Abstract
AIM: To investigate the effects of resistin-like molecule β (RELMβ) over-expression on the invasion, metastasis and angiogenesis of gastric cancer cells.
METHODS: Human RELMβ encoding expression vector was constructed and transfected into the RELMβ lowly-expressed gastric cancer cell lines SGC-7901 and MKN-45. Gene expression was measured by Western blotting, reverse transcription polymerase chain reaction (PCR) and real-time quantitative PCR. Cell proliferation was measured by 2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetry, colony formation and 5-ethynyl-20-deoxyuridine incorporation assays. The in vitro migration, invasion and metastasis of cancer cells were measured by cell adhesion assay, scratch assay and matrigel invasion assay. The angiogenic capabilities of cancer cells were measured by tube formation of endothelial cells.
RESULTS: Transfection of RELMβ vector into SGC-7901 and MKN-45 cells resulted in over-expression of RELMβ, which did not influence the cellular proliferation. However, over-expression of RELMβ suppressed the in vitro adhesion, invasion and metastasis of cancer cells, accompanied by decreased expression of matrix metalloproteinase-2 (MMP-2) and MMP-9. Moreover, transfection of RELMβ attenuated the expression of vascular endothelial growth factor and in vitro angiogenic capabilities of cancer cells.
CONCLUSION: Over-expression of RELMβ abolishes the invasion, metastasis and angiogenesis of gastric cancer cells in vitro, suggesting its potentials as a novel therapeutic target for gastric cancer.
Keywords: Resistin-like molecule β, Gastric cancer, Invasion, Metastasis, Angio-genesis
INTRODUCTION
Gastric cancer is one of the most common cancer types in the world[1]. In spite of the standardization of surgical techniques and multimodal therapy, the postoperative survival of patients with advanced gastric cancer still remains very low[1]. Recent evidence shows that goblet cell-derived proteins, such as intestinal trefoil factor (ITF) and mucin 2 (MUC2), participate in the pathogenesis of gastric cancer[2-6]. As a member of trefoil peptide family that is expressed exclusively in the goblet cells of small intestine and colon[7], ITF is over-expressed in several cancer tissues including gastric cancer[2,7], and promotes tumor cell invasion and angiogenesis[7-9]. Blocking ITF expression via an antisense strategy suppresses the in vitro growth and tumorigenicity of gastric cancer cells[3], suggesting that ITF may serve as a potential target in the control of gastrointestinal cancer progression. Similarly, MUC2 is expressed in the goblet cells of colon, small intestine and airways[10], and is aberrantly expressed in gastric cancer[4,5]. Measuring the MUC2 transcriptional levels is a sensitive and specific approach to detect lymph node micrometastasis in gastric cancer patients[6]. These results suggest that goblet cell-specific proteins may be involved in the progression of gastric cancer, which are potential targets for regulating the invasion, metastasis and angiogenesis of gastric cancer.
Resistin-like molecule β (RELMβ), also known as Found in Inflammatory Zone 2 (FIZZ2), belongs to a family of resistin-like cytokine molecules consisting of small and cysteine-rich secretory proteins[11]. As a novel goblet cell-specific protein that is abundantly expressed in proximal and distal colon[11,12], RELMβ is induced by intestinal microbial colonization, and plays a key role in epithelial barrier function and integrity[12,13]. In addition, RELMβ functions not only as a Th2 cytokine immune effector but also as an inhibitor of chemotaxis of parasites, through interfering with parasite nutrition by directly binding to the chemosensory components of parasites[13]. Recent evidence shows that RELMβ has the potentials to contribute to the airway remodeling in diseases such as asthma[14], and is involved in the pathogenesis of fibrotic lung diseases as a Th2-associated multifunctional mediator[15] and the development of scleroderma-associated pulmonary hypertension[16]. However, the role of RELMβ in cancer development still remains unclear.
Our previous studies have indicated that RELMβ is over-expressed in a majority of human colon cancer tissues[17], and in the metaplastic epithelium of Barrett’s esophagus and associated dysplasia[18]. Moreover, RELMβ is aberrantly expressed in the goblet cells of intestinal metaplasia and cytoplasm of cancer cells in gastric cancer tissues, which is positively correlated with tumor differentiation and longer overall survival, and inversely correlated with tumor infiltration and lymph node metastasis, indicating the value of RELMβ in predicting the outcomes of gastric cancer patients[19]. In this study, to further elucidate the exact role of RELMβ in the progression of gastric cancer, we investigated the effects of RELMβ over-expression on the RELMβ lowly-expressed gastric cancer cells. We found that over-expression of RELMβ attenuated the invasion, metastasis and angiogenesis of cancer cells, suggesting the anti-tumor role of RELMβ in the progression of gastric cancer.
MATERIALS AND METHODS
Cell culture
Human gastric cancer cell lines SGC-7901 and MKN-45 were obtained from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Human endothelial cell line HUVEC (CRL-1730) was purchased from American Type Culture Collection (Rockville, MD, United States). The cells were grown in RPMI1640 medium (Life Technologies, Inc., Gaithersburg, MD, United States) supplemented with 10% fetal bovine serum (FBS, Life Technologies, Inc., Gaithersburg, MD, United States), penicillin (100 U/mL) and streptomycin (100 μg/mL). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Vector construction and transfection
Full-length RELMβ cDNA was amplified from human colon tissues, subcloned between the restrictive sites Hind III and Bam HI of pcDNA3.1/Zeo(+) (Invitrogen, Carlsbad, CA, United States), and validated by sequencing. The primers used for the RELMβ cDNA amplification were 5’-CGCCCAAGCTTATGGGGCCGTCCTCTTGC-3’ (forward) and 5’-CGCGGATCCTCAGGTCAGGTGGCAGCA-3’ (reverse). The recombinant pcDNA 3.1-RELMβ or empty vector (mock) was transfected into SGC-7901 and MKN-45 cells with Lipofectamine 2000 (Life Technologies, Inc., Gaithersburg, MD, United States), according to the manufacturer’s instructions. To monitor the transfection efficiency, the cancer cells were co-transfected with pEGFP-N1 (Clontech, Mountair View, CA, United States).
