Abstract
Purpose
This study was designed to examine the effects of high fat (HF) diet and subsequent exercise training (Ex) on coronary arteries of an animal model of early stage coronary artery disease (CAD). We hypothesized that HF diet would induce early stage disease and promote a pro-atherogenic coronary phenotype, while Ex would blunt disease progression and induce a healthier anti-inflammatory environment reflected by increased expression of antioxidant capacity and decreased expression of inflammatory markers in both the macro and microvasculature of the coronary circulation.
Methods
Immunohistochemistry in left anterior descending (LAD) and right coronary arteries (RCA), and immunoblots in LAD and left ventricular (LV) arterioles were used to characterize effects of HF diet and Ex on the progression of coronary atherosclerosis.
Results
Our results revealed that HF diet promoted a pro-atherogenic coronary endothelial cell phenotype as evidenced by the endothelial expression of inflammatory and oxidative stress markers. Ex did not significantly alter any of these immunohistochemical markers in conduit arteries; however, Ex did increase antioxidant protein content in LV arterioles.
Conclusions
We conclude that, at this early stage of CAD, Ex did not seem to modify vascular cell phenotypes of conduit coronary arteries from pro- to a more favorable anti-atherogenic status; however, Ex increased antioxidant protein content in coronary arterioles. These findings also support the idea that endothelial phenotype expression follows different patterns in the macro and microvasculature of the coronary circulation.
Keywords: Coronary Macro and Microvasculature, Physical Activity, eNOS, Inflammation, Oxidative Stress
INTRODUCTION
Coronary 2artery disease (CAD) remains the major cause of mortality in developing countries and atherosclerosis is the most important single factor contributing to this disease burden (34). Inflammation and increased levels of oxidative stress are known to disrupt normal endothelial function, thereby promoting endothelial dysfunction (ED) and leading to the development of cardiovascular disease (CVD) (3, 18). Indeed, the initial stage of atherogenesis consists of ED, which occurs early in the progression of CAD prior to the formation of structural atherosclerotic changes (27).
H3ypercholesterolemia, a well-known risk factor for atherosclerosis, has been reported to promote ED of coronary arteries in human (4, 5) and animal studies (39, 44) leading to a pro-inflammatory phenotype of the endothelium. 4Although mechanisms for the detrimental effects of hypercholesterolemia on endothelial function are not yet fully established, there is a substantial body of evidence indicating that disruption of the nitric oxide synthase (NOS) pathway (3) and/or reduced availability of nitric oxide (NO) contribute (1). Furthermore, the increased production of reactive oxygen species (ROS), in particular superoxide, commonly found in association with hypercholesterolemia may result in the reduction of NO (3). Interestingly, hypercholesterolemia has also been shown to induce phenotypic changes in the microcirculation that are consistent with oxidative and nitrosative stress and an inflammatory response (37).
It is important to recognize that conduit arteries and arterioles differ significantly in function and local environment and appear to be differentially affected by atherosclerosis. Indeed, assessment of endothelial function in the macro and microvasculature has been documented by some researchers to provide differing information of risk of CVD (16, 38).
Regular physical activity has been associated with a chronic anti-inflammatory effect (20) which is mediated principally by the increase of NO bioavailability. We recently proposed that ED is one component of a pro-atherogenic endothelial cell phenotype that contributes to development of atherosclerosis and that exercise training (Ex) produces beneficial effects on CVD in part by producing an anti-atherogenic endothelial cell phenotype, signaled by increases in shear stress and wall stretch in the arteries during exercise bouts (23). Similarly, Thompson et al. (39), having demonstrated that endothelial-dependent relaxation (EDR) was decreased in coronary arteries of a porcine model of early stage CAD, indicated that the contribution of NO to EDR was decreased in the coronary arteries (39). Additionally, Ex of high fat (HF) diet pigs restored coronary EDR with an apparent increase in NO bioavailability and decreased prostanoid constriction (39). The results of the study of Thompson et al. (39) suggested that these changes in EDR were associated with slowed progression of CAD but this suggestion could not be rigorously evaluated from their data.
Thus the primary purpose of the present study was to characterize the effects of HF diet and Ex on the progression of coronary atherosclerosis in an animal model of diet-induced, early stage CAD. In addition, the present study was intended to determine whether the changes mediated by HF diet or Ex, though dissimilar in effect, would be uniformly represented in conduit coronary arteries (macrovasculature) and arterioles (microvasculature) of this early model of CAD. Disease progression and coronary phenotypes were assessed by measuring expression of markers of disease with the combination of histopathology, immunohistochemistry and immunoblot analysis of coronary artery tissue/cells. We hypothesized that HF diet would promote a pro-atherogenic coronary phenotype; whilst Ex would mediate a more favorable anti-inflammatory environment reflected by increased expression of antioxidant capacity and decreased expression of inflammatory markers, on both the coronary macro and microvasculature.
METHODS
Experimental Animals
Thirty two adult, male Yucatan miniature swine, 8–12 months of age, 38–55 kg body weight (Charles River, Maine and Sinclair Research Farm, Columbia, MO) were used with protocols approved by the Institutional Animal Care and Use Committee at the University of Missouri. Pigs were housed in rooms maintained at 20–23° C with a 12:12 hour light-dark cycle. In order to evaluate the changes associated to a HF diet, we decided to include also a normal fat (NF) diet group. Half of the pigs (n=16) were provided a NF diet (Purina Lab Mini-pig Chow; calories provided by fat were 8%). The other half (n=16) were fed a HF diet (calories provided by fat were 46%) as reported previously (19, 39, 41). The HF or NF diet was maintained for a period of 20 to 24 weeks, which promote changes in plasma lipids similar to those reported previously for this model (39, 41, 44). This study was conducted in conformance with the policy statement of the American College of Sports Medicine on research with experimental animals.
At the beginning of the study pigs were familiarized with running on a motorized treadmill and randomly assigned into exercise trained (NFEx, n=8; HFEx, n=8) or cage confined/sedentary (NFSed, n=8; HFSed, n=8) groups and had ad libitum access to water. Following one month on diet, the Ex pigs completed the 16- to 20-wk endurance training program described previously (24, 29). Briefly, intensity and duration of exercise bouts increased steadily so that by week 10 of training the pigs ran 85 min/day, 5 days/wk. The 85-min training bouts consisted of a 5-min warm-up, a 15-min sprint run at 5 to 7 mph, a 60-min endurance run at 4 to 5 mph, and a 5-min cool-down. On the day of sacrifice, pigs were anesthetized with intramuscular ketamine (35 mg/kg)-xylazine (2.25 mg/kg), and intravenous thiopental (25 mg/kg) for deep anesthesia. Finally, the efficacy of training was assessed by comparing heart-to-body weight ratios and skeletal muscle oxidative capacity of Sed and Ex swine. At the time of sacrifice, muscle samples were taken from the triceps brachii and deltoid, frozen in liquid nitrogen, and stored at 270°C until processed for spectrophotometric determination of citrate synthase activity (35).
