Abstract
AIM: To verify the usefulness of FibroQ for predicting fibrosis in patients with chronic hepatitis C, compared with other noninvasive tests.
METHODS: This retrospective cohort study included 237 consecutive patients with chronic hepatitis C who had undergone percutaneous liver biopsy before treatment. FibroQ, aspartate aminotransferase (AST)/alanine aminotransferase ratio (AAR), AST to platelet ratio index, cirrhosis discriminant score, age-platelet index (API), Pohl score, FIB-4 index, and Lok’s model were calculated and compared.
RESULTS: FibroQ, FIB-4, AAR, API and Lok’s model results increased significantly as fibrosis advanced (analysis of variance test: P < 0.001). FibroQ trended to be superior in predicting significant fibrosis score in chronic hepatitis C compared with other noninvasive tests.
CONCLUSION: FibroQ is a simple and useful test for predicting significant fibrosis in patients with chronic hepatitis C.
Keywords: Liver fibrosis, Noninvasive test, FibroQ, Aspartate aminotransferase, Alanine aminotransferase, FIB-4 index, Aspartate aminotransferase to platelet ratio index, Lok’s model, Cirrhosis discriminant score, Pohl score
INTRODUCTION
Viral hepatitis C is one of the most common liver diseases in the world, affecting an estimated 200 million individuals[1], with a particularly high prevalence in Southern Taiwan[2]. Approximately 60%-80% of infected individuals develop chronic hepatitis[2], and patients with higher degrees of fibrosis may progress rapidly to cirrhosis and hepatocellular carcinoma. Approximately 20% of patients with chronic hepatitis C advance to cirrhosis, and 5% of them develop hepatocellular carcinoma[3,4].
Knowledge of the extent of liver fibrosis is important for the clinical management of chronic hepatitis C. Patients with no fibrosis or with only portal fibrosis at the time of diagnosis have more favorable outcomes and a lower chance of reaching end-stage liver disease than patients with severe fibrosis or cirrhosis[5-8]. The probability of developing cirrhosis and/or other unfavorable outcomes is closely related to fibrosis stage, therefore, liver biopsy is recommended prior to antiviral treatment. However, liver biopsy adds expense, requires an experienced clinician, and may cause complications, including mortality in 0.018% of patients[9]. In addition, sampling errors and inter- and intraobserver variations may lead to understaging of cirrhosis, particularly macronodular cirrhosis[10-13]. Hence, several noninvasive tests have been proposed to assess the severity of hepatic fibrosis as an alternative to liver biopsy. As reported by Akkaya et al[14], alanine aminotransferase (ALT) levels in patients with hepatitis C virus (HCV) infection correlate with periportal bridging/necrosis, and Lu et al[15] have reported that thrombocytopenia is a surrogate for cirrhosis. Furthermore, aspartate aminotransferase (AST)-to-platelet ratio index (APRI)[16] and AST/ALT ratio (AAR)[17], cirrhosis discriminant score (CDS)[18], age-platelet index (API)[19], Pohl score[20], FIB-4 index[21], and Lok’s model[22] are well-known parameters that are based on routine laboratory data, and are therefore readily available in clinical practice (Table 1). These parameters have been reported to predict the presence of significant fibrosis and extensive fibrosis in some patients[14-23].
Table 1.
Fibrosis tests composed of laboratory parameters
| Fibrosis test | Calculation |
| AAR | AST/ALT |
| APRI | [(AST/ULN)/platelet count (109/L)] × 100 |
| FibroQ | (10 × age × AST × PT INR)/(PLT × ALT) |
| CDS | Platelet count (109/L): > 340 = 0; 280-339 = 1; 220-279 = 2; 160-219 = 3; 100-159 = 4; 40-99 = 5; < 40 = 6 |
| ALT/AST ratio: > 1.7 = 0; 1.2-1.7 = 1; 0.6-1.19 = 2; < 0.6 = 3 | |
| API | INR: < 1.1 = 0; 1.1-1.4 = 1; > 1.4 = 2 CDS is the sum of the above (possible value, 0-11) |
| Age (yr): < 30 = 0; 30-39 = 1; 40-49 = 2; 50-59= 3; 60-69 = 4; > 70 = 5 | |
| Platelet count (109/L): ≥ 225 = 0; 200-224 = 1; 175-199 = 2; 150-174 = 3; 125-149 = 4; ≤ 125 = 5 | |
| API is the sum of the above (possible value, 0-10) | |
| Pohl score | Positive: AAR ≥ 1 and platelet count < 150 × 109/L |
| FIB-4 index | [Age (yr) × AST (U/L)]/[platelet count (109/L) × ALT (U/L) 1/2] |
| Lok’s model | Log odds (predicting cirrhosis) = -5.56 to 0.0089 × platelet (× 103/mm3) + 1.26 × AST/ALT ratio + 5.27 × PT INR Predicted probability = exp (log odds)/[1 + exp (log odds)] |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; API: Age-platelet index; CDS: Cirrhosis discriminant score; ULN: Upper limit of normal; PLT: Posterolateral thoracotomy; PT-INR: Prothrombin time international normalized ratio.