Western blotting
Western blotting was performed as previously descri-bed[20], with antibodies specific for RELMβ (Abcam Inc, Cambridge, MA, United States), matrix metalloproteinase-2 (MMP-2), MMP-9, v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1), vascular endothelial growth factor (VEGF) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, United States). ECL substrate kit (Amersham, Piscataway, NJ, United States) was used for the chemilu-minscent detection of signals with autoradiography film (Amersham).
Reverse transcription polymerase chain reaction and real-time quantitative reverse transcription polymerase chain reaction
The reverse transcription reactions were conducted with Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, United States). The polymerase chain reaction (PCR) amplification was performed with the primer sets indicated in Table 1. Real-time PCR with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, United States) was performed using ABI Prism 7700 Sequence Detector (Applied Biosystems). The fluorescent signals were collected during extension phase, Ct values of the sample were calculated, and the transcript levels were analyzed by 2-∆∆Ct method.
Table 1.
Primers sets used for reverse transcription polymerase chain reaction and real-time reverse transcription polymerase chain reaction
| Primer set | Primers | Sequence | Product size (bp) |
| RELMβ | Forward | 5’-ATGGGGCCGTCCTCTTGCCTCC-3’ | 336 |
| Reverse | 5’-TCAGGTCAGGTGGCAGCAGCG-3’ | ||
| MMP-2 | Forward | 5’-CCAAAACGGACAAAGAGT-3’ | 275 |
| Reverse | 5’-ATCAGGTGTGTAGCCAAT-3’ | ||
| MMP-9 | Forward | 5’-CAGAGATGCGTGGAGAGT-3’ | 220 |
| Reverse | 5’-TCTTCCGAGTAGTTTTGG-3’ | ||
| Ets1 | Forward | 5’-TTCACTAAAGAACAGCAAC-3’ | 205 |
| Reverse | 5’-TGTCCCCAACAAAGTCTG-3’ | ||
| VEGF | Forward | 5’-GGCAGAATCATCACGAAG-3’ | 276 |
| Reverse | 5’-TGTGCTGTAGGAAGCTCA-3’ | ||
| GAPDH | Forward | 5’- AGAAGGCTGGGGCTCATTTG-3’ | 258 |
| Reverse | 5’- AGGGGCCATCCACAGTCTTC-3’ |
RT-PCR: Reverse transcription polymerase chain reaction; RELMβ: Resistin-like molecule β; MMP: Matrix metalloproteinase-2; Ets1: E26 oncogene homolog 1; VEGF: Vascular endothelial growth factor; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
MTT colorimetric assay
Cancer cells were cultured in 96-well plates at 5 × 103 cells per well and transfected with pcDNA3.1-RELMβ or empty vector (mock). After transfection for 24 h, 72 h and 120 h, cell viability was monitored by the 3-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma, St. Louis, MO, United States) colorimetric assay[20]. All experiments were done with 6-8 wells per experiment and repeated at least three times.
Colony formation assay
Seventy-two hours after transfection, the cells were seeded at a density of 300/mL on 35-mm dishes. Colony formation assay was performed as previously described[20]. Positive colony formation (more than 50 cells/colony) was counted. The survival fraction of cells was expressed as the ratio of plating efficiency of treated cells to that of untreated control cells.
EdU incorporation assay
Cancer cells were cultured in 96-well plates at 5 × 103 cells per well, transfected with pcDNA3.1-RELMβ or empty vector (mock) for 72 h, then exposed to 50 μmol/L of 5-ethynyl-20-deoxyuridine (EdU, Ribobio, China) for additional 4 h at 37 °C. The cells were fixed with 4% formaldehyde for 15 min and treated with 0.5% Triton X-100 for 20 min at room temperature. After washing with phosphate buffered saline for three times, the cells of each well were reacted with 100 μL of 1 × Apollo® reaction cocktail for 30 min. Subsequently, the DNA contents of cells in each well were stained with 100 μL of Hoechst 33342 (5 μg/mL) for 30 min and visualized under a fluorescent microscope.
Cell adhesion assay
Seventy-two hours after transfection, 2 × 104 cancer cells were inoculated into each well of 96-well plates that were precoated with 100 μL of 20 μg/mL matrigel (BD Biosciences, Franklin Lakes, NJ, United States), and incubated at 37 °C in serum-free complete medium (pH 7.2) for 2 h. Cell adhesion was measured as previously described[20]. And 0%, 20%, 50% and 100% of inoculated cells were directly fixed in 4% paraformaldehyde 2 h after inoculation.
Scratch migration assay
Cancer cells were cultured in 24-well plates and transfected with pcDNA3.1-RELMβ or empty vector (mock). Seventy-two hours after transfection, the cells were scraped with the fine end of 1-mL pipette tips (Time 0). Scratch migration assay was performed as previously described[20]. Remodeling was measured as diminishing distance across the induced injury and normalized to the 0 h control.