5Immunohistochemistry
Samples of right coronary artery (RCA), left anterior descending coronary artery (LAD), and left ventricular (LV) myocardium (containing arterioles) were dissected and immersed in 10% formalin for a minimum of 24 hours. The RCA and LAD samples were taken consistently from the same location in all pigs. The proximal segments (less than 0.5 cm in length) from both RCA and LAD were fixed for histology and immunohistochemistry analysis. In the case of the LAD, the proximal segment was taken just after the LAD/left circumflex coronary artery bifurcation. The proximal segment of the RCA was dissected from its aortic origin, especially from the ostium and above the origin of the first acute marginal artery. LV arterioles were dissected from the LV-apex in all pigs. Then, samples were processed routinely to paraffin embedment. Five μm sections were cut with an automated microtome (Microm), floated onto positively charged slides (Thermo Fischer Scientific), and deparaffinized. Histological assessment of atherosclerosis (grading of the lesion) in coronary arteries were performed on five micron sections stained with Verhoeff's method for elastin (32). The intima-media thickness (IMT) was measured at the point of greatest thickness in sections from the standardized samples sites, as previously described (41). Then, we performed three consecutive measurements from the intima to the external elastic lamina; these measurements were averaged and reported as IMT. For immunohistochemistry the slides were steamed in citrate buffer at pH 6.0 (target retrieval solution S1699, DAKO, Carpenteria, CA) for 30 min to achieve antigen retrieval and then cooled for 20 min at room temperature. The slides were stained manually with sequential Tris buffer and water wash steps performed after each protocol step. Sections were incubated with avidin biotin two-step blocking solution (Vector SP-2001) to inhibit background staining, and in 3% hydrogen-peroxide to inhibit endogenous peroxidase. Non-serum protein block (Dako X0909) was applied to inhibit nonspecific protein binding. The source and dilutions of the primary antibodies used are listed as supplementary material (data online “Supplemental Table 1”). All the primary antibodies were diluted using antibody diluent (Dako S0809), and were incubated with the tissue sections overnight at 4°C. After appropriate washing steps were completed the sections were incubated with biotinylated anti-mouse or rabbit link secondary antibody in PBS containing 15 mM sodium azide and peroxidase-labeled streptavidin (Dako LSAB+ kit, peroxidase, K0690). Diaminobenzidine (Dako #K3468) applied for 5 min allowed visualization of primary antibody staining. Sections were counterstained with Mayer's hematoxylin for 1 min, dehydrated, and cover-slipped. For negative controls, histological sections were prepared as described above but incubation in primary antibody was replaced with Tris buffer. Sections were examined using an Olympus BX40 photomicroscope (Olympus, Melville, NY) and photographed with a Spot Insight digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI). Image Pro Plus (version 6.2.0.424, Media Cybernetics, Inc., Silver Spring, MD) was used to identify and quantify the positive area of staining. The term “immunoreactivity” was defined as the presence of antibody, “positive staining”, on the different samples. The staining intensity was evaluated and described by our pathologist using a scale from grade 0 through grade 3: a) absence of staining (grade 0), b) faint immunoreactivity or focal staining (grade 1), c) very slight immunoreactivity or intermediate staining (grade 2), and d) diffuse immunireactivity or strong staining (grade 3). Positive controls were used for these assessments. It is important to mention that the sections selected in each vessel were taken from standardized sites to ensure anatomical consistency. Briefly, the complete cross-section of each vessel, LAD's and RCA's, was visually examined by a well-trained pathologist that was blinded to the animal condition. Three separate and different sections from the intima-media boundary were identified, selected and then photographed for later analysis. These sections were: one overlying an area that exhibited the greater positive staining, another overlying an area with less staining than the first one, and finally, a section overlying an area with even less and/or no staining than the previous two. Subsequently, the areas of positive staining of the vessel within the photomicrograph were identified using segmentation for 24 BIT RGB images. The histogram based segmentation for each channel was set up as follows; red: 0–164, green: 0–150, and blue: 0–150 pixel intensity. These settings were consistently used throughout the whole experiment. Percent staining of the vessel was calculated from the data for positive staining and total area.
Immunoblot analysis
Segments of LAD and LV arterioles were carefully dissected from LV myocardium, fat, and connective tissue while the hearts were maintained at 0 – 4°C. A segment of no less than 1 cm in length from the middle to distal portion of the LAD's was used for immunoblot analysis. Arterioles were isolated from myocardium (LV-apex) by dissecting along the length of small arteries to the smallest branches. Five arterioles, ranging from 50–100μ diameter and 1000–1700μm in length were collected and frozen at −70C. Diameter and length of arterioles were measured with eyepiece micrometer using Olympus SZ60 microscope at 63X power. Endothelial cells were scraped from conduit coronary arteries as described previously (2). Briefly, immediately following death, arteries were opened longitudinally and pinned. Laemmli buffer was applied on the endothelial surface and a blade was used to remove the endothelial cell layer by gentle scraping. All samples of arteries and arterioles were solubilized in 20 μL Laemmli buffer (22), boiled, and sonicated for 2 min and subjected to SDS-PAGE under reducing conditions. Proteins were transferred to PVDF membrane (Hybond-ECL, Amersham) and blocked (1 hr at 25 °C) with 5 % non-fat milk in TBS-tween (20 mM Tris HCl, 137 mM NaCl, and 0.1 % Tween 20).
