Abstract
Endothelial adaptations to exercise training are not exclusively conferred within the active muscle beds. Herein, we summarize key studies that have evaluated the impact of chronic exercise on the endothelium of vasculatures perfusing nonworking skeletal muscle, brain, viscera, and skin, concluding with discussion of potential mechanisms driving these endothelial adaptations.
The beneficial effects of physical activity and exercise training in prevention and treatment of cardiovascular disease are not entirely mediated by the reduction of systemic risk factors (71, 100, 141, 195). Indeed, current data suggest that at least 40% of the cardiovascular risk reduction associated with exercise cannot be accounted for by the modification of established or emerging risk factors (71, 100, 141, 195). In view of this risk reduction gap, it is likely that exercise exerts direct effects on the vascular wall of arteries, a concept recently referred to as vascular conditioning (65, 71, 195). The vascular effects of chronic exercise may include structural (angiogenesis and remodeling) and functional adaptations, the latter involving phenotypic alterations of vascular smooth muscle and endothelial cells. Of particular interest is the possible direct impact of physical activity on sustaining or restoring a normal/healthy endothelial phenotype (65, 69, 93, 114, 115, 127, 144, 195). In fact, it is this favorable effect of exercise on the endothelium that may explain, at least in part, the well established association between physical activity and reduced cardiovascular events and mortality (11, 149, 155, 162, 207).
During exercise, the metabolic demands of contracting muscle cause vasodilation and increased blood flow in the working muscle tissue. As a result, it is believed that shear stress is elevated in the vasculatures supplying blood to the active cardiac and skeletal muscle (116). It is commonly accepted that repeated episodes of elevated shear stress represent the primary physiological signal for endothelial adaptations to exercise training (65, 69, 93, 114, 116, 127, 195). The concept that vascular adaptations are mediated by shear stress-dependent mechanisms is indeed supported by cell and organ culture (18, 21, 22, 40, 171, 180) as well as in vivo animal (117, 133) and human (67, 78, 148, 198) experiments. For example, data from rats (117, 133) suggest that exercise training-induced adaptations in skeletal muscle arteries are related to muscle fiber type and recruitment patterns. That is, exercise-induced vascular adaptations occur, spatially, in the area of muscle tissue with the greatest relative increase in fiber activity and presumably shear stress during exercise training bouts. Moreover, recent data in humans (198) indicate that brachial artery endothelial adaptations to handgrip exercise training can be abolished when the increases in conduit artery shear associated with each bout of handgrip exercise are experimentally prevented.
Intriguingly, however, adaptations to exercise training have also been observed in vasculatures perfusing tissues that do not exhibit increased metabolism during exercise and that, as a result, are exposed to a lesser elevation in blood flow (or no increase at all) (109–111, 212). These data suggest the possibility that regular exercise may also exert a nonspecific, systemic effect on endothelial function/phenotype and that such effects may partially occur through shear stress-independent mechanisms. However, it should be noted that, even in the absence of increased mean shear stress, changes in shear profiles (i.e., waveforms) occur (63, 64, 66, 181, 194), and therefore the role of shear stress as a systemic modulator of endothelial health with exercise training cannot be ruled out.
Several reviews (65, 69, 93, 114, 115, 127, 144, 195) have described the vascular effects of exercise training in the coronary and skeletal muscle circulations and a debate remains on whether these adaptations extend to regions less active during exercise bouts (70, 192). In this regard, although previous reports have suggested the presence of a systemic influence of exercise on the vasculature (69, 70, 72, 93, 195), we are unaware of a review exclusively focused on endothelial adaptations in blood vessels of tissues beyond the active muscle beds. Therefore, the main purpose of the present review is to examine those key studies that have evaluated the impact of chronic exercise on the endothelium in noncontracting tissues. Specifically, we describe whether endothelial adaptations occur with exercise training in vasculatures of various tissues that have smaller increases (or no increase) in activity (“inactive”) during exercise and identify or speculate whether these vessels are exposed to changes in shear stress during bouts of physical activity. Furthermore, based on this information, a hypothesis is formulated regarding alternative signals (i.e., circumferential stretch, circulating humoral factors) that may act independently or synergistically with shear forces in the modulation of systemic endothelial adaptations to chronic exercise.
Effects of Exercise Training on the Endothelium of Noncontracting Tissues
“Inactive” Skeletal Muscle Circulation
Important evidence endorsing the concept that exercise training exerts systemic effects on the arterial endothelium derives from human studies evaluating endothelial function in arteries that perfuse limbs that would be considered nonworking during training bouts (70, 192). Many, although not all (45, 53, 61, 105, 143, 161, 190, 191), interventional studies have demonstrated that lower-limb exercise training (e.g., cycling or walking) is associated with vascular adaptations in the arms (28, 46, 62, 85, 103, 118, 122, 124–126, 164, 197, 202, 203, 205, 206). Such vascular adaptations in the upper limbs occur even when subjects are instructed to not grip the handle bars or railings (124–126, 202, 203, 205, 206) to minimize arm activity during exercise bouts. Remarkably, the favorable chronic effects of exercise training in the nonworking limbs are manifested in both the conduit arteries (i.e., brachial and radial) (28, 118, 122, 124, 164, 197, 202, 203, 205, 206) as well as resistance arteries (46, 62, 85, 103, 125, 126). In these studies, conduit artery endothelial adaptations have been examined non-invasively via flow-mediated dilation, whereas resistance vessel endothelial adaptations have typically been assayed by measuring forearm blood flow responses to intrabrachial artery infusions of endothelium-dependent vasodilators such as acetylcholine (ACh).
Given that 50–80% of resting forearm blood flow is directed to the skeletal muscle (36), the exercise training-mediated increase in forearm vascular responsiveness to intrabrachial artery ACh infusion has been interpreted as evidence of endothelial adaptation in the nonworking skeletal muscle vasculature. However, it should be noted that intra-arterial infusions of ACh has also been shown to increase skin blood flow, although the precise distribution of changes in limb conductance during ACh infusion is not known (54, 178). In light of the evidence that exercise training improves endothelial responsiveness to local (intradermal) administration of ACh in the skin (10) (see Cutaneous Circulation section), it is possible that increases in whole forearm blood flow responses to ACh following exercise training may be explained, at least in part, by endothelial adaptations conferred within the cutaneous circulation. Therefore, further research is necessary to dissect out what portion of the increase in forearm vascular responsiveness to ACh associated with lower-limb exercise training is reflective of endothelial adaptations within the noncontracting skeletal muscle.
There is evidence from animal studies supporting the concept that exercise training induces adaptations in the endothelium of vessels perfusing nonworking skeletal muscle. Studies in rats (109–111, 212) demonstrate that exercise training produces vascular changes in skeletal muscles (i.e., spinotrapezius) that exhibit no increase in metabolic activity or blood flow during treadmill exercise (146). For example, Lash et al. (111) demonstrated that 16 wk of exercise training increases ACh-induced vasodilation in arterioles of spinotrapezius muscle of healthy rats. These results have been confirmed by Xiang and colleagues (212) examining the effect of exercise training in lean and obese Zucker rats. They found that 4–5 wk of exercise improved ACh-induced vasodilation in spinotrapezius arterioles in both groups of rats, with the greatest effect found in the obese animals (212). The trend for a greater improvement of endothelial function in disease states is also supported by human studies (69, 127, 144, 195). In this regard, available literature makes it apparent that the beneficial effects of leg exercise on endothelial function of resistance arteries and conduit arteries of nonworking human limbs are most notable in subject populations with preexisting cardiovascular risk factors or disease; that is, in individuals with compromised basal vascular function (69, 127, 144, 195). It is plausible that there is an interaction between endothelial health and exercise training such that the endothelium is more amenable to exercise when impairment or disease is present (93, 127, 144, 154).