Matrigel invasion assay
The Boyden chamber technique (transwell analysis) was applied as previously described[20]. Briefly, 72 h after transfection, homogeneous single cell suspensions (1 × 105 cells/well) were added to the upper chambers and allowed to invade for 24 h at 37 °C in a CO2 incubator. The migrated cells were counted according to the published criteria[21].
Tube formation assay
Fifty microliters of growth factor-reduced matrigel were polymerized on 96-well plates. HUVECs were serum starved in RPMI1640 medium for 24 h, suspended in RPMI1640 medium preconditioned with pcDNA3.1-RELM- or empty vector-transfected SGC-7901 or MKN-45 cells, added to the matrigel-coated wells at the density of 5 × 104 cells/well, and incubated at 37 °C for 18 h. Tube formation was visualized using a Leitz inverted microscope equipped with a Sony color digital DXC-S500 camera. Anti-angiogenic activity was detected by measuring the length of tube walls formed in the discrete endothelial cells in each well compared with the controls.
Statistical analysis
Unless otherwise stated, all data were shown as mean ± SE. Statistical significance (P < 0.05) was determined by t test or analysis of variance (ANOVA) followed by assessment of differences using SigmaStat 2.03 software (Jandel, Erkrath, Germany).
RESULTS
Transient transfection-mediated over-expression of RELMβ in gastric cancer cells
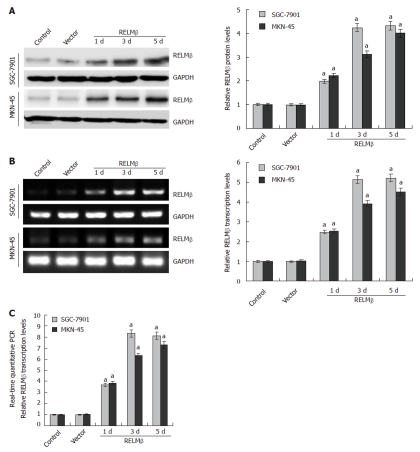
To examine the effects of RELMβ over-expression on human gastric cancer, the RELMβ cDNA was amplified from human colon tissues, subcloned into pcDNA3.1/Zeo(+) and validated by sequencing. Gastric cancer SGC-7901 and MKN-45 cells were transfected with pcDNA3.1-RELM or empty vector (mock). The transfection efficiency was monitored by co-transfection with the enhanced green fluorescent protein (EGFP) reporter vector pEGFP-N1. Seventy-two hours after transfection, EGFP expressed within the cytoplasm of cancer cells, with the transfection efficiency around 60% (data not shown). The protein and mRNA expression of RELMβ was examined by Western blotting, reverse transcription polymerase chain reaction (RT-PCR), and real-time quantitative RT-PCR. As shown in Figure 1A-C, low RELMβ protein and mRNA could be detected in the parental SGC-7901 and MKN-45 cells, and transient transfection of empty vector did not affect the expression levels of RELMβ (P > 0.05). However, RELMβ was significantly increased in the pcDNA3.1-RELMβ-transfected cells (P < 0.01). These results indicated that the eukaryotic vector for RELMβ used in this study was efficient in up-regulating the expression of RELMβ in gastric cancer cells.
Figure 1.
Transient transfection-mediated over-expression of resistin-like molecule β in gastric cancer cells. A: Western blotting indicated that low resistin-like molecule β (RELMβ) protein was detected in the parental SGC-7901 and MKN-45 cells, and transient transfection of the empty vector (mock) did not affect the expression levels of RELMβ. However, transient transfection of pcDNA3.1-RELMβ for 24 h, 72 h and 120 h resulted in increased RELMβ expression; B: 24 h, 72 h and 120 h after transfection, reverse transcription polymerase chain reaction (RT-PCR) indicated the increased RELMβ transcription levels in pcDNA3.1-RELMβ transfected SGC-7901 and MKN-45 cells, but not in mock group; C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 24 h, 72 h and 120 h resulted in upregulation of RELMβ transcription levels in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant increase compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
Over-expression of RELMβ did not affect the in vitro cell proliferation of gastric cancer cells
The effects of RELMβ over-expression on proliferation of SGC-7901 and MKN-45 cells were measured by MTT colorimetric assay. We found that transfection of pcDNA 3.1-RELMβ or empty vector (mock) did not affect the cell proliferation when compared with the parental cells (P > 0.05, Figure 2A). In addition, colony formation and EdU incorporation assays further revealed that over-expression of RELMβ did not influence the proliferation of cultured SGC-7901 and MKN-45 cells (P > 0.05, Figure 2B and C). These results indicated that over-expression of RELMβ did not affect the in vitro proliferation of gastric cancer cells.
Figure 2.
Upregulating resistin-like molecule β expression did not affect the in vitro proliferation of gastric cancer cells. A: In 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay, transfection of pcDNA3.1-resistin-like molecule β (RELMβ) or empty vector (mock) for 24 h, 72 h and 120 h, did not affect the cell proliferation, when compared with the parental SGC-7901 and MKN-45 cells (P > 0.05); B: Colony formation assay indicated that 72 h after transfection, over-expression of RELMβ did not affect the in vitro proliferation of SGC-7901 and MKN-45 cells (P > 0.05); C: 5-ethynyl-20-deoxyuridine incorporation assay revealed that 72 h after transfection, over-expression of RELMβ did not influence the proliferation of cultured SGC-7901 and MKN-45 cells (P > 0.05). Triplicate experiments were performed with essentially identical results.