Protein content of endothelial NOS (eNOS), superoxide dismutase (SOD-1, SOD-2) and extracellular SOD (ecSOD), catalase, phospho-eNOS, AKT and phospho-AKT, HSP90, Arginase-1, Rac-1, and CAV-1 in whole artery, scraped endothelial cells (enriched endothelial cell sample) and smooth muscle (remainder of artery after endothelial cell scraping) were assessed with immunoblot analysis as described previously (2). The same techniques were used to determine the expression of oxidant stress markers by measuring immunoreactivity for malondialdehyde (MDA) and nitrotyrosine. Total protein content was measured utilizing the NanoOrange protein assay. Protein samples from the whole vessel segment (5 μg/lane), endothelial scrapes (2.5 μg/lane), arterioles (2 μg/lane) and smooth muscle (5 μg/lane) were loaded on separate 12 lane 5–12% NuPage Bis Tris gradient gels, electrophoresed at 200 volts for 50 min, and transferred at 34V for 60 min to a polyvinylidene difluoride membrane (Hybond-ECL, Amersham). Artery samples were loaded on the same gel in an alternating pattern so that each gel contained equal samples for Ex and Sed pigs, so all comparisons could be made on the gel. Blots were incubated overnight (25 °C) with the primary antibody against eNOS (1:1000; BD Transduction), SOD-1 (1:5000; Stressgen), SOD-2 (1:2000; Stressgen), ecSOD (1:500; Upstate/Millipore), catalase (1:5000; Sigma), phospho-eNOS-pS1177 (1:250; BD Transduction), AKT (1:500; Cell Signalling), phospho-AKT (1:250; Cell Signalling), Arginase-1 (1:1000; BD Transduction), COX-1 (1:1000; Santa Cruz), COX-2 (1:500; Cayman Chemical), VCAM-1 (1:250; Santa Cruz), Rac-1 (1:1000; Cytoskeleton), HSP90 (1:1000; BD Transduction), CAV-1 (1:250; BD Transduction), MDA (1:1000; Abcam), and nitrotyrosine (1:1000; Chemicon). This was followed by one hour incubation with a secondary antibody (1:2,500; horseradish peroxidase-conjugated anti-mouse; Sigma Chemical). Western blot data were expressed relative to NFSed values, unless otherwise indicated. Analysis of protein was performed with chemiluminescence and quantified by densitometry using NIH Image software (National Institute of Health, Bethesda, MD) or Kodak 4000R Imager and Molecular Imagery Software.
Statistical Analysis
The data is presented as means ± SE, unless otherwise indicated. Differences between mean values of body weight, heart weight, heart weight/body weight ratio, and citrate synthase activity were evaluated via two-way ANOVA using SuperANOVA software. Immunohistochemistry and immunoblot data analysis was done using a Mixed procedure in SAS V9 (SAS Institute Inc. Cary, NC, USA). For immunoblot analysis each Western blot was treated as a block and the statistical design was considered to be a randomized block design. The treatments within the blocks consisted of the two between subject factors (diet and activity) and the within subject factor (vessel). Log transformation was utilized when data was not normally distributed. As a precautionary step, non-parametric analysis was done to guard against the assumption of normal error terms. To examine the effects of the treatment combinations, a Friedman's test (immunoblot) and a Kruskal-Wallis test (immunohistochemistry) were performed. Cochran-Mantel-Haenszel methodology was used to examine the effect of treatment. P values of <0.05 were considered significant.
RESULTS
Pig characteristics
Body weights were significantly greater in the HF pigs than in NF (p<0.01), but Ex did not significantly alter weights in either NF or HF groups; NFSed, 44.5 ± 2.2 kg; NFEx, 39.6 ± 1 kg; HFSed, 49.6 ± 2.2 kg; and HFEx, 46.1 ± 2 kg. Average heart weight-to-body weight ratio (HW/BW) was significantly increased by Ex in both groups (p<0.0001); NFSed, 4.3 ± 0.1 g/kg; NFEx, 5.2 ± 0.2 g/kg; HFSed, 3.9 ± 0.1 g/kg; and HFEx, 4.9 ± 0.1 g/kg. As we have reported previously (25, 26), the Ex program implemented in this study also increased citrate synthase activity by 40 to 60 % in the medial, lateral, long and accessory heads of the triceps brachi muscle and by 60 % in the deltoid muscle of NFEx and HFEx pigs, which confirms the shift in skeletal muscle oxidative capacity that characterizes effective Ex.
6Histopathologic and Immunohistochemical characteristics of the LADs and arterioles
NF LADs exhibited normal morphology with a thick muscular media and a tunica intima with a single endothelial cell layer (Figure 1A). The intima was distinctly different in many LADs and RCAs from HFEx and HFSed pigs as it was expanded by accumulations of foam cells producing intimal thickening and formation of classical fatty streaks by gross examination (Figure 1A). The fatty streaks, in the coronary arterial trees exhibiting them, were located primarily at branch points along the larger conduit arteries. Because foam cells were frequently observed in HF LADs and RCAs, below we describe immunoreactivity of endothelial, smooth muscle, and foam cells in HF LADs and RCAs. At this early stage of disease development, intima media thickness was not significantly different between NF and HF LADs. We did not observe foam cells in coronary arterioles of either NF or HF pigs.
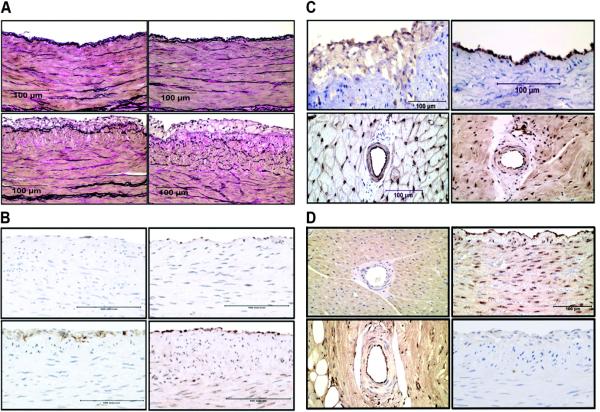
FIGURE 1.
A. Demonstrates accumulation of foam cells in high fat diet animals. Representative photographs of left anterior descending (LAD) coronary arteries stained with Verhoeff's technique (VVG) for elastin from normal fat diet sedentary (NFSed), upper left panel, and exercise (NFEx), upper right panel; high fat diet sedentary (HFSed), lower left panel, and exercise (HFEx), lower right panel. Note the presence of foam cells in the HFEx and HFSed arteries. B. Demonstrates inflammatory changes by immunohistochemistry (IHC) with high fat diet. Representative photographs of LAD coronary arteries stained for scavenger receptor antigen antibody (SRA) from NFSed, upper left panel, and HFSed, lower left panel. Representative MDA immunoreactivity of LAD coronary arteries from NFSed, upper right panel, and HFSed, lower right panel. C. Demonstrates patterns of distribution of IHC staining in coronary arteries and arterioles. Representative photograph of LAD stained for cyclooxygenase 2 (COX-2) from HFEx, upper left panel. Representative photograph of coronary arterioles stained for Caveolin-1 (CAV-1) from HFEx, lower left panel; note positive immunoreactivity in endothelium and smooth muscle. Representative photograph of LAD stained for phospho-eNOS from NFEx, upper right panel; note strong positive staining in endothelium of LAD. Representative photograph of coronary arterioles stained for superoxide dismutase 1 (SOD-1), lower right panel; note positive immunoreactivity in endothelium and smooth muscle for SOD-1. D. Demonstrates distribution of IHC staining in coronary vessels vs. surrounding muscle. Representative photograph of coronary arterioles stained for superoxide dismutase 2 (SOD-2) from HFSed, upper left panel; SOD-2 staining is less in arterioles than in myocardium. Representative photograph showing positive catalase immunoreactivity in coronary arterioles from HFSed, lower left panel; note positive immunoreactivity in endothelium and smooth muscle and that endothelium and myocardium have greater immunoreactivity than does smooth muscle. Representative photograph showing positive extracellular superoxide dismutase (ecSOD) immunoreactivity in right coronary artery (RCA), upper right panel; note positive immunoreactivity in endothelium and smooth muscle. Representative photograph showing positive vascular cell adhesion molecule-1 (VCAM-1) immunoreactivity of LAD, lower right panel.