Recently, we proposed a novel index, FibroQ[23], which is calculated from common laboratory test results that include prothrombin time international normalized ratio (PT INR), platelet count, AST, ALT, and age, as 10 × (age × AST × PT INR)/(ALT × platelet count) to predict significant fibrosis. In a previous study, we enrolled 140 patients with hepatitis B virus (HBV) and HCV infection. To focus on HCV, 113 of these patients were included in the 237 patients in the present study. The aims of the present study were to assess the value of the FibroQ index and to determine its threshold values to differentiate significant fibrosis. We also compared the discriminatory performance of FibroQ to that of AAR, APRI, CDS, API, Pohl score, FIB-4 index, and Lok’s model.
MATERIALS AND METHODS
We retrospectively studied 250 consecutive treatment-naïve patients with chronic HCV infection that was confirmed by the presence of anti-HCV antibody by enzyme immunoassay methods (Abbott Architect I 2000; Abbott, Champaign, IL, United States) as recorded in the departmental files of the Department of Gastroenterology, Chang Gung Memorial Hospital, Chiayi, between May 2005 and December 2008. All patients were Taiwanese. The research study was approved by the Clinical Research Sub-committee of the hospital. Patients with the following conditions were excluded from the study: those co-infected with human immunodeficiency virus or HBV, and those with alcohol consumption in excess of 20 g/d, hepatocellular carcinoma, liver transplantation, antiviral or immunosuppressive therapy, metabolic liver disease, insufficient liver tissue for fibrosis staging, or recent warfarin or other anticoagulant usage. Thirteen patients were excluded due to incomplete data on liver function tests or platelet count within 1 mo before the date of biopsy. The clinical data were reviewed, and the following parameters were recorded: sex, age, AST, ALT, platelet count, PT INR, hemoglobin, white blood cell count, serum creatinine, free thyroxine, thyroid-stimulating hormone, and bilirubin. Liver biopsies were performed by hepatologists and were interpreted by a single pathologist using the Metavir fibrosis score[10]. Significant liver fibrosis and extensive liver fibrosis were defined as Metavir fibrosis scores of ≥ 2 (F2, F3 and F4) and ≥ 3 (F3 and F4), respectively.
Statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, United States). Patient characteristics were represented as the mean ± SD. Bivariate Spearman’s rank correlation coefficient (rs) was calculated to measure the relationship between the clinical variables and degree of fibrosis. Receiver operating characteristic (ROC) curves were constructed for each test. To evaluate the diagnostic accuracies of the simple fibrosis prediction tests, their sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the ROC curve (AUC) were calculated. In tests of significance, two-sided P < 0.05 was considered significant.
RESULTS
Patient characteristics
The demographics of the 237 patients and standard laboratory tests around the time of liver biopsy are summarized in Table 2. The mean age of the 237 patients (135 male and 102 female) was 54.3 ± 11.6 years. The AST range was 21-348 U/L (mean value, 101.2 ± 56.0 U/L). The ALT range was 24-637 U/L (mean value, 156.3 ± 92.5 U/L). The platelet count range was 60-373 × 103/μL (mean value, 170.9 ± 55.1 × 103/μL).
Table 2.
Clinical characteristics of 237 patients with chronic hepatitis C
| Male/female | 135/102 |
| Age (yr) | 54.3 ± 11.6 (19-76) |
| AST (U/L) | 101.2 ± 56.0 (21-348) |
| ALT (U/L) | 156.3 ± 92.5 (24-637) |
| PT INR | 1.049 ± 0.080 (0.88-1.34) |
| PLT (× 103/μL) | 170.9 ± 55.1 (60-373) |
| Hemoglobin (g/L) | 14.4 ± 1.37 (9.0-18.0) |
| WBC count (x 109/L) | 5.85 ± 1.73 (2.8-16.7) |
| Creatinine (mg/dL) | 0.94 ± 0.23 (1.0-2.0) |
| FT4 (μg/dL) | 1.12 ± 0.19 (1.0-2.0) |
| TSH (mU/mL) | 2.06 ± 2.13 (0.1-23.0) |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PLT: Posterolateral thoracotomy; PT-INR: Prothrombin time international normalized ratio; WBC: White blood cell; FT4: Free thyroxine; TSH: Thyroid stimulating hormone.
