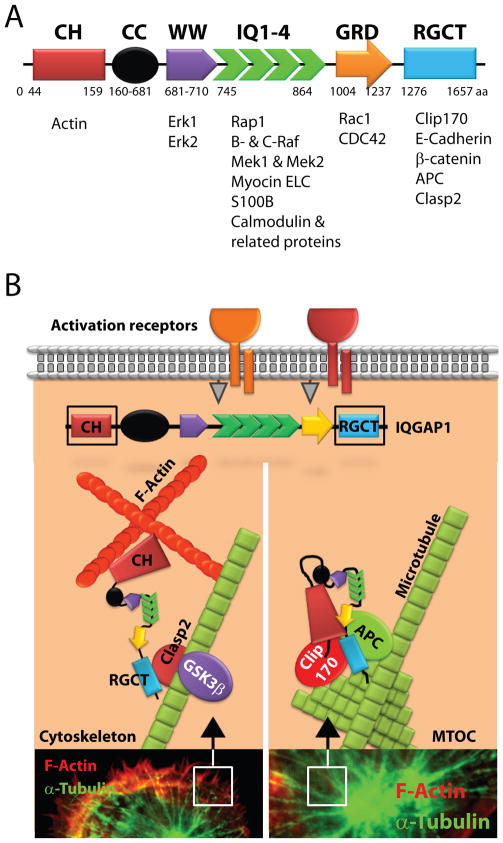
IQGAP1 is composed of multiple protein recognition-motifs (Figure 1A). As the name denotes, a unique domain in IQGAP1 contains sequence homology to the Ras GTPase-activating proteins (Ras-GAP). However, this domain lacks GAP activity and thereby is unable to regulate Ras-GTP or Rap-GTP hydrolysis. The N-terminal calponin homology (CH) domain of IQGAP1 binds to actin (27,28) while the IQ domain recruits calmodulin (29). The WW domain, with two highly conserved tryptophans, is an interaction module for proline-rich ligands (30) and binds to Erk1/2 (31). The functional role of the coiled-coil domain is not known; however, it has a presumptive α-helical domain with significant sequence homology to myosins.
Figure 1. Protein structure and interacting partners of IQGAP1.
A) IQGAP1 is a 190 kDa protein that contains at least six distinct protein-interacting domains. The calponin homology (CH) domain binds to polymerized filamentous actin. The function or the interacting partners of the coiled-coil (CC) domain has yet to be defined. The two highly conserved tryptophan-containing (WW) domains recruit Erk1/2. Isoleucine/glutamine-containing (IQ) domain is a binding domain for multiple proteins including Rap1a, Rap1b, Mek1, Mek2, myosin ELC, Rafs, S100B and Ca2+-independent interaction of calmodulin and its related proteins. Ras-GAP domain (GRD) interacts with small GTPases Cdc42 and Rac1. Ras-GAP C-terminus domain (RGCT) interacts with the microtubule-binding protein Clip-170, β-catenin, E-cadherin, Clasp2 and APC. B) The CH domain of IQGAP1 interacts with polymerized F-actin. Further, IQGAP1 can delay the hydrolysis of Cdc42 and stabilize its interaction with WASp/Arp2/3 complex. IQGAP1 also plays important roles in linking actin meshwork with plus-ends of microtubules through Clasp2. The role of IQGAP1 in the formation and function of MTOC is not well understood. Both Clip-170 and APC may facilitate the organization and multimerization of γ-tubulin molecules that form the core of the centrosome and MTOC.
The IQ domain is a tandem repeat of four IQ motifs that mediates interactions with MEK1/2, myosin essential light chains (9), S100B (a Zn2+ and Ca2+-binding protein) (32), calmodulin (27,29,33) and calmodulin-related proteins (Figure 1A) (34). Pathmanathan et al (35) demonstrated that the first IQ domain recruited myosin essential light chain Mlc1sa, while the first and the fourth interacted with myosin light chain, Mlc1p, from yeast. The first and second IQ domains were responsible for interacting with S100B. The C-terminal end of IQGAP1 engages with Cdc42-GTP (29), Rac1-GTP (33), E-cadherin (12), β-catenin (36) and APC (37). IQGAP1 also has the ability to bind to Rap1 (38), B-Raf or C-Raf (39), Mek1/2 (40) and Erk1/2 (31). Ras-GAP domain (GRD) interacts with small GTPases such as Cdc42 (29,33), Rac1 (33) and TC10 (41). This GAP domain lacks the ability to hydrolyze the bound GTP. Crystal structure of this region indicates that the GRD domain of IQGAP family possesses a conserved threonine instead of the catalytic ‘arginine finger’ described in functional Ras GAPs that is obligatory for GTP hydrolysis (42). The Ras-GAP C-terminus domain (RGCT) interacts with the microtubule-binding protein Clip170 (43), β-catenin (44), E-cadherin (12), APC, and Clasp2 (Figure 1A) (37). Other proteins such as AKAP79 have been shown to regulate calcium flux via PKA by directly binding to a C-terminal domain of IQGAP1 (45,46). Thus, IQGAP1 can potentially regulate cell polarization, transcription, actin and microtubule function, MAPK cascade, and Ca2+/calmodulin signaling (47,48). While these studies demonstrate the critical cellular functions of IQGAP1, the immunological relevance of many of these protein interactions and the effector regulations by IQGAP1 has yet to be defined.