7Immunohistochemical Characterization of Vascular Cell Phenotypes
Immunohistochemical staining patterns for these markers were subjectively evaluated primarily to discern the patterns and distribution of these proteins in the vessels and surrounding tissue. Quantification of percent staining was not performed for COX-1 and -2, eNOS, CAV-1, SOD-1, and SOD-2 rather the major intent of immunohistochemistry here was to determine the cell types in which the markers were expressed on. Faint immunoreactivity for COX-1 was apparent in the endothelium of NF LADs but not arterioles of NF pigs. Only very slight COX-2 immunoreactivity was present in endothelium of LAD and LV arterioles of NF pigs. Importantly, COX-2 immunoreactivity was present in both endothelium and foam cells in HF LADs (Figure 1C), but not in arterioles of HF pigs. COX-1 and COX-2 immunoreactivity did not appear to be altered by Ex in NF or HF pigs. We observed that macrophage foam cells in conduit coronary arteries of HF pigs exhibit immunoreactivity for eNOS, CAV-1, SOD-1, and SOD-2 confirming previous observations (39).
Immunoreactivity to eNOS, phospho-eNOS (Figure 1C), and AKT was restricted to the endothelium of LADs, without detectable effects of diet or Ex. Coronary arterioles exhibited immunoreactivity for eNOS but not for phospho-eNOS or AKT. Failure to detect phospho-eNOS or AKT immunoreactivity in arterioles in formalin-fixed, paraffin-embedded myocardium may be the result of the signal (abundance of protein) being insufficient. Immunoreactivity for catalase (Figure 1D) was observed in the endothelium and smooth muscle of LADs of both NF and HF pigs. Catalase expression appeared greatest in endothelium of arterioles and smooth muscle expressed less catalase than myocardium (Figure 1D). In LV arterioles, positive immunoreactivity for CAV-1 and SOD-1 was also observed in the endothelial and smooth muscle layers (Figure 1C). Immunoreactivity for SOD-2 in arterioles while detectable was faint compared to the staining in the myocardium (Figure 1D). Immunoreactivity for ecSOD was apparent in endothelium and smooth muscle of conduit arteries (Figure 1D).
Immunohistochemistry: Markers of Inflammation and/or oxidative stress
VCAM-1 staining was faint in the coronary arteries of HF pigs and slightly greater in the endothelium but some staining was also detected in smooth muscle (Figure 1D). There was no detectable VCAM-1 staining in arteries of NF pigs. Ex did not appear to influence VCAM-1 expression as there were no significant differences in VCAM-1 staining between HFSed and HFEx in the LAD (HFSed, 0.19 ± 0.28; HFEx, 0.09 ± 0.05, p=0.30) or RCA (HFSed, 0.09 ± 0.08; HFEx, 0.09 ± 0.06, p=0.99).
There was significantly more SRA staining in RCA and LAD arteries of the HF groups than in NF (the main effect of diet was highly significant, p<0.0001) (Table 1 and Figure 1B), and this activity was primarily focused in foam cells located in the intima. In the HF group, Ex tended to increase SRA staining in both the RCA and LAD however, this was not statistically significant. Neither diet nor Ex altered nitrotyrosine immunohistochemical staining in conduit arteries or LV arterioles, indicating similar amounts of oxidative stress in the coronary arteries of all groups. There were no differences in nitrotyrosine staining in the RCA and LAD across HF and NF groups (Table 1).
Table 1.
Immunohistochemistry staining levels (% area of staining).
| Antibody | Vessel | NFSed (n=8) | NFEx (n=8) | HFSed (n=8) | HFEx (n=8) |
|---|---|---|---|---|---|
| SRA | LAD | 0.09 (0.003, 0.31) | 0.08 0.009, 0.23) | 0.65† (0.04, 1.34) | 2.58* (0.44, 4.65) |
| RCA | 0.1 (0, 0.24) | 0.05 (0.004, 0.13) | 0.37† (0.21, 2.99) | 0.58* (0.17, 3.13) | |
| MDA | LAD | 3.14 (1.04, 4.36) | 1.37 (0.1, 4.69) | 3.64 (0.15, 7.46) | 4.22 (2.92, 9.04) |
| RCA | 3.40 (0.30, 5.27) | 2.87 (0.06, 4.87) | 5.40 (0.16, 11.77) | 5.13 (2.46, 8.96) | |
| Nitrotyrosine | LAD | 6.97 (1.82, 13.61) | 5.49 (3.74, 17.17) | 6.80 (3.04, 11.20) | 7.81 (2.48, 8.84) |
| RCA | 4.83 (2.22, 8.05) | 2.88 (0.64, 9.29) | 6.31 (3.14, 21.35) | 7.57 (0.31, 11.08) |
Values are medians; lower and upper quartiles in parentheses (quartile range is the difference between these two); n, number of animals. They were obtained from normal fat diet sedentary (NFSed); normal fat diet exercise (NFEx); high fat diet sedentary (HFSed); and high fat diet sedentary (HFSed) swine. Left anterior descending coronary artery (LAD); right coronary artery (RCA); scavenge receptor A (SRA); malondialdehyde (MDA).
p<0.0001 vs. NFEx;
vs. NFSed.
MDA staining tended to be greater in endothelium of HF than in NF coronary arteries; however, this difference was not significant (Table 1 and Figure 1B). While Ex tended to decrease MDA staining in both RCA and LAD of the HF pigs this was not a statistically significant effect. In summary, immunohistochemistry revealed that arteries of the HF groups exhibited signs of early stage coronary disease as reflected in; a) increased inflammation reflected in increased SRA staining (presence of macrophages) and detectable VCAM-1 expression, as well as b) increased oxidant stress (MDA staining) in HF arteries than in NF arteries. Evaluation of these markers of inflammation and oxidative stress revealed no difference in staining across groups of LV arterioles.