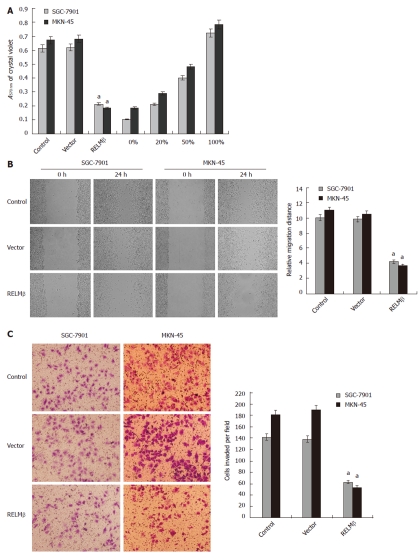
Over-expression of RELMβ attenuated the adhesion, migration and invasion of gastric cancer cells in vitro
Since the adhesion, migration and invasion are three critical steps involved in metastasis, and RELMβ expression in gastric cancer is correlated with tumor infiltration and lymph node metastasis[19], we examined the effects of RELMβ over-expression on these characteristics in cultured gastric cancer cells. In the adhesion assay, SGC-7901 and MKN-45 cells transfected with pcDNA3.1-RELMβ exhibited markedly reduced ability in adhesion to the precoated matrigel, when compared with parental cells (P < 0.01, Figure 3A). However, the cells transfected with empty vector (mock) had similar adhesive abilities as parental cells (Figure 3A). In addition, transfection of pcDNA3.1-RELMβ into SGC-7901 and MKN-45 cells resulted in an impaired migration capacity (P < 0.01), when compared with the parental and mock cells as evidenced by scratch migration assay (Figure 3B). Moreover, over-expression of RELMβ abolished the invasion capabilities of SGC-7901 and MKN-45 cells, when compared with the parental and mock cells as evidenced by transwell analysis (P < 0.01, Figure 3C). These results suggested that over-expression of RELMβ suppressed the adhesion, invasion and metastasis of gastric cancer cells in vitro.
Figure 3.
Over-expression of resistin-like molecule β attenuated the adhesion, migration and invasion of gastric cancer cells in vitro. A: In the adhesion assay, SGC-7901 and MKN-45 cells transfected with pcDNA3.1-resistin-like molecule β (RELMβ) for 72 h exhibited markedly reduced ability in adhesion to the precoated matrigel, when compared with parental cells. However, the cells transfected with empty vector (mock) had a similar adhesive ability as parental cells; B: Scratch migration assay indicated that transfection of pcDNA3.1-RELMβ into SGC-7901 and MKN-45 cells for 72 h resulted in an impaired migration capacity, when compared with the parental cells and mock group; C: Transwell analysis indicated that transfection of pcDNA3.1-RELMβ for 72 h abolished the invasive capabilities of SGC-7901 and MKN-45 cells, when compared with the parental and mock cells. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
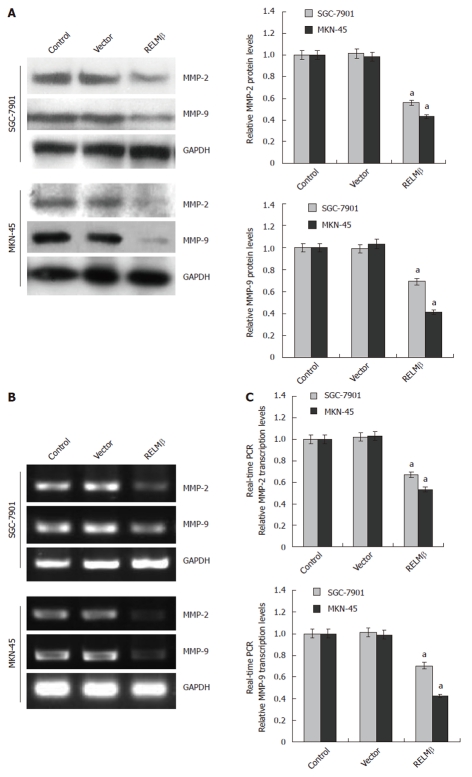
Over-expression of RELMβ decreased the expression of MMP-2 and MMP-9 in gastric cancer cells
To explore the mechanisms underlying RELMβ-mediated suppression on the adhesion, invasion and metastasis of gastric cancer cells, the protein and mRNA expression of MMP-2 and MMP-9 were examined by Western blotting, RT-PCR and real-time quantitative RT-PCR. As shown in Figure 4A-C, the expression of MMP-2 and MMP-9 was significantly decreased in the pcDNA3.1-RELMβ-transfected SGC-7901 and MKN-45 cells (P < 0.01) as compared with the parental cells. However, transient transfection of the empty vector (mock) did not affect the expression level of MMP-2 or MMP-9. These results indicated that over-expression of RELMβ attenuated the expression of MMP-2 and MMP-9 in gastric cancer cells.
Figure 4.
Over-expression of resistin-like molecule β decreased the expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in gastric cancer cells. A: Western blotting indicated that 72 h after transfection, over-expression of resistin-like molecule β (RELMβ) abolished the expression of matrix metalloproteinase (MMP)-2 and MMP-9 in SGC-7901 and MKN-45 cells. However, transfection of empty vector (mock) did not influence their expression; B: Reverse transcription polymerase chain reaction (RT-PCR) indicated the decreased MMP-2 and MMP-9 transcription levels in SGC-7901 and MKN-45 cells transfected with pcDNA3.1-RELMβ for 72 h, but not in mock group; C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 72 h resulted in decreased transcription levels of MMP-2 and MMP-9 in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
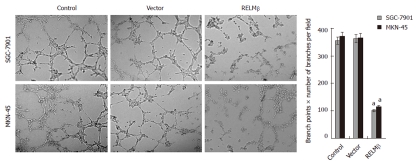
Over-expression of RELMβ inhibited the in vitro angiogenesis of gastric cancer cells
We further investigated the effects of RELMβ over-expression on the in vitro angiogenic capabilities of SGC-7901 and MKN-45 cells. As shown in Figure 5, extensive tube formation of endothelial cells was observed in parental and mock cells. However, when the endothelial cells were treated with the medium preconditioned with pcDNA3.1-RELMβ-transfected SGC-7901 or MKN-45 cells, the tube formation was significantly suppressed (P < 0.01, Figure 5). These results indicated that over-expression of RELMβ remarkably decreased the angiogenesis of gastric cancer cells in vitro.