IQGAP1 regulates actin/tubulin cytoskeleton and MTOC formation
The actin cytoskeletal structure functions as a regulator of signaling and cell integrity. IQGAP1 can directly interact with F-actin and regulate the actin meshwork formation (6,9,27,28,43,48–83). In particular, the N-terminal CH domain of IQGAP1 directly interacts with the actin meshwork (Figure 1B) (37,84). This CH motif of IQGAP1 and IQGAP2 are similar to the F-actin-binding domains present in members of the spectrin, filamin, and fimbrin families. Detailed biochemical analyses have revealed that IQGAP1 using its CH domain interacts with F-actin in a way that is critical to regulate the polymerization of actin (28,52). It has been demonstrated that the purified IQGAP1 can directly bind to F-actin and cross-link the actin filaments into irregular, interconnected bundles that exhibit gel-like properties (85). In addition, IQGAP1 can also interact with various proteins that are involved in cytoskeletal reorganization (43,52,69). This includes Cdc42 and Rac1 (33,49,85), APC (86), CLIP-170 (87), Clasp2 (88), and EB1 (89). APC that has been well characterized to regulate the polarized cell migration can also directly interact with IQGAP1 (37).
Cdc42 and Rac1 belong to the Rho family of small guanosine-3-phosphatases and play a significant role in regulating the cellular cytoskeleton (43,90). In recent years, the requirement of IQGAP1 in Cdc42 and Rac1-mediated actin polymerization has been well established. Indeed, the GRD domain of IQGAP1 directly recruits small GTPases such as Cdc42 (29,33), Rac1 (33) and TC10 (41). Importantly, Cdc42-GTP and Rac1-GTP but not RhoA (6,85) or Ras (33) have been shown to interact with the GRD domains of IQGAP1 and IQGAP2. Thus, it appears that the activated Cdc42-GTP will function as a linker between the WASp/Arp2/3 complex and IQGAP1/APC/Clip170/Clasp2 complexes. The ability of the N-terminal region of IQGAP1 (1–216 aa) to directly interact with F-actin (49) brings additional questions on the precise functional role played by IQGAP1. Is IQGAP1 critical for forming the actin meshwork using polymerized actin filaments? The ability of IQGAP1 to cross-link actin was augmented by guanosine 5′-(3-O-thio)triphosphate (GTPgammaS).GST-Cdc42 but not by GDP.GST-Cdc42 (91). This augmentation occurred by a preferential oligomerization of IQGAP1 by GTPgammaS.GST-Cdc42. These findings reveal that the oligomerization of IQGAP1 is a crucial step mediated by Cdc42 in regulating the cross-linking of filamentous beta or gamma actin (49).
Other quantitative co-localization studies have shown that IQGAP1 also plays a significant role in the co-localization of N-WASp in close proximity to Arp2/3 complex in lamellipodial structures (84). In addition, co-immunoprecipitation, pull-down and kinetic assays demonstrate that the C-terminal half of IQGAP1 activated N-WASp by interacting with its BR-CRIB domain similar to that of Cdc42, while the N-terminal half of IQGAP1 antagonizes this activation by association with a C-terminal region of IQGAP1 through intramolecular interactions (84). Thus, a structural change in the IQGAP1 protein can function as an auto-regulatory switch that when turned ‘on’ can activate N-WASp resulting in the stimulation of actin assembly in an Arp2/3-dependent manner. The shape and morphology of dendritic arbors of neurons depend on the plus-end tracking protein Clip-170 and IQGAP1 (81). This study demonstrates that a direct interaction of mTOR kinase with Clip-170 is required for the formation of the Clip-170/IQGAP1 complex. This complex is capable of regulating the actin/tubulin cytoskeletons in primary hippocampal and cortical neurons. Thus, IQGAP1 can function as a focal point for feedback interactions between the actin and microtubule cytoskeletal systems. One of the first evidences that IQGAP1 could play a major role in the reorganization of cytoskeleton came from Kaibuchi’s laboratory (43). This study showed that the activated Rac1/Cdc42, IQGAP1, and CLIP-170 form a tripartite complex. Although these studies provide a mechanistic explanation of how IQGAP1 plays a critical role in the regulation of tubulin multimerization and microtubule remodeling, its unique role on MTOC is not well understood. Evidence on the role of IQGAP1 on MTOC reorientation came from studies by Watanabe et al (37). Recent studies from the Malarkannan laboratory demonstrate that the size and shape of MTOC could be regulated by IQGAP1 via the small GTPase, Rap1b (8). Rap1b has been shown to directly interact with IQGAP1 (38). Lack of Rap1b did not affect the formation of the MTOC. However, the size and the length of MTOCs were proportionately much larger in NK cells that lacked Rap1b. Lack of Rap1b results in reduced ERK1/2 phosphorylation primarily due to an impairment in the sequential phosphorylation of B-Raf/C-Raf→MEK1/2→ERK1/2 signaling pathway that requires the presence of the IQGAP1 scaffold (8). Other studies by Kanwar et al demonstrate that the knockdown of IQGAP1 in the NK cell line YTS resulted in the inability of MTOC to reorient, while the ability of YTS cells to form conjugates with target cells was preserved (92). Lack of IQGAP1 did not grossly affect the development of T cells in the thymus. However, in the absence of IQGAP1, T cells fail to accumulate F-actin or polarize their MTOC toward anti-CD3-coated beads (93).