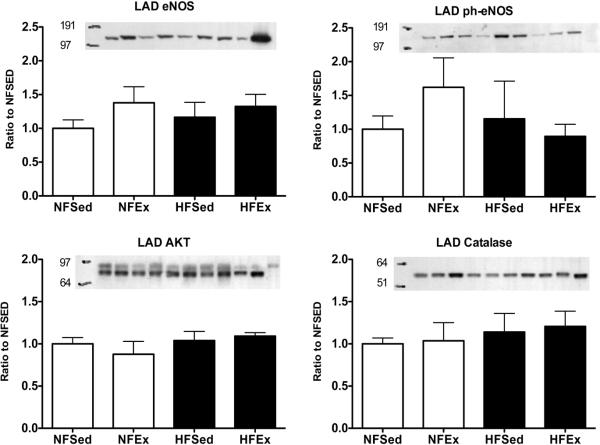
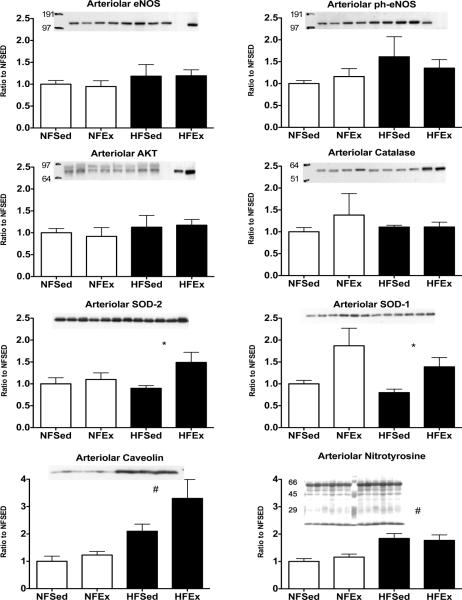
8Immunoblot Analysis of Whole LADs and Coronary Arterioles
Immunoblot analyses of whole LAD artery ring homogenates indicate that eNOS protein expression was not altered by the HF diet; however, Ex tended to increase eNOS content. Neither HF diet nor Ex had significant effects on phospho-eNOS, AKT, or catalase protein content of LADs. These findings are summarized in figure 2. The effect of HF diet and Ex on eNOS, phospho-eNOS, AKT, catalase, SOD-1, SOD-2, CAV-1, and nitrotyrosine expression in LV arterioles is summarized in Figure 3. Interestingly, Ex had a significant effect of increasing expression of SOD-1 and SOD-2 only in LV arterioles from HF diet pigs. In addition, we found that HF diet promotes a significant increase in arteriolar content of CAV-1 and nitrotyrosine. Unfortunately, we were not able to detect immunoreactivity for COX-1, COX-2, or ecSOD in immunoblots of coronary arteriolar tissue of NFEx or HFEx.
FIGURE 2.
Immunoblot analysis of expression of markers related to nitric oxide (NO) production/ bioavailability in whole left anterior descending (LAD) coronary arteries from normal fat diet sedentary (NFSed) and exercise (NFEx); high fat diet sedentary (HFSed) and exercise (HFEx). Representative blots are presented at the top of each set of mean values presented as bar graphs. The band sequence on the sample blots, from left to right, for eNOS, AKT, and Catalase is: NFSed, NFEx, HFSed, HFEx, NFSed, NFEx, HFEx, HFSed, Std, and Std. For ph-eNOS the sequence is: NFSed, NFEx, HFSed, HFEx, NFSed, NFEx, HFSed, HFEx, Std, and Std. The sequence of the immunoblots, from top to bottom, is as follow; endothelial nitric oxide synthase (eNOS); phospho-eNOS protein content expressed relative to total eNOS content; AKT; and Catalase. Values are means ± SE. Data are normalized so that NF-SED values were set to 1.0. There were no statistically significant diet or exercise effects in these data.
FIGURE 3.
Immunoblot analysis of expression of markers related to nitric oxide (NO) production/ bioavailability in coronary arterioles from normal fat diet sedentary (NFSed) and exercise (NFEx); high fat diet sedentary (HFSed) and exercise (HFEx). Representative blots are presented at the top of each set of mean values presented as bar graphs. The band sequence on the sample blots, from left to right, for eNOS, ph-eNOS, AKT, and Catalase is: NFSed, NFEx, HFSed, HFEx, NFSed, NFEx, HFSed, HFEx, Std, and Std. For SOD-2, SOD-1, Caveolin, and nitrotyrosine the sequence is: 3 samples of NFSed, 3 samples of NFEx, 3 samples of HFSed, and 3 samples of HFEx. The sequence of the immunoblots, from top to bottom, is as follow; endothelial nitric oxide synthase (eNOS); phospho-eNOS content expressed relative to total eNOS; AKT; catalase; SOD-2; SOD-1; Caveolin; and Nitrotyrosine. Values are means ± SE. Data are normalized so that NFSed values were set to 1.0. * = HFEx greater than HFSed p value < 0.05. # = HF diet values different from NF values.
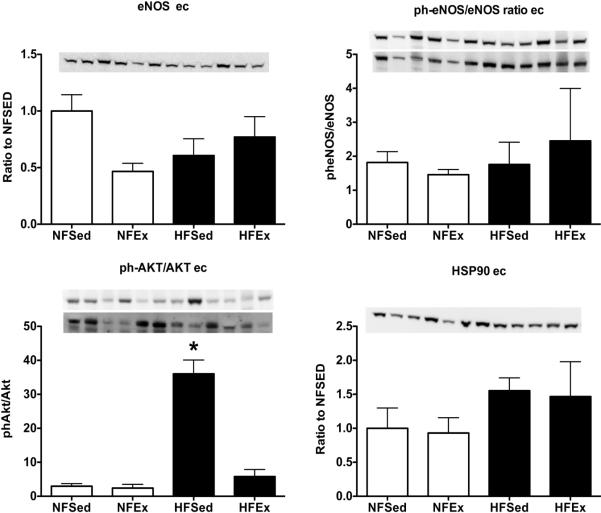
Immunoblot Analysis of LAD Endothelial Cell Scrapes and Smooth Muscle
Since our immunohistochemistry findings indicated that all three cell types express some of our proteins of interest; in a subset of 3 pigs of each group, NF and HF, we measured protein content in samples enriched for endothelial cell proteins by scraping endothelial cells (ECs) from the arteries (Figure 4) as described previously (2). We completed immunoblot analysis on the scraped ECs samples and the smooth muscle cell (SMC) samples (remaining wall). There were no statistically significant differences among groups for eNOS, phospho-eNOS/eNOS ratio or HSP90; nevertheless, HSP90 tended to be increased in the HF groups. Phospho-AKT expression appeared to be increased in HFSed but not in the HFEx. Expression of SOD-1, SOD-2, ecSOD and catalase were similar among groups in ECs and SMC samples (data not shown). SOD-1 protein content was present in both ECs and SMC samples; however, content was higher in EC samples. On the contrary, the amounts of SOD-2, ecSOD, and catalase were substantially greater in SMC fraction than in ECs. Arginase-1 expression was similar among groups in endothelium and smooth muscle (data not shown). Also, Rac-1 reactivity was increased in endothelium enriched samples of HF LADs and exercise tended to increase it in the NF pigs (data not shown). Finally there were no group differences for MDA reactivity in LAD ECs or SMC samples.