Figure 5.
Over-expression of resistin-like molecule β inhibited the in vitro angiogenic capabilities of gastric cancer cells. Extensive tube formation of endothelial cells was observed in parental and empty vector (mock) groups. However, when the endothelial cells were treated by the medium preconditioned with pcDNA3.1-resistin-like molecule β (RELMβ)-transfected SGC-7901 or MKN-45 cells, the tube formation was suppressed. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
RELMβ attenuated the expression of VEGF, but not Ets1 in gastric cancer cells
Since Ets1 is one of the most important transcription fa-ctors to promote tumor angio-genesis[22], and based on the evidence that resistin, a member of the RELM family, influences the VEGF expression in cancer cells[23], we hypothesized that RELMβ might affect its expression in gastric cancer cells. The expression levels of Ets1 and VEGF were examined by Western blotting, RT-PCR and real-time quantitative RT-PCR. As shown in Figure 6A-C, the protein and mRNA levels of Ets1 and VEGF could be detected in the parental SGC-7901 and MKN-45 cells, and transient transfection of the empty vector (mock) did not affect their expression levels. However, VEGF, but not Ets1, was significantly decreased in the pcDNA3.1-RELMβ-transfected cells (P < 0.01). These results indicated that over-expression of RELMβ attenuated the expression of VEGF in gastric cancer cells.
Figure 6.
Over-expression of resistin-like molecule β decreased the expression of vascular endothelial growth factor, but not v-ets erythroblastosis virus E26 oncogene homolog 1, in gastric cancer cells. A: Western blotting indicated that 72 h after transfection, over-expression of resistin-like molecule β (RELMβ) abolished the expression of vascular endothelial growth factor (VEGF), but not v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1), in SGC-7901 and MKN-45 cells. Moreover, transfection of empty vector (mock) did not influence the expression of VEGF and Ets1; B: Reverse transcription polymerase chain reaction (RT-PCR) indicated the decreased VEGF transcription levels in SGC-7901 and MKN-45 cells transfected with pcDNA3.1-RELMβ for 72 h, but not in mock group. Moreover, the Est1 transcription levels were not influenced by transfection of pcDNA3.1-RELMβ or empty vector (mock); C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 72 h resulted in decreased transcription levels of VEGF, but not of Ets1, in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant decrease from parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Resistin-like molecules/found in inflammatory zone (RELM/FIZZ) gene family consists of four members, including resistin, RELMα, RELMβ and RELMγ, which exhibit unique distribution patterns in mammalian species[11]. Resistin, a small and cysteine-rich protein hormone secreted from adipose tissue, is named for its ability to induce insulin resistance[24]. RELMα is expressed in several tissues including white adipose tissue and lung, and participates in the regulation of inflammatory process[25,26]. RELMβ is highly conserved in all examined mammalian species, and its expression is tightly restricted to intestinal goblet cells, from where it is secreted apically into the intestinal lumen as a homodimer[11]. RELMγ is expressed in mouse spleen, bone marrow and intestine, and may play a role in promyelocytic differentiation[27,28]. Currently, although most studies have focused on the roles of RELMβ in intestinal defense against parasitic nematode infection and colonic inflammation[29], the functions of RELMβ remain to be further elucidated. Interestingly, recent evidences reveal the close relationship between resistin and prostate cancer[30], gastric cancer[31], colorectal cancer[32,33], breast cancer[34,35], and endometrial cancer[36]. It has been indicated that resistin induces cell proliferation of prostate cancer through phosphatidyl-inositol 3-kinase (PI-3K)/Akt signaling pathways[37]. In addition, transfection of RELMγ into promyelocytic HL60 cells resulted in increased proliferation rate and an altered response to retinoic acid-induced granulocytic differentiation[27]. Thus, these findings indicate the potential role of RELM/FIZZ gene family in the progression of cancer.
Our previous studies have revealed that RELMβ is virtually absent in normal gastric mucosa, whereas gastric cancer exhibits aberrant RELMβ expression[19]. Patients showing positive RELMβ expression have a significantly longer overall survival than those with negative expression, indicating the prognostic value of RELM-β in predicting the outcomes of gastric cancer[19]. Current literatures show conflicting results regarding the role of RELMβ in cell proliferation[38,39]. McVay et al[38] reported that RELMβ did not alter colonic epithelial proliferation or barrier function in the dextran sodium sulfate-induced model of murine colonic injury. In cultured lung adenocarcinoma A549 cells, transfection of a RELMβ encoding expression vector resulted in increased proliferation via the PI-3K pathway[39]. In this study, we found low expression levels of RELMβ in the poorly or moderately differentiated gastric cancer cell lines SGC-7901 and MKN-45. Unexpectedly, over-expression of RELMβ did not affect the proliferation of SGC-7901 and MKN-45 cells as evidenced by MTT colorimetry, colony formation and EdU incorporation assays. We believe that the effects of RELMβ on cell proliferation varied among different cancer types. In our previous studies, we have observed the correlation between the intensity of RELMβ and metastatic index heparanase, one of the key enzymes involved in the invasion and metastasis of gastric cancer[19]. We found that in primary gastric cancer tissues, lower RELMβ intensity was correlated with higher heparanase expression[19]. In this study, we chose the RELMβ lowly-expressed gastric cancer cell lines as models, and demonstrated that over-expression of RELMβ resulted in attenuated adhesion, migration and invasion, three important steps for cancer metastasis.