IQGAP1 provides the scaffold for the sequential phosphorylation of Pak→Raf→Mek→Erk1/2
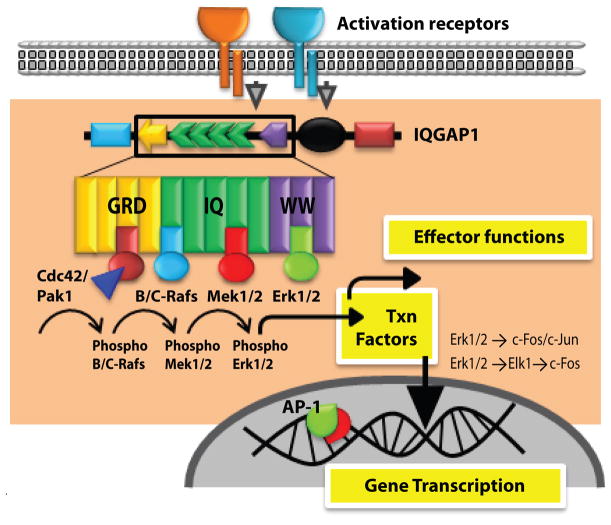
Activation and phosphorylation of Erk1/2 constitute a key signaling event in all lymphocyte subsets. Erk1/2 phosphorylation regulates development and effector functions of T, B and NK cells (8,94,95). MAPKs, in particular Erk1/2, have an important role in regulating the generation of IFN-γ, GM-CSF, MIP-1α, MIP-1β and RANTES and cytotoxicity in NK cells (8,96). MAPKs have also been shown to play a central role in the cytotoxic granule exocytosis from NK cells (97,98). Therefore, their phosphorylation and subsequent subcellular compartmentalization has to be tightly regulated to achieve intended outcomes. Multiple scaffolding proteins including IQGAP1 (31,99), KSR1 (100,101), MP1 (102,103) and β-arrestins (104-107) have been shown to regulate Erk1/2 activation in lymphocytes. Recent studies by Awasthi et al demonstrate that IQGAP1 plays an important role in regulating the Rap1b-GTP→Vav1→Cdc42→ Pak→B-Raf/C-Raf→Mek1/2→Erk1/2 signaling pathway in NK cells (Figure 2) (8). Through its scaffolding function, IQGAP1 has the ability to recruit and sequentially regulate the activations of B-Raf (39), Mek1/2 (40) and Erk1/2 (31). Thus, IQGAP1 can play a significant role in the cytokine/chemokine gene transcriptions and other effector functions of lymphocytes. Each member of this sequential phosphorylation of protein kinase cascade namely B-Raf, Mek1/2, Erk1/2 have been shown to bind directly to IQGAP1 in vitro and in intact cells (12).
Figure 2. IQGAP1 scaffold regulates Erk1/2 phosphorylation.
Sequential activation of Cdc42-Pak1-B/C-Raf-Mek1/2-Erk1/2 occurs on the IQGAP1 scaffold. The GRD domain, the four IQ repeats and the WW domain of the IQGAP1 are involved in the recruitment and phosphorylation of Cdc42, Rafs and Mek1/2 and Erk1/2, respectively. Membrane proximal signaling from activation receptors activate the small GTPase Cdc42 that is recruited to the GRD domain of IQGAP1. This in turn results in the recruitment and activation of Pak1. Although no direct binding of Pak1 to IQGAP1 has been described, it can bind to Rafs and it is critical for the conformational change and activation of B- or C- Rafs. Rafs are recruited to the IQ domains of the IQGAP1. Activation of Rafs results in the recruitment and phosphorylation of Mek1/2 to the IQ domains. Activated Mek1/2 is essential for the recruitment and phosphorylation of Erk1/2 that have the ability to bind to the WW domains of the IQGAP1.
At the start of this sequence, receptor-mediated signaling activates Fyn, a Src family PTK, to phosphorylate Vav-1 (108). Activated Vav-1 is one of the major GEFs required for Cdc42 or Rac1 activation that results in the conversion of GDP into GTP forms. The active Cdc42-GTP uses its second β-strand and a region of a peptide loop between the first α-helix and switch I region to bind the PBD46 motif of Pak with very high affinity (109). Studies have also indicated that Cdc42 exhibits differential binding patterns to Pak1, WASp and IQGAP1 (110). Independently, it has been shown that the activated Cdc42-GTP used the ‘insert region’ (111) and a part of the switch I domain (112) to interact with IQGAP1.