Correlations between fibrosis stage and fibrosis-predicting models
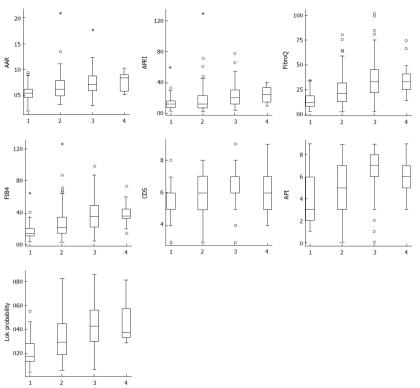
Correlations between routine blood tests, fibrosis-pre-dicting models, and histological fibrosis stage are summarized in Table 3. The highest correlation was observed for FibroQ (rs = 0.444), Lok’s model (rs = 0.430), and FIB-4 (rs = 0.429) (P < 0.001). Figure 1 shows the box-plots of fibrosis scores according to Metavir fibrosis stage. Weaker correlations were also found between other scores and histological fibrosis stage, especially for API (rs = 0.360), AAR (rs = 0.341), and APRI (rs = 0.322) (P < 0.001).
Table 3.
Correlation of histological fibrosis severity with the variables
| Bivariate Spearman’s rank correlation coefficient (95% CI) | P value | |
| Age (yr) | 0.232 (0.108-0.349) | < 0.001 |
| AST (U/L) | 0.188 (0.062-0.308) | 0.004 |
| ALT (U/L) | -0.028 (-0.155 to 0.100) | 0.666 |
| PT INR | 0.337 (0.219-0.445) | < 0.001 |
| PLT (× 103/μL) | -0.326 (-0.435 to -0.207) | < 0.001 |
| Hb | -0.176 (-0.297 to -0.050) | 0.005 |
| WBC | -0.053 (-0.179 to 0.075) | 0.404 |
| Cr | -0.152 (-0.274 to -0.025) | 0.02 |
| FT4 | -0.167 (-0.288 to -0.040) | 0.014 |
| TSH | 0.065 (-0.063 to 0.191) | 0.341 |
| Bil(T) | 0.135 (0.008-0.258) | 0.04 |
| AAR | 0.341 (0.223-0.449) | < 0.001 |
| APRI | 0.322 (0.203-0.431) | < 0.001 |
| FibroQ | 0.444 (0.336-0.541) | < 0.001 |
| FIB-4 | 0.429 (0.319-0.528) | < 0.001 |
| CDS | 0.185 (0.059-0.305) | 0.004 |
| API | 0.360 (0.244-0.466) | < 0.001 |
| Lok’s model | 0.430 (0.320-0.528) | < 0.001 |
| Pohl score | 0.144 (0.017-0.267) | 0.027 |
We used Fisher’s Z-transform to compute asymmetric confidence limits for the Spearman's rank correlation coefficients. Hb: Hemoglobin; Bil(T): Total bilirubin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PLT: Posterolateral thoracotomy; PT-INR: Prothrombin time international normalized ratio; WBC: White blood cell; FT4: Free thyroxine; TSH: Thyroid stimulating hormone; AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; API: Age-platelet index; CDS: Cirrhosis discriminant score.
Figure 1.
Score values according to Metavir fibrosis stages. Each outlier value is represented by a small circle symbol (o) in the Box Plot graph. If an outlier is more than 3 times the inter-quartile range away from Q1 or Q3, it is classified as an extreme outlier, asterisk sign (*). The top and bottom of each box are the 25th and the 75th percentiles. The line through the box is the median, and the errors bars are the 5th and 95th percentiles. AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score.
There were 41 (17.3%) patients with Metavir stage F1 fibrosis, 85 (35.9%) with F2, 98 (41.4%) with F3, and 13 (5.5%) with F4. AAR, FibroQ, FIB-4, API, and Lok’s model results increased significantly as the fibrosis advanced (Table 4, analysis of variance test: P < 0.001).
Table 4.
Correlation between fibrosis score and aspartate aminotransferase-to-platelet ratio index, aspartate aminotransferase/alanine aminotransferase ratio, and FibroQ, FIB-4, cirrhosis discriminant score, age-platelet index, Lok’s model, Pohl score
| Metavir fibrosis score | F1 | F2 | F3 | F4 |
| Patient number (%) | 41 (17.3) | 85 (35.9) | 98 (41.4) | 13 (5.5) |
| AAR | 0.566 ± 0.162ce | 0.673 ± 0.250 | 0.749 ± 0.222 | 0.776 ± 0.175 |
| APRI | 1.433 ± 1.040a | 1.919 ± 1.904 | 2.320 ± 1.390 | 2.508 ± 1.102 |
| FibroQ | 1.485 ± 0.855gi | 2.532 ± 1.660m | 3.563 ± 2.056 | 3.581 ± 1.812 |
| FIB-4 | 1.79 ± 1.13gim | 2.79 ± 2.03 | 3.75 ± 1.97 | 3.82 ± 1.57 |
| CDS | 5.61 ± 1.16 | 5.69 ± 1.19 | 6.14 ± 1.12 | 6.15 ± 1.41 |
| API | 3.95 ± 2.06gim | 5.25 ± 2.24 | 6.40 ± 2.29 | 6.15 ± 1.63 |
| Lok’s model | 0.22 ± 0.12kmo | 0.32 ± 0.18 | 0.43 ± 0.19 | 0.46 ± 0.18 |
| Pohl score | 0 | 0.01 ± 0.11 | 0.08 ± 0.28 | 0.04 ± 0.19 |
P < 0.05, F1 vs F3;
P < 0.01, F1 vs F3;
P < 0.05, F1 vs F4;
P < 0.05, F1 vs F2;
P < 0.01, F1 vs F3 and F4;
P < 0.01, F1 vs F2, F3 and F4;
P < 0.01, F2 vs F3;
P < 0.05, F2 vs F4. AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score.