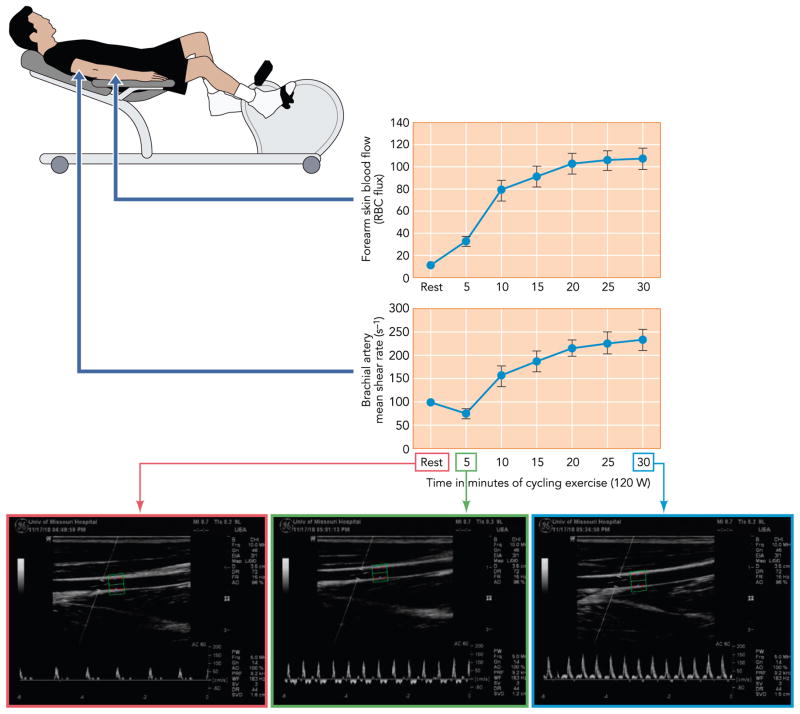
The mechanisms by which endothelial adaptations are manifested in the nonworking arms of individuals undergoing leg training have not been fully elucidated. During the last decade, research has focused on examining changes in blood flow and shear rate magnitude and patterns in the upper limbs as potential signals for adaptations to training. It is now established that, at the onset of lower-limb dynamic exercise (i.e., first ~10 min), the brachial artery is exposed to a marked increase in retrograde and oscillatory shear (63, 64, 66, 181, 194) likely as a result of the increase in downstream resistance (181). As exercise continues, blood flow to the forearm is increased and primarily directed to the skin to serve thermoregulatory needs (97, 152) (see Cutaneous Circulation). Cutaneous vasodilation (and the subsequent decrease in forearm vascular resistance) observed during prolonged exercise results in a significant augmentation of shear stress in the upstream conduit arteries (i.e., brachial and radial) (153, 181, 188, 196) and the partial removal of the initial increase in retrograde and oscillatory shear (181) (FIGURE 1). These observations stimulate the hypothesis that the favorable vascular adaptations occurring in the conduit arteries feeding the nonworking limbs (28, 118, 122, 124, 164, 197, 202, 203, 205, 206) may be mediated by the same signal through which conduit arteries perfusing the active limbs are believed to adapt; that is, repeated bouts of elevated shear stress (65, 69, 116, 127, 195, 198). Green and coworkers (196) recently addressed this premise using an acute exercise paradigm. They bilaterally evaluated brachial artery endothelium-dependent dilation before and after a 30-min bout of cycling exercise. In one arm, the increase in brachial artery shear rate during exercise was prevented by the application of a forearm cuff at 60 mmHg. The authors found that brachial artery vasomotor function was improved after cycling exercise in the uncuffed, but not cuffed, arm (196), hence suggesting that a single episode of increased shear at the conduit artery of the nonworking limb produces endothelial adaptations. Recent data from the same group further support the argument that shear stress is capable of inducing conduit artery adaptations in humans even when the hyperemic stimulus is independent of exercise (i.e., local heating) (148).
FIGURE 1. Forearm skin blood flow and brachial artery mean shear rate during prolonged cycling exercise.
Increase in forearm skin blood flow and brachial artery mean shear rate during prolonged cycling exercise (top and middle) [data adapted from Simmons et al. (181)]. Bottom: original Doppler ultrasound images from a representative subject illustrate the remarkable changes in blood velocity profiles from rest (left) to early (middle) and prolonged (right) exercise.
In summary, extensive evidence in rodents indicates that exercise training induces favorable endothelial adaptations in arteries of nonworking skeletal muscle, whereas evidence in humans support that these adaptations occur in nonworking limbs that contain muscles not involved in the exercise bout. In humans, the mechanisms for conduit artery vascular adaptations may be attributed to elevated shear stress (induced by an increase in blood flow directed to the skin), whereas the mechanisms responsible for adaptations in non-recruited skeletal muscle resistance arteries remain unclear. It is plausible that, during exercise, the marked sympathetically mediated arteriolar vasoconstriction in the nonworking skeletal muscle may result in actual increases in shear, which in turn may act as a signal to the endothelium.
Cerebral Circulation
It is well established that regular physical activity is associated with reduced cerebrovascular events (e.g., stroke) (119, 120, 128, 174, 208). Although the mechanisms responsible for these vasoprotective effects of exercise are largely unknown, it is reasonable to propose that exercise may exert its beneficial effects on the cerebral circulation by reducing carotid plaque burden and instability, hence decreasing the risk of emboli. There is a large body of evidence indicating that exercise training improves endothelial function (52) and decreases arterial stiffness (57, 177, 187) in the carotid artery. In addition, although less compelling, there is also evidence that physical activity is associated with reduced carotid artery intima-media thickness (101, 136, 201), an index of pre-clinical atherosclerosis. Importantly, exercise training has been shown to alter composition of carotid artery plaque and reduce its vulnerability to rupture (166). Furthermore, there is also evidence that exercise training exerts direct protective effects on the cerebral circulation. Gertz et al. (60) reported that, 4 wk after brain ischemia, mice that exercise trained exhibited increased resting cerebral blood flow in the ischemic lesion as well as better functional and cognitive outcomes. Importantly, the protective effects of physical activity were abolished in animals either co-treated with a NOS inhibitor or in animals lacking eNOS gene expression (eNOS−/−) (60). Similarly, Endres et al. (55) demonstrated that 3 wk of exercise training led to an increase in resting cerebral blood flow and a reduction of cerebral infarct size in wild-type, but not eNOS−/−, mice. Together, these data unequivocally demonstrate that eNOS upregulation is critical in enhancing postischemic recovery of cerebral function and increasing cerebral collateral flow and/or cerebral resistance to ischemia. Studies in rats also indicate that protein expression and endothelium-dependent vasodilation of cerebral arteries and arterioles can be modulated with exercise training. Mayhan et al. found that 6–10 wk of exercise training increased eNOS protein expression and NO-dependent dilation in the basilar artery (132) and cerebral (pial) arterioles (130) in diabetic rats. The same group of investigators evaluated the influence of exercise training on reversing the deleterious effects of cigarette smoking in the cerebral circulation (131). They found that exercise training restored adenosine-induced vasodilation in cerebral (pial) arterioles in nicotine-treated rats, whereas no effects of exercise were observed in healthy control (untreated) rats (131). Furthermore, the increase in superoxide ion production associated with the chronic exposure to nicotine was reduced by exercise training, possibly as a result of an increase in SOD-1 protein expression (131). Recently, in a large cross-sectional study (n = 307), Ainslie et al. (1) provided evidence in humans that resting blood flow velocity in the middle cerebral artery is elevated by habitual exercise across different ages, independent of possible confounding variables. It is possible that increases in cerebral blood flow associated with training (1) are mediated, at least in part, by local changes within the vasculature. The observation that habitual physical activity increases resting cerebral blood flow velocity is in contrast to the consistent finding that exercise training does not alter resting muscle blood flow (8, 68, 129, 156, 200). We speculate this incongruence between vascular beds may be explained by regional differences in basal sympathetic tone following exercise training. In this regard, recent data suggest that the skeletal muscle and cerebral circulations are differentially regulated by the sympathetic nervous system during acute exercise (81).
It is important to emphasize that exercise training-induced vascular adaptations in the cerebral circulation are accompanied by improvements in functional and cognitive outcomes as well (94). Indeed, results of longitudinal studies indicate that exercise training can provide a positive effect on human cognitive abilities (106), potentially counteracting declines in cerebral tissue density (33) and increasing brain volume in aging humans (34). Recent data from the Framingham Heart Study indicates that decreasing cardiac function, even at normal cardiac index levels, is associated with accelerated brain aging (95). Of note, there is growing evidence that cerebrovascular dysfunction plays a role not only in vascular causes of cognitive impairment but also in Alzheimer’s disease (89). In fact, novel data implies that there may be a causal link between endothelium-derived NO deficiency and Alzheimer’s disease (27).
Although the mechanisms by which regular physical activity induces beneficial effects in the cerebral vasculature have not been entirely elucidated, it is possible that repeated exposure to increases in blood flow and shear stress in specific regions of the brain during exercise plays a role. Indeed, there is consistent evidence that blood flow is increased in some areas of the brain during physical activity (55, 58, 82–84, 90, 98, 99, 142, 151). Blood flow is primarily directed to those areas that are intensely activated during exercise (e.g., motor and sensory cortex, and cerebellum-cerebral cortex) (56). Interestingly, the increase in cerebral blood flow appears to be related to the intensity of the activity up to ~60% of V̇O2max, an intensity after which flow decreases toward baseline levels (83, 142). The increase in cerebral blood flow seems to be linked to the local vasodilator action of metabolites and/or perhaps to other local effects produced by increased neural activity during exercise (113, 150, 167, 182), whereas the limitation of hyperemia above 60% of V̇O2max may result from decreased arterial PCO2 (13).
In summary, the studies presented herein provide evidence that exercise training is able to promote favorable adaptations in the cerebral vasculature, blood vessels which are not viewed as belonging to exercising tissue. The signals mediating these adaptations are unclear at this time, but the increase in shear stress associated with regional increases in cerebral blood flow during acute exercise should be considered as a prime candidate.