Specifically, the switch I domain (amino acids 29–55) served as the binding site for Pak1, while the determinants outside this region (amino acids 84–120 and 157–191) were required for the binding of IQGAP1 (and WASp). Synthetic peptide analogs from the PBD46 motif of Pak partly prevented the ability of Cdc42 binding to IQGAP1. This demonstrates IQGAP1, Pak and WASp may form complexes that may interact with Cdc42 in a synchronized fashion (112). The next substrate in the sequential activation on the IQGAP1 scaffold is Raf, which requires the direct binding of Pak1 for inducing conformational changes and phosphorylation of Serine338 (Raf-1) or Serine445 (B-Raf) (113,114). Under resting conditions, both Raf-1 and B-Raf contain an N-terminal autoinhibitory domain that interacts with its catalytic domain (115,116). However, upon activation, H-Ras (116) or another Ras family member, Rap1b (8) can directly interact with Rafs and relieve this autoinhibition.
Although, these detailed studies explain the mechanism of Raf activations, the temporal kinetics of how Cdc42/Pak1 complex transfers Pak1 to B- or Raf-1 or the precise sequence in which small GTPases mitigate their autoinhibition on the IQGAP1 scaffold have yet to be determined. B- or Raf-1 phosphorylate Mek1/2, which has been shown to be recruited to the IQGAP1 scaffold (40). IQGAP1-null cells are unable to augment the EGF-mediated Raf phosphorylation of Mek1/2 confirming the critical role of this scaffold (39,117). Although both Mek1 and Mek2 equally bind to IQGAP1, only Erk2 is predominantly recruited and phosphorylated via this sequential activation (31).
IQGAP1 forms a master signalosome
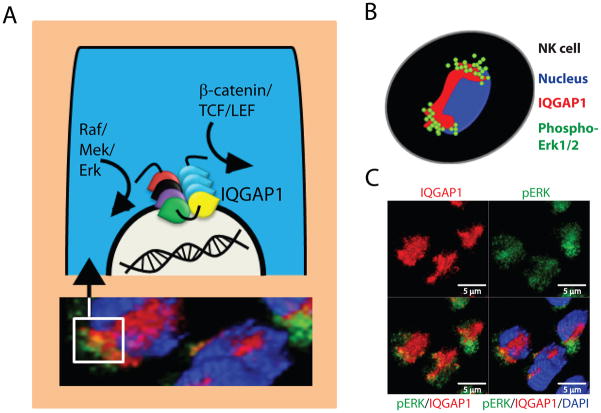
Mechanistic insights of how IQGAP1 functions as a scaffolding protein during Erk1/2 activation or β-catenin-mediated gene transcriptions are fundamental in understanding signaling processes in lymphocytes. More importantly, the transient spatio-temporal organization of the signaling events by IQGAP1 will provide novel insights and will help to develop additional cellular paradigms. Recently, Awasthi et al have demonstrated that IQGAP1 can form a unique signalosome in the perinuclear region of NK cells to coordinate the phosphorylation of Erk1/2 (Figure 3) (8). A significant quantity of phospho-ERK1/2 was co-localized with IQGAP1 in these activated NK cells. This novel IQGAP1-mediated signalosome enforced abundant but transient phosphorylation of Erk1/2 and thereby the effector functions of NK cells. In non-stimulated NK cells, phospho-Erk1/2 was not detectable and the IQGAP1 was distributed throughout the cytoplasm with slight accumulation around the perinuclear region. After 15 min of activation, Erk1/2 phosphorylation was evident in NK cells and after 30 min of activation the distribution pattern of an IQGAP1 was drastically altered with a strong accumulation around the nucleus in NK cells.
Figure 3. Role of IQGAP1 in maestrosome formation.
A) IQGAP1 can compartmentalize and coordinate the signaling processes of multiple activation cascades that include the MAPK and β-catenin/TCF/LEF pathways. Evidence indicates that during the activation of MAPK pathway, IQGAP1 forms large macromolecular scaffolding structures. We name this IQGAP1-based master signalosome, ‘maestrosome’ to denote its macromolecular size. B) Activation of NK cells through NKG2D and the resulting Erk1/2 phosphorylation provide proof-of-principle for the formation of a maestrosome around the nucleus. C) Confocal images of NK cells 30 minutes post NKG2D-mediated activation. These cells were stained for IQGAP1, phospho-Erk1/2 and nuclei (DAPI). Before activation, IQGAP1 was distributed throughout the cytoplasm (not shown and Ref #2). However, after activation the distribution pattern of an IQGAP1 was drastically altered with a strong accumulation around the nucleus. Additionally, considerable quantities of phospho-Erk1/2 co-localized around IQGAP1. These findings demonstrate the role of IQGAP1 in the formation of a maestrosome around the nucleus that regulates the MAPK pathway.