ROC curve analysis
ROC curves evaluating the diagnostic accuracies of FibroQ, AAR, APRI, CDS, API, Pohl score, FIB-4 index, and Lok’s model were constructed and superimposed (Figure 2) to determine which score would have the most clinical utility to predict significant fibrosis (≥ F2). The AUC (95% CI) using the procedures described by Hanley and McNeil[24] was greatest for FibroQ (0.789, 0.720-0.857), then FIB-4 (0.785, 0.686-0.830), followed by Lok’s model (0.768, 0.695-0.840), API (0.739, 0.660-0.818), AAR (0.709, 0.626-0.792), APRI (0.651, 0.566-0.736), CDS (0.580, 0.485-0.674), and Pohl score (0.523, 0.429-0.617) (Table 5). The AUC of FibroQ was significantly higher than those of AAR, APRI, CDS, and Pohl score (P < 0.05).
Figure 2.
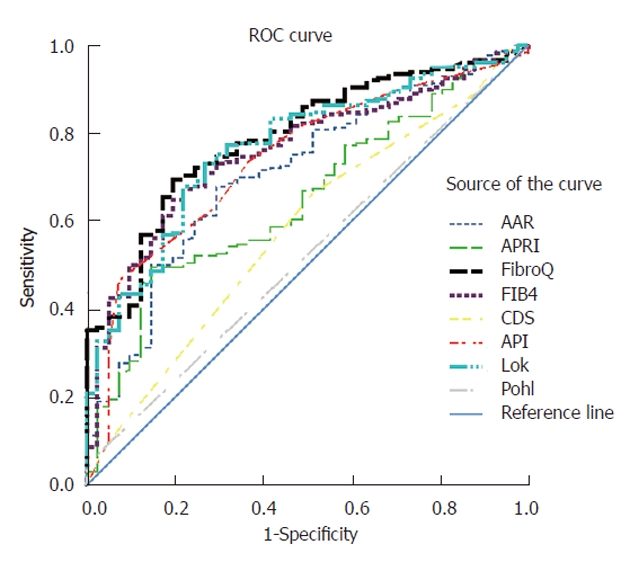
Receiver operating characteristic curves of simple noninvasive tests evaluated for prediction of significant fibrosis (F2, F3 and F4). AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score; ROC: Receiver operating characteristic.
Table 5.
Performance of simple fibrosis prediction tests for significant fibrosis (F2, F3 and F4) and extensive fibrosis (F3 and F4)
| Metavir fibrosis score vs | AUC (F2, F3, F4)(95% CI) | AUC (F3, F4)(95% CI) |
| AAR | 0.709 (0.626-0.792) | 0.675 (0.607-0.743) |
| APRI | 0.651 (0.566-0.736) | 0.681 (0.613-0.749) |
| FibroQ | 0.789 (0.720-0.857) | 0.728 (0.662-0.793) |
| FIB-4 | 0.785 (0.686-0.830) | 0.725 (0.659-0.791) |
| CDS | 0.580 (0.485-0.674) | 0.609 (0.537-0.680) |
| API | 0.739 (0.660-0.818) | 0.696 (0.628-0.764) |
| Lok’s model | 0.768 (0.695-0.840) | 0.721 (0.656-0.786) |
| Pohl score | 0.523 (0.429-0.617) | 0.532 (0.458-0.606) |
AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score; AUC: Area under the receiver operating characteristic curve.
To predict extensive fibrosis (≥ F3), ROC curves for FibroQ, AAR, APRI, CDS, API, Pohl score, FIB-4 index, and Lok’s model were also constructed and superimposed to determine which score would have the most clinical utility (Figure 3). The AUC curves (95% CI) using the procedures described by Hanley and McNeil[24] were greatest for FibroQ (0.728, 0.662-0.793), then FIB-4 (0.725, 0.659-0.791), followed by Lok’s model (0.721, 0.656-0.786), API (0.696, 0.628-0.764), APRI (0.681, 0.613-0.749), AAR (0.675, 0.607-0.743), CDS (0.609, 0.537-0.680), and Pohl score (0.532, 0.458-0.606) (Table 5). The AUC of FibroQ was significantly higher than those of CDS or Pohl score (P < 0.05).
Figure 3.