Splanchnic and Renal Circulations
Blood flow to the splanchnic and renal vasculatures is markedly reduced during dynamic exercise as a function of exercise intensity (2, 73, 76, 86, 160, 173, 213). Data from animal and human studies suggest that exercise training results in an attenuated reduction of blood flow to both the splanchnic and renal vasculatures at a given absolute exercise intensity (3, 30, 147, 163, 173). Rowell et al. (173) first described this effect on total splanchnic flow in humans via measures of indocyanine green clearance before and after training. More recent animal studies have demonstrated that the attenuated reduction of splanchnic blood flow after training occurs in most, if not all, splanchnic vasculatures (3, 47, 147). The training-induced adaptations in the splanchnic and renal vasculature may be sensitive to the type of training since adaptations in the renal arteries has been reported following endurance treadmill training but not following high-intensity interval sprint training in rats (147).
The training-induced attenuation of splanchnic and renal vasoconstriction during exercise is likely mediated by both local and systemic adaptations. Centrally, exercise training-induced reductions in peripheral sympathetic outflow, which mediates visceral constriction during exercise, likely accounts for a large part of the attenuated splanchnic and renal vasoconstriction during exercise after training (5, 31, 47, 77, 87, 145, 165, 176, 193). Indeed, endurance training and daily exercise reduce renal sympathetic nerve activity during exercise due, in part, to enhanced cardiac baroreflex inhibition of sympathetic outflow to the mesenteric and renal circulation in rabbits (47, 48). Additionally, local adaptations within the vascular wall also appear to contribute to the training-induced attenuation of visceral constriction during exercise. Most, but not all (134, 186), in vitro studies examining the splanchnic and renal vasculature have revealed enhanced ACh-induced vasodilation and reduced vasoconstriction to various agonists (nor-epinephrine, phenylephrine, prostaglandin F2α, KCl) related, at least in part, to enhanced production of and/or sensitivity to endogenous endothelial dilators (i.e., nitric oxide, prostacyclin, etc.) (4, 19, 20, 25, 42, 43, 74, 92, 112, 214). Mechanistically, the training-induced enhancement of mesenteric vasodilation to ACh appears to be accounted for by an enhanced endothelium-derived hyperpolarizing factor-dependent component of ACh dilation and subsequent activation of calcium activated K+ channels (19, 214); however, other unexamined pathways may be involved as well. These adaptations have been reported in both conduit arteries and the microcirculation of the splanchnic and renal vasculatures (4, 19, 25, 43, 112, 134). Furthermore, similar training-induced changes in local vascular reactivity occur in both healthy and diseased animals (i.e., obesity, hypertension) (4, 19, 20, 25, 42, 43, 74, 92, 112, 214). Further research is necessary to determine the extent to which findings concerning training effects on visceral circulations from animal studies can be extrapolated to humans.
Taken together, these studies suggest that exercise training induces local vascular adaptations of the splanchnic and renal vasculatures that likely act to enhance nutrient absorption and utilization during exercise in the trained organism. Augmented cardiac output and mean arterial pressure during exercise coupled with visceral vasoconstriction (i.e., reduction in diameter) may lead to increases in shear stress despite the reduction of blood flow (160). Thus the signal for these chronic adaptations may be the repetitive bouts of increased shear stress occurring during acute exercise. However, the precise levels of shear stress present in the splanchnic and renal macro and microvasculatures during exercise have not been fully characterized.
Cutaneous Circulation
Endurance exercise training for a period of weeks to months results in cutaneous vascular adaptations characterized by increased endothelial-mediated vasomotor function. The main evidence for these adaptations derives from studies in which regional forearm skin blood flow responses were assessed during local administration of endothelium-dependent (ACh) and -independent (sodium nitroprusside) vasodilators or during local heating. For example, in a cross-sectional study, Kvernmo et al. (108) demonstrated that forearm skin blood flow responses to ACh were greater in elite endurance athletes than in control subjects who performed regular physical activity. In contrast, responses to sodium nitroprusside were not different between groups. Similarly, Roche et al. (172) recently reported an enhanced skin blood flow response to local heating in physically active children (aged 13–15 yr) compared with age-matched (less active) controls. Using a longitudinal study design, Wang (204) evaluated endothelium-dependent and -independent cutaneous vascular responses in sedentary but otherwise healthy males before and after 8 wk of exercise training. After completing the exercise training period, endothelium-dependent cutaneous vascular responsiveness was increased, whereas endothelium-independent responses were unchanged. Interestingly, when subjects were then detrained for 8 wk, the training-mediated changes in vascular responsiveness were abolished (204). Similar detraining results have been shown when normally active subjects are confined to bedrest for 14–56 days (39, 44). Furthermore, it appears that regular exercise during bedrest prevents the decline in vascular responsiveness and maximal vasodilation in the skin (39, 44). Thus a number of studies and experimental models of increased and decreased physical activity support the notion that cutaneous vascular responsiveness, particularly to endothelium-dependent stimulation, is altered by physical activity. Increased physical activity is associated with increased cutaneous endothelial vasomotor responsiveness, whereas decreased physical activity is associated with decreased cutaneous vasomotor function.
It appears that the beneficial effects of exercise training on the cutaneous vascular endothelium are sufficient to stave off the progression toward microcirculatory dysfunction associated with aging and disease. Evidence for this comes from studies demonstrating that older individuals who exercise regularly have enhanced endothelial vasomotor function in the cutaneous circulation compared with their sedentary, age-matched counterparts (10). In fact, cutaneous microvascular endothelial function in physically active older persons is not different from that in recreationally active young individuals (10, 189). Similar effects of exercise on cutaneous microvascular function have been demonstrated in patients with Type 2 diabetes (32) and chronic venous disease (104). Thus it appears that regular exercise training increases cutaneous microvascular (endothelial) function in health and disease.
It is important to note that many of the studies discussed above (44, 104, 108, 189, 204) have examined the effect of physical activity/inactivity on microvascular function in the skin using iontrophoretic application of ACh. Today, it is recognized that results obtained with this technique to test endothelial function of skin blood vessels must be interpreted cautiously (37, 38). Skin blood flow responses to ACh iontophoresis are only partly NO mediated and largely mediated by prostanoids (12, 51, 88). In addition, ACh iontophoresis induces an axon reflex that contributes to the increase in cutaneous blood flow (9). Nevertheless, Black et al. (10) used microdialysis for direct intradermal delivery of ACh to study chronic adaptations to exercise training and found similar effects to those reported by others (44, 104, 108, 189, 204).
The mechanisms responsible for the beneficial adaptations in the cutaneous vascular endothelium in response to exercise training have not been identified. The cutaneous circulation plays a pivotal role in the dissipation of heat during dynamic exercise, which is generated as the most abundant by-product of metabolism (96). As such, sevenfold increases in skin blood flow are not uncommon in subjects exercising at a moderate intensity in thermoneutral conditions (181) (FIGURE 1). In view of the cutaneous hyperemia associated with exercise, changes in hemodynamic forces are primary candidates for mechanisms through which beneficial adaptations are produced in response to exercise training. In this regard, a recent study by Green et al. (67) demonstrated that chronic forearm heating, which provides a similar hyperemic stimulus to that occurring during exercise, improves cutaneous microvascular vasodilator function. To investigate the mechanism of this beneficial effect, skin blood flow in one arm (but not the other) was manipulated by inflation of a proximal forearm cuff, resulting in significant attenuation of the forearm hyperemia during heating sessions. Interestingly, the arm in which hyperemia was blunted exhibited no adaptation to chronic heating, suggesting that repetitive increases in tissue perfusion are responsible for cutaneous vascular adaptations in this setting. Because changes in tissue perfusion are often related to changes in vascular wall shear stress, these results stimulate the possibility that shear stress signals endothelial adaptation in the cutaneous circulation and further suggest that increases in shear may be responsible for cutaneous vascular adaptations to exercise training. However, it is currently unclear how changes in bulk blood flow during forearm heating (or exercise) translate to alterations in shear stress in the cutaneous microcirculation. Therefore, further investigation is necessary to clarify the role of hemodynamic forces in the cutaneous (endothelial) vascular adaptations resulting from chronic endurance exercise training.
Exercise-Induced Signals for Endothelial Adaptations in Noncontracting Tissues
Hemodynamic Forces
Although it is universally recognized that shear stress is a primary signal for exercise training-induced endothelial adaptations in active muscle beds, the literature summarized above appears to suggest that shear may also be a signal for endothelial adaptations in vasculatures perfusing noncontracting tissues. The concept that shear can signal the endothelium to alter its phenotype/function is well supported by in vitro (18, 21, 22, 40, 171, 180) and in vivo (15, 67, 138, 139, 196, 198) data and has been the subject of several reviews (21, 26, 40, 75, 116, 171). It is important to highlight that, during exercise, the signal triggering endothelial adaptations in blood vessels perfusing tissues outside actively contracting muscle may not only be increased mean shear stress but also the alteration in shear profiles (63, 64, 66, 181, 194) that result from hemodynamic changes (e.g., heart rate and pressure) during exercise. In this respect, Canty and Schwarz (16) demonstrated in conscious dogs that cardiac pacing at 200 beats/min produced a near doubling in coronary blood flow that resulted in epicardial artery dilation that was NO mediated. In contrast, a tripling in coronary blood flow induced by adenosine was equally effective in producing epicardial artery dilation; however, this dilation was not NO dependent. These observations suggest that alterations in the frequency of cyclic shear, and hence the profile of the shear waveform, may activate highly different signaling pathways than do increases in average shear stress. More research is warranted to isolate the influence of shear patterns from other exercise-related signals to fully evaluate the hypothesis that exercise-induced acute changes in shear waveforms modulate endothelial health systemically with training.