FIGURE 4.
Immunoblot analysis of expression of markers related to NO production/ bioavailability in samples of left anterior descending (LAD) coronary artery endothelial cell scrapes from normal fat diet sedentary (NFSed) and exercise (NFEx); high fat diet sedentary (HFSed) and exercise (HFEx). Representative blots are presented at the top of each set of mean values presented as bar graphs. The band sequence on the sample blots, from left to right, for all four antibodies is in the following order: NFEx, NFSed, HFEx, and HFSed repeated 3 times per gel. Data are expressed relative to NFSed values for endothelial nitric oxide synthase (eNOS) content of LAD endothelial cells. Phospho-eNOS content expressed relative to total eNOS content in LAD endothelial cells (upper sample blot: ph-eNOS; lower sample blot: eNOS after stripping for ph-eNOS). Phospho-AKT content expressed relative to total AKT content in LAD endothelial cells (upper sample blot: ph-AKT; lower sample blot: AKT after stripping for ph-AKT). Heat shock protein 90 (HSP90) content LAD endothelial cells. Values are means ± SE. * = HFSed value significantly greater than other groups p < 0.05.
DISCUSSION
The present study was designed to examine the effects of HF diet and subsequent Ex on coronary vessels of an animal model of diet-induced, early stage CAD. Additionally, we evaluated whether the changes mediated by HF diet or Ex would reveal uniform distribution in conduit coronary arteries and arterioles. Our hypothesis was that HF diet would induce early stage disease and promote a pro-atherogenic coronary phenotype, while Ex would blunt disease progression and induce a healthier anti-inflammatory environment reflected by increased expression of antioxidant capacity and decreased expression of inflammatory markers on both the macro and microvasculature of the coronary circulation.
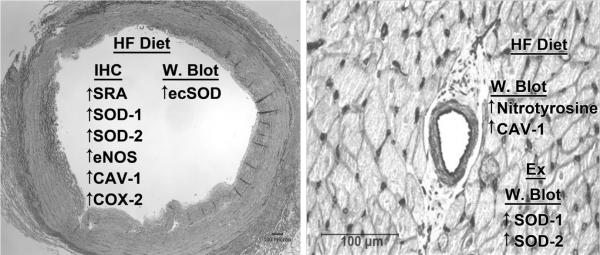
The important findings of this study are summarized in Figure 5. Briefly, these include: a) HF diet promoted the appearance of SRA positive foam cells in coronary vessels, RCA and/or LAD, but not in LV arterioles; b) Ex did not appear to significantly alter the number of foam cells in RCA or LAD of HF pigs; c) RCA and LAD macrophage foam cells were immunoreactive for eNOS, CAV-1, SOD-1, SOD-2, catalase, COX-2, and SRA; d) HF diet appears to increase COX-2 in both endothelial and foam cells of coronary vessels; however Ex did not alter the expression of COX-1, COX-2 in endothelial or foam cells; e) immunoblot data demonstrated that ecSOD was expressed in endothelium and smooth muscle of LADs; f) HF diet and Ex did not significantly modify the amount of eNOS, phospho-eNOS, AKT or catalase protein detected by immunoblot in either LADs or LV arterioles; g) HF diet increased CAV-1 and nitrotyrosine content in LV arterioles; h) Ex increased the expression of SOD-1 and SOD-2 in arterioles.
FIGURE 5.
Summary of the immunohistochemistry (IHC) and western blot (W. Blot) changes induced by high fat (HF) diet and/or exercise training (Ex) on conduit coronary artery (on the left) and coronary arteriole (on the right) of this animal model of early stage coronary artery disease. As shown on the left, IHC revealed that HF diet caused increased expression of a number of oxidant related markers and eNOS, while immunoblot revealed increased ecSOD in conduit coronary arteries. There were not effects of Ex on gene expression in conduit arteries revealed by either IHC or W. Blot. As illustrated on the right, IHC did not reveal any changes in gene expression in coronary arterioles, whereas immunoblot analysis revealed increased oxidative stress (nitrotyrosine) and CAV-1 in HF diet pigs. Ex increased expression of SOD-1 and SOD-2. Note: the immunohistochemical staining for SOD-1, SOD-2, eNOS, CAV-1, and COX-2 in the conduit coronary artery was observed in macrophage foam cells.
Perhaps the most important, and the most surprising, finding of this study is that Ex did not appear to alter progression of coronary disease at this early stage of CAD. Current epidemiologic literature (17, 30) indicates that decreases progression and/or stimulates regression of atherosclerosis in end stage CAD, thus, we expected to find blunted disease progression in Ex HF diet animals. Our hypothesis was also stimulated by previously observed improvement in EDR following training (39, 44). The results of the current study may indicate that either Ex has beneficial effects on disease progression only in more advanced stage of disease or that the beneficial effects of Ex are not mediated by alterations in disease progression, as reflected in the measures used in this study, but perhaps has beneficial effects through altered hemostasis, improved cardiac resistance to ischemia, and/or functional change in the vascular cells. Another possible explanation is that Ex was only one component of lifestyle interventions used in the studies of Hambrecht et al. (17, 30). Thus, it is difficult to determine the specific beneficial effects of exercise in these experiments (40). In present experiments the HF pigs remained on the high fat diet during exercise training. Literature from animal models also presents a mixed answer to the question of the effects of Ex on atherosclerosis progression/regression. For instance, the effect of Ex per se on atherosclerosis in animal models remains equivocal as rodent, rabbit, swine, and non-human primate studies have shown both positive (21, 28, 31, 45) and negligible (43, 46) effects of exercise, independent of dietary intervention. The notion that this controversy could be the result of interactions between diet and exercise is supported by a recent ApoE null mouse study in which effects of exercise on aortic atherosclerosis was examined alone and in combination with a low-fat, high protein diet (6). Cesar et al. (6) reported that aortic plaque accumulation was inhibited only by combined exercise and diet; neither Ex nor diet alone had an effect. Our results support the consistent observation that Ex induces classic signs of training induced adaptation in spite of the fact that Ex did not have beneficial effects on CAD progression (43).
Furthermore, in a porcine model of restenosis following percutaneous transluminal coronary angiography (PTCA), Fleenor and Bowles (11) concluded that Ex decreased coronary re-stenosis lesion size and altered the composition of the extracellular matrix of the wall of the coronary arteries. Ex also appeared to decrease vascular disease in the non-stent and peri-stent regions of coronary arteries treated with PTCA and stents in Ossabaw pigs, a model of type 2 diabetes (7). Of interest, the beneficial effects of Ex were not observed by Fleenor and Bowles (11) in the left circumflex coronary artery which had not been treated with PTCA. These results suggest that PTCA and/or stenting of coronary arteries may alter the effects of Ex on lesion progression, if true this observation suggests the speculation that these interventional procedures alter shear stress in the coronary arteries during exercise. In summary, current literature indicates that in large mammals Ex does not alter progression/regression of CAD, but there is evidence of beneficial effects of exercise in coronary arteries that have been treated with PTCA and/or intravascular stents.