Both non-stimulated and NKG2D-activated NK cells contained comparable levels of total ERK1/2 proteins (8). It is also important to note that IQGAP1 can oligomerize to form macromolecular structures (118). This study demonstrates that IQGAP1 exists as a combination of monomers, dimers, and larger oligomers. The self- association region was mapped to amino acids 763–863 of IQGAP1 that contains the four IQ domains. Since IQGAP1 can bind to B-Raf (39), Mek1/2 (40) and Erk1/2 (31), a structured master signalosome is indispensable for the generation of transient but optimal functions of kinases. Such an ordered regulation of kinases could be an essential and integral part of the IQGAP1-mediated master signalosome. Existence and function of such IQGAP1-based signalosomes must be further investigated in T and B cells.
Conclusion and future directions
Recent studies have highlighted the central regulatory functions played by the IQGAP1 scaffold. Multiple protein partners have been identified and described to interact with IQGAP1; however, the molecular relevance of many of these interactions has yet to be defined. Additionally, the fact that the knockout mice for IQGAP1 have only exhibited modest defects in the immune and cellular functions warrant more careful and detailed future analyses. The functional relevance of many of these interactions in immune cells is still under study. Activation of Erk1/2 through the IQGAP1 scaffold has been well established in multiple cell types. These studies also provide a molecular model that can be used to further explore the spacetime kinetics of signaling events in lymphocytes. IQGAP1-based signalosomes are exciting molecular structures. Irrespective of recent studies focusing on its formation and possible functions, much of the biochemical basis and broader functional relevance of these signalosomes remain unknown. The precise spatiotemporal organization and recruitment of distinct signaling molecules to the IQGAP1 scaffold must be investigated in further detail. Future studies can identify drug targets in the IQGAP1 protein itself or among the myriad of IQGAP-interacting proteins that can be used in tumor treatments. This is of particular significance since IQGAP1 has also been reported to be over expressed in transformed cells.
Acknowledgments
This work was supported in part by R01 A1064828 (S.M.), Hyundai Scholars Program (M.T.), Rebecca Jean Slye Endowment (M.T.), Midwest Athletes Against Childhood Cancer (MACC) Fund, and by the Clinical & Translational Science Institute of Southeastern Wisconsin (NIH UL1RR031973) (M.T.). We thank Tina Heil for the critical reading of this manuscript.
Reference List
- 1.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem. 1994;269:20517–20521. [PubMed] [Google Scholar]
- 3.Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt VA, Scudder L, Devoe CE, Bernards A, Cupit LD, Bahou WF. IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood. 2003;101:3021–3028. doi: 10.1182/blood-2002-09-2807. [DOI] [PubMed] [Google Scholar]
- 5.Cupit LD, V, Schmidt A, Miller F, Bahou WF. Distinct PAR/IQGAP expression patterns during murine development: implications for thrombin-associated cytoskeletal reorganization. Mamm Genome. 2004;15:618–629. doi: 10.1007/s00335-004-2370-8. [DOI] [PubMed] [Google Scholar]
- 6.Brill S, Li S, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, Snijders AJ. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol. 1996;16:4869–4878. doi: 10.1128/mcb.16.9.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nojima H, Adachi M, Matsui T, Okawa K, Tsukita S, Tsukita S. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat Cell Biol. 2008;10:971–978. doi: 10.1038/ncb1757. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi A, Samarakoon A, Chu H, Kamalakannan R, Quilliam LA, Chrzanowska-Wodnicka M, White GC, Malarkannan S. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. J Exp Med. 2010;207:1923–1938. doi: 10.1084/jem.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissbach L, Bernards A, Herion DW. Binding of myosin essential light chain to the cytoskeleton-associated protein IQGAP1. Biochem Biophys Res Commun. 1998;251:269–276. doi: 10.1006/bbrc.1998.9371. [DOI] [PubMed] [Google Scholar]
- 10.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 13.Grundemann C, Bauer M, Schweier O, von ON, Lassing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int Immunol. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzkopff S, Grundemann C, Schweier O, Rosshart S, Karjalainen KE, Becker KF, Pircher H. Tumor-associated E-cadherin mutations affect binding to the killer cell lectin-like receptor G1 in humans. J Immunol. 2007;179:1022–1029. doi: 10.4049/jimmunol.179.2.1022. [DOI] [PubMed] [Google Scholar]
- 17.Banh C, Fugere C, Brossay L. Immunoregulatory functions of KLRG1 cadherin interactions are dependent on forward and reverse signaling. Blood. 2009;114:5299–5306. doi: 10.1182/blood-2009-06-228353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, Mariuzza RA. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, gli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 20.Kutlesa S, Wessels JT, Speiser A, Steiert I, Muller CA, Klein G. E-cadherin-mediated interactions of thymic epithelial cells with CD103+ thymocytes lead to enhanced thymocyte cell proliferation. J Cell Sci. 2002;115:4505–4515. doi: 10.1242/jcs.00142. [DOI] [PubMed] [Google Scholar]