Several considerations should be taken into account when viewing the hypothesis that shear stress is an exercise-induced signal for endothelial adaptations in nonworking tissues. Shear stress (4ηQ̇/ΠR3; where η = viscosity, Q̇ = blood flow, R = internal radius of the artery) is directly related to blood flow and viscosity but inversely related to arterial diameter. Accordingly, given that vascular tone (and hence diameter) is constantly regulated by central and local factors (e.g., shear stress), changes in blood flow through a given vessel do not always correspond with alterations in shear stress. In this regard, the extent to which enhanced blood flow or viscosity results in increased shear stress may be dependent on the caliber of the vessel and/or its ability to dilate in response to shear. Contrary to conduit arteries, given the remarkable capacity of arterioles to dilate and constrict, it is unclear to what degree changes in blood flow in the microvasculature translate into alterations in shear. As new in vivo imaging techniques emerge, future research should characterize the actual magnitudes and profiles of shear stress in the microcirculation during rest and exercise.
Importantly, shear stress may not be the only hemodynamic exercise-induced signal for systemic endothelial adaptations. Endothelial cells are also exposed to stress from distention of arteries caused by relaxation of smooth muscle in the wall or by increased transmural pressure across the arterial wall. Since endothelial cells are exposed to cyclic distention within each cardiac cycle and during exercise the frequency and magnitude of this distention is augmented, cyclic strain should be considered as a potential exercise-induced signal. In this regard, Awolesi et al. (6, 7) have shown that cyclic strain increases transcription of eNOS in cultured endothelial cells. Similarly, it is demonstrated that distention of isolated arteries is a stimulus for increased expression of the eNOS gene (116, 209, 216). However, it is important to note that cyclic strain has also been associated with increased production of reactive oxygen species and expression of adhesion molecules including VCAM-1, ICAM-1, E-selectin, and MCP-1 (23, 24, 210, 211, 215). Although chronic exposure of endothelial cells to increased cyclic strain (as occurs with hypertension) may produce detrimental adaptations, based on the classical physiological concept of hormesis, it is plausible that recurring periods of exercise-induced cyclic strain and consequent oxidative stress may increase the tolerance of endothelial cells to withstand subsequent doses and hence stimulate a long-term protective effect. However, this is a hypothesis that needs to be tested. It is also possible that cyclic strain is most favorable to the endothelium when combined with increased shear stress. Interestingly, in vitro data suggest that changes in endothelial gene expression in response to shear stress and cyclic strain vary according to whether the two forces are applied separately or simultaneously (199).
Circulating Factors
In addition to evidence in the literature that exercise-induced adaptations of the endothelium result from increases in shear stress and/or cyclic strain (116), there is also growing evidence suggesting that changes in chemical signaling (i.e., hormones, cytokines, adipokines) may contribute to systemic benefits of chronic exercise on endothelial cells. Hemodynamic forces may interact with anti-atherogenic mediators such as insulin, adiponectin, and IL-6 and with inflammatory cytokines (pro-atherogenic mediators) in determination of endothelial cell phenotype/function. It appears that substances that signal remodeling and altered phenotype of endothelial and smooth muscle cells are also released in response to increased shear stress (80, 116, 121, 123).
With the acceptance that adipose tissue is an active metabolic organ that releases a growing number of adipokines (leptin, adiponectin, resistin, etc.) it seems reasonable to consider the hypothesis that chronic physical activity may have beneficial effects systemically on endothelial cell phenotype/function by modifying release of such adipokines from adipose tissue (17, 175). There is a growing consensus that the protective effect of exercise against vascular diseases may to some extent be ascribed to an anti-inflammatory effect of regular physical activity (157, 170). However, further research is required to fully evaluate the role of adipose tissue in the inflammatory processes generated in the peripheral circulation as well as the ability of exercise training to attenuate such vascular inflammation signaled from adipose tissue.
In this context, recent results indicate that exercise training alters gene expression in adipose tissue differently, i.e., has greater effects on some types of adipose tissue than others (35). Company et al. (35) recently examined the effects of exercise training on expression of 18 selected genes in the adipose tissue of pigs. These results indicated that exercise training had significant effects on expression of inflammatory cytokines and inflammatory mediators in epicardial and visceral adipose but not in subcutaneous adipose tissue (35). This, combined with observations that perivascular adipose tissue of normal pigs has an anti-contractile effect on coronary arteries, which is not apparent in the perivascular adipose tissue of pigs with advanced coronary artery disease (14, 169, 185), supports the following hypothesis: exercise training may restore/maintain normal phenotype of cells in adipose tissue resulting in a healthier adipokine release, thereby signaling optimal regulation of endothelial cell gene expression. Similar effects of perivascular adipose tissue may contribute to the beneficial influence of exercise training in non-coronary arteries as well.
In line with the acceptance of adipose tissue as an endocrine organ, Pedersen and colleagues (157, 158) have advocated that skeletal muscle should also be viewed as an endocrine organ. In this regard, Pedersen et al. (157, 158) suggest that cytokines and other peptides that are produced, expressed, and released by muscle fibers and exert endocrine effects should be classified as “myokines.” The discovery that myokines are released during muscle contraction leads to the interesting hypothesis that the effect of regular physical activity on endothelial cell phenotype may be partially mediated by the repeated exposures of the endothelium to such circulating factors. The prototype myokine is IL-6 (157). During exercise, IL-6 is markedly increased in the circulation in both humans (79, 157–159) and animals (91) and precedes the appearance of other cytokines. IL-6 exerts favorable metabolic effects including fat oxidation and lipolysis in adipose tissue and furthermore augments insulin sensitivity in skeletal muscle (157). Importantly, there is evidence to support that IL-6 exerts anti-inflammatory effects by inducing production and release of anti-inflammatory cytokines, IL-10 and IL-1ra, and inhibiting TNF-α (157, 179, 183). Therefore, it is plausible that the release of IL-6 and/or other myokines from contracting muscle during exercise sets the stage for an anti-inflammatory environment that, over time, may contribute to the protective influence of habitual physical activity on vascular wall inflammation systemically.
Another putative signal for systemic endothelial adaptations that can be modulated with exercise training is insulin. There are numerous beneficial effects of insulin on endothelial cells, including the stimulation of endothelial-mediated vasodilation (184), increased production of NO (107), decreased production of reactive oxygen species (50), increased expression of antioxidant genes (59), and decreased expression of adhesion factors such as VCAM-1 (168). Conversely, loss of insulin signaling in endothelial cells accelerates atherosclerosis (168). Furthermore, although the systemic release of insulin is reduced during acute exercise bouts, it appears that chronic exercise is capable of increasing the sensitivity of endothelial vasomotor responses to insulin (137). These combined results support the hypothesis that exercise training may exert a systemic effect on endothelial phenotype and function in part through sensitizing the endothelium to the beneficial effects of insulin. Indeed, both insulin (at rest) and shear stress (during exercise) signal vasodilation in endothelial cells through the phospho-Akt/eNOS pathway; thus it is plausible that insulin and shear stress have synergistic effects on endothelial gene expression. That is, the effects of insulin on the endothelium may be enhanced following repeated exposures to increased shear stress.
The duration of exercise bouts generally ranges between 30 and 120 min. Therefore, the endothelium is likely exposed to increased shear stress and/or cyclic strain for a relatively short portion of a day (2–10% of 24 h). However, exercise may alter circulating factors beyond the duration of the exercise bout and therefore represent a significant stimulus to the vasculature. As work progresses in this area, we must consider the kinetics of these responses as well as the sequence of changes in endothelial gene expression following each exercise bout. This information will be important as we begin to establish signals responsible for changes in endothelial cell gene expression in the arteries feeding active muscle and those perfusing non-contracting tissues.