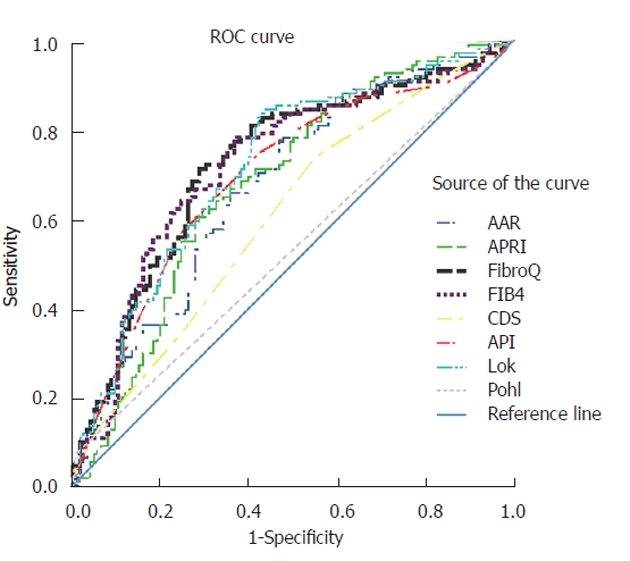
Receiver operating characteristic curves of simple noninvasive tests evaluated for prediction of extensive fibrosis (F3 and F4). AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score; ROC: Receiver operating characteristic.
Sensitivity, specificity, positive predictive value, and negative predictive value
Table 6 shows the performance of FibroQ, AAR, APRI, CDS, API, Pohl score, FIB-4, and Lok’s model at various cutoff levels for the prediction of significant fibrosis (F2, F3 and F4) and extensive fibrosis (F3 and F4). To compare our results with those of previous reports, the sensitivity, specificity, PPV, and NPV of the simple fibrosis prediction tests were calculated using cutoff values exactly as originally described[16,17,21-23]. Using a cutoff value of the FibroQ score of > 1.6 to predict the presence of significant fibrosis resulted in a sensitivity of 77.6%, specificity of 65.9%, PPV of 91.6%, and NPV of 38.0% in 166 (70%) of the 237 patients. With AAR, the cutoff levels to predict the presence (AAR > 1.0) of significant fibrosis had a sensitivity of 8.16%, specificity of 100%, PPV of 100%, and NPV of 18.5%. Using APRI, the cutoff values to predict the presence (APRI > 1.5) or absence (APRI < 0.5) of significant fibrosis had a sensitivity of 56.6% and 96.9%, specificity of 58.5% and 9.7%, PPV of 86.7% and 83.7%, and NPV of 22.0% and 40.0%, respectively. In Lok’s model, the cutoff values to predict the presence (Lok’s model > 0.5) or absence (Lok’s model < 0.2) of extensive fibrosis had a sensitivity of 37.8% and 88.3%, specificity of 88.1% and 37.3%, PPV of 73.7% and 55.4%, and NPV of 61.7% and 78.3%, respectively. At a cutoff of FIB-4 < 1.45, the NPV to exclude extensive fibrosis (F3 and F4) was 75.9% with a sensitivity of 87.4%. A cutoff of FIB-4 > 3.25 had a PPV of 70.1% and a specificity of 77%.
Table 6.
Diagnostic accuracy of simple fibrosis prediction tests for significant and extensive fibrosis
| Score | Cut-off value | (%) | Significant fibrosis (F2, F3 and F4) | Extensive fibrosis (F3 and F4) | ||||||
| Sen | Spe | PPV | NPV | Sen | Spe | PPV | NPV | |||
| FibroQ | > 0.6 | 94.9 | 96.4 | 12.2 | 84.0 | 41.7 | 97.3 | 7.14 | 48.0 | 75.0 |
| > 1.2 | 79.7 | 85.7 | 48.8 | 88.9 | 41.7 | 90.1 | 29.4 | 52.9 | 77.1 | |
| > 1.4 | 74.3 | 80.1 | 53.7 | 89.2 | 36.1 | 86.5 | 36.5 | 54.5 | 75.4 | |
| > 1.6 | 70.0 | 77.6 | 65.9 | 91.6 | 38.0 | 85.6 | 43.7 | 57.2 | 77.5 | |
| > 1.8 | 66.2 | 74.0 | 70.7 | 92.4 | 36.3 | 83.8 | 49.2 | 59.2 | 77.5 | |
| > 2.0 | 60.8 | 69.4 | 80.5 | 94.4 | 35.5 | 82.0 | 57.9 | 63.2 | 78.5 | |
| > 2.6 | 44.3 | 51.0 | 87.8 | 95.2 | 27.3 | 64.9 | 73.8 | 68.6 | 70.5 | |
| AAR | > 0.4 | 97.0 | 95.4 | 12.2 | 83.9 | 35.7 | 96.4 | 7.1 | 47.8 | 69.2 |
| > 0.6 | 61.2 | 67.9 | 70.7 | 91.7 | 31.5 | 77.5 | 53.2 | 59.3 | 72.8 | |
| > 0.8 | 28.7 | 31.6 | 85.4 | 91.2 | 20.7 | 36.9 | 78.6 | 60.3 | 58.6 | |
| > 1.0 | 6.8 | 8.16 | 100 | 100 | 18.5 | 10.8 | 96.8 | 75.0 | 55.2 | |
| APRI | > 0.5 | 95.8 | 96.9 | 9.7 | 83.7 | 40.0 | 99.1 | 7.1 | 48.5 | 90.0 |
| > 1 | 72.6 | 75.5 | 41.5 | 86.0 | 26.2 | 87.4 | 40.5 | 56.4 | 78.5 | |