- 21.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schon MP, Schon M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol. 2002;119:190–193. doi: 10.1046/j.1523-1747.2002.17973.x. [DOI] [PubMed] [Google Scholar]
- 24.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z, Schmidt V, Hogan PG, Sacks DB, Rao A. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci U S A. 2011;108:11381–11386. doi: 10.1073/pnas.1019711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho YD, Joyal JL, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- 28.Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- 29.Joyal JL, Annan RS, Ho YD, Huddleston ME, Carr SA, Hart MJ, Sacks DB. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J Biol Chem. 1997;272:15419–15425. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- 30.Macias MJ, Wiesner S, Sudol M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett. 2002;513:30–37. doi: 10.1016/s0014-5793(01)03290-2. [DOI] [PubMed] [Google Scholar]
- 31.Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. J Biol Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- 32.Mbele GO, Deloulme JC, Gentil BJ, Delphin C, Ferro M, Garin J, Takahashi M, Baudier J. The zinc- and calcium-binding S100B interacts and co-localizes with IQGAP1 during dynamic rearrangement of cell membranes. J Biol Chem. 2002;277:49998–50007. doi: 10.1074/jbc.M205363200. [DOI] [PubMed] [Google Scholar]
- 33.Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 34.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathmanathan S, Elliott SF, McSwiggen S, Greer B, Harriott P, Irvine GB, Timson DJ. IQ motif selectivity in human IQGAP1: binding of myosin essential light chain and S100B. Mol Cell Biochem. 2008;318:43–51. doi: 10.1007/s11010-008-9855-9. [DOI] [PubMed] [Google Scholar]
- 36.Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Jeong HW, Li Z, Brown MD, Sacks DB. IQGAP1 binds Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- 39.Ren JG, Li Z, Sacks DB. IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci U S A. 2007;104:10465–10469. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr Biol. 1998;8:1151–1160. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- 42.Kurella VB, Richard JM, Parke CL, Lecour LF, Jr, Bellamy HD, Worthylake DK. Crystal structure of the GTPase-activating protein-related domain from IQGAP1. J Biol Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 44.Briggs MW, Li Z, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002;277:7453–7465. doi: 10.1074/jbc.M104315200. [DOI] [PubMed] [Google Scholar]
- 45.Nauert JB, Rigas JD, Lester LB. Identification of an IQGAP1/AKAP79 complex in beta-cells. J Cell Biochem. 2003;90:97–108. doi: 10.1002/jcb.10604. [DOI] [PubMed] [Google Scholar]
- 46.Logue JS, Whiting JL, Tunquist B, Sacks DB, Langeberg LK, Wordeman L, Scott JD. AKAP220 organizes signaling elements that impact cell migration. J Biol Chem. 2011 doi: 10.1074/jbc.M111.277756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 48.Brown MD, Bry L, Li Z, Sacks DB. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J Biol Chem. 2007;282:30265–30272. doi: 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- 49.Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Velasco R, Lanning CC, Williams CL. The activation of Rac1 by M3 muscarinic acetylcholine receptors involves the translocation of Rac1 and IQGAP1 to cell junctions and changes in the composition of protein complexes containing Rac1, IQGAP1, and actin. J Biol Chem. 2002;277:33081–33091. doi: 10.1074/jbc.M202664200. [DOI] [PubMed] [Google Scholar]
- 51.Katata T, Irie K, Fukuhara A, Kawakatsu T, Yamada A, Shimizu K, Takai Y. Involvement of nectin in the localization of IQGAP1 at the cell-cell adhesion sites through the actin cytoskeleton in Madin-Darby canine kidney cells. Oncogene. 2003;22:2097–2109. doi: 10.1038/sj.onc.1206255. [DOI] [PubMed] [Google Scholar]
- 52.Mateer SC, Morris LE, Cromer DA, Bensenor LB, Bloom GS. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton. 2004;58:231–241. doi: 10.1002/cm.20013. [DOI] [PubMed] [Google Scholar]
- 53.Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K. Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol Biol Cell. 2004;15:1065–1076. doi: 10.1091/mbc.E03-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166:237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe T, Noritake J, Kaibuchi K. Roles of IQGAP1 in cell polarization and migration. Novartis Found Symp. 2005;269:92–101. [PubMed] [Google Scholar]
- 56.Nuriya M, Oh S, Huganir RL. Phosphorylation-dependent interactions of alpha-Actinin-1/IQGAP1 with the AMPA receptor subunit GluR4. J Neurochem. 2005;95:544–552. doi: 10.1111/j.1471-4159.2005.03410.x. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 58.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A. 2005;102:9814–9819. doi: 10.1073/pnas.0504166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 60.Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 61.Nakajima E, Suzuki K, Takahashi K. Mitotic dissociation of IQGAP1 from Rac-bound beta1-integrin is mediated by protein phosphatase 2A. Biochem Biophys Res Commun. 2005;326:249–253. doi: 10.1016/j.bbrc.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Emadali A, Muscatelli-Groux B, Delom F, Jenna S, Boismenu D, Sacks DB, Metrakos PP, Chevet E. Proteomic analysis of ischemia-reperfusion injury upon human liver transplantation reveals the protective role of IQGAP1. Mol Cell Proteomics. 2006;5:1300–1313. doi: 10.1074/mcp.M500393-MCP200. [DOI] [PubMed] [Google Scholar]