A final explanation of why Ex did not alter disease progression in the present study is that it is possible that the beneficial effects occurred in the coronary arteries at a location we did not sample. Thus, if Ex has beneficial effects on disease progression and/or coronary phenotype specifically in the areas where lesions are developing, the failure to see a beneficial effect could have resulted from sampling strategy. We consistently sampled coronary arteries from the same anatomical location, we did not select samples specifically from areas with ongoing CAD, so it is possible, yet unlikely that Ex had beneficial effects in other segments of the coronary arteries, not sampled in our design.
Effects of High Fat Diet on Phenotype of Vascular Cells
The results of this study reveal that, in addition to the decreased EDR reported previously (44); there are modest changes in endothelial cell phenotype present in the conduit and arteriolar coronary arteries in this porcine model of early stage CAD. These findings confirm previous work from our laboratory (39, 41). Indeed, similar to Thompson et al. (39) we found that foam cells and fatty streaks are present in conduit coronary arteries of male pigs on this diet. Thus based on these results we conclude that the animals used in the present study exhibited an early stage of atherosclerotic vascular disease comparable to Stary Stage I–III (36).
The immunohistochemistry data demonstrated a significant difference between RCAs and LADs of NF compared to HF diet pigs. LADs and RCAs from pigs receiving HF diet contained macrophage foam cells in addition to endothelial and smooth muscle cells (Figure 1A). This is demonstrated by the SRA immunoreactivity of the macrophage foam cells (Figure 1B). These macrophage foam cells were also immunoreactive for eNOS. The expression of eNOS by activated macrophages has also been reported in rats (8) and humans (42). Macrophage foam cells in the HF LADs also were immunoreactive for SOD-1 as we have previously reported (39), and would contribute to the SOD-1 detected by immunoblots of whole artery samples. Immunohistochemistry indicates that these foam cells also express CAV-1, SOD-2, and COX-2. Endothelial and smooth muscle cells of RCA and LAD of NF and HF pigs also express eNOS, CAV-1, SOD-1, and SOD-2. We did not see noteworthy immunoreactivity for COX-2 in NF LADs or in HF LADs that did not appear to contain foam cells. The foam cells observed in the wall of diseased arteries which express a number of markers of importance to endothelial cell function (eNOS, SOD-1, SOD-2, CAV-1 or COX-2) should be considered when using immunoblots of whole arteries to examine endothelial gene expression in diseased arteries.
The expression of CAV-1 in endothelium, smooth muscle, and foam cells of HF LADs is a very interesting finding. CAV-1 not only plays an important role in macrophage lipid metabolism (13) but also has been proposed to have both pro- and anti-atherogenic properties (12). In addition, CAV-1 has been reported to be preferentially expressed in smooth muscle cells of the LAD as compared to the internal mammary artery of non-diseased pigs (33). It is known that in endothelial cells the interaction between eNOS and CAV-1 reduces eNOS activity (14, 15), and that hypercholesterolemia increases CAV-1 expression in cultured endothelial cells (9, 10).
SRA positive macrophages were not noted in the walls of LV arterioles of animals on HF diet. However, HF diet produced a significant increase in arteriolar content of nitrotyrosine (Figure 3). Our findings indicated that CAV-1 protein content was increased in both smooth muscle and endothelial cells of LV arterioles from HFSed animals. Previously, we have reported that CAV-1 expression was increased in HFSed LADs relative to the content in NF diet LADs and that both smooth muscle and endothelial cells express CAV-1 (39). Similarly, in this study we observed that CAV-1 is expressed in both smooth muscle and endothelial cells of LV arterioles (Figure 1C). This strongly suggests that endothelial cells of HF LADs (39) and LV arterioles express more CAV-1 than NF diet pigs. These findings are consistent with the interpretation that HF diet may cause decreased NO release and decreased EDR by increasing expression of CAV-1 which binds NOS protein and decreases the activity of eNOS (9, 15).
Effects of Exercise Training on Vascular Cell Phenotype
Ex did not significantly alter the number of foam cells seen in LAD of HF pigs or the level of expression of eNOS, SOD-1, SOD-2, CAV-1 or COX-2 in foam cells. Ex also did not appear to change CAV-1 expression in HF coronary arteries. Immunoblot analysis indicated that neither HF diet nor Ex altered LV arteriolar protein content for eNOS, phospho-eNOS, AKT or catalase (Figure 3). In contrast, Ex induced a significant increase in LV arteriolar expression of SOD-1 and SOD-2 (Figure 3). Henderson et al. (19) reported that Ex of HF diet pigs resulted in an increase in eNOS expression in LV arterioles. One significant difference between the two studies is the procedures used in loading the arteriolar samples on the gels. Henderson et al. (19) controlled for total protein in their arteriolar samples only by measuring arteriolar diameters and lengths were as in the present study, protein content was determined and equal amounts of protein were loaded on each lane. It is not apparent how this technical difference could provide such different results so we do not know why these previous data appear to conflict with current results.
In whole LAD samples (Figure 2) we observed no effect of Ex on eNOS, AKT or catalase content confirming results of Woodman et al. (44) who reported that Ex did not alter expression of eNOS, Cav-1, SOD-1 or SOD-2 in LAD of HF diet fed pigs and Thompson et al. (39) reported that Ex only increased expression of SOD-1 of HF LADs. Because our present results, as well as those of Woodman et al. (44) and Thompson et al. (39) indicate that some of these markers are expressed in non-endothelial cells in coronary arteries, we analyzed protein content in LAD endothelial scrapes. These results suggest that HF diet increased phospho-AKT, HSP90 (Figure 4), and SOD-2, but not SOD-1. In addition, these data suggest that Ex significantly reduced the effect of the HF diet on phospho-AKT protein content in LADs. In the endothelial enriched endothelial scrape samples there were no statistically significant HF diet or Ex effects on eNOS, MDA, SOD-1, ecSOD, catalase or arginase-1 protein contents. Thus, similarly to the whole LAD samples, there were only modest changes in expression of these markers produced by HF diet and/or Ex.