Summary
It is becoming increasingly recognized that endothelial adaptations to exercise training are not solely conferred within the active muscle beds. Indeed, herein, we have summarized evidence that regular physical activity can alter endothelial phenotype and function in vasculatures perfusing the nonworking skeletal muscle, brain, viscera, and skin. Although limited, there is also recent evidence that exercise training can alter the vasculature perfusing bone (49), adipose tissue (41), and male reproductive organs (29). Although the mechanisms driving exercise training-induced endothelial adaptations beyond the active muscle beds are not established at this time, we propose hemodynamic forces (i.e., shear stress and cyclic strain) and/or circulating factors released from adipose tissue and skeletal muscle as likely signals responsible for the endothelial adaptations in these vasculatures (FIGURE 2). Given that localized exercise training (i.e., single arm or leg) does not exert systemic effects on the vasculature (102, 135, 140), it is possible that there is a threshold for hemodynamic forces and circulatory factors that may only be reached when exercise involves large muscle groups. Further research is warranted to shed light on our understanding of the exercise-mediated signals for endothelial adaptation and to determine how these signals synergistically interact in the systemic modulation of endothelial phenotype and function.
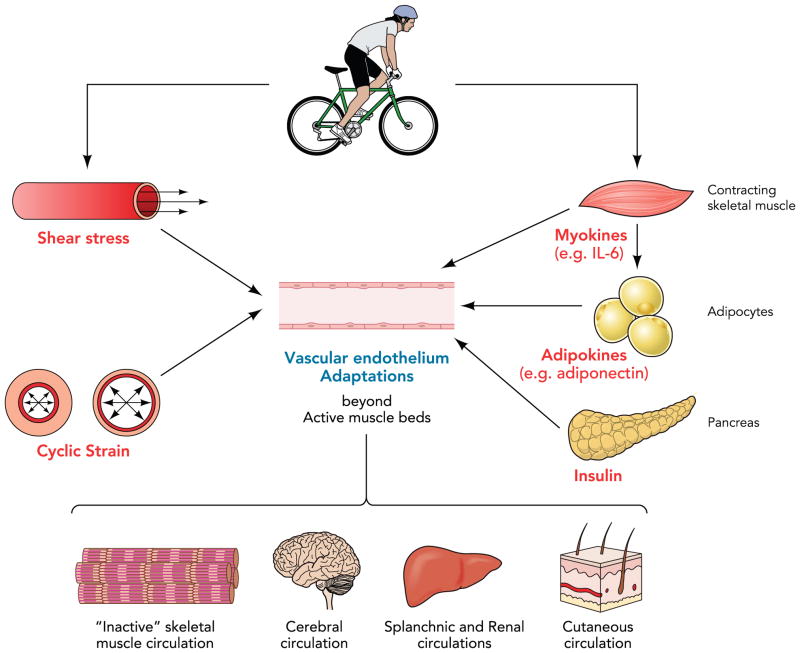
FIGURE 2. Summary of proposed mechanisms by which exercise training may alter endothelial cell phenotype and function beyond the active muscle beds.
During a bout of exercise, endothelial gene expression can be regulated by changes in the magnitude and profiles of shear stress and cyclic strain as well as by circulating factors such as myokines released from contracting skeletal muscle. Exercise training may also restore/maintain normal phenotype of adipocytes resulting in a healthier adipokine release, thereby signaling expression of healthy endothelial cell genes. In addition, exercise training may exert a systemic effect on endothelial phenotype and function in part through sensitizing the endothelium to the beneficial effects of insulin.
Acknowledgments
J. Padilla is supported by American Heart Association Grant 11POST5080002; G. H. Simmons is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant T32-AR-048523; M. H. Laughlin is supported by National Heart, Lung, and Blood Institute Grants P01-HL-052490 and HL-36088.
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol. 1984;246:H59–H68. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- 4.Arvola P, Wu X, Kahonen M, Makynen H, Riutta A, Mucha I, Solakivi T, Kainulainen H, Porsti I. Exercise enhances vasorelaxation in experimental obesity associated hypertension. Cardiovasc Res. 1999;43:992–1002. doi: 10.1016/s0008-6363(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 5.Ashkar E. Effects of bilateral splanchnicectomy on circulation during exercise in dogs. Acta Physiol Latinoam. 1973;23:171–177. [PubMed] [Google Scholar]
- 6.Awolesci MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awolesci MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439–444. [PubMed] [Google Scholar]
- 8.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 9.Berghoff M, Kathpal M, Kilo MJ, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–788. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- 10.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008;586:3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair SN, Morris JN. Healthy hearts–and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Boutsiouki P, Clough GF. Modulation of microvascular function following low-dose exposure to the organophosphorous compound malathion in human skin in vivo. J Appl Physiol. 2004;97:1091–1097. doi: 10.1152/japplphysiol.00123.2004. [DOI] [PubMed] [Google Scholar]
- 13.Brooks GA, Fahey TD, Baldwin KM. Human Bioenergetics and Its Applications. New York: McGrawn-Hill; 2005. Exercise Physiology. [Google Scholar]
- 14.Bunker A, Laughlin MH. Influence of exercise and perivascular adipose tissue on coronary artery vasomotor function in a familial hypercholesterolemic porcine atherosclerosis model. J Appl Physiol. 2010;108:490–497. doi: 10.1152/japplphysiol.00999.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield AB, Miller CW, Lumb WV, Mcleod FD, Nelson AW, Histand MB. Inverse effect of chronically elevated blood flow on atherogenesis in miniature swine. Atherosclerosis. 1977;26:215–224. doi: 10.1016/0021-9150(77)90104-6. [DOI] [PubMed] [Google Scholar]
- 16.Canty JM, Schwartz JS. Nitric oxide mediates flow-dependent epicardial coronary vasodilation to changes in pulse frequency but not mean flow in conscious dogs. Circulation. 1994;89:375–384. doi: 10.1161/01.cir.89.1.375. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 19.Chen SJ, Wu CC, Yen MH. Exercise training activates large-conductance calcium-activated K+ channels and enhances nitric oxide production in rat mesenteric artery and thoracic aorta. J Biomed Sci. 2001;8:248–255. doi: 10.1007/BF02256598. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Collins HL, DiCarlo SE. Daily exercise enhances acetylcholine-induced dilation in mesenteric and hindlimb vasculature of hypertensive rats. Clin Exp Hypertens. 1999;21:353–376. doi: 10.3109/10641969909068670. [DOI] [PubMed] [Google Scholar]
- 21.Cheng C, Crom R, Haperen R, Helderman F, Gourabi BM, Damme LCA, Kirschbaum SW, Slager CJ, Steen AFW, Krams R. The role of shear stress in atherosclerosis. Cell Biochem Biophys. 2004;41:279–294. doi: 10.1385/cbb:41:2:279. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 23.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31:125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension. 1996;28:386–391. doi: 10.1161/01.hyp.28.3.386. [DOI] [PubMed] [Google Scholar]
- 25.Chies AB, de Oliveira AM, Pereira FC, de Andrade CR, Correa FM. Phenylephrine-induced vasoconstriction of the rat superior mesenteric artery is decreased after repeated swimming. J Smooth Muscle Res. 2004;40:249–258. doi: 10.1540/jsmr.40.249. [DOI] [PubMed] [Google Scholar]
- 26.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Ann Med. 2009;41:19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 27.Chu Y, Heistad DD. NO answer to Alzheimer’s Disease? Circ Res. 2010;107:1400–1402. doi: 10.1161/CIRCRESAHA.110.234450. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 29.Claudino MA, Delbin MA, Franco-Penteado CF, Priviero FB, De Nucci G, Antunes E, Zanesco A. Exercise training ameliorates the impairment of endothelial and nitrergic corpus cavernosum responses in diabetic rats. Life Sci. 2011;88:272–277. doi: 10.1016/j.lfs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Clausen J, Klausen K, Rasmussen B, Trap-Jensen J. Central and peripheral circulatory changes after training of the arms or legs. Am J Physiol. 1973;225:675–682. doi: 10.1152/ajplegacy.1973.225.3.675. [DOI] [PubMed] [Google Scholar]
- 31.Coker RH, Krishna MG, Lacy DB, Allen EJ, Wasserman DH. Sympathetic drive to liver and nonhepatic splanchnic tissue during heavy exercise. J Appl Physiol. 1997;82:1244–1249. doi: 10.1152/jappl.1997.82.4.1244. [DOI] [PubMed] [Google Scholar]
- 32.Colberg SR, Stansberry KB, McNitt PM, Vinik AI. Chronic exercise is associated with enhanced cutaneous blood flow in Type 2 diabetes. J Diabetes Complications. 2002;16:139–145. doi: 10.1016/s1056-8727(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 33.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fit-ness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 34.Colombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 35.Company JM, Booth FW, Laughlin MH, Arce-Esquivel AA, Sacks HS, Bahouth SW, Fain JN. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J Appl Physiol. 2010;109:1904–1912. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol. 1955;128:258–267. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cracowski J, McCord GR, Minson CT. How to investigate skin endothelial dysfunction in diabetes. J Diabetes Complications. 2006;20:133–135. doi: 10.1016/j.jdiacomp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Cracowski J, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Crandall CG, Shibasaki M, Wilson TE, Cui J, Levine BD. Prolonged head-down tilt exposure reduces maximal cutaneous vasodilator and sweating capacity in humans. J Appl Physiol. 2003;94:2330–2336. doi: 10.1152/japplphysiol.00790.2002. [DOI] [PubMed] [Google Scholar]
- 40.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature Clin Practice Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis RTI, McCullough D, Dominguez JMI, Behnke BJ. Effects of aging and exercise training on alpha-adrenergic vasomotor responses of rat adipose tissue arterioles. Med Sci Sports Exerc. 2010;42 [Google Scholar]
- 42.de Moraes C, Davel A, Rossoni L, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol. 2008;8:12. doi: 10.1186/1472-6793-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Moraes R, Gioseffi G, Nobrega AC, Tibirica E. Effects of exercise training on the vascular reactivity of the whole kidney circulation in rabbits. J Appl Physiol. 2004;97:683–688. doi: 10.1152/japplphysiol.00923.2003. [DOI] [PubMed] [Google Scholar]