- 63.Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi K, Nakajima E, Suzuki K. Involvement of protein phosphatase 2A in the maintenance of E-cadherin-mediated cell-cell adhesion through recruitment of IQGAP1. J Cell Physiol. 2006;206:814–820. doi: 10.1002/jcp.20524. [DOI] [PubMed] [Google Scholar]
- 65.Mataraza JM, Li Z, Jeong HW, Brown MD, Sacks DB. Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal. 2007;19:1857–1865. doi: 10.1016/j.cellsig.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci. 2007;120:658–669. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- 67.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown MD, Bry L, Li Z, Sacks DB. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J Biol Chem. 2008;283:35212–35222. doi: 10.1074/jbc.M803477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai-Yageta M, Recchi C, Le DG, Sibarita JB, Daviet L, Camonis J, Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usatyuk PV, I, Gorshkova A, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem. 2009;284:15339–15352. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, Yuan CX, Bloom GS, Hua X. Menin interacts with IQGAP1 to enhance intercellular adhesion of beta-cells. Oncogene. 2009;28:973–982. doi: 10.1038/onc.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osman M. An emerging role for IQGAP1 in regulating protein traffic. ScientificWorldJournal. 2010;10:944–953. doi: 10.1100/tsw.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skandalis SS, Kozlova I, Engstrom U, Hellman U, Heldin P. Proteomic identification of CD44 interacting proteins. IUBMB Life. 2010;62:833–840. doi: 10.1002/iub.392. [DOI] [PubMed] [Google Scholar]
- 75.Umemoto R, Nishida N, Ogino S, Shimada I. NMR structure of the calponin homology domain of human IQGAP1 and its implications for the actin recognition mode. J Biomol NMR. 2010;48:59–64. doi: 10.1007/s10858-010-9434-8. [DOI] [PubMed] [Google Scholar]
- 76.Hong D, Chen HX, Yu HQ, Liang Y, Wang C, Lian QQ, Deng HT, Ge RS. Morphological and proteomic analysis of early stage of osteoblast differentiation in osteoblastic progenitor cells. Exp Cell Res. 2010;316:2291–2300. doi: 10.1016/j.yexcr.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bridge DR, Novotny MJ, Moore ER, Olson JC. Role of host cell polarity and leading edge properties in Pseudomonas type III secretion. Microbiology. 2010;156:356–373. doi: 10.1099/mic.0.033241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelikan-Conchaudron A, Le CC, Didry D, Carlier MF. The IQGAP1 Protein Is a Calmodulin-regulated Barbed End Capper of Actin Filaments: POSSIBLE IMPLICATIONS IN ITS FUNCTION IN CELL MIGRATION. J Biol Chem. 2011;286:35119–35128. doi: 10.1074/jbc.M111.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neel NF, Sai J, Ham AJ, Sobolik-Delmaire T, Mernaugh RL, Richmond A. IQGAP1 Is a Novel CXCR2-Interacting Protein and Essential Component of the “Chemosynapse”. PLoS ONE. 2011;6:e23813. doi: 10.1371/journal.pone.0023813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2011;300:L840–L850. doi: 10.1152/ajplung.00404.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swiech L, Blazejczyk M, Urbanska M, Pietruszka P, Dortland BR, Malik AR, Wulf PS, Hoogenraad CC, Jaworski J. CLIP-170 and IQGAP1 cooperatively regulate dendrite morphology. J Neurosci. 2011;31:4555–4568. doi: 10.1523/JNEUROSCI.6582-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim H, White CD, Sacks DB. IQGAP1 in microbial pathogenesis: Targeting the actin cytoskeleton. FEBS Lett. 2011;585:723–729. doi: 10.1016/j.febslet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boyer O, Benoit G, Gribouval O, Nevo F, Tete MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, Grunfeld JP, Legendre C, Joly D, Rieu P, Mohsin N, Hannedouche T, Moal V, Gubler MC, Broutin I, Mollet G, Antignac C. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:239–245. doi: 10.1681/ASN.2010050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le CC, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, Didry D, Le D, Egile C, Carlier MF, Kroschewski R. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J Biol Chem. 2007;282:426–435. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- 85.Bashour AM, Fullerton AT, Hart MJ, Bloom GS. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tirnauer JS. A new cytoskeletal connection for APC: linked to actin through IQGAP. Dev Cell. 2004;7:778–780. doi: 10.1016/j.devcel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Gundersen GG. Microtubule capture: IQGAP and CLIP-170 expand the repertoire. Curr Biol. 2002;12:R645–R647. doi: 10.1016/s0960-9822(02)01156-9. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, Galjart N, Kaibuchi K. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122:2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 89.Zhang T, Zaal KJ, Sheridan J, Mehta A, Gundersen GG, Ralston E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J Cell Sci. 2009;122:1401–1409. doi: 10.1242/jcs.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mataraza JM, Briggs MW, Li Z, Frank R, Sacks DB. Identification and characterization of the Cdc42-binding site of IQGAP1. Biochem Biophys Res Commun. 2003;305:315–321. doi: 10.1016/s0006-291x(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 91.Zhang B, Wang ZX, Zheng Y. Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors. Comparison of kinetic properties of Cdc42 binding to the Cdc42-interactive domains. J Biol Chem. 1997;272:21999–22007. doi: 10.1074/jbc.272.35.21999. [DOI] [PubMed] [Google Scholar]