| > 1.5 | 54.0 | 56.6 | 58.5 | 86.7 | 22.0 | 69.4 | 59.5 | 60.2 | 68.8 | |
| > 2 | 39.7 | 45.4 | 87.8 | 94.7 | 25.2 | 55.0 | 73.8 | 64.9 | 65.0 | |
| CDS | > 5 | 88.6 | 88.8 | 12.2 | 82.9 | 18.5 | 92.8 | 15.1 | 49.0 | 70.4 |
| > 6 | 63.7 | 66.3 | 48.8 | 86.1 | 23.3 | 73.9 | 45.2 | 54.3 | 66.3 | |
| > 7 | 29.1 | 30.6 | 78.0 | 87.0 | 19.0 | 34.2 | 75.4 | 55.1 | 56.5 | |
| > 8 | 8.9 | 9.7 | 95.1 | 90.5 | 18.1 | 12.6 | 94.4 | 66.7 | 55.1 | |
| API | > 4 | 75.9 | 81.6 | 51.2 | 88.9 | 36.8 | 87.4 | 34.1 | 53.9 | 75.4 |
| > 5 | 67.1 | 73.5 | 63.4 | 90.6 | 33.3 | 82.0 | 46.0 | 57.2 | 74.4 | |
| > 6 | 57.4 | 63.3 | 70.7 | 91.2 | 28.7 | 74.8 | 57.9 | 61.0 | 72.3 | |
| > 7 | 39.7 | 46.4 | 92.7 | 96.8 | 26.6 | 56.8 | 75.4 | 67.0 | 66.4 | |
| Pohl score | Positive | 3.8 | 4.59 | 100 | 100 | 18.0 | 7.2 | 99.2 | 88.9 | 54.8 |
| FIB-4 | > 1.45 | 75.5 | 81.6 | 53.7 | 89.4 | 37.9 | 87.4 | 34.9 | 54.2 | 75.9 |
| > 2 | 62.0 | 69.4 | 73.2 | 92.5 | 33.3 | 81.1 | 54.8 | 61.2 | 76.7 | |
| > 2.5 | 53.6 | 61.2 | 82.9 | 94.5 | 30.9 | 74.8 | 65.1 | 65.4 | 74.5 | |
| > 3 | 46.0 | 52.6 | 85.4 | 94.5 | 27.3 | 66.7 | 72.2 | 67.9 | 71.1 | |
| > 3.25 | 40.9 | 47.4 | 90.2 | 95.9 | 26.4 | 61.3 | 77.0 | 70.1 | 69.3 | |
| Lok’s model | > 0.2 | 74.7 | 81.6 | 58.5 | 90.4 | 40.4 | 88.3 | 37.3 | 55.4 | 78.3 |
| > 0.4 | 35.0 | 40.8 | 92.7 | 96.4 | 24.7 | 51.4 | 79.4 | 68.7 | 64.9 | |
| > 0.5 | 24.1 | 28.6 | 97.6 | 98.2 | 22.2 | 37.8 | 88.1 | 73.7 | 61.7 | |
AAR: AST/ALT ratio; APRI: AST-to-platelet ratio index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; API: Age-platelet index; CDS: Cirrhosis discriminant score; Sen: Sensitivity; Spe: Specificity; PPV: Positive predictive value; NPV: Negative predictive value.
Since lower cutoffs were originally described to exclude significant fibrosis, specific attention must be paid to NPVs that ranged from 18.0% to 41.7%. For the same cutoffs, NPVs to exclude extensive fibrosis showed superior performance compared with performance on significant fibrosis cases, ranging from 54.8% to 90.0%. For example, a FibroQ < 1.4, which was observed in 25.7% of patients, excluded significant fibrosis with 36.1% certainty and excluded extensive fibrosis with 75.4% certainty. The best predictive value was observed for positive Pohl score, but this cutoff selected only 3.8% of patients.
DISCUSSION
To assess the pathological grade and stage of chronic viral hepatitis, liver biopsy is necessary. However, liver biopsy is invasive, costly, and has its own limitations[11,25]. Hence, several noninvasive tests combining biological parameters have been proposed to attempt to predict the degree of fibrosis, with the objective of replacing liver biopsy[20,26-29]. There are also some noninvasive tests, such as PIIIP (N -terminal peptide of type III procollagen)[29], fibrometer[26], Hepascore[30], FibroTest[31], and Forn’s score[27]. The current study excludes these tests due to their expense (e.g., procollagen level), because they can only be checked in the laboratory (e.g., hyaluronic acid), or because they are not included in the routine monitoring and investigations of patients with chronic liver disease (e.g., haptoglobin or cholesterol).