- 44.Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol. 2007;293:H3159–H3164. doi: 10.1152/ajpheart.00591.2007. [DOI] [PubMed] [Google Scholar]
- 45.Demopoulos MDL, Bijou MDR, Fergus MDI, Jones RNM, Strom MDFJ, LeJemtel MDTH. Exercise training in patients with severe congestive heart failure: enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol. 1997;29:597–603. doi: 10.1016/s0735-1097(96)00526-8. [DOI] [PubMed] [Google Scholar]
- 46.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 47.DiCarlo SE, Bishop VS. Regional vascular resistance during exercise: role of cardiac afferents and exercise training. Am J Physiol Heart Circ Physiol. 1990;258:H842–H847. doi: 10.1152/ajpheart.1990.258.3.H842. [DOI] [PubMed] [Google Scholar]
- 48.DiCarlo SE, Stahl LK, Bishop VS. Daily exercise attenuates the sympathetic nerve response to exercise by enhancing cardiac afferents. Am J Physiol Heart Circ Physiol. 1997;273:H1606–H1610. doi: 10.1152/ajpheart.1997.273.3.H1606. [DOI] [PubMed] [Google Scholar]
- 49.Dominguez JM, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–819. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durand S, Tartas M, Bouye P, Koitka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004;561:811–819. doi: 10.1113/jphysiol.2004.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dziekan G, Myers J, Goebbels U, Muller P, Reinhart W, Ratti R, Hafeli W, Dubach P. Effects of exericse training on limb blood flow in patients with reduced ventricular function. Am Heart J. 1998;136:22–30. doi: 10.1016/s0002-8703(98)70177-2. [DOI] [PubMed] [Google Scholar]
- 54.Ellis LB, Weiss S. A study of the cardiovascular responses in man to the intravenous and intra-arterial injection of acetylcholine. J Pharmacol Exp Ther. 1932:235–252. [Google Scholar]
- 55.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 56.Evarts EV, Thach WT. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–498. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- 57.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-B1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman DB, Friberg L, Mitchell JH, Secher NH. Effect of axillary blockade on regional cerebral blood flow during static handgrip. J Appl Physiol. 1991;71:651–656. doi: 10.1152/jappl.1991.71.2.651. [DOI] [PubMed] [Google Scholar]
- 59.Geraldes P, Yagi K, Ohshiro Y, He Z, Maeno Y, Yamamoto-Hiraoka J, Rask-Madsen C, Chung SW, Perrella MA, King GL. Selective regulation of Heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem. 2008;283:34327–34336. doi: 10.1074/jbc.M807036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 61.Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, O’Malley C, Keaney JF, Balady GJ. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- 62.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans. Role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 63.Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O’Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;283:H899–H907. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- 64.Green D, Cheetham C, Reed C, Dembo L, O’Driscoll G. Assessment of brachial artery blood flow across the cariac cycle: retrograde flows during cycle ergometry. J Appl Physiol. 2002;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- 65.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 66.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O’Driscoll JG, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588:1571–1577. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green DJ, Fowler DT, O’Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol. 1996;81:943–948. doi: 10.1152/jappl.1996.81.2.943. [DOI] [PubMed] [Google Scholar]
- 69.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green DJ, Maiorana AJ, Cable NT. Point: exercise training does induce vascular adaptations beyond the active muscle beds. J Appl Physiol. 2008;105:1002–1004. doi: 10.1152/japplphysiol.90570.2008. [DOI] [PubMed] [Google Scholar]
- 71.Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008;105:766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green DJ, Spence A, Halliwill JR, Cable NT, Thijssen DHJ. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96:57–70. doi: 10.1113/expphysiol.2009.048694. [DOI] [PubMed] [Google Scholar]
- 73.Grimby G. Renal clearances during prolonged supine exercise at different loads. J Appl Physiol. 1965;20:1294–1298. doi: 10.1152/jappl.1965.20.1.137. [DOI] [PubMed] [Google Scholar]
- 74.Hagg U, Andersson I, Naylor AS, Gronros J, Jonsdottir IH, Bergstrom G, Gan LM. Voluntary physical exercise-induced vascular effects in spontaneously hypertensive rats. Clin Sci (Lond) 2004;107:571–581. doi: 10.1042/CS20040171. [DOI] [PubMed] [Google Scholar]
- 75.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haidet GC, Parsons D. Reduced exercise capacity in senescent beagles: an evaluation of the periphery. Am J Physiol Heart Circ Physiol. 1991;260:H173–H182. doi: 10.1152/ajpheart.1991.260.1.H173. [DOI] [PubMed] [Google Scholar]
- 77.Hales JR, Ludbrook J. Baroreflex participation in redistribution of cardiac output at onset of exercise. J Appl Physiol. 1988;64:627–634. doi: 10.1152/jappl.1988.64.2.627. [DOI] [PubMed] [Google Scholar]
- 78.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 79.Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity. 2008;16:578–584. doi: 10.1038/oby.2007.87. [DOI] [PubMed] [Google Scholar]
- 80.Harrison DG, Sayegh H, Ohara Y, Inoue N, Venema RC. Regulation of expression of the endothelial cell nitric oxide synthase. Clin Exp Pharmacol Physiol. 1996;23:251–255. doi: 10.1111/j.1440-1681.1996.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 81.Hartwich D, Fowler KL, Wynn LJ, Fisher JP. Differential responses to sympathetic stimulation in the cerebral and brachial circulations during rhythmic handgrip exercise in humans. Exp Physiol. 2010;95:1089–1097. doi: 10.1113/expphysiol.2010.054387. [DOI] [PubMed] [Google Scholar]
- 82.Heckmann JG, Brown CM, Cheregi M, Hilz MJ, Neundorfer B. Delayed cerebrovascular autoregulatory response to ergometer exercise in normotensive elderly humans. Cerebrovasc Dis. 2003;16:423–429. doi: 10.1159/000072567. [DOI] [PubMed] [Google Scholar]
- 83.Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- 84.Herholz K, Buskies W, Rist M, Pawlik G, Hollmann W, Heiss WD. Regional cerebral blood flow in man at rest and during exercise. J Neurol. 1987;234:9–13. doi: 10.1007/BF00314001. [DOI] [PubMed] [Google Scholar]
- 85.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 86.Hohimer AR, Hales JR, Rowell LB, Smith OA. Regional distribution of blood flow during mild dynamic leg exercise in the baboon. J Appl Physiol. 1983;55:1173–1177. doi: 10.1152/jappl.1983.55.4.1173. [DOI] [PubMed] [Google Scholar]
- 87.Hohimer AR, Smith OA. Decreased renal blood flow in the baboon during mild dynamic leg exercise. Am J Physiol Heart Circ Physiol. 1979;236:H141–H150. doi: 10.1152/ajpheart.1979.236.1.H141. [DOI] [PubMed] [Google Scholar]
- 88.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogensis of dementia. Acta Neuropathol (Berl) 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol. 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- 91.Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148:3758–3764. doi: 10.1210/en.2006-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jansakul C, Hirunpan P. Effects of exercise training on responsiveness of the mesenteric arterial bed to phenylephrine and KCl in male rats. Br J Pharmacol. 1999;127:1559–1566. doi: 10.1038/sj.bjp.0702697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc. 2006;38:445–454. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jedrziewski MK, Ewbank DC, Wang H, Trojanowski JQ. Exercise and cognition: results from the National Long Term Care Survey. Alzheimer’s & Dementia. 2010;6:448–455. doi: 10.1016/j.jalz.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson JM, Proppe DW. The Handbook of Physiology. Environmental Physiology. Vol. 1. Bethesda, MD: Am. Physiol. Soc; 1996. Cardiovascular adjustments to heat stress; pp. 215–243. sect. 4, chapt. 11. [Google Scholar]
- 97.Johnson JM, Rowel LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol. 1975;39:920–924. doi: 10.1152/jappl.1975.39.6.920. [DOI] [PubMed] [Google Scholar]
- 98.Jorgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol. 1992;73:1825–1830. doi: 10.1152/jappl.1992.73.5.1825. [DOI] [PubMed] [Google Scholar]
- 99.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol. 1992;72:1123–1132. doi: 10.1152/jappl.1992.72.3.1123. [DOI] [PubMed] [Google Scholar]
- 100.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kadoglou NPE, Iliadis F, Liapis CD. Exercise and carotid atherosclerosis. Eur J Vasc Endovasc Surg. 2008;35:264–272. doi: 10.1016/j.ejvs.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 102.Katz SD, Yuen J, Bijou R, Lejemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. 1997;82:1488–1492. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]
- 103.Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- 104.Klonizakis M, Tew G, Michaels J, Saxton J. Exercise training improves cutaneous microvascular endothelial function in post-surgical varicose vein patients. Microvasc Res. 2009;78:67–70. doi: 10.1016/j.mvr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Kobayaskhi N, Tsuruya Y, Iwasawa T, Ikeda N, Hasimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, Saito M. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J. 2003;67:505–510. doi: 10.1253/circj.67.505. [DOI] [PubMed] [Google Scholar]