In summary, the findings of the present study reveal that this model of early stage CAD involves appearance of macrophage foam cells (SRA positive cells) in the walls of the large coronary arteries. Importantly, we also found that these foam cells were positive for eNOS, CAV-1, SOD-1, SOD-2, catalase, and COX-2 protein expression. As we have previously hypothesized, HF diet promoted a pro-atherogenic coronary phenotype as evident by the endothelial expression of inflammatory and oxidative stress markers VCAM-1, SRA and MDA. Contrary to our hypothesis, Ex did not significantly alter any of these immunohistochemical markers of early stage coronary disease, inflammation or oxidative stress. Nevertheless, Ex did increase SOD-1 and SOD-2 immunoreactivity of LV arterioles, but did not alter arterial endothelial expression of any proteins which we examined with immunoblot.
Thus, at this early stage of CAD, Ex did not seem to shift vascular cell phenotypes from pro- to a more favorable anti-atherogenic status in this model. In addition, these findings support the idea that endothelial phenotype expression follows different patterns in the macro and microvasculature of the coronary circulation. While these results are interesting and provocative, further studies are required as it is possible that the techniques used in this study are not sensitive enough to resolve more subtle changes in vascular cell phenotype produced by Ex. Additionally, it is possible that Ex induces changes in genes other than the ones selected for this study. In conclusion, the results of this study support our hypothesis that HF diet produces a pro-atherogenic coronary endothelial cell phenotype, but results do not support that Ex signals the expression of an anti-atherogenic endothelial cell phenotype in coronary conduit arteries and arterioles in this model of early stage CAD.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr. Richard Madsen for statistical analysis of the results, Dr. Isabelle Masseau for kindly providing us the VCAM-1 antibody used in this study, and the expert technical assistance of Pam Thorne, Denise Holiman, Jennifer Casati, Ann Melloh, David Harah, and Tammy Strawn.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Grants: This work was supported by National Heart, Lung, and Blood Institute Grant HL-52490.
Disclosure of funding received for this work from National Institutes of Health (NIH).
Footnotes
Disclosures: These authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors, or any of the author's academic institutions or employers.
REFERENCES
- 1.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 2.Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol. 2010;298:H1889–1901. doi: 10.1152/ajpheart.01252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 4.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88(6):2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 6.Cesar L, Suarez SV, Adi J, Adi N, Vazquez-Padron R, Yu H, Ma Q, Goldschmidt Clermont PJ, Agatston A, Kurlansky P, Webster KA. An essential role for diet in exercise-mediated protection against dyslipidemia, inflammation and atherosclerosis in ApoE/ mice. PloS one. 2011;6:e17263. doi: 10.1371/journal.pone.0017263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovascular research. 2010;85:631–640. doi: 10.1093/cvr/cvp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ermert M, Ruppert C, Gunther A, Duncker HR, Seeger W, Ermert L. Cell-specific nitric oxide synthase-isoenzyme expression and regulation in response to endotoxin in intact rat lungs. Lab Invest. 2002;82(4):425–441. doi: 10.1038/labinvest.3780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest. 1999;103(6):897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res. 2001;88(2):129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- 11.Fleenor BS, Bowles DK. Exercise training decreases the size and alters the composition of the neointima in a porcine model of percutaneous transluminal coronary angioplasty (PTCA) J Appl Physiol. 2009;107:937–945. doi: 10.1152/japplphysiol.91444.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23(7):1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 13.Gargalovic P, Dory L. Caveolins and macrophage lipid metabolism. J Lipid Res. 2003;44(1):11–21. doi: 10.1194/jlr.r200005-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280(2):F193–206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 15.Govers R, van der Sluijs P, van Donselaar E, Slot JW, Rabelink TJ. Endothelial nitric oxide synthase and its negative regulator caveolin-1 localize to distinct perinuclear organelles. J Histochem Cytochem. 2002;50(6):779–788. doi: 10.1177/002215540205000604. [DOI] [PubMed] [Google Scholar]
- 16.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 17.Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, Schlierf G, Kubler W, Schuler G. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22(2):468–477. doi: 10.1016/0735-1097(93)90051-2. [DOI] [PubMed] [Google Scholar]
- 18.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 19.Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol. 2004;97(3):1159–1168. doi: 10.1152/japplphysiol.00261.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 21.Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB., Jr. Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. NEnglJMed. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67(3):1140–1149. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90(2):501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam VK. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003;95(1):250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 28.Link RP, Pedersoli WM, Safanie AH. Effect of exercise on development of atherosclerosis in swine. Atherosclerosis. 1972;15:107–122. doi: 10.1016/0021-9150(72)90044-5. [DOI] [PubMed] [Google Scholar]
- 29.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89(5):2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- 30.Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, Zimmermann R, Kubler W. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96(8):2534–2541. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- 31.Okabe T, Kishimoto C, Murayama T, Yokode M, Kita T. Effects of exercise on the development of atherosclerosis in apolipoprotein E-deficient mice. Experimental and clinical cardiology. 2006;11:276–279. [PMC free article] [PubMed] [Google Scholar]
- 32.Prophet EBMB, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology. American Registry of Pathology; Washington DC: 1992. p. 279. [Google Scholar]
- 33.Qin M, Zeng Z, Zheng J, Shah PK, Schwartz SM, Adams LD, Sharifi BG. Suppression subtractive hybridization identifies distinctive expression markers for coronary and internal mammary arteries. Arterioscler Thromb Vasc Biol. 2003;23(3):425–433. doi: 10.1161/01.ATV.0000059303.94760.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 35.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 36.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20(5):1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 37.Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33(8):1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 38.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 39.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96(3):1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 40.Thompson PA. Exercise for patients with coronary artery and/or coronary heart disease. In: Thompson PA, editor. Exercise and Sports Cardiology. McGraw-Hill; New York: 2001. pp. 354–370. [Google Scholar]
- 41.Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol. 2005;86(5):335–345. doi: 10.1111/j.0959-9673.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Straaten JF, Postma DS, Coers W, Noordhoek JA, Kauffman HF, Timens W. Macrophages in lung tissue from patients with pulmonary emphysema express both inducible and endothelial nitric oxide synthase. Mod Pathol. 1998;11(7):648–655. [PubMed] [Google Scholar]
- 43.Williams JK, Kaplan JR, Suparto IH, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:864–871. doi: 10.1161/01.ATV.0000067934.12783.6A. [DOI] [PubMed] [Google Scholar]
- 44.Woodman CR, Turk JR, Rush JW, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J Appl Physiol. 2004;96(3):1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- 45.Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol. 2003;95:1194–1200. doi: 10.1152/japplphysiol.00282.2003. [DOI] [PubMed] [Google Scholar]
- 46.Young CG, Knight CA, Vickers KC, Westbrook D, Madamanchi NR, Runge MS, Ischiropoulos H, Ballinger SW. Differential effects of exercise on aortic mitochondria. Am J Physiol Heart Circ Physiol. 2005;288:H1683–1689. doi: 10.1152/ajpheart.00136.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.