We believe that an ideal noninvasive test for assessing liver fibrosis should be reliable, reproducible, and based on readily available tests and parameters. APRI, AAR, FibroQ, FIB-4, CDS, API, Lok’s model, and Pohl score fulfilled these criteria, therefore, we compared these measures for evaluation of patients with chronic hepatitis C. The aim was to validate the usefulness of these simple tests in a community hospital in an area with hyperendemic HCV infection.
In addition, bias of the biopsy examination is also acknowledged. Regev et al[32] have reported discordances in fibrosis stage in one third of patients when right and left liver lobes were compared. As a liver biopsy specimen from a cirrhotic liver is often fragmented, the inadequate size of biopsy samples can lead to an underestimation of fibrosis as reported by Colloredo et al[33] and Bedossa et al[11]. Therefore, some cases diagnosed as F3 may have had cirrhosis (F4). Biopsy length and fragmentation were not recorded or compared in our study; however, a recent study has found that these variables did not affect the performance of their model[34]. Furthermore, we should notice that four different staging systems were used in the original studies for liver fibrosis tests. AAR results were based on the Scheuer system; APRI and FIB-4 were based on the Ishak system; FibroQ, API, and Pohl’s score were based on the Metavir system; and CDS and Lok’s model were based on a modified Knodell system. The different staging systems using either a 5-stage (F0-F4) or a 7-stage (F0-F6) scale prevented us from comparing fibrosis scores more precisely.
Although the current study was retrospective, it had some advantages over previous studies. First, the patients were all from a treatment-naïve population with HCV infection as the only problem. Second, we compared all fibrosis markers that combined routine blood tests rather than markers only available in the laboratory. Third, all histological assessment was performed blindly by one pathologist, thus preventing interobserver bias in fibrosis staging. Finally, using a 5-stage fibrosis scoring system in this study resulted in a lower tendency to induce interpretation error than would be the case with a 7-stage scoring system[22].
We divided the patients in two different ways to obviate liver biopsy. The first grouping was F1 vs F2, F3 and F4, the second was F1 and F2 vs F3 and F4. The rationale for the F1 vs F2, F3 and F4 grouping was that there was no need for treatment in patients with mild fibrosis. Patients with severe fibrosis or cirrhosis need screening for hepatocellular carcinoma, esophageal varices, and complications of portal hypertension, therefore, we made a second patient grouping of F1 and F2 vs F3 and F4. Applying the cutoff values, we were able to discriminate patients according to two relevant thresholds of fibrosis based on the needs for clinical treatment decision-making.
The result of our current study showed that Pohl score and CDS had high specificity but low sensitivity, and their AUCs were not statistically different from 0.5. In addition, FibroQ, FIB-4, and Lok’s model showed the best performance characteristics. The AUCs for predicting significant fibrosis were 0.789, 0.785 and 0.768, respectively. The AUCs for predicting extensive fibrosis were 0.728, 0.725 and 0.721, respectively[21-23]. To evaluate the accuracy of the fibrosis index, we also checked the percentage of patients that were correctly classified according to the stage of fibrosis. Using FibroQ results below the lower cutoff value (0.6) and above the higher cutoff value (1.6), 157 of 178 patients (88.2%) were correctly identified as having or not having significant fibrosis, which was a better performance than in the original study on the use of FibroQ[23]. Using FIB-4, 72.3% of the 155 patients with FIB-4 values outside 1.45-3.25 would be correctly classified, and liver biopsy could be avoided in 65.4% of patients, which is slightly lower than the value of 71% reported by Sterling et al[21]. Using Lok’s model with a cutoff of 0.2 to exclude extensive fibrosis, only 11.7% of those with extensive fibrosis were misclassified. Using a cutoff of 0.5, only 11.9% of those without extensive fibrosis had a score > 0.5. These results are compatible with those reported by Cheung et al[22]. Using the same cutoff values, 7.8% and 14.8% of patients were misclassified in their study.
In summary, the current study demonstrated that FibroQ, FIB-4, and Lok’s model were simple methods that correlated well with the stages of fibrosis in patients with chronic hepatitis C. FibroQ showed a trend to be superior to the other modalities evaluated. Further prospective studies involving larger numbers of patients are warranted to validate the usefulness of FibroQ in clinical practice.
COMMENTS
Background
Viral hepatitis C is one of the most common liver diseases in the world, affecting an estimated 200 million individuals. Knowledge of the extent of liver fibrosis is important for the clinical management of chronic hepatitis C. Liver biopsy is recommended prior to antiviral treatment. However, liver biopsy may cause complications, including mortality in 0.018% of patients. Hence, several noninvasive tests have been proposed to assess the severity of hepatic fibrosis.
Research frontiers
Aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and AST/alanine aminotransferase (ALT) ratio (AAR), cirrhosis discriminant score (CDS), age-platelet index (API), Pohl score, FIB-4 index, and Lok’s model are well-known parameters that are based on routine laboratory data and are therefore readily available in clinical practice.