MMPs are a family of enzymes that proteolytically degrade various components of the extracellular matrix (ECM), and are closely correlated with tumor invasive and metastatic potentials[40]. MMP-2 and MMP-9 participate in the degradation of basement membrane and the remodeling of ECM[41], and appear to promote tumor initiation, invasion, and metastasis[42]. Tumor cells can synthesize and secret large amounts of MMP-2 and MMP-9 in a paracrine and/or autocrine manner to stimulate angiogenesis[41]. Previous studies show that high levels of MMP-2 and/or MMP-9 have a significant correlation with the invasion and metastasis of gastric cancer[43,44], and are associated with poor prognosis[44]. In this study, we found that over-expression of RELMβ inhibited the expression of MMP-2 and MMP-9 in gastric cancer cells, which at least in part, contributed to the RELM-mediated suppression of migration and invasion of cancer cells.
Angiogenesis, the process of new capillary formation from pre-existing vessels to provide oxygen and nutrients to tumor, plays an essential role in invasion and metastasis of malignancies[45]. Previous studies indicate that resistin increases in vitro angiogenesis in human coronary artery endothelial cells and umbilical vein endothelial cells[46]. As a mouse homolog of RELMβ, hypoxia-induced mitogenic factor (HIMF) is found to promote angiogenesis and participate in pulmonary vascular remodeling and fibrotic lung disease[47-49]. RELMβ is expressed in the lung tissue of patients with sclerodermaassociated pulmonary hypertension[16], and recombinant RELMβ induces the proliferation and activation of extracellular signal regulated kinase 1/2 (ERK1/2) in primary cultured human pulmonary endothelial and smooth muscle cells[16]. However, the influence of RELMβ on the angiogenic capabilities of cancer cells still remains exclusive.
In the current study, we demonstrated the anti-angiogenic properties of RELMβ in gastric cancer cells. It has been established that VEGF is secreted by most tumor cells, and plays a determinant role in regulating tumor angiogenesis through inducing cell proliferation, differentiation, and migration of vascular endothelial cells[50]. VEGF induces the formation of new vessels by targeting VEGF receptor 2 signaling pathway, and benefits primary tumor growth and metastasis[51]. Thus, targeting constitutive VEGF and/or its receptors has been an attractive approach for cancer therapy. Our results further showed that over-expression of RELMβ inhibited the expression of VEGF in gastric cancer cells. Based on our recent evidence that recombinant RELMβ protein possesses anti-angiogenic effects via decreasing the proliferation, migration, and tube formation of human umbilical vein endothelial HUVEC cells (data not shown), we believe that RELMβ is of potential values as a novel therapeutic target for human gastric cancer.
The mechanisms underlying RELMβ expression in gastric cancer still remains exclusive. Previous evidence indicates that a region between -418 and -588 in the human RELMβ promoter contains two potential caudal type homeobox (CDX) binding sites[52]. Moreover, CDX-2, but not CDX-1, binds to the human RELMβ promoter and thereby transactivates RELMβ expression in a goblet cell-specific fashion[52]. However, our preliminary findings indicate that CDX-2 does not transactivate the RELMβ expression in cultured gastric cancer cells (data not shown). The constitutive expression of RELMβ in gastric cancer tissues with or without intestinal metaplasia[19] suggests that other transcription factors are involved in the regulation of RELMβ expression in gastric cancer, which warrants further investigations.
In summary, for the first time, we have demonstrated that over-expression of RELMβ can efficiently inhibit the invasion, metastasis and angiogenesis of gastric cancer cells. It is likely that the RELMβ over-expression depresses the expression of MMP-2 and MMP-9, thus inhibiting the invasion and metastasis of gastric cancer. In addition, transfection of RELMβ suppresses the VEGF expression, which may result in decreased angiogenesis of gastric cancer cells. These results suggest a potential strategy for gastric cancer therapy via modulating or regulating the RELMβ expression. Further knocking down the RELMβ expression in RELMβ highly-expressed cell lines and in vivo studies are warranted to investigate the role of RELMβ in the development and progression of gastric cancer.
COMMENTS
Background
According to the previous studies of the authors, the aberrant expression of resistin-like molecule β (RELMβ), an intestinal goblet cell-specific protein, in gastric cancer tissues, is positively correlated with tumor differentiation and longer overall survival, and inversely correlated with tumor infiltration and lymph node metastasis. However, the exact roles and underlying mechanisms of RELMβ in the progression of gastric cancer still remain unknown.
Research frontiers
Although most studies of RELMβ have focused on its function in intestinal defense against parasitic nematode infection of the intestine and colonic inflammation, increasing attention has been paid to the role of RELMβ in tumor biology. The authors in their previous studies have demonstrated that RELMβ is a biomarker of intestinal metaplasia in Barrett’s esophagus, and over-expressed in gastric cancer and colon cancer. However, no study has yet investigated the exact role of RELMβ in invasion, metastasis and angiogenesis of gastric cancer.