- 106.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fit-ness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 107.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 108.Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol. 1998;79:30–36. doi: 10.1007/s004210050469. [DOI] [PubMed] [Google Scholar]
- 109.Lash JM. Exercise training enhances adrenergic constriction and dilation in the rat spinotrapezius muscle. J Appl Physiol. 1998;85:168–174. doi: 10.1152/jappl.1998.85.1.168. [DOI] [PubMed] [Google Scholar]
- 110.Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol. 1992;72:2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- 111.Lash JM, Bohlen HG. Time- and order-dependent changes in functional and NO-mediated dilation during exercise training. J Appl Physiol. 1997;82:460–468. doi: 10.1152/jappl.1997.82.2.460. [DOI] [PubMed] [Google Scholar]
- 112.Lash JM, Reilly T, Thomas M, Bohlen HG. Adrenergic and pressure-dependent vascular regulation in sedentary and trained rats. Am J Physiol Heart Circ Physiol. 1993;265:H1064–H1073. doi: 10.1152/ajpheart.1993.265.4.H1064. [DOI] [PubMed] [Google Scholar]
- 113.Lassen NA. Control of cerebral circulation in health and disease. Circ Res. 1974;34:749–760. doi: 10.1161/01.res.34.6.749. [DOI] [PubMed] [Google Scholar]
- 114.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–1144. [PubMed] [Google Scholar]
- 115.Laughlin B, Joseph MH. Wolfe Memorial Lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc. 2004;36:352–362. doi: 10.1249/01.mss.0000117114.02875.5c. [DOI] [PubMed] [Google Scholar]
- 116.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588– 600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96:233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 118.Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Artheriosclerosis Thrombosis Vasc Biol. 2000;20:551–555. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- 119.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 120.Lee IM, Hennekens CH, Berger K, Buring JE, Manson JE. Exercise and risk of stroke in male physicians. Stroke. 1999;30:1–6. doi: 10.1161/01.str.30.1.1. [DOI] [PubMed] [Google Scholar]
- 121.Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation: alternations and adaptations in coronary artery disease. Prog Cardiovasc Dis. 2006;48:270–284. doi: 10.1016/j.pcad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 122.Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. N Engl J Med. 2001;315:1046–1051. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 123.Luscher TF, Vanhoutte PM. The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL: CRC Press; 1990. pp. 1–162. [Google Scholar]
- 124.Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic exercise and resistance exercise training on vascular function in Type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 125.Maiorana A, O’Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green DJ. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H1999–H2005. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- 126.Maiorana A, O’Driscoll G, Dembo L, Goodman C, Taylor R, Green D. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sport Exerc. 2001;33:2022–2028. doi: 10.1097/00005768-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 127.Maiorana A, O’Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- 128.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 129.Martin WH, Kohrt WM, Malley MT, Korte E, Stoltz S. Exercise training enhances leg vasodilatory capacity of 65-yr-old men and women. J Appl Physiol. 1990;69:1804–1809. doi: 10.1152/jappl.1990.69.5.1804. [DOI] [PubMed] [Google Scholar]
- 130.Mayhan WG, Arrick DM, Patel KP, Sun H. Exercise training normalizes impaired NOS-dependent responses of cerebral arterioles in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300:H1013–H1020. doi: 10.1152/ajpheart.00873.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mayhan WG, Arrick DM, Sun H, Patel KP. Exercise training restores impaired dilator responses of cerebral arterioles during chronic exposure to nicotine. J Appl Physiol. 2010;109:1109–1114. doi: 10.1152/japplphysiol.00564.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol. 2004;96:1730–1737. doi: 10.1152/japplphysiol.01185.2003. [DOI] [PubMed] [Google Scholar]
- 133.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol. 2005;98:753–761. doi: 10.1152/japplphysiol.01263.2003. [DOI] [PubMed] [Google Scholar]
- 134.McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training on responses of peripheral and visceral arteries in swine. J Appl Physiol. 1996;80:216–225. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- 135.McGowan CL, Visocchi A, Faulkner M, Verduyn R, Rakobowchuk M, Levy AS, McCartney N, Mac-Donald MJ. Isometric handgrip training improves local flow-mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2007;99:227–234. doi: 10.1007/s00421-006-0337-z. [DOI] [PubMed] [Google Scholar]
- 136.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48:1865–1870. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 137.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol. 2010;109:1203–1210. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller VM, Burnett JC. Modulation of NO and endothelin by chronic increases in blood flow in canine femoral arteries. Am J Physiol Heart Circ Physiol. 1992;263:H103–H108. doi: 10.1152/ajpheart.1992.263.1.H103. [DOI] [PubMed] [Google Scholar]
- 139.Miller VM, Vanhoutte PM. Enhanced release of endothelium-derived relaxing factor(s) by chronic increases in blood flow. Am J Physiol Heart Circ Physiol. 1988;255:H446–H451. doi: 10.1152/ajpheart.1988.255.3.H446. [DOI] [PubMed] [Google Scholar]
- 140.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects on one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- 141.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moraine JJ, Lamotte M, Berre J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol. 1993;67:35–38. doi: 10.1007/BF00377701. [DOI] [PubMed] [Google Scholar]
- 143.Motohiro M, Yuasa F, Hattori T, Sumimoto T, Takeuchi M, Kaida M, Jikuhara T, Hikosaka M, Sugiura T, Iwasaka T. Cardiovascular adaptations to exercise training after uncomplicated acute myocardial infarction. Am J Phys Med Rehabil. 2005;84:684–691. doi: 10.1097/01.phm.0000171167.31010.f4. [DOI] [PubMed] [Google Scholar]
- 144.Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113–123. doi: 10.1111/j.0001-6772.2003.01253.x. [DOI] [PubMed] [Google Scholar]
- 145.Mueller PJ, O’Hagan KP, Skogg KA, Buckwalter JB, Clifford PS. Renal hemodynamic responses to dynamic exercise in rabbits. J Appl Physiol. 1998;85:1605–1614. doi: 10.1152/jappl.1998.85.5.1605. [DOI] [PubMed] [Google Scholar]
- 146.Musch TI, Poole DC. Blood flow response to treadmill running in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 1996;271:H2730–H2734. doi: 10.1152/ajpheart.1996.271.6.H2730. [DOI] [PubMed] [Google Scholar]
- 147.Musch TI, Terrell JA, Hilty MR. Effects of high-intensity sprint training on skeletal muscle blood flow in rats. J Appl Physiol. 1991;71:1387–1395. doi: 10.1152/jappl.1991.71.4.1387. [DOI] [PubMed] [Google Scholar]
- 148.Naylor LH, Carter H, Fitzsimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol. 2011;300:H664–H669. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 149.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systemic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 150.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol. 2009;107:1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 151.Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans O, Secher NH, Raven PB. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1526–H1531. doi: 10.1152/ajpheart.00979.2004. [DOI] [PubMed] [Google Scholar]
- 152.Ooue A, Ichinose T, Inoue Y, Nishiyasu T, Koga S, Kondo N. Changes in blood flow in conduit artery and veins of the upper arm during leg exercise in humans. Eur J Appl Physiol. 2008;103:367–373. doi: 10.1007/s00421-008-0706-x. [DOI] [PubMed] [Google Scholar]
- 153.Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vasc Med. 2008;13:105–111. doi: 10.1177/1358863x07086671. [DOI] [PubMed] [Google Scholar]
- 154.Padilla J, Newcomer SC, Simmons GH, Kreutzer KV, Laughlin MH. Long-term exercise training does not alter brachial and femoral artery vasomotor function and endothelial phenotype in healthy pigs. Am J Physiol Heart Circ Physiol. 2010;299:H379–H385. doi: 10.1152/ajpheart.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paffenbarger RSJ, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 156.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104:655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- 157.Pedersen BK. The diseasome of physical inactivity: and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 159.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M. The metabolic role of IL-6 produced during exercise: is IL-6 and exercise factor? Proc Nutr Soc. 2004;63:263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 160.Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol. 1998;513:907–913. doi: 10.1111/j.1469-7793.1998.907ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pierce GL, Schofield RS, Casey DP, Hamlin SA, Hill JA, Braith RW. Effects of exercise training on forearm and calf vasodilation and proinflammatory markers in recent heart transplant patients: a pilot study. Eur J Cardiovasc Prev Rehabil. 2008;15:10–18. doi: 10.1097/HJR.0b013e3282f0b63b. [DOI] [PubMed] [Google Scholar]