- 92.Kanwar N, Wilkins JA. IQGAP1 involvement in MTOC and granule polarization in NK-cell cytotoxicity. Eur J Immunol. 2011;41:2763–2773. doi: 10.1002/eji.201040444. [DOI] [PubMed] [Google Scholar]
- 93.Gorman JA, GTBD IQGAP1 mediates lymphocyte cytoskeletal polarization and peripheral effector functions. The Journal of Immunology. 2009;182 [Google Scholar]
- 94.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Yankee TM, Clark EA. Signaling through the B cell antigen receptor in developing B cells. Rev Immunogenet. 2000;2:185–203. [PubMed] [Google Scholar]
- 96.Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med. 2008;205:2419–2435. doi: 10.1084/jem.20072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li C, Ge B, Nicotra M, Stern JN, Kopcow HD, Chen X, Strominger JL. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sbroggio M, Carnevale D, Bertero A, Cifelli G, De BE, Mascio G, Hirsch E, Bahou WF, Turco E, Silengo L, Brancaccio M, Lembo G, Tarone G. IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res. 2011;91:456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu W, Fantl WJ, Harrowe G, Williams LT. Regulation of the MAP kinase pathway by mammalian Ksr through direct interaction with MEK and ERK. Curr Biol. 1998;8:56–64. doi: 10.1016/s0960-9822(98)70020-x. [DOI] [PubMed] [Google Scholar]
- 101.Roy F, Laberge G, Douziech M, Ferland-McCollough D, Therrien M. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 2002;16:427–438. doi: 10.1101/gad.962902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma C, Vomastek T, Tarcsafalvi A, Catling AD, Schaeffer HJ, Eblen ST, Weber MJ. MEK partner 1 (MP1): regulation of oligomerization in MAP kinase signaling. J Cell Biochem. 2005;94:708–719. doi: 10.1002/jcb.20344. [DOI] [PubMed] [Google Scholar]
- 103.Mouchel-Vielh E, Bloyer S, Salvaing J, Randsholt NB, Peronnet F. Involvement of the MP1 scaffold protein in ERK signaling regulation during Drosophila wing development. Genes Cells. 2008;13:1099–1111. doi: 10.1111/j.1365-2443.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 104.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 106.Jafri F, El-Shewy HM, Lee MH, Kelly M, Luttrell DK, Luttrell LM. Constitutive ERK1/2 activation by a chimeric neurokinin 1 receptor-beta-arrestin1 fusion protein. Probing the composition and function of the G protein-coupled receptor “signalsome”. J Biol Chem. 2006;281:19346–19357. doi: 10.1074/jbc.M512643200. [DOI] [PubMed] [Google Scholar]
- 107.Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klussmann E, Houslay MD, Baillie GS. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem. 2009;284:11425–11435. doi: 10.1074/jbc.M806395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang J, Tilly D, Altman A, Sugie K, Grey HM. T-cell receptor antagonists induce Vav phosphorylation by selective activation of Fyn kinase. Proc Natl Acad Sci U S A. 2000;97:10923–10929. doi: 10.1073/pnas.97.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo W, Sutcliffe MJ, Cerione RA, Oswald RE. Identification of the binding surface on Cdc42Hs for p21-activated kinase. Biochemistry. 1998;37:14030–14037. doi: 10.1021/bi981352+. [DOI] [PubMed] [Google Scholar]
- 110.Li R, Debreceni B, Jia B, Gao Y, Tigyi G, Zheng Y. Localization of the PAK1-, WASP-, and IQGAP1-specifying regions of Cdc42. J Biol Chem. 1999;274:29648–29654. doi: 10.1074/jbc.274.42.29648. [DOI] [PubMed] [Google Scholar]
- 111.Zong H, Kaibuchi K, Quilliam LA. The insert region of RhoA is essential for Rho kinase activation and cellular transformation. Mol Cell Biol. 2001;21:5287–5298. doi: 10.1128/MCB.21.16.5287-5298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCallum SJ, Wu WJ, Cerione RA. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J Biol Chem. 1996;271:21732–21737. doi: 10.1074/jbc.271.36.21732. [DOI] [PubMed] [Google Scholar]
- 113.King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 114.Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–4405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- 115.Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem. 2003;278:11221–11226. doi: 10.1074/jbc.M210318200. [DOI] [PubMed] [Google Scholar]
- 116.Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- 117.Ren JG, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and B-Raf signaling. J Biol Chem. 2008;283:22972–22982. doi: 10.1074/jbc.M804626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren JG, Li Z, Crimmins DL, Sacks DB. Self-association of IQGAP1: characterization and functional sequelae. J Biol Chem. 2005;280:34548–34557. doi: 10.1074/jbc.M507321200. [DOI] [PubMed] [Google Scholar]