Innovations and breakthroughs
In this study, the authors demonstrate, for the first time, that over-expression of RELMβ can efficiently inhibit the invasion, metastasis and angiogenesis of gastric cancer cells. It is likely that the RELMβ over-expression depresses the expression of matrix metalloproteinase (MMP)-2 and MMP-9, thus inhibiting the invasion and metastasis of gastric cancer. In addition, transfection of RELMβ suppresses the vascular endothelial growth factor expression, which may result in decreased angiogenesis of gastric cancer cells.
Applications
RELMβ expression is a useful prognostic factor for predicting the outcomes of gastric cancer patients. The effects of RELMβ over-expression on the invasion, metastasis and angiogenesis of gastric cancer cells suggest a potential strategy for gastric cancer treatment via modulating or regulating the RELMβ expression. Further knocking down the RELMβ expression in RELMβ highly-expressed cell lines and in vivo studies are warranted to investigate the role of RELMβ in the development and progression of gastric cancer.
Terminology
RELMβ is a recently described goblet cell-specific protein that belongs to the resistin-like molecules, or found in inflammatory zone (RELM/FIZZ) gene family and functions as a critical immune-effector molecule in the expulsion of gastrointestinal tract nematodes.
Peer review
The authors reported a study elucidating the effects of RELMβ over-expression on the invasion, metastasis and angiogenesis of gastric cancer cell lines. It revealed that transient transfection of RELMβ into low-expressing gastric cancer cell lines resulted in attenuated adhesion, migration and invasion, three important steps for cancer metastasis. In addition, over-expression of RELMβ suppressed the angiogenic capabilities of cancer cells. The results are original and may represent a novel strategy for the treatment of gastric cancer.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30200284, No. 30600278, No. 30772359, No. 81071997 and No. 81072073; Program for New Century Excellent Talents from Universities, No. NCET-06-0641; Scientific Research Fund for the Returned Overseas Chinese Scholars, No. 2008-889; Fundamental Research Funds for the Central Universities, No. 2010JC025
Peer reviewers: Henning Schulze-Bergkamen, National Centre for Tumor Diseases, University of Heidelberg, Im Neuenheimer Feld 350, Heidelberg 69120, Germany; Ki Baik Hahm, Professor, Gachon Graduate School of Medicine, Lee Gil Ya Cancer and Diabetes Institute, Incheon 406-840, South Korea
S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
References
- 1.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 2.Yamachika T, Werther JL, Bodian C, Babyatsky M, Tatematsu M, Yamamura Y, Chen A, Itzkowitz S. Intestinal trefoil factor: a marker of poor prognosis in gastric carcinoma. Clin Cancer Res. 2002;8:1092–1099. [PubMed] [Google Scholar]
- 3.Chan MW, Chan VY, Leung WK, Chan KK, To KF, Sung JJ, Chan FK. Anti-sense trefoil factor family-3 (intestinal trefoil factor) inhibits cell growth and induces chemosensitivity to adriamycin in human gastric cancer cells. Life Sci. 2005;76:2581–2592. doi: 10.1016/j.lfs.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Lee HW, Yang DH, Kim HK, Lee BH, Choi KC, Choi YH, Park YE. Expression of MUC2 in gastric carcinomas and background mucosae. J Gastroenterol Hepatol. 2007;22:1336–1343. doi: 10.1111/j.1440-1746.2007.04939.x. [DOI] [PubMed] [Google Scholar]
- 5.Leteurtre E, Zerimech F, Piessen G, Wacrenier A, Leroy X, Copin MC, Mariette C, Aubert JP, Porchet N, Buisine MP. Relationships between mucinous gastric carcinoma, MUC2 expression and survival. World J Gastroenterol. 2006;12:3324–3331. doi: 10.3748/wjg.v12.i21.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoda H, Yamamoto K, Kushima R, Okabe H, Tani T. Detection of lymph node micrometastasis in gastric cancer by MUC2 RT-PCR: usefulness in pT1 cases. J Surg Oncol. 2004;88:63–70. doi: 10.1002/jso.20143. [DOI] [PubMed] [Google Scholar]
- 7.Emami S, Rodrigues S, Rodrigue CM, Le Floch N, Rivat C, Attoub S, Bruyneel E, Gespach C. Trefoil factor family (TFF) peptides and cancer progression. Peptides. 2004;25:885–898. doi: 10.1016/j.peptides.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Rivat C, Rodrigues S, Bruyneel E, Piétu G, Robert A, Redeuilh G, Bracke M, Gespach C, Attoub S. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) -- and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res. 2005;65:195–202. [PubMed] [Google Scholar]
- 9.Emami S, Le Floch N, Bruyneel E, Thim L, May F, Westley B, Rio M, Mareel M, Gespach C. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J. 2001;15:351–361. doi: 10.1096/fj.00-0355com. [DOI] [PubMed] [Google Scholar]
- 10.Allen A, Hutton DA, Pearson JP. The MUC2 gene product: a human intestinal mucin. Int J Biochem Cell Biol. 1998;30:797–801. doi: 10.1016/s1357-2725(98)00028-4. [DOI] [PubMed] [Google Scholar]
- 11.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang C, Meng Q, Wu H, Eid G, Zhang G, Zhang X, Yang S, Huang K, Lee TH, Corrigan CJ, et al. Resistin-like molecule-β (RELM-β) is a human airways remodelling mediator. Eur Respir J. 2012;39:458–466. doi: 10.1183/09031936.00107811. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, et al. FIZZ2/RELM-β induction and role in pulmonary fibrosis. J Immunol. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009;41:553–561. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng LD, Tong QS, Weng MX, He J, Lv Q, Pu JR, Jiang GS, Cai JB, Liu Y, Hou XH. Enhanced expression of resistin-like molecule beta in human colon cancer and its clinical significance. Dig Dis Sci. 2009;54:274–281. doi: 10.1007/s10620-008-0355-2. [DOI] [PubMed] [Google Scholar]