- 162.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 163.Proctor DN, Miller JD, Dietz NM, Minson CT, Joyner MJ. Reduced submaximal leg blood flow after high-intensity aerobic training. J Appl Physiol. 2001;91:2619–2627. doi: 10.1152/jappl.2001.91.6.2619. [DOI] [PubMed] [Google Scholar]
- 164.Pullin CH, Bellamy MF, Bailey D, Ashton M, Davies B, Williams S, Goodfellow J, Wilson JF, Lewis MJ. Time course of changes in endothelial function following exercise in habitually sedentary men. J Exerc Physiol. 2004;7:14–22. [Google Scholar]
- 165.Puvi-Rajasingham S, Smith GD, Akinola A, Mathias CJ. Hypotensive and regional haemodynamic effects of exercise, fasted and after food, in human sympathetic denervation. Clin Sci (Lond) 1998;94:49–55. doi: 10.1042/cs0940049. [DOI] [PubMed] [Google Scholar]
- 166.Pynn M, Schafer K, Konstantinides S, Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation. 2004;109:386–392. doi: 10.1161/01.CIR.0000109500.03050.7C. [DOI] [PubMed] [Google Scholar]
- 167.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 168.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metabolism. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH. Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc. 2007;39:2125–2134. doi: 10.1249/mss.0b013e318156e9df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ribeiro F, Alves AJ, Duarte JA, Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol. 2010;141:214–221. doi: 10.1016/j.ijcard.2009.09.548. [DOI] [PubMed] [Google Scholar]
- 171.Ridger V, Krams R, Carpi A, Evans PC. Hemodynamic parameters regulating vascular inflammation and atherosclerosis: a brief update. Biomed Pharmacother. 2008;62:536–540. doi: 10.1016/j.biopha.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 172.Roche DM, Rowland TW, Garrard M, Marwood S, Unnithan VB. Skin microvascular reactivity in trained adolescents. Eur J Appl Physiol. 2010;108:1201–1208. doi: 10.1007/s00421-009-1328-7. [DOI] [PubMed] [Google Scholar]
- 173.Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest. 1964;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 175.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 176.Sadowski J, Gellert R, Kurkus J, Portalaska E. Denervated and intact kidney responses to exercise in the dog. J Appl Physiol. 1981;51:1618–1624. doi: 10.1152/jappl.1981.51.6.1618. [DOI] [PubMed] [Google Scholar]
- 177.Seals DR. Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev. 2003;31:68–72. doi: 10.1097/00003677-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 178.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 179.Shephard RJ. Cytokine responses to physical activity, with particular reference to IL-6: sources, actions, and clinical implications. Crit Rev Immunol. 2002;22:165–182. [PubMed] [Google Scholar]
- 180.Siasos G, Tousoulis D, Siasou Z, Stefanadis C, Papavassiliou AG. Shear stress, protein kinases and atherosclerosis. Curr Med Chem. 2007;14:1567–1572. doi: 10.2174/092986707780831087. [DOI] [PubMed] [Google Scholar]
- 181.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol. 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 183.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 184.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stepp DW. Origins of vascular disease: is fat where it’s at? J Appl Physiol. 2010;108:475–476. doi: 10.1152/japplphysiol.01434.2009. [DOI] [PubMed] [Google Scholar]
- 186.Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- 187.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeZouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 188.Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sport Exerc. 2006;38:81–85. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- 189.Tew G, Klonizakis M, Saxton J. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. Eur J Appl Physiol. 2010;109:173–181. doi: 10.1007/s00421-009-1342-9. [DOI] [PubMed] [Google Scholar]
- 190.Thijssen DH, Ellenkamp R, Smits P, Hopman MT. Rapid vascular adaptations to training and detraining in persons with spinal cord injury. Arch Phys Med Rehabil. 2006;87:474–481. doi: 10.1016/j.apmr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 191.Thijssen DH, Heesterbeek P, van Kuppevelt DJ, Duysens J, Hopman MT. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc. 2005;37:1112–1118. doi: 10.1249/01.mss.0000170126.30868.fb. [DOI] [PubMed] [Google Scholar]
- 192.Thijssen DH, Hopman MT. Counterpoint: exercise training does not induce vascular adaptations beyond the active muscle beds. J Appl Physiol. 2008;105:1004–1006. doi: 10.1152/japplphysiol.90570.2008a. [DOI] [PubMed] [Google Scholar]
- 193.Thijssen DH, Steendijk S, Hopman MT. Blood redistribution during exercise in subjects with spinal cord injury and controls. Med Sci Sports Exerc. 2009;41:1249–1254. doi: 10.1249/MSS.0b013e318196c902. [DOI] [PubMed] [Google Scholar]
- 194.Thijssen DHJ, Dawson EA, Black MA, Hopman MTE, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc. 2009;41:1072–1079. doi: 10.1249/MSS.0b013e3181923957. [DOI] [PubMed] [Google Scholar]
- 195.Thijssen DHJ, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MTE, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108:845–875. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003–5012. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 199.Toda M, Yamamoto K, Shimizu N, Syotaro O, Kumagaya S, Igarashi T, Kamiya A, Ando J. Differential gene responses in endothelial cells exposed to a combination of shear stress and cyclic stretch. J Biotechnol. 2008;133:239–244. doi: 10.1016/j.jbiotec.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 200.Volianitis S, Yoshiga CC, Nissen P, Secher NH. Effect of fitness on arm vascular and metabolic resonses to upper body exercise. Am J Physiol Heart Circ Physiol. 2004;286:H1736. doi: 10.1152/ajpheart.01001.2003. [DOI] [PubMed] [Google Scholar]
- 201.Volkmann ER, Grossman JM, Sahakian LJ, Skaggs BJ, Fitzgerald J, Ragavendra N, Charles-Schoeman C, Chen W, Gorn A, Karpouzas G, Weisman M, Wallace DJ, Hahn BH, McMahon M. Low physical activity is associated with proinflammatory high-density lipoprotein and increased subclinical atherosclerosis in women with systemic lupus erythematosus. Arthritis Care Res. 2010;62:258–265. doi: 10.1002/acr.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Walsh JH, Best M, Maiorana AJ, Taylor RR, O’Driscoll GJ, Green DJ. Exercise improves conduit vessel endothelial function in CAD patients. J Appl Physiol. 2003;285:20–25. doi: 10.1152/japplphysiol.00012.2003. [DOI] [PubMed] [Google Scholar]
- 203.Walsh JH, Yong G, Cheetham C, Watts GF, O’Driscoll GJ, Taylor RR, Green DJ. Effect of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J. 2003;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. [DOI] [PubMed] [Google Scholar]
- 204.Wang J. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. Eur J Appl Physiol. 2005;93:429–434. doi: 10.1007/s00421-004-1176-4. [DOI] [PubMed] [Google Scholar]
- 205.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O’Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 206.Watts K, Beye P, Siafarikas A, O’Driscoll G, Jones TW, Davis EA, Green DJ. Effects of exercise training on vascular function in obese children. J Pediatr. 2004;144:620–625. doi: 10.1016/j.jpeds.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 207.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RSJ, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese man. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 208.Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 209.Woodman CR, Trott DW, Laughlin MH. Short-term increases in intraluminal pressure reverse age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries. J Appl Physiol. 2007;103:1172–1179. doi: 10.1152/japplphysiol.00416.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 211.Wung BS, Cheng JJ, Shyue SK, Wang DL. NO modulates monocyte chemotactic protein-1 expression in endothleial cells under cyclic strain. Arterioscler Thromb Vasc Biol. 2001;21:1941–1947. doi: 10.1161/hq1201.099428. [DOI] [PubMed] [Google Scholar]
- 212.Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R987–R991. doi: 10.1152/ajpregu.00702.2004. [DOI] [PubMed] [Google Scholar]
- 213.Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol. 1993;75:1334–1340. doi: 10.1152/jappl.1993.75.3.1334. [DOI] [PubMed] [Google Scholar]
- 214.Yen MH, Yang JH, Sheu JR, Lee YM, Ding YA. Chronic exercise enhances endothelium-mediated dilation in spontaneously hypertensive rats. Life Sci. 1995;57:2205–2213. doi: 10.1016/0024-3205(95)02127-5. [DOI] [PubMed] [Google Scholar]
- 215.Yun JK, Anderson JM, Ziats NP. Cyclic-strain-induced endothelial cell expression of adhesion molecules and their roles in monocyte-endothelial interaction. J Biomed Mater Res. 1999;44:87–97. doi: 10.1002/(sici)1097-4636(199901)44:1<87::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 216.Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–355. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]