Innovations and breakthroughs
Recently, the authors proposed a novel index, FibroQ, which was calculated from common laboratory test results that included prothrombin time international normalized ratio (PT INR), platelet, AST, ALT, and age, calculated as 10 × (age × AST × PT INR)/(ALT × platelet count) to predict significant fibrosis. FibroQ trended to be superior in predicting significant fibrosis score in chronic hepatitis C compared with other noninvasive tests.
Applications
FibroQ is a simple and useful noninvasive test for predicting significant fibrosis in patients with chronic hepatitis C.
Terminology
FibroQ, APR, AAR, CDS, API, Pohl score, FIB-4 index, and Lok’s model are well-known parameters that are based on routine laboratory data and are reported to predict the presence of liver significant fibrosis and extensive fibrosis.
Peer review
The study is of particular practical medical interest. The results provide sufficient evidence that the FibroQ correlates with significant liver fibrosis in patients with hepatitis C virus.
Footnotes
Supported by Clinical Study Project XMRP, No. CMRPG 690081, from Chiayi Chang Gung Memorial Hospital
Peer reviewer: Luigi Muratori, MD, PhD, Assistant Professor, Department of Clinical Medicine, University of Bologna, via Massarenti, 9, Bologna 40138, Italy
S- Editor Sun H L- Editor Kerr C E- Editor Li JY
References
- 1.Hepatitis C--global prevalence (update) Wkly Epidemiol Rec. 2000;75:18–19. [PubMed] [Google Scholar]
- 2.Seeff LB. Natural history of hepatitis C. Am J Med. 1999;107:10S–15S. doi: 10.1016/s0002-9343(99)00374-5. [DOI] [PubMed] [Google Scholar]
- 3.EASL International Consensus Conference on hepatitis C. Paris, 26-27 February 1999. Consensus statement. J Hepatol. 1999;31 Suppl 1:3–8. [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 5.Bonino F, Oliveri F, Colombatto P, Coco B, Mura D, Realdi G, Brunetto MR. Treatment of patients with chronic hepatitis C and cirrhosis. J Hepatol. 1999;31 Suppl 1:197–200. doi: 10.1016/s0168-8278(99)80401-2. [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology. 1997;26:122S–127S. doi: 10.1002/hep.510260721. [DOI] [PubMed] [Google Scholar]
- 7.Kaserer K, Fiedler R, Steindl P, Müller CH, Wrba F, Ferenci P. Liver biopsy is a useful predictor of response to interferon therapy in chronic hepatitis C. Histopathology. 1998;32:454–461. doi: 10.1046/j.1365-2559.1998.00413.x. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 9.Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C: risks, benefits, and costs. JAMA. 1998;280:2088–2093. doi: 10.1001/jama.280.24.2088. [DOI] [PubMed] [Google Scholar]
- 10.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 11.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–1355. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]
- 14.Akkaya O, Kiyici M, Yilmaz Y, Ulukaya E, Yerci O. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J Gastroenterol. 2007;13:5481–5485. doi: 10.3748/wjg.v13.i41.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu SN, Wang JH, Liu SL, Hung CH, Chen CH, Tung HD, Chen TM, Huang WS, Lee CM, Chen CC, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107:2212–2222. doi: 10.1002/cncr.22242. [DOI] [PubMed] [Google Scholar]
- 16.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 17.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 18.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 19.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 20.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 22.Cheung RC, Currie S, Shen H, Bini EJ, Ho SB, Anand BS, Hu KQ, Wright TL, Morgan TR. Can we predict the degree of fibrosis in chronic hepatitis C patients using routine blood tests in our daily practice? J Clin Gastroenterol. 2008;42:827–834. doi: 10.1097/MCG.0b013e318046ea9a. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YY, Tung SY, Lee IL, Lee K, Shen CH, Wei KL, Chang TS, Chuang CS, Wu CS, Lin YH. FibroQ: an easy and useful noninvasive test for predicting liver fibrosis in patients with chronic viral hepatitis. Chang Gung Med J. 2009;32:614–622. [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 26.Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 27.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 28.Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775–782. doi: 10.1016/j.jhep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Trocme C, Leroy V, Sturm N, Hilleret MN, Bottari S, Morel F, Zarski JP. Longitudinal evaluation of a fibrosis index combining MMP-1 and PIIINP compared with MMP-9, TIMP-1 and hyaluronic acid in patients with chronic hepatitis C treated by interferon-alpha and ribavirin. J Viral Hepat. 2006;13:643–651. doi: 10.1111/j.1365-2893.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 30.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 31.Rossi E, Adams L, Prins A, Bulsara M, de Boer B, Garas G, MacQuillan G, Speers D, Jeffrey G. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem. 2003;49:450–454. doi: 10.1373/49.3.450. [DOI] [PubMed] [Google Scholar]
- 32.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 33.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 34.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]