- 18.Zheng L, Tong Q, Weng M, He J, Lv Q, Wu Z, Du Z, Mei H, Hou X. Expression of resistin-like molecule beta in Barrett’s esophagus: a novel biomarker for metaplastic epithelium. Dig Dis Sci. 2010;55:32–39. doi: 10.1007/s10620-009-0719-2. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, Weng M, He J, Yang X, Jiang G, Tong Q. Expression of resistin-like molecule beta in gastric cancer: its relationship with clinicopathological parameters and prognosis. Virchows Arch. 2010;456:53–63. doi: 10.1007/s00428-009-0861-4. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Jiang G, Mei H, Pu J, Dong J, Hou X, Tong Q. Small RNA interference-mediated gene silencing of heparanase abolishes the invasion, metastasis and angiogenesis of gastric cancer cells. BMC Cancer. 2010;10:33. doi: 10.1186/1471-2407-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 22.Sato Y, Teruyama K, Nakano T, Oda N, Abe M, Tanaka K, Iwasaka-Yagi C. Role of transcription factors in angiogenesis: Ets-1 promotes angiogenesis as well as endothelial apoptosis. Ann N Y Acad Sci. 2001;947:117–123. [PubMed] [Google Scholar]
- 23.Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D’Asta M, Caforio L, Caruso A. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006;189:691–699. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- 24.Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 25.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stütz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschläger M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 27.Schinke T, Haberland M, Jamshidi A, Nollau P, Rueger JM, Amling M. Cloning and functional characterization of resistin-like molecule gamma. Biochem Biophys Res Commun. 2004;314:356–362. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- 28.Gerstmayer B, Küsters D, Gebel S, Müller T, Van Miert E, Hofmann K, Bosio A. Identification of RELMgamma, a novel resistin-like molecule with a distinct expression pattern. Genomics. 2003;81:588–595. doi: 10.1016/s0888-7543(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 29.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Housa D, Vernerova Z, Heracek J, Cechak P, Rosova B, Kuncova J, Haluzik M. Serum resistin levels in benign prostate hyperplasia and non-metastatic prostate cancer: possible role in cancer progression. Neoplasma. 2008;55:442–446. [PubMed] [Google Scholar]
- 31.Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, Kato K, Hamaguchi T, Shimada Y. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44:685–690. doi: 10.1007/s00535-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S, Kato K, Hamaguchi T, Shimada Y. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101:1286–1291. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sălăgeanu A, Tucureanu C, Lerescu L, Caraş I, Pitica R, Gangurà G, Costea R, Neagu S. Serum levels of adipokines resistin and leptin in patients with colon cancer. J Med Life. 2010;3:416–420. [PMC free article] [PubMed] [Google Scholar]
- 34.Sun CA, Wu MH, Chu CH, Chou YC, Hsu GC, Yang T, Chou WY, Yu CP, Yu JC. Adipocytokine resistin and breast cancer risk. Breast Cancer Res Treat. 2010;123:869–876. doi: 10.1007/s10549-010-0792-4. [DOI] [PubMed] [Google Scholar]
- 35.Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121. doi: 10.3346/jkms.2007.22.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hlavna M, Kohut L, Lipkova J, Bienertova-Vasku J, Dostalova Z, Chovanec J, Vasku A. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58:124–128. doi: 10.4149/neo_2011_02_124. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, Kim MK, Kim W, Myung SC. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108:E77–E83. doi: 10.1111/j.1464-410X.2010.09813.x. [DOI] [PubMed] [Google Scholar]
- 38.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renigunta A, Hild C, Rose F, Klepetko W, Grimminger F, Seeger W, Hänze J. Human RELMbeta is a mitogenic factor in lung cells and induced in hypoxia. FEBS Lett. 2006;580:900–903. doi: 10.1016/j.febslet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 41.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubben FJ, Sier CF, van Duijn W, Griffioen G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB, Verspaget HW. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br J Cancer. 2006;94:1035–1040. doi: 10.1038/sj.bjc.6603041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torii A, Kodera Y, Uesaka K, Hirai T, Yasui K, Morimoto T, Yamamura Y, Kato T, Hayakawa T, Fujimoto N, et al. Plasma concentration of matrix metalloproteinase 9 in gastric cancer. Br J Surg. 1997;84:133–136. [PubMed] [Google Scholar]
- 45.Kakeji Y, Maehara Y, Sumiyoshi Y, Oda S, Emi Y. Angiogenesis as a target for gastric cancer. Surgery. 2002;131:S48–S54. doi: 10.1067/msy.2002.119304. [DOI] [PubMed] [Google Scholar]
- 46.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Tong Q, Zheng L, Li B, Wang D, Huang C, Matuschak GM, Li D. Hypoxia-induced mitogenic factor enhances angiogenesis by promoting proliferation and migration of endothelial cells. Exp Cell Res. 2006;312:3559–3569. doi: 10.1016/j.yexcr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res. 2006;7:37. doi: 10.1186/1465-9921-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Matuschak GM, Li D. Participation of the PI-3K/Akt-NF-kappa B signaling pathways in hypoxia-induced mitogenic factor-stimulated Flk-1 expression in endothelial cells. Respir Res. 2006;7:101. doi: 10.1186/1465-9921-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 51.Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97:978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang ML, Shin ME, Knight PA, Artis D, Silberg DG, Suh E, Wu GD. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1074–G1